Preparation of Polyvinyl Alcohol–Chitosan Nanocellulose–Biochar Nanosilver Composite Hydrogel and Its Antibacterial Property and Dye Removal Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fish Scale Carbon Composite Material (C-Ag) Preparation
2.3. Nanocellulose Extraction
2.4. Preparation of C/CTS/PVA/CNF Hydrogel
2.5. C/CTS/PVA/CNF Hydrogel Characteristics
2.6. Antibacterial Testing
2.7. Optimization C/CTS/PVA/CNF Hydrogel Composite and Its Antibacterial Ability
2.8. Swelling Ratio, Moisture Content, Moisture Adsorption, and Water Loss of C/CTS/PVA/CNF Hydrogel
2.9. Dye Removal by C/CTS/PVA/CNF Hydrogel
3. Results and Discussion
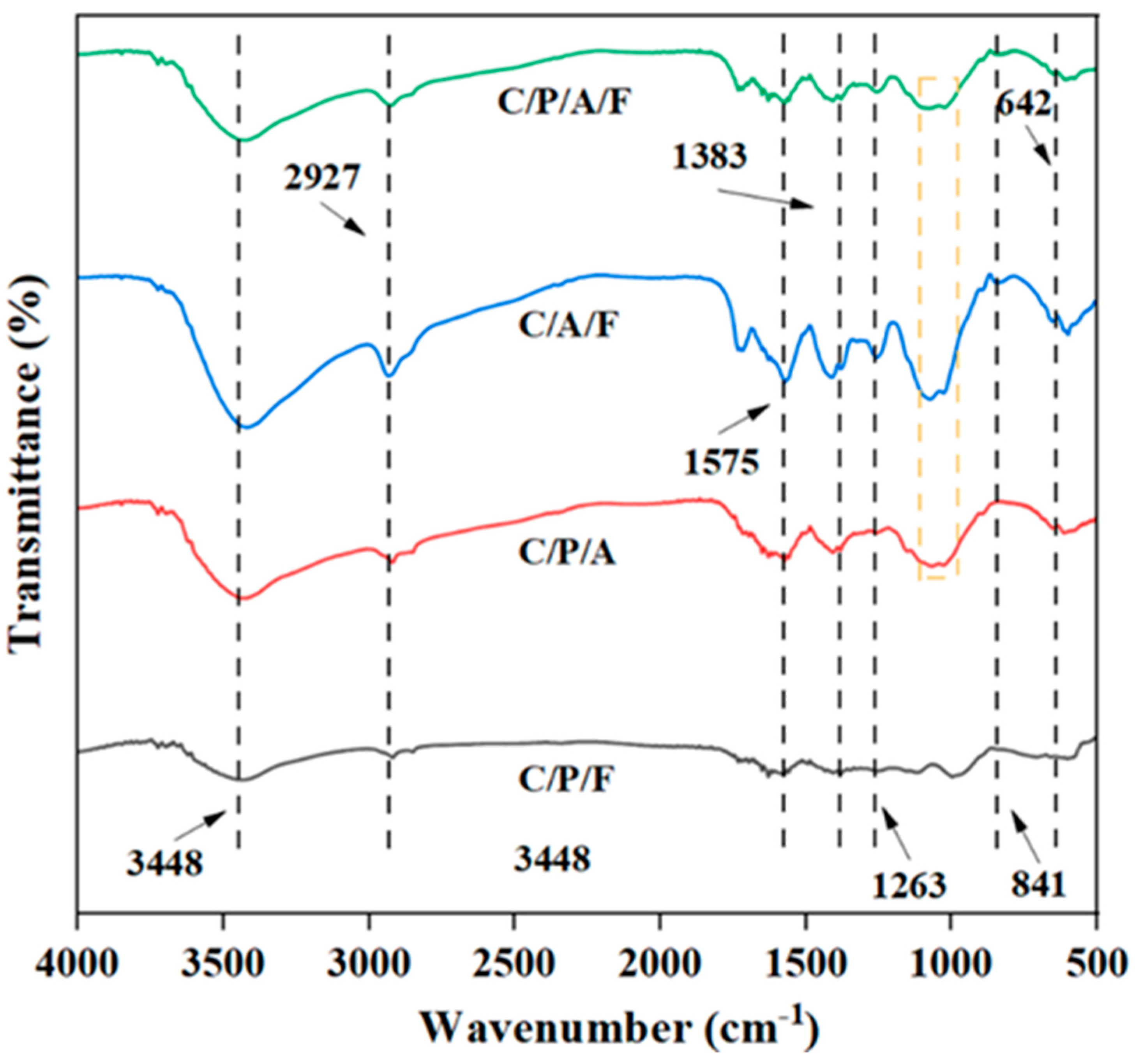
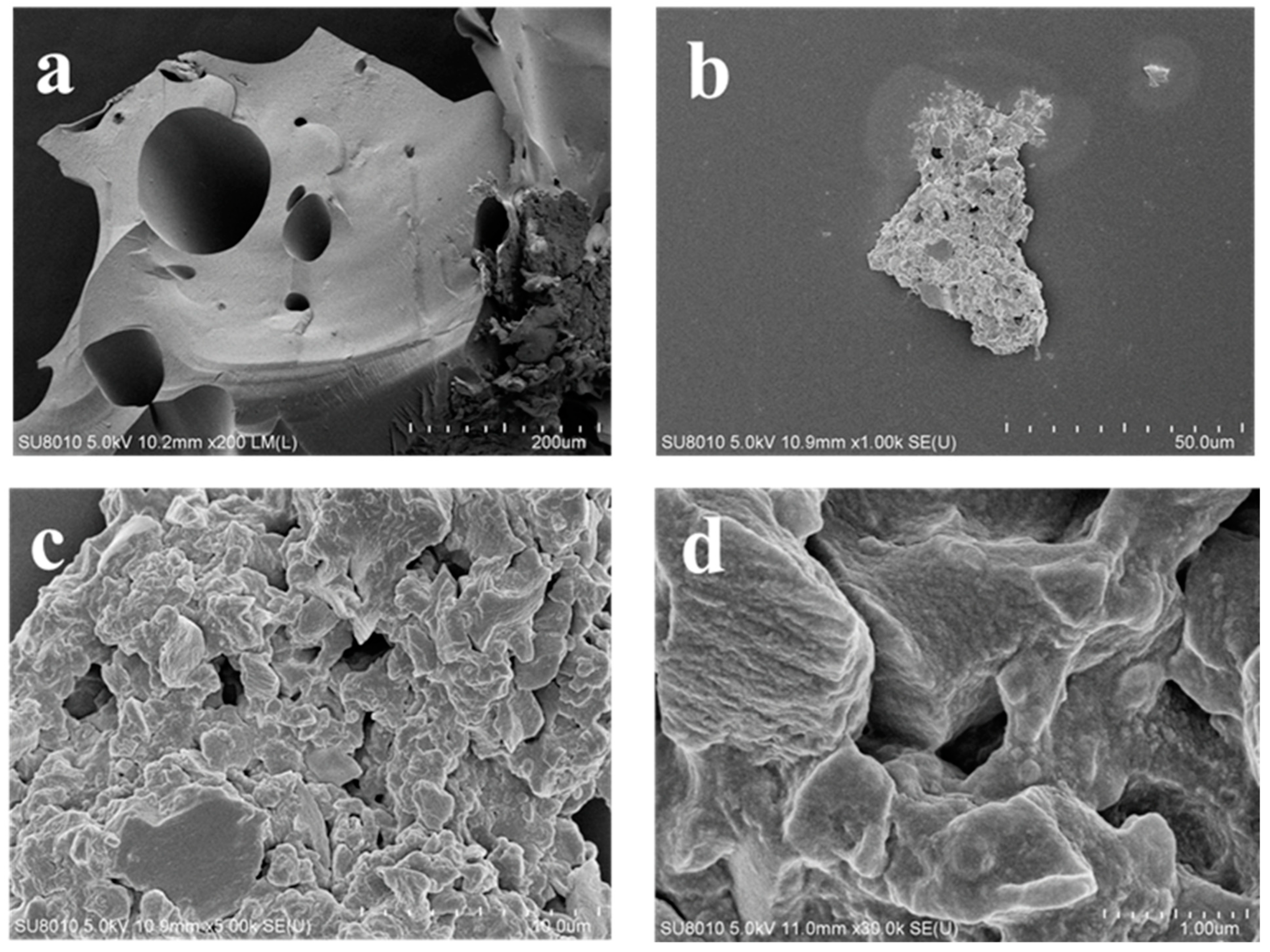
3.1. Characterization of C/CTS/PVA/CNF Hydrogel Composite
3.2. Antimicrobial Property
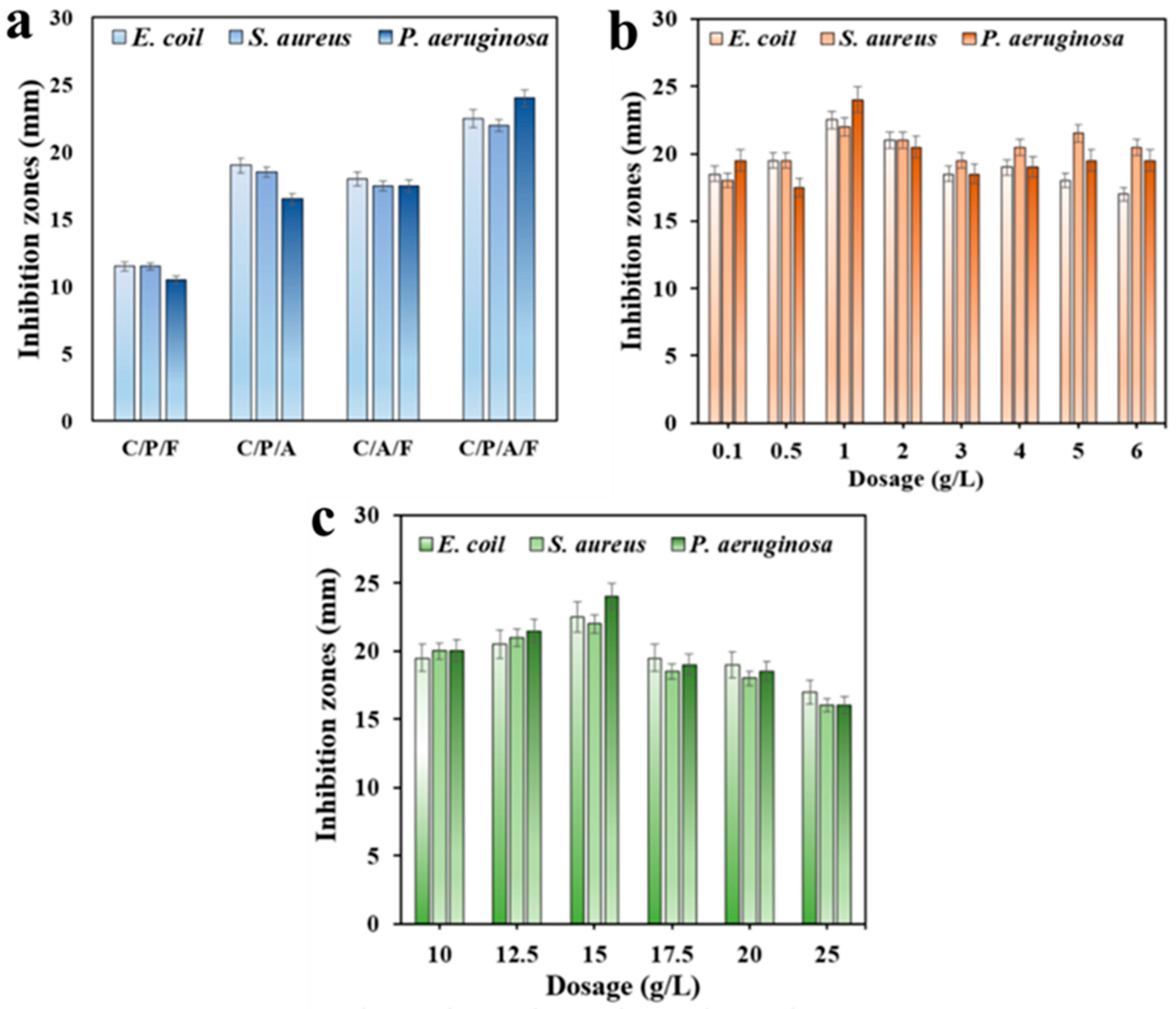
3.2.1. Effect of CST and C-Ag
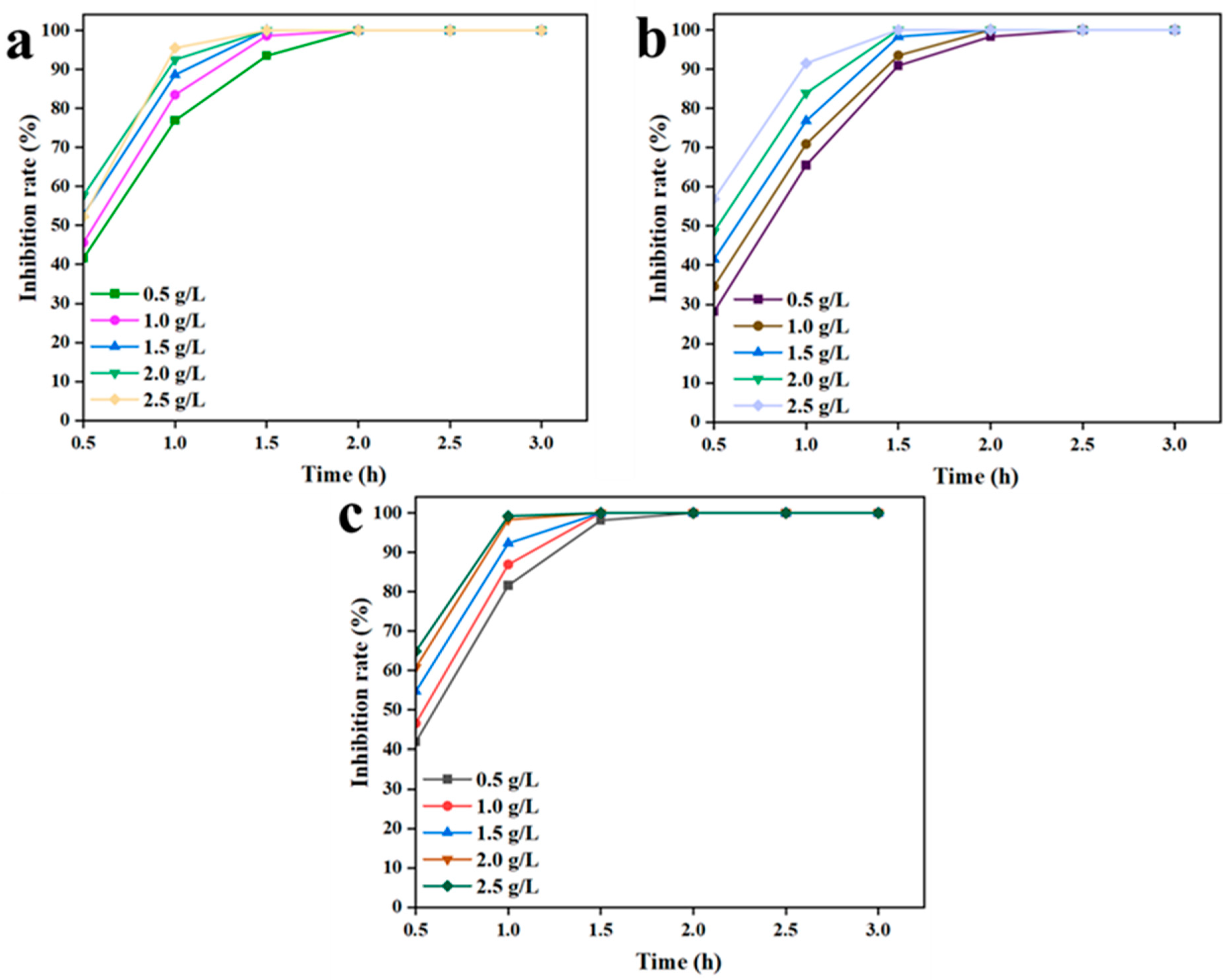
3.2.2. Effect of C/CTS/PVA/CNF Hydrogel Dosage and Incubation Time
3.3. Stability and Reusability of C/CTS/PVA/CNF Hydrogels
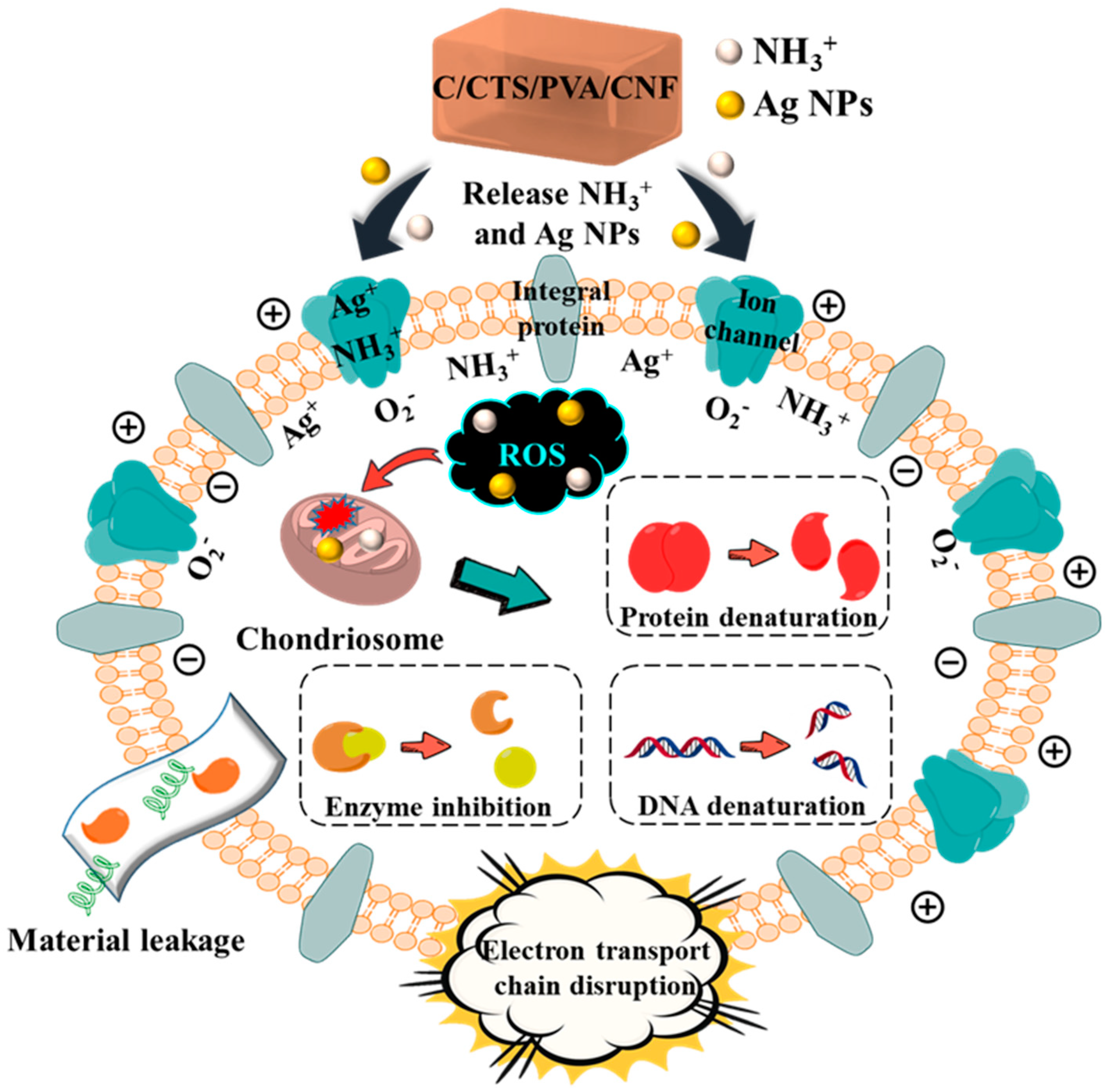
3.4. Synergistic Antibacterial Mechanism of C/CTS/PVA/CNF Hydrogels
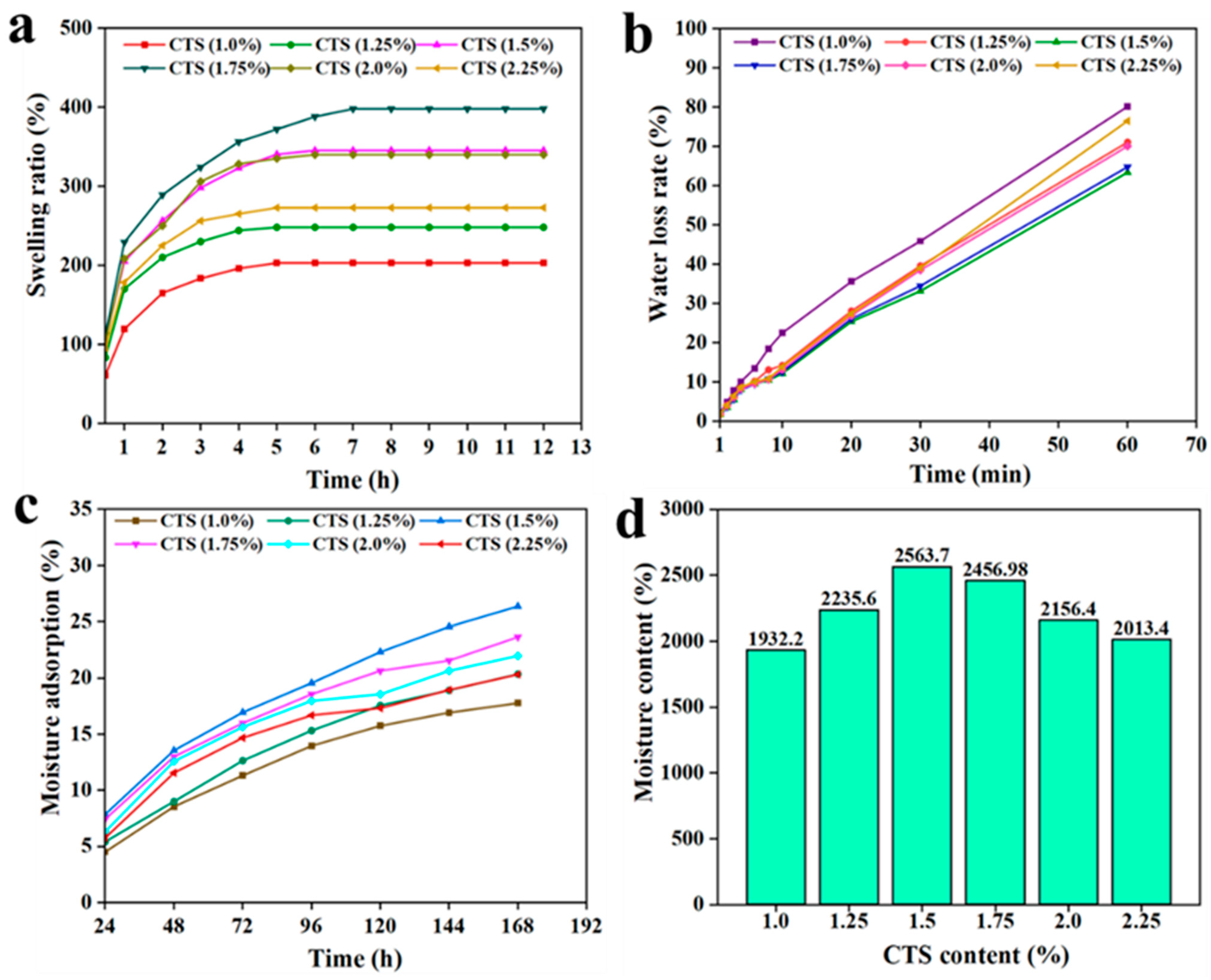
3.5. Swelling Ratio, Moisture Adsorption, Water Loss, and Moisture Content of C/CTS/PVA/CNF Hydrogels
3.6. C/CTS/PVA/CNF Hydrogel Dye-Removal Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Q.; Zhang, H.; Ma, M.; He, Y.; Jia, J.; Hu, Q.; Gong, Y. Critical assessment of protozoa contamination and control measures in mass culture of the diatom Phaeodactylum tricornutum. Bioresour. Technol. 2022, 359, 127460. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Meng, L.; Lou, Y.; Yan, Z.; Xi, J.; Bian, H.; Wu, W.; Xiao, H. Fabrication of multifunctional air filter paper with flame retardant, antibacterial and hydrophobic properties. J. Environ. Chem. Eng. 2023, 11, 111540. [Google Scholar] [CrossRef]
- Hezma, A.M.; Shaltout, W.A.; Kabary, H.A.; El-Bahy, G.S.; Abdelrazzak, A.B. Fabrication, characterization and adsorption investigation of nano zinc oxide–sodium alginate beads for effective removal of chromium (VI) from aqueous solution. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1400–1408. [Google Scholar] [CrossRef]
- Kazeminava, F.; Arsalani, N.; Ahmadi, R.; Kafil, H.S.; Geckeler, K.E. A facile approach to incorporate silver nanoparticles into solvent-free synthesized PEG-based hydrogels for antibacterial and catalytical applications. Polym. Test. 2021, 101, 106906. [Google Scholar] [CrossRef]
- Phatai, P.; Prachumrak, N.; Kamonwannasit, S.; Kamcharoen, A.; Roschat, W.; Phewphong, S.; Futalan, C.M.; Khemthong, P.; Butburee, T.; Youngjan, S.; et al. Zinc-Silver doped mesoporous hydroxyapatite synthesized via ultrasonic in combination with sol-gel method for increased antibacterial activity. Sustainability 2022, 14, 11756. [Google Scholar] [CrossRef]
- Zhang, Z.; He, Y. Synthesis and characteristics of a fish scale-based biochar–nanosilver antibacterial material. Processes 2023, 11, 1992. [Google Scholar] [CrossRef]
- Jiang, W.; Cai, Y.; Liu, D.; Yu, X.; Wang, Q. Enhanced adsorption performance of oxytetracycline in aqueous solutions by Mg-Fe modified suaeda-based magnetic biochar. Environ. Res. 2024, 241, 117662. [Google Scholar] [CrossRef]
- Cai, Q.; Bai, X.; Pu, J. Author Correction: Adaptive VAlCN-Ag composite and VAlCN/VN-Ag multilayer coatings intended for applications at elevated temperature. J. Mater. Sci. 2022, 57, 15674–15675. [Google Scholar] [CrossRef]
- Oliveira, Í.M.; de Jesus, R.A.; Nascimento, V.R.S.; Bilal, M.; Iqbal, H.M.N.; Ferreira, L.F.R.; Cestari, A.R. Bioremediation potential of Dicentrarchus labrax fish scales for dye-based emerging contaminants by ANN–GA hybrid modeling. Bioprocess Biosyst. Eng. 2022, 45, 1189–1200. [Google Scholar] [CrossRef]
- Xia, D.; Liu, Y.; Cheng, X.; Gu, P.; Chen, Q.; Zhang, Z. Temperature-tuned fish-scale biochar with two-dimensional homogeneous porous structure: A promising uranium extractant. Appl. Surf. Sci. 2022, 591, 153136. [Google Scholar] [CrossRef]
- Kesarwani, V.; Rai, V.K. Impact of AgNPs on the optical thermometry and stability of bismuth modified tellurium-tungstate upconverting glass. J. Non-Cryst. Solids 2023, 603, 122129. [Google Scholar] [CrossRef]
- Yang, D.; Fan, B.; Sun, G.; He, Y.-C.; Ma, C. Ultraviolet blocking ability, antioxidant and antibacterial properties of newly prepared polyvinyl alcohol-nanocellulose-silver nanoparticles-ChunJian peel extract composite film. Int. J. Biol. Macromol. 2023, 252, 126427. [Google Scholar] [CrossRef] [PubMed]
- Hariram, M.; Vivekanandhan, S.; Ganesan, V.; Muthuramkumar, S.; Rodriguez-uribe, A.; Mohanty, A.K.; Misra, M. Tecoma stans flower extract assisted biogenic synthesis of functional Ag-Talc nanostructures for antimicrobial applications. Bioresour. Technol. Rep. 2019, 7, 100298. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, L.; Zhong, L.; Zhou, Y.; Xue, J.; Li, Y. Preparation of an antibacterial chitosan-coated biochar-nanosilver composite for drinking water purification. Carbohydr. Polym. 2019, 219, 290–297. [Google Scholar] [CrossRef]
- Cui, J.; Liang, Y.; Yang, D.; Liu, Y. Facile fabrication of rice husk based silicon dioxide nanospheres loaded with silver nanoparticles as a rice antibacterial agent. Sci. Rep. 2016, 6, 21423. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, Y.; Chen, L.; Wang, R.; Wang, X.; Miao, Y.; Ji, X.; Bian, H.; Dai, H. Diisocyanate modifiable commercial filter paper with tunable hydrophobicity, enhanced wet tensile strength and antibacterial activity. Carbohydr. Polym. 2020, 248, 116791. [Google Scholar] [CrossRef]
- Potaś, J.; Szymańska, E.; Winnicka, K. Challenges in developing of chitosan—Based polyelectrolyte complexes as a platform for mucosal and skin drug delivery. Eur. Polym. J. 2020, 140, 110020. [Google Scholar] [CrossRef]
- Singh, S.; Arputharaj, E.; Dahms, H.U.; Patel, A.K.; Huang, Y.L. Chitosan-based nanocomposites for removal of Cr(VI) and synthetic food colorants from wastewater. Bioresour. Technol. 2022, 351, 127018. [Google Scholar] [CrossRef]
- Sekhavat Pour, Z.; Ghaemy, M. Removal of dyes and heavy metal ions from water by magnetic hydrogel beads based on poly(vinyl alcohol)/carboxymethyl starch-g-poly(vinyl imidazole). RSC Adv. 2015, 5, 64106–64118. [Google Scholar] [CrossRef]
- Su, J.F.; Li, G.Q.; Wen, Q.; Xue, L.; Chen, C.L.; Huang, T.L. Highly efficient nitrate and phosphorus removal and adsorption of tetracycline by precipitation in a chitosan/polyvinyl alcohol immobilized bioreactor. Bioprocess Biosyst. Eng. 2020, 43, 1761–1771. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Saha, N.; Brodnjak, U.V.; Sáha, P. Bacterial cellulose and guar gum based modified PVP-CMC hydrogel films: Characterized for packaging fresh berries. Food Packag. Shelf Life 2019, 22, 100402. [Google Scholar] [CrossRef]
- Ma, S.; Shi, W.; Li, H.; Zhang, Y. Biomimetic mineralization of nacre-inspired multiple crosslinked PVA/CaAlg/SiO2 membrane with simultaneously enhanced mechanical and separation properties. Int. J. Biol. Macromol. 2023, 234, 123650. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Froese, A.G.; Sparling, R. Cross-feeding and wheat straw extractives enhance growth of Clostridium thermocellum-containing co-cultures for consolidated bioprocessing. Bioprocess Biosyst. Eng. 2021, 44, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-W.; Li, F.-Y.; Li, J.-F.; Li, Y.-L.; Xu, J.; Xie, Q.; Chen, S.; Guo, A.-F. Novel treatments for compatibility of plant fiber and starch by forming new hydrogen bonds. J. Clean. Prod. 2018, 185, 357–365. [Google Scholar] [CrossRef]
- Kumar Trivedi, A.; Kumar, A.; Gupta, M.K. Extraction of nanocellulose from wheat straw and its characterization. Mater. Today Proc. 2023, 78, 48–54. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, T.; Liu, K.; Zhang, M.; Zhao, Q.; Liang, Q.; Si, C. Nanocellulose-based advanced materials for flexible supercapacitor electrodes. Ind. Crops Prod. 2023, 204, 117378. [Google Scholar] [CrossRef]
- Srivastava, K.R.; Dixit, S.; Pal, D.B.; Mishra, P.K.; Srivastava, P.; Srivastava, N.; Hashem, A.; Alqarawi, A.A.; Abd_Allah, E.F. Effect of nanocellulose on mechanical and barrier properties of PVA–banana pseudostem fiber composite films. Environ. Technol. Innov. 2021, 21, 101312. [Google Scholar] [CrossRef]
- Bai, H.; Liang, Z.; Wang, D.; Guo, J.; Zhang, S.; Ma, P.; Dong, W. Biopolymer nanocomposites with customized mechanical property and exceptionally antibacterial performance. Compos. Sci. Technol. 2020, 199, 108338. [Google Scholar] [CrossRef]
- Luo, C.; Guo, A.; Zhao, Y.; Sun, X. A high strength, low friction, and biocompatible hydrogel from PVA, chitosan and sodium alginate for articular cartilage. Carbohydr. Polym. 2022, 286, 119268. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.; Kapil, S.; Sachar, S.; Sharma, S. Design of experiment based methodical optimization and green syntheses of hybrid patchouli oil coated silver nanoparticles for enhanced antibacterial activity. Curr. Res. Green Sustain. Chem. 2020, 3, 100016. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Bhardwaj, N.K.; Choudhary, V. Synthesis and characterization of methylcellulose/PVA based porous composite. Carbohydr. Polym. 2012, 88, 1364–1372. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Maidannyk, V.; Miao, S. Effect of concentrations of alginate, soy protein isolate and sunflower oil on water loss, shrinkage, elastic and structural properties of alginate-based emulsion gel beads during gelation. Food Hydrocoll. 2020, 108, 105998. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, Z.; He, Y. Antibacterial effect of polyvinyl alcohol/biochar–nano silver/sodium alginate gel beads. Processes 2023, 11, 2330. [Google Scholar] [CrossRef]
- Feng, Q.; Fan, B.; He, Y. Antibacterial, antioxidant, Cr (VI) adsorption and dye adsorption effects of biochar-based silver nanoparticles-sodium alginate-tannic acid composite gel beads. Int. J. Biol. Macromol. 2024, 271, 132453. [Google Scholar] [CrossRef]
- Xiang, X.; Yi, X.; Zheng, W.; Li, Y.; Zhang, C.; Wang, X.; Chen, Z.; Huang, M.; Ying, G.G. Enhanced biodegradation of thiamethoxam with a novel polyvinyl alcohol (PVA)/sodium alginate (SA)/biochar immobilized Chryseobacterium sp. H5. J. Hazard. Mater. 2023, 443, 130247. [Google Scholar] [CrossRef]
- Salunke, A.S.; Salunke, S.T.; Deokate, R.J.; Kale, B.B. Tuning of photoluminescence behavior of gold coated chitosan-polyvinyl alcohol binding with graphene quantum dots. Mater. Today Proc. 2022, 62, 1752–1757. [Google Scholar] [CrossRef]
- Bian, H.; Chen, L.; Dong, M.; Wang, L.; Wang, R.; Zhou, X.; Wu, C.; Wang, X.; Ji, X.; Dai, H. Natural lignocellulosic nanofibril film with excellent ultraviolet blocking performance and robust environment resistance. Int. J. Biol. Macromol. 2021, 166, 1578–1585. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against Gram-positive and Gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef]
- Dhandapani, P.; Santhoshkumar, M.; Narenkumar, J.; AlSalhi, M.S.; Kumar, P.A.; Devanesan, S.; Kokilaramani, S.; Rajasekar, A. Bio-approach: Preparation of RGO-AgNPs on cotton fabric and interface with sweat environment for antibacterial activity. Bioprocess Biosyst. Eng. 2022, 45, 1825–1837. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, L.; Ma, H.; Zhang, H.; Guo, L.H. Quantitative analysis of reactive oxygen species photogenerated on metal oxide nanoparticles and their bacteria toxicity: The role of superoxide radicals. Environ. Sci. Technol. 2017, 51, 10137–10145. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Fan, B.; He, Y.C. UV-blocking, antibacterial, corrosion resistance, antioxidant, and fruit packaging ability of lignin-rich alkaline black liquor composite film. Int. J. Biol. Macromol. 2024, 275, 133344. [Google Scholar] [CrossRef] [PubMed]
- Janani, B.; Okla, M.K.; Abdel-Maksoud, M.A.; AbdElgawad, H.; Thomas, A.M.; Raju, L.L.; Al-Qahtani, W.H.; Khan, S.S. CuO loaded ZnS nanoflower entrapped on PVA-chitosan matrix for boosted visible light photocatalysis for tetracycline degradation and anti-bacterial application. J. Environ. Manag. 2022, 306, 114396. [Google Scholar] [CrossRef] [PubMed]
- Masilan, K.; Neethiselvan, N.; Shakila, R.J.; Muralidharan, N.; Karthy, A.; Ravikumar, T.; Parthiban, F. Investigation on the coacervation of fish scale gelatin hydrogel with seafood waste hydrolysates for the development of artificial fish bait: Physico-chemical, thermodynamic, and morpho-structural properties. J. Indian Chem. Soc. 2022, 99, 100783. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Zhang, L.; Hu, Z.; Zhong, L.; Xue, J. Preparation and bacteriostatic research of porous polyvinyl alcohol/biochar/nanosilver polymer gel for drinking water treatment. Sci. Rep. 2021, 11, 12205. [Google Scholar] [CrossRef]
- Thakur, K.; Kalia, S.; Kaith, B.S.; Pathania, D.; Kumar, A.; Thakur, P.; Knittel, C.E.; Schauer, C.L.; Totaro, G. The development of antibacterial and hydrophobic functionalities in natural fibers for fiber-reinforced composite materials. J. Environ. Chem. Eng. 2016, 4, 1743–1752. [Google Scholar] [CrossRef]
- Qi, D.; Zhao, S.; Zhang, H.; Liu, B.; She, P.; Yue, X. Development of high-strength porous polyetheretherketone foam/nanosilver antibacterial composites for the prevention of postoperative infections in bone repair. Compos. Commun. 2022, 31, 101127. [Google Scholar] [CrossRef]
- Velidandi, A.; Pabbathi NP, P.; Dahariya, S.; Baadhe, R.R. Green synthesis of novel Ag–Cu and Ag–Znbimetallic nanoparticles and their in vitro biological, eco-toxicity and catalytic studies. Nano-Struct. Nano-Objects 2021, 26, 100687. [Google Scholar] [CrossRef]
- Ahmed, B.; Hashmi, A.; Khan, M.S.; Musarrat, J. ROS mediated destruction of cell membrane, growth and biofilms of human bacterial pathogens by stable metallic AgNPs functionalized from bell pepper extract and quercetin. Adv. Powder Technol. 2018, 29, 1601–1616. [Google Scholar] [CrossRef]
- Jiang, B.; Tian, C.; Song, G.; Pan, Q.; Wang, Z.; Shi, L.; Qiao, Y.; Fu, H. A green route to synthesize novel Ag/C antibacterial agent. Mater. Res. Bull. 2012, 47, 458–463. [Google Scholar] [CrossRef]
- Aryan, N.; Behpour, M.; Benvidi, A.; Jookar Kashi, F.; Azimzadeh, M.; Zare, H.R. Evaluation of sodium alendronate drug released from TiO2 nanoparticle doped with hydroxyapatite and silver–strontium for enhancing antibacterial effect and osteoinductivity. Mater. Chem. Phys. 2023, 295, 126934. [Google Scholar] [CrossRef]
- Vityazev, F.V.; Khramova, D.S.; Saveliev, N.Y.; Ipatova, E.A.; Burkov, A.A.; Beloserov, V.S.; Belyi, V.A.; Kononov, L.O.; Martinson, E.A.; Litvinets, S.G.; et al. Pectin-glycerol gel beads: Preparation, characterization and swelling behaviour. Carbohydr. Polym. 2020, 238, 116166. [Google Scholar] [CrossRef] [PubMed]
- Sethi, V.; Kaur, M.; Thakur, A.; Rishi, P.; Kaushik, A. Unravelling the role of hemp straw derived cellulose in CMC/PVA hydrogel for sustained release of fluoroquinolone antibiotic. Int. J. Biol. Macromol. 2022, 222, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Rezagholizade-shirvan, A.; Fathi Najafi, M.; Behmadi, H.; Masrournia, M. Preparation of nano-composites based on curcumin/chitosan-PVA-alginate to improve stability, antioxidant, antibacterial and anticancer activity of curcumin. Inorg. Chem. Commun. 2022, 145, 110022. [Google Scholar] [CrossRef]
- Bhat, V.G.; Masti, S.P.; Narasagoudr, S.S.; Chougale, R.B.; Kumar, P.; Vantamuri, A.B. Development and characterization of Chitosan/Guar gum/Gum ghatti bionanocomposites with in situ silver nanoparticles. Chem. Data Collect. 2023, 44, 101009. [Google Scholar] [CrossRef]
- Mathew, S.; Jayakumar, A.; Kumar, V.P.; Mathew, J.; Radhakrishnan, E.K. One-step synthesis of eco-friendly boiled rice starch blended polyvinyl alcohol bionanocomposite films decorated with in situ generated silver nanoparticles for food packaging purpose. Int. J. Biol. Macromol. 2019, 139, 475–485. [Google Scholar] [CrossRef]
- Li, Q.; Lu, F.; Zhou, G.; Yu, K.; Lu, B.; Xiao, Y.; Dai, F.; Wu, D.; Lan, G. Silver inlaid with gold nanoparticle/chitosan wound dressing enhances antibacterial activity and porosity, and promotes wound healing. Biomacromolecules 2017, 18, 3766–3775. [Google Scholar] [CrossRef]
- Aghaei, F.; Tangestaninejad, S.; Bahadori, M.; Moghadam, M.; Mirkhani, V.; Mohammadpoor-Baltork, I.; Khalaji, M.; Asadi, V. Green synthesize of nano-MOF-ethylcellulose composite fibers for efficient adsorption of Congo red from water. J. Colloid Interface Sci. 2023, 648, 78–89. [Google Scholar] [CrossRef]
- Chen, L.; Mi, B.; He, J.; Li, Y.; Zhou, Z.; Wu, F. Functionalized biochars with highly-efficient malachite green adsorption property produced from banana peels via microwave-assisted pyrolysis. Bioresour. Technol. 2023, 376, 128840. [Google Scholar] [CrossRef]
- Qiu, J.; Feng, Y.; Zhang, X.; Jia, M.; Yao, J. Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: Adsorption performance and mechanisms. J. Colloid Interface Sci. 2017, 499, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, G.; Pannipara, M.; Deviga, G.; Mariappan, M.; Al-Sehemi, A.G.; Anthony, S.P. Natural tea extract coated porous MOF nano/microparticles for highly enhanced and selective adsorption of cationic dyes from aqueous medium. J. Mol. Liq. 2024, 394, 123747. [Google Scholar] [CrossRef]
- Liu, W.; Lou, T.; Wang, X. Enhanced dye adsorption with conductive polyaniline doped chitosan nanofibrous membranes. Int. J. Biol. Macromol. 2023, 242, 124711. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Y.; He, Y.C.; Ma, C. Synthesis of furoic acid from biomasses by sequential catalysis with fish scale-rice husk-based heterogeneous chemocatalyst and dehydrogenase biocatalyst. Ind. Crops Prod. 2023, 202, 117033. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Zhang, Z. A versatile EDTA and chitosan bi-functionalized magnetic bamboo biochar for simultaneous removal of methyl orange and heavy metals from complex wastewater. Environ. Pollut. 2022, 293, 118517. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Sun, L.; Li, G.; Temmink, H.; Rijnaarts, H.H.M. Granule-based immobilization and activity enhancement of anammox biomass via PVA/CS and PVA/CS/Fe gel beads. Bioresour. Technol. 2020, 309, 123448. [Google Scholar] [CrossRef]
- Rodwihok, C.; Suwannakeaw, M.; Charoensri, K.; Wongratanaphisan, D.; Woon Woo, S.; Kim, H.S. Alkali/zinc-activated fly ash nanocomposites for dye removal and antibacterial applications. Bioresour. Technol. 2021, 331, 125060. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biomass type effect on biochar surface characteristic and adsorption capacity relative to silver and copper. Fuel 2020, 278, 118168. [Google Scholar] [CrossRef]
- Bhuyan, M.M.; Jophous, M.; Jeong, J.H. Preparation of pectin–acrylamide–(vinyl phosphonic acid) hydrogel and its selective adsorption of metal ions. Polym. Bull. 2023, 80, 4625–4641. [Google Scholar] [CrossRef]

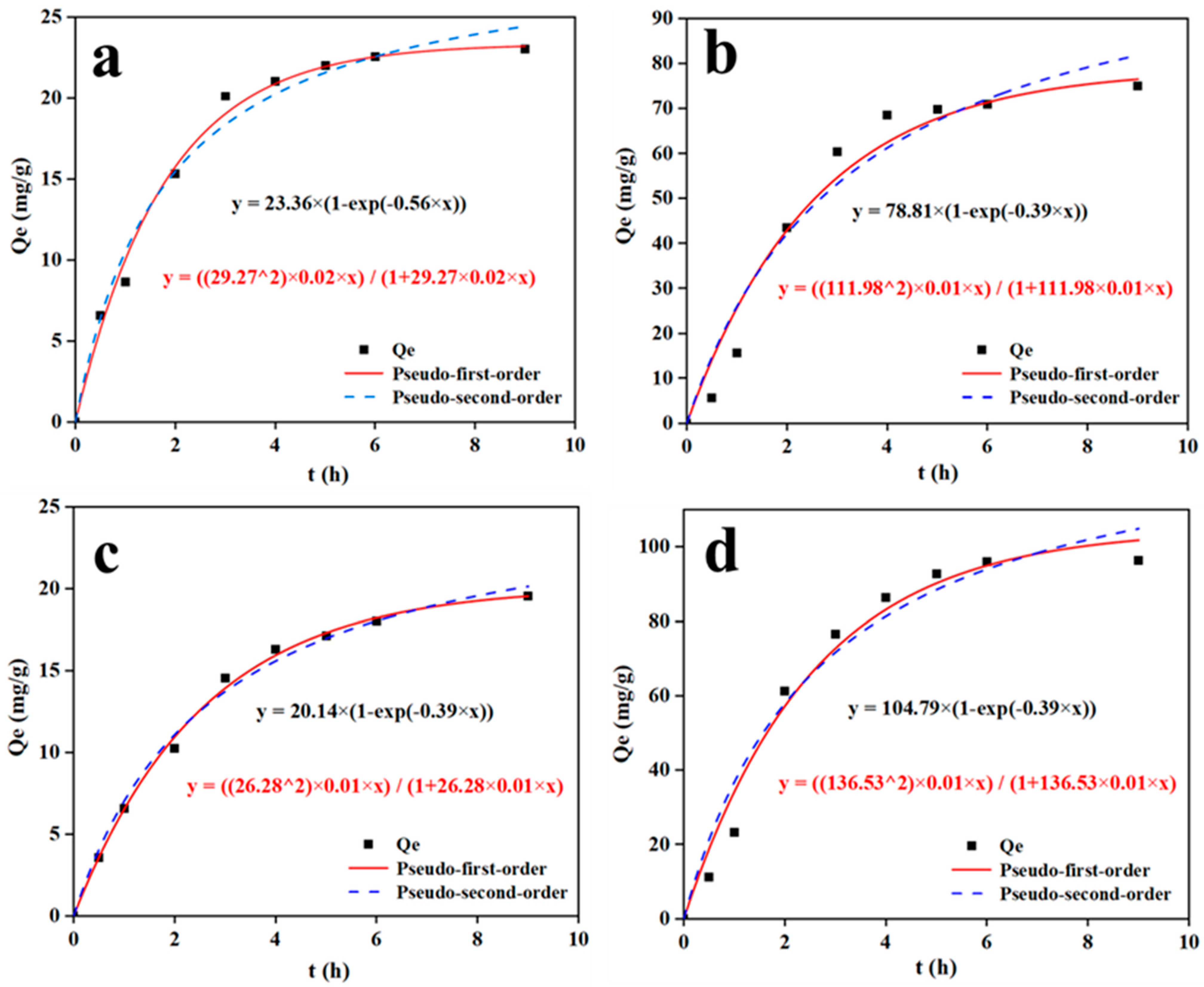
| Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||||
|---|---|---|---|---|---|---|---|
| qe exp (mg/g) | qe cal (mg/g) | k1 (min−1) | R2 | qe cal (mg/g) | k2 (g/mg·min) | R2 | |
| MB | 23.01 | 23.21 | 0.56 | 0.99 | 24.38 | 0.02 | 0.98 |
| MG | 75.01 | 76.54 | 0.39 | 0.97 | 81.31 | 0.01 | 0.95 |
| MO | 19.56 | 19.55 | 0.16 | 0.99 | 20.14 | 0.01 | 0.99 |
| CR | 96.32 | 101.81 | 0.39 | 0.99 | 104.91 | 0.01 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Zhang, Z.; He, Y.; Jiang, Y. Preparation of Polyvinyl Alcohol–Chitosan Nanocellulose–Biochar Nanosilver Composite Hydrogel and Its Antibacterial Property and Dye Removal Capacity. Processes 2024, 12, 2277. https://doi.org/10.3390/pr12102277
Xie L, Zhang Z, He Y, Jiang Y. Preparation of Polyvinyl Alcohol–Chitosan Nanocellulose–Biochar Nanosilver Composite Hydrogel and Its Antibacterial Property and Dye Removal Capacity. Processes. 2024; 12(10):2277. https://doi.org/10.3390/pr12102277
Chicago/Turabian StyleXie, Licheng, Zhichao Zhang, Yucai He, and Yan Jiang. 2024. "Preparation of Polyvinyl Alcohol–Chitosan Nanocellulose–Biochar Nanosilver Composite Hydrogel and Its Antibacterial Property and Dye Removal Capacity" Processes 12, no. 10: 2277. https://doi.org/10.3390/pr12102277
APA StyleXie, L., Zhang, Z., He, Y., & Jiang, Y. (2024). Preparation of Polyvinyl Alcohol–Chitosan Nanocellulose–Biochar Nanosilver Composite Hydrogel and Its Antibacterial Property and Dye Removal Capacity. Processes, 12(10), 2277. https://doi.org/10.3390/pr12102277







