Metal Acetate-Enhanced Microwave Pyrolysis of Waste Textiles for Efficient Syngas Production
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Experimental Apparatuses and Pyrolysis Performance Evaluation
2.3. Characterization
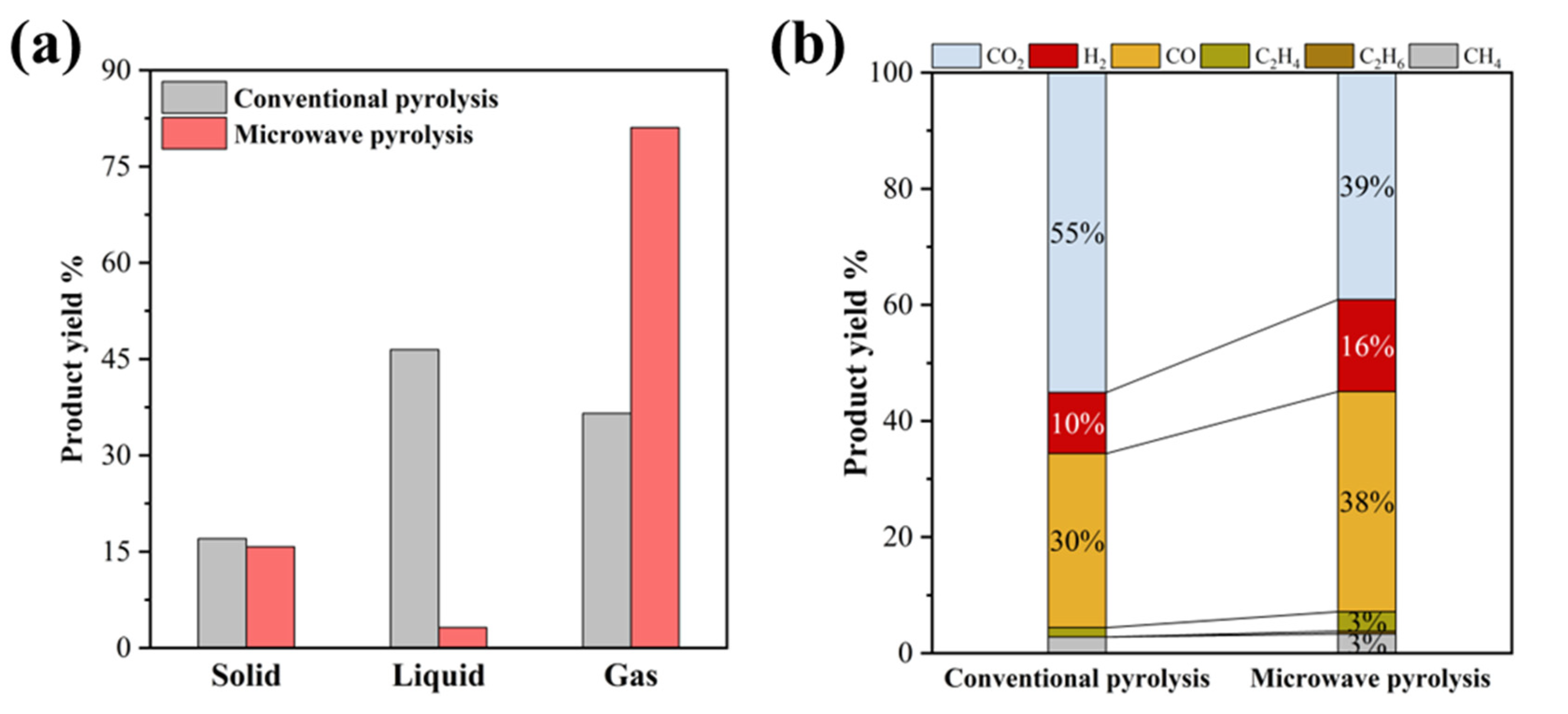
3. Results and Discussion
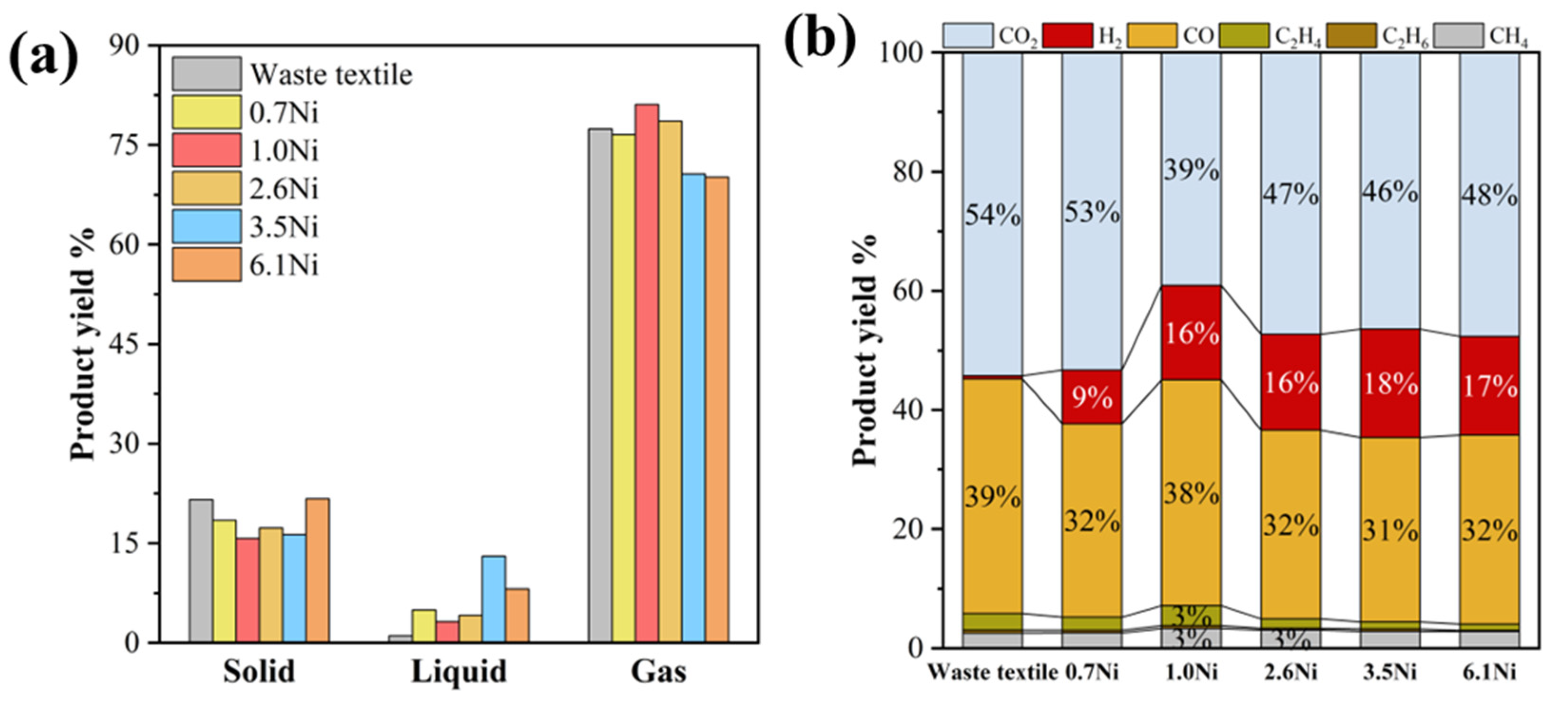
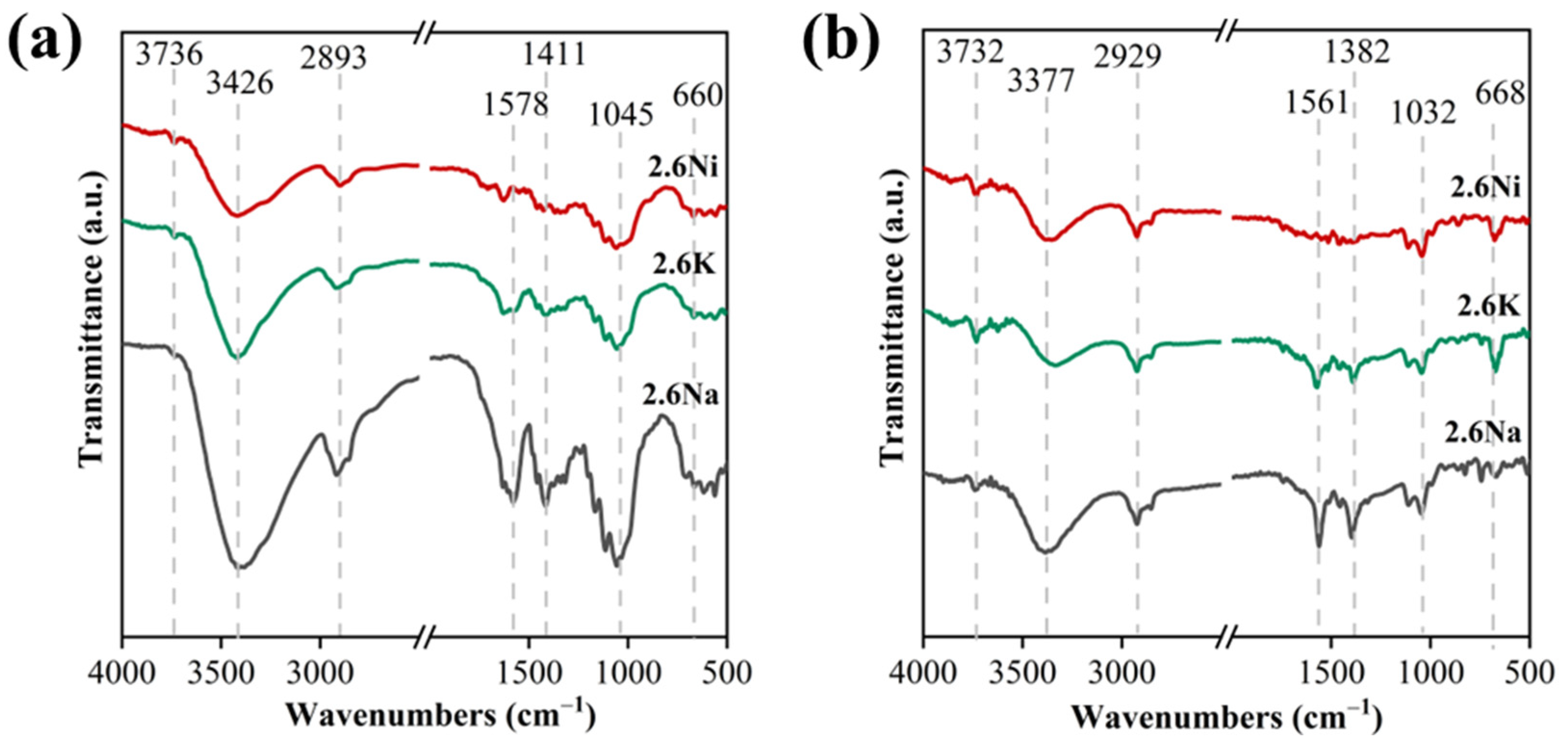
3.1. Optimization of Metal Acetate Types and Contents for Microwave Pyrolysis of Waste Textiles
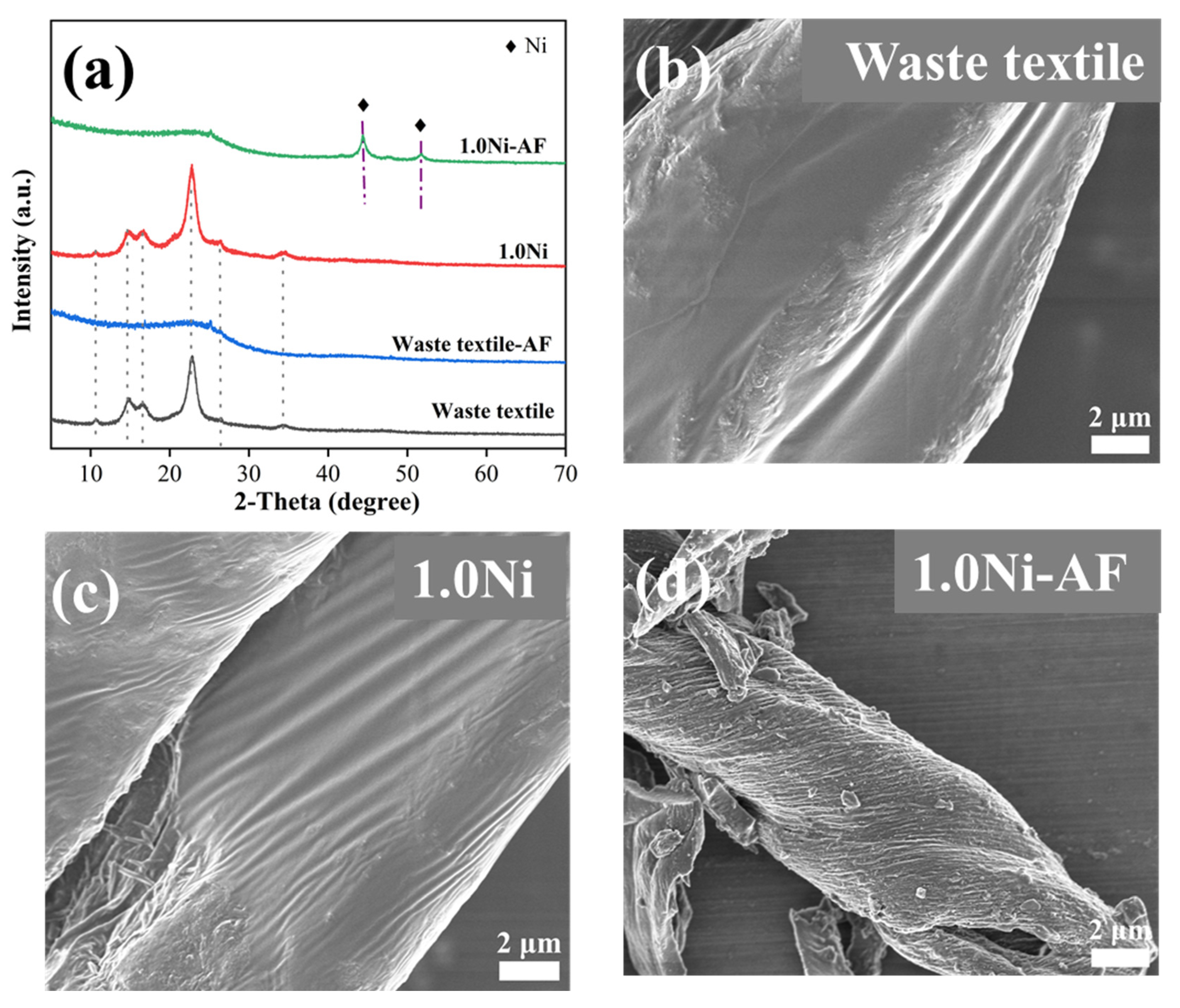
3.2. Zero-Valent Nickel-Enhanced Microwave Pyrolysis of Waste Textiles to Produce Syngas
3.3. Evaluation of Carbon Emission Reduction and Environmental Implication
- (1)
- Reduce fossil fuel dependence: The use of waste textiles impregnated with metal salts and microwave-directed pyrolysis to generate hydrogen-containing combustible gas could effectively replace traditional fossil fuels (e.g., coal and oil). Hydrogen, a clean energy source whose combustion product is only water, significantly reduces the emissions of carbon dioxide and other harmful gases. This shift helps slow global warming and reduces air pollution, thereby supporting carbon reduction targets.
- (2)
- Improve the resource efficiency of waste textiles: Treating waste textiles through microwave thermal pyrolysis technology not only effectively disposes of waste textiles and reduces the environmental burden caused by landfills and incineration, but also transforms waste textiles into high-value-added hydrogen and other usable by-products. It improves the resource efficiency of waste materials, reduces carbon emissions during waste treatment, and reduces greenhouse gases at the source.
- (3)
- Reduce the carbon footprint of traditional waste treatment: Traditional waste textile treatment methods such as incineration and landfill usually release a large amount of greenhouse gases such as carbon dioxide. In contrast, the technology of metal salt impregnation and microwave pyrolysis can more efficiently convert waste textiles into hydrogen-containing renewable gases, which reduces the direct greenhouse gas emissions in the waste treatment process. In addition, the higher energy conversion efficiency of this method can further reduce the carbon footprint due to the energy conversion process.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nanda, S.; Berruti, F. A Technical Review of Bioenergy and Resource Recovery from Municipal Solid Waste. J. Hazard. Mater. 2021, 403, 123970. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.; Chopra, S.S.; Cakin, E.; Li, X.; Lin, C.S.K. Environmental Life Cycle Assessment of Textile Bio-Recycling—Valorizing Cotton-Polyester Textile Waste to Pet Fiber and Glucose Syrup. Resour. Conserv. Recycl. 2020, 161, 104989. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, J.; Geng, Y.; Tian, Y.; Jiang, P. Energy-Related GHG Emissions of the Textile Industry in China. Resour. Conserv. Recycl. 2017, 119, 69–77. [Google Scholar] [CrossRef]
- Andini, E.; Bhalode, P.; Gantert, E.; Sadula, S.; Vlachos, D.G. Chemical Recycling of Mixed Textile Waste. Sci. Adv. 2024, 10, eado6827. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Wang, Y.; Liu, R.; Zou, J.; Yu, X.; Liu, Y.; Zhi, C.; Meng, J. Textile Production by Additive Manufacturing and Textile Waste Recycling: A Review. Environ. Chem. Lett. 2024, 22, 1929–1987. [Google Scholar] [CrossRef]
- Dong, J.; Tang, Y.; Nzihou, A.; Chi, Y.; Weiss-Hortala, E.; Ni, M.; Zhou, Z. Comparison of Waste-to-Energy Technologies of Gasification and Incineration Using Life Cycle Assessment: Case Studies in Finland, France and China. J. Clean. Prod. 2018, 203, 287–300. [Google Scholar] [CrossRef]
- Khan, S.; Anjum, R.; Raza, S.T.; Ahmed Bazai, N.; Ihtisham, M. Technologies for Municipal Solid Waste Management: Current Status, Challenges, and Future Perspectives. Chemosphere 2022, 288, 132403. [Google Scholar] [CrossRef]
- Hong, J.; Chen, Y.; Wang, M.; Ye, L.; Qi, C.; Yuan, H.; Zheng, T.; Li, X. Intensification of Municipal Solid Waste Disposal in China. Renew. Sustain. Energy Rev. 2017, 69, 168–176. [Google Scholar] [CrossRef]
- Cao, C.; Cheng, Y.; Hu, H.; Wang, H.; Liu, S.; Hu, M.; Li, X.; Yao, H. Products Distribution and Sulfur Fixation during the Pyrolysis of CaO Conditioned Textile Dyeing Sludge: Effects of Pyrolysis Temperature and Heating Rate. Waste Manag. 2022, 153, 367–375. [Google Scholar] [CrossRef]
- Yin, M.; Bi, D.; Zhao, W.; Liu, J.; Zhao, A.; Jiang, M. Production of Bio-Oil and Biochar by the Nitrogen-Rich Pyrolysis of Cellulose with Urea: Pathway of Nitrile in Bio-Oil and Evolution of Nitrogen in Biochar. J. Anal. Appl. Pyrolysis 2023, 174, 106137. [Google Scholar] [CrossRef]
- Zhu, Y.; Huo, G.; Yang, W.; Liu, H.; Zhang, W.; Cheng, W.; Yang, H.; Wang, Z.; Jin, Y.; Zhao, H. Catalytic Pyrolysis of Duckweed with Phosphoric Acid: Pyrolysis Behavior and Kinetics Analysis. J. Anal. Appl. Pyrolysis 2024, 177, 106384. [Google Scholar] [CrossRef]
- Ren, X.; Shanb Ghazani, M.; Zhu, H.; Ao, W.; Zhang, H.; Moreside, E.; Zhu, J.; Yang, P.; Zhong, N.; Bi, X. Challenges and Opportunities in Microwave-Assisted Catalytic Pyrolysis of Biomass: A Review. Appl. Energy 2022, 315, 118970. [Google Scholar] [CrossRef]
- Ge, S.; Yek, P.N.Y.; Cheng, Y.W.; Xia, C.; Wan Mahari, W.A.; Liew, R.K.; Peng, W.; Yuan, T.-Q.; Tabatabaei, M.; Aghbashlo, M.; et al. Progress in Microwave Pyrolysis Conversion of Agricultural Waste to Value-Added Biofuels: A Batch to Continuous Approach. Renew. Sustain. Energy Rev. 2021, 135, 110148. [Google Scholar] [CrossRef]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Wan Mahari, W.A.; Lee, X.Y.; Han, C.S.; Vo, D.-V.N.; Van Le, Q.; et al. Valorization of Biomass Waste to Engineered Activated Biochar by Microwave Pyrolysis: Progress, Challenges, and Future Directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Huang, Y.F.; Kuan, W.H.; Lo, S.L.; Lin, C.F. Total Recovery of Resources and Energy from Rice Straw Using Microwave-Induced Pyrolysis. Bioresour. Technol. 2008, 99, 8252–8258. [Google Scholar] [CrossRef]
- Parvez, A.M.; Wu, T.; Afzal, M.T.; Mareta, S.; He, T.; Zhai, M. Conventional and Microwave-Assisted Pyrolysis of Gumwood: A Comparison Study Using Thermodynamic Evaluation and Hydrogen Production. Fuel Process. Technol. 2019, 184, 1–11. [Google Scholar] [CrossRef]
- Kim, D.; Kim, G.; Oh, D.Y.; Seong, K.-W.; Park, K.Y. Enhanced Hydrogen Production from Anaerobically Digested Sludge Using Microwave Assisted Pyrolysis. Fuel 2022, 314, 123091. [Google Scholar] [CrossRef]
- Freitas, L.C.; Barbosa, J.R.; da Costa, A.L.C.; Bezerra, F.W.F.; Pinto, R.H.H.; Carvalho Junior, R.N. de From Waste to Sustainable Industry: How Can Agro-Industrial Wastes Help in the Development of New Products? Resour. Conserv. Recycl. 2021, 169, 105466. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; He, C.; Ruan, R.; Yu, Z.; Jiang, L.; Zeng, Z.; Wu, Q. A Review on Selective Production of Value-Added Chemicals via Catalytic Pyrolysis of Lignocellulosic Biomass. Sci. Total Environ. 2020, 749, 142386. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhu, N.; Li, F.; Lin, H.; Liang, C.; Dang, Z.; Zou, Y. Hydrogen-Rich Syngas Production from Waste Textile Gasification Coupling with Catalytic Reforming under Steam Atmosphere. Processes 2024, 12, 1790. [Google Scholar] [CrossRef]
- Kwon, D.; Yi, S.; Jung, S.; Kwon, E.E. Valorization of Synthetic Textile Waste Using CO2 as a Raw Material in the Catalytic Pyrolysis Process. Environ. Pollut. 2021, 268, 115916. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Li, H.; Dai, Y.; Wang, C.-H. Impact of Temperature on the Activity of Fe-Ni Catalysts for Pyrolysis and Decomposition Processing of Plastic Waste. Chem. Eng. J. 2021, 408, 127268. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, K.; Zhang, Y.; Wang, F.; Yang, F.; Cheng, F. Mechanism of Gas Production under Microwave/Conventional Pyrolysis of Sewage Sludge: Mechanism of Microwave Energy Action on Oxygen-Containing Functional Groups. Chem. Eng. J. 2023, 464, 142511. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Chen, Z.; Liu, B.; Ma, W.; Tang, Z.; Chen, Y.; Yang, H.; Chen, H. Study on the Effect Mechanism of Ca and Mg with Different Anion on Lignocellulosic Biomass Pyrolysis. J. Anal. Appl. Pyrolysis 2024, 177, 106335. [Google Scholar] [CrossRef]
- Baidya, T.; Cattolica, R.J.; Seiser, R. High Performance Ni-Fe-Mg Catalyst for Tar Removal in Producer Gas. Appl. Catal. A Gen. 2018, 558, 131–139. [Google Scholar] [CrossRef]
- Lu, Q.; Yuan, S.; Li, J.; Chen, X.; Li, K.; Xie, X.; Fu, X.; He, Z. Influence of Mg and Co Addition on Fe Based Catalyst for In-Situ Biomass Pyrolysis. J. Anal. Appl. Pyrolysis 2023, 169, 105832. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Influence of Inorganic Salts on the Primary Pyrolysis Products of Cellulose. Bioresour. Technol. 2010, 101, 4646–4655. [Google Scholar] [CrossRef]
- Shang, S.; Guo, C.; Lan, K.; Li, Z.; He, W.; Qin, Z.; Li, J. Hydrogen-Rich Syngas Production via Catalytic Gasification of Sewage Sludge and Wheat Straw Using Corn Stalk Char-Supported Catalysts. BioResources 2020, 15, 4294–4313. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Tatariants, M.; Abdelnaby, M.A.; Tuckute, S.; Kliucininkas, L. A Sustainable Bioenergy Conversion Strategy for Textile Waste with Self-Catalysts Using Mini-Pyrolysis Plant. Energy Convers. Manag. 2019, 196, 688–704. [Google Scholar] [CrossRef]
- Eskandari, S.; Tate, G.; Leaphart, N.R.; Regalbuto, J.R. Nanoparticle Synthesis via Electrostatic Adsorption Using Incipient Wetness Impregnation. ACS Catal. 2018, 8, 10383–10391. [Google Scholar] [CrossRef]
- Stoyanova, M.; Schneider, M.; Pohl, M.M.; Rodemerck, U. Direct Conversion of Ethylene to Propene over Ni Impregnated by Incipient Wetness on Silica-Alumina. Catal. Commun. 2017, 92, 65–69. [Google Scholar] [CrossRef]
- Chen, S.; Wang, T.; Song, W.; Tang, Z.; Cao, Z.; Chen, H.; Lian, Y.; Hu, X.; Zheng, W.; Lian, H. A Novel Particulate Matter Sampling and Cell Exposure Strategy Based on Agar Membrane for Cytotoxicity Study. Chemosphere 2022, 300, 134473. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, N.; Feng, M.; Li, G.; Zhang, W.; An, T. Near-Infrared Light Induced Adsorption–Desorption Cycle for VOC Recovery by Integration of Metal–Organic Frameworks with Graphene Oxide Nanosheets. Environ. Sci. Nano 2022, 9, 1858–1868. [Google Scholar] [CrossRef]
- Meshksar, M.; Farsi, M.; Rahimpour, M.R. Effect of Ni Active Site Position and Synthesis Route on Activity, Stability, and Morphology of Ce Promoted Ni/Al2O3 Catalyst for Clean H2 Production. J. Environ. Chem. Eng. 2022, 10, 108471. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, Y.; Williams, P.T.; Yang, H.; Chen, H. Co-Production of Hydrogen and Carbon Nanotubes from Real-World Waste Plastics: Influence of Catalyst Composition and Operational Parameters. Appl. Catal. B Environ. 2018, 221, 584–597. [Google Scholar] [CrossRef]
- Ochoa, A.; Barbarias, I.; Artetxe, M.; Gayubo, A.G.; Olazar, M.; Bilbao, J.; Castaño, P. Deactivation Dynamics of a Ni Supported Catalyst during the Steam Reforming of Volatiles from Waste Polyethylene Pyrolysis. Appl. Catal. B Environ. 2017, 209, 554–565. [Google Scholar] [CrossRef]
- Hwang, H.; Oh, S.; Cho, T.S.; Choi, I.G.; Choi, J.W. Fast Pyrolysis of Potassium Impregnated Poplar Wood and Characterization of Its Influence on the Formation as Well as Properties of Pyrolytic Products. Bioresour. Technol. 2013, 150, 359–366. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Journal of Analytical and Applied Pyrolysis Review on the Catalytic Effects of Alkali and Alkaline Earth Metals (AAEMs) Including Sodium, Potassium, Calcium and Magnesium on the Pyrolysis of Lignocellulosic Biomass and on the Co-Pyrolysis of Coal With. J. Anal. Appl. Pyrolysis 2022, 163, 105479. [Google Scholar] [CrossRef]
- Jia, X.; Wei, Y.; Si, R.; Wang, C.; Guo, P.; Ji, J. Catalytic Production of H2-Rich Syngas from Cellulose Pyrolysis under Nickel Metal Fog with Molten Carbonates. Int. J. Hydrogen Energy 2024, 72, 991–1000. [Google Scholar] [CrossRef]
- Buffoni, I.N.; Pompeo, F.; Santori, G.F.; Nichio, N.N. Nickel Catalysts Applied in Steam Reforming of Glycerol for Hydrogen Production. Catal. Commun. 2009, 10, 1656–1660. [Google Scholar] [CrossRef]
- Choya, A.; de Rivas, B.; No, M.L.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Dry Reforming of Methane over Sub-Stoichiometric NiAl2O4-Mediated Ni/Al2O3 Catalysts. Fuel 2024, 358, 130166. [Google Scholar] [CrossRef]
- Du, J.; Gao, J.; Gu, F.; Zhuang, J.; Lu, B.; Jia, L.; Xu, G.; Liu, Q.; Su, F. A Strategy to Regenerate Coked and Sintered Ni/Al2O3 Catalyst for Methanation Reaction. Int. J. Hydrogen Energy 2018, 43, 20661–20670. [Google Scholar] [CrossRef]
- Rubi, R.V.C.; Allayban, J.P.O.; Deduque, D.A.B.; Mena, J.A.B.; Robles, N.M.D.; Rubio, B.D.; Roque, E.C.; Joy Janaban, P.; Olay, J.G. Production and Characterization of Activated Carbon from Pyrolysis Biochar of Cellulosic Cotton-Based Textile Wastes. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Zakarauskas, K.; Striūgas, N.; Mohamed, A. A New Strategy for Using Lint-Microfibers Generated from Clothes Dryer as a Sustainable Source of Renewable Energy. Sci. Total Environ. 2021, 762, 143107. [Google Scholar] [CrossRef]
- Ling, C.; Shi, S.; Hou, W.; Yan, Z. Separation of Waste Polyester/Cotton Blended Fabrics by Phosphotungstic Acid and Preparation of Terephthalic Acid. Polym. Degrad. Stab. 2019, 161, 157–165. [Google Scholar] [CrossRef]
- Dinga, C.D.; Wen, Z. China’s Green Deal: Can China’s Cement Industry Achieve Carbon Neutral Emissions by 2060? Renew. Sustain. Energy Rev. 2022, 155, 111931. [Google Scholar] [CrossRef]
- Zhang, S.-C.; Yang, X.-Y.; Xu, W.; Fu, Y.-J. Contribution of Nearly-Zero Energy Buildings Standards Enforcement to Achieve Carbon Neutral in Urban Area by 2060. Adv. Clim. Chang. Res. 2021, 12, 734–743. [Google Scholar] [CrossRef]


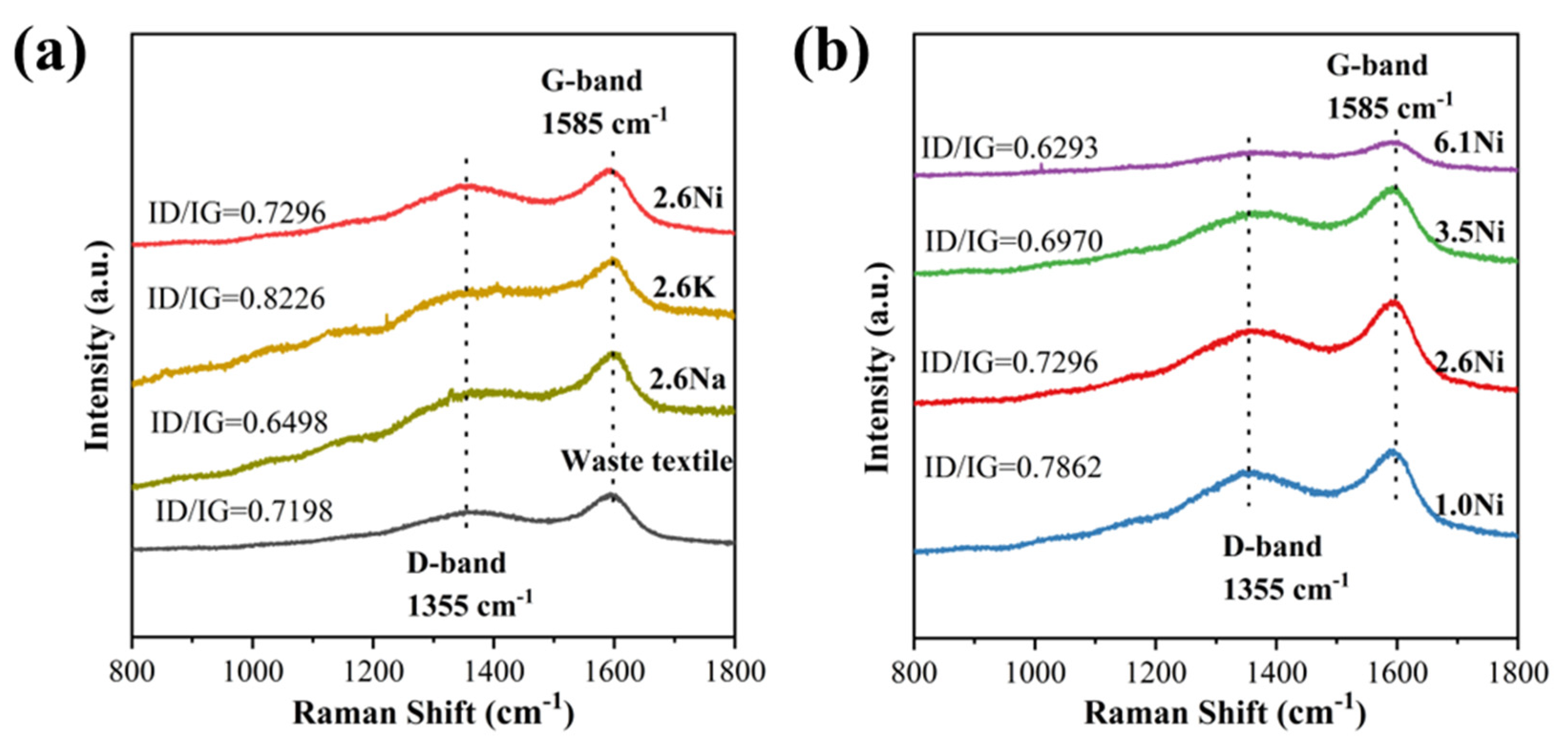

| Materials | Waste Textile | 2.6Na | 2.6K | 1.0Ni | 2.6Ni | 3.5Ni | 6.1Ni |
|---|---|---|---|---|---|---|---|
| ID/IG | 0.7198 | 0.6498 | 0.8226 | 0.7862 | 0.7296 | 0.6970 | 0.6293 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Wu, L.; Li, F.; Xiong, W.; Yao, P.; Zhang, Y.; Li, X. Metal Acetate-Enhanced Microwave Pyrolysis of Waste Textiles for Efficient Syngas Production. Processes 2024, 12, 2505. https://doi.org/10.3390/pr12112505
Zhang B, Wu L, Li F, Xiong W, Yao P, Zhang Y, Li X. Metal Acetate-Enhanced Microwave Pyrolysis of Waste Textiles for Efficient Syngas Production. Processes. 2024; 12(11):2505. https://doi.org/10.3390/pr12112505
Chicago/Turabian StyleZhang, Bo, Lei Wu, Fei Li, Wuwan Xiong, Peiyu Yao, Yang Zhang, and Xiang Li. 2024. "Metal Acetate-Enhanced Microwave Pyrolysis of Waste Textiles for Efficient Syngas Production" Processes 12, no. 11: 2505. https://doi.org/10.3390/pr12112505
APA StyleZhang, B., Wu, L., Li, F., Xiong, W., Yao, P., Zhang, Y., & Li, X. (2024). Metal Acetate-Enhanced Microwave Pyrolysis of Waste Textiles for Efficient Syngas Production. Processes, 12(11), 2505. https://doi.org/10.3390/pr12112505





