Ignition Characteristics and Flame Behavior of Automotive Lubricating Oil on Hot Surfaces
Abstract
:1. Introduction
2. Research Methods
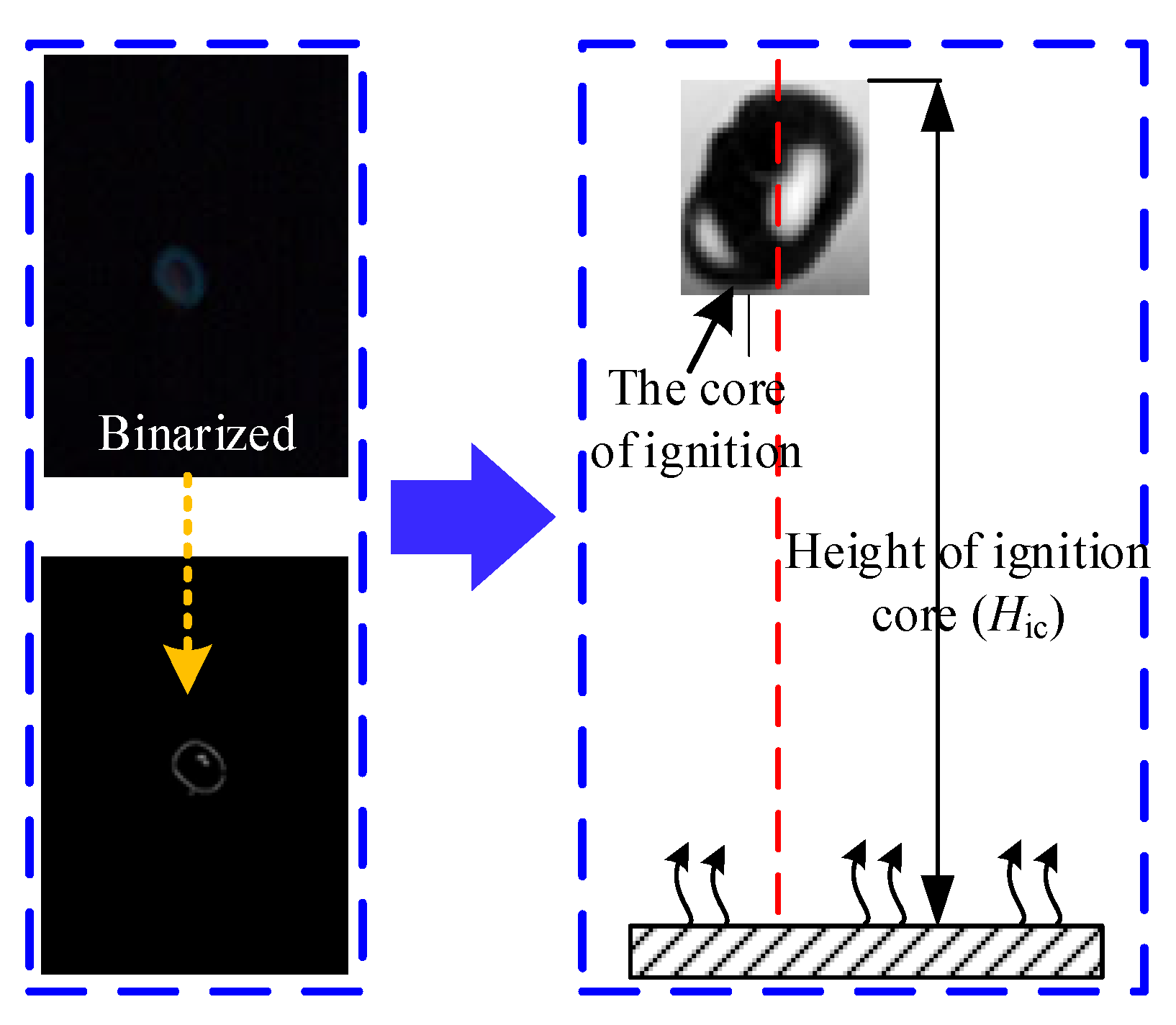
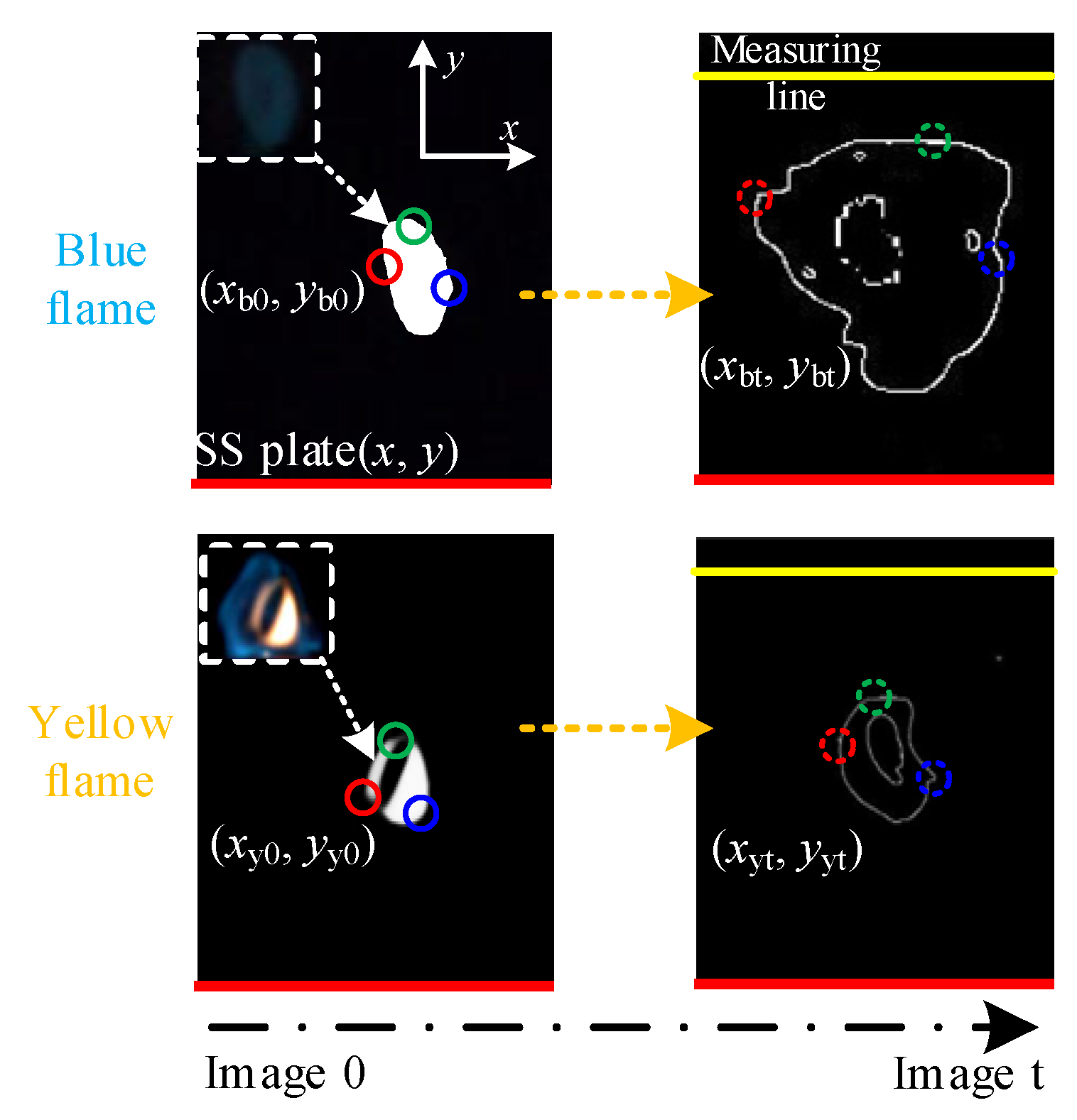
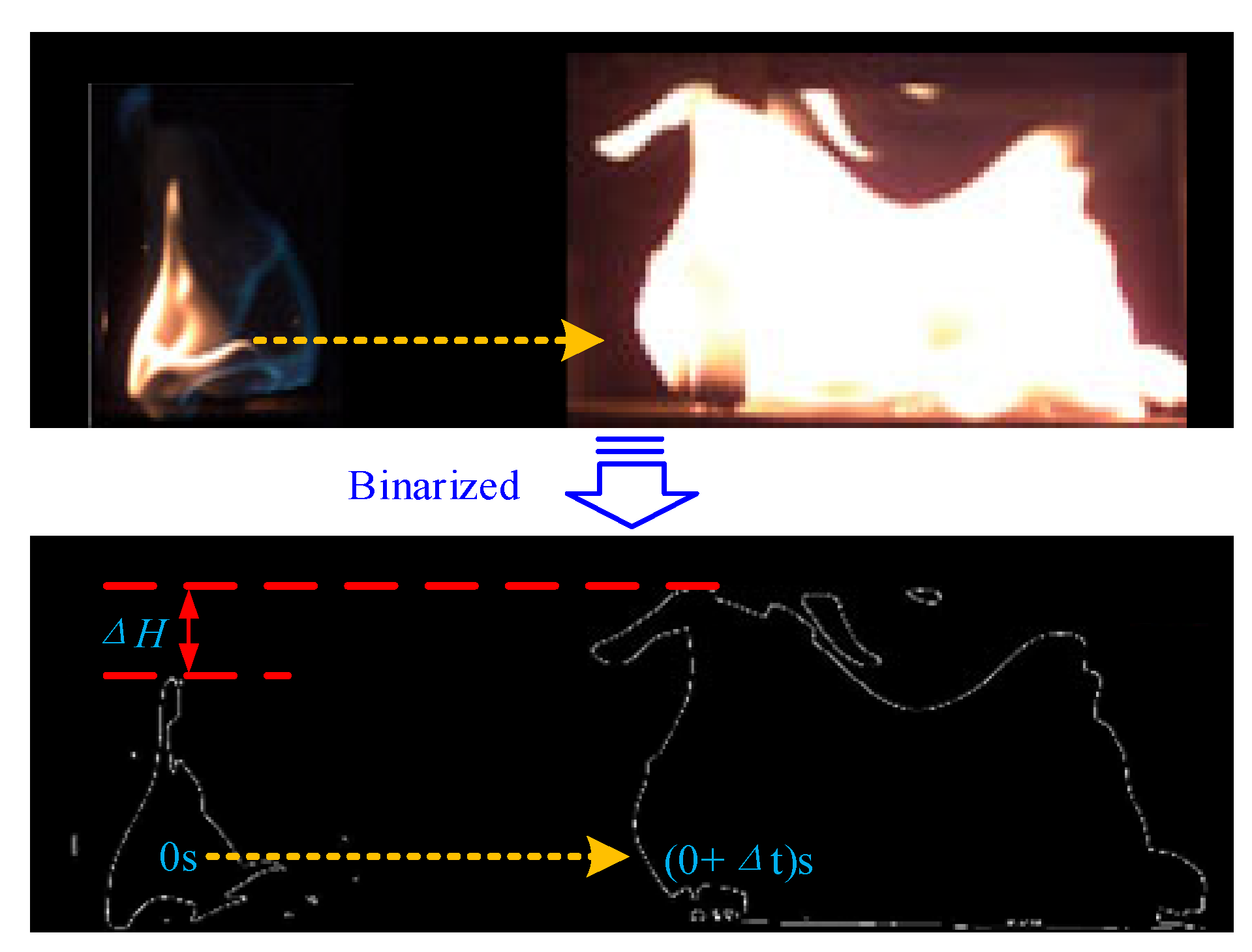
2.1. Flame Image Processing Methods
2.2. Calculation of Flame Height
2.3. Calculation of Flame Propagation Velocity
2.4. Calculation of Flame Temperature
3. Experimental Results and Discussion
3.1. Specimen
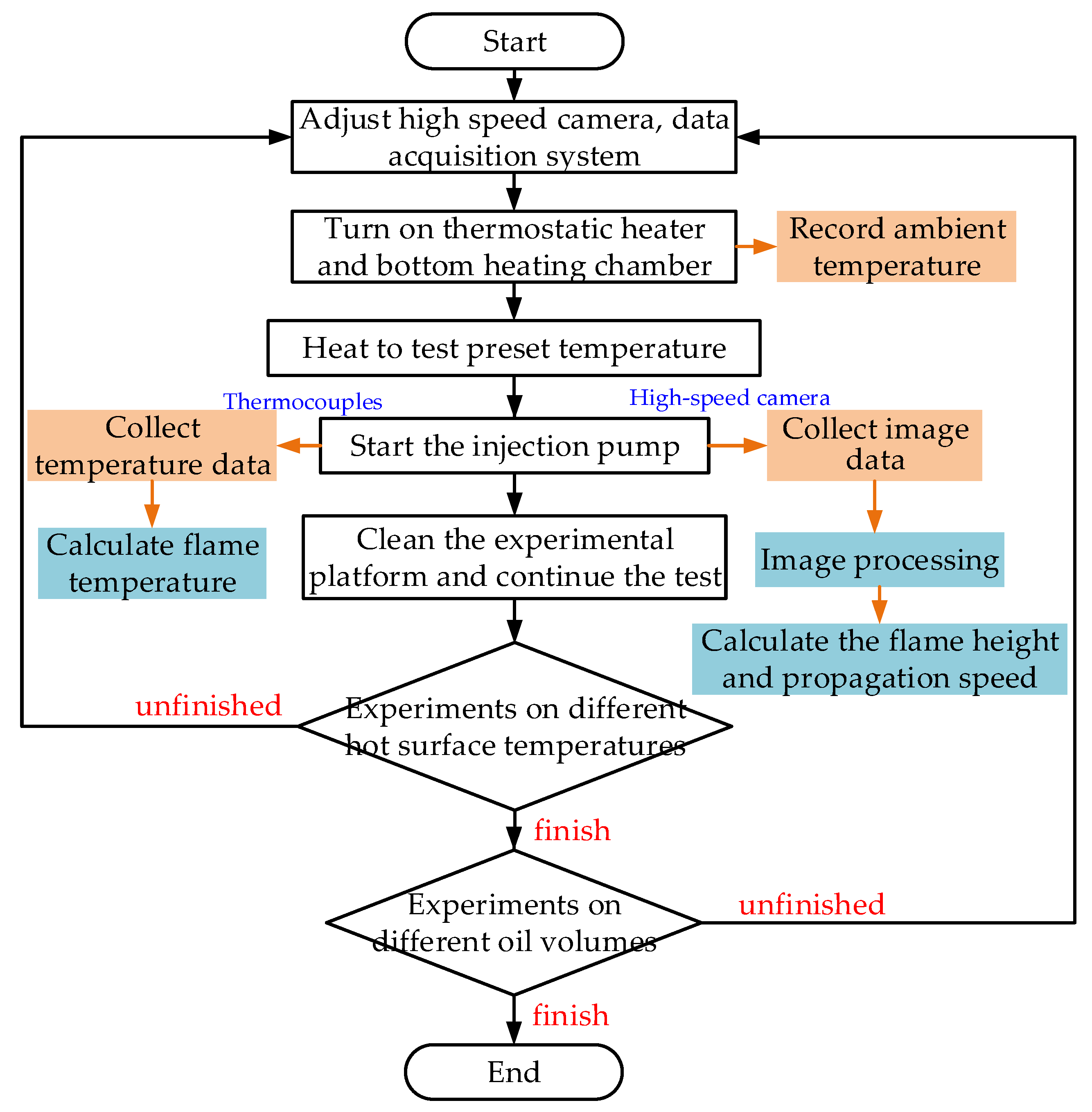
3.2. Test System
3.3. Test Process
4. Results
4.1. Ignition Temperature
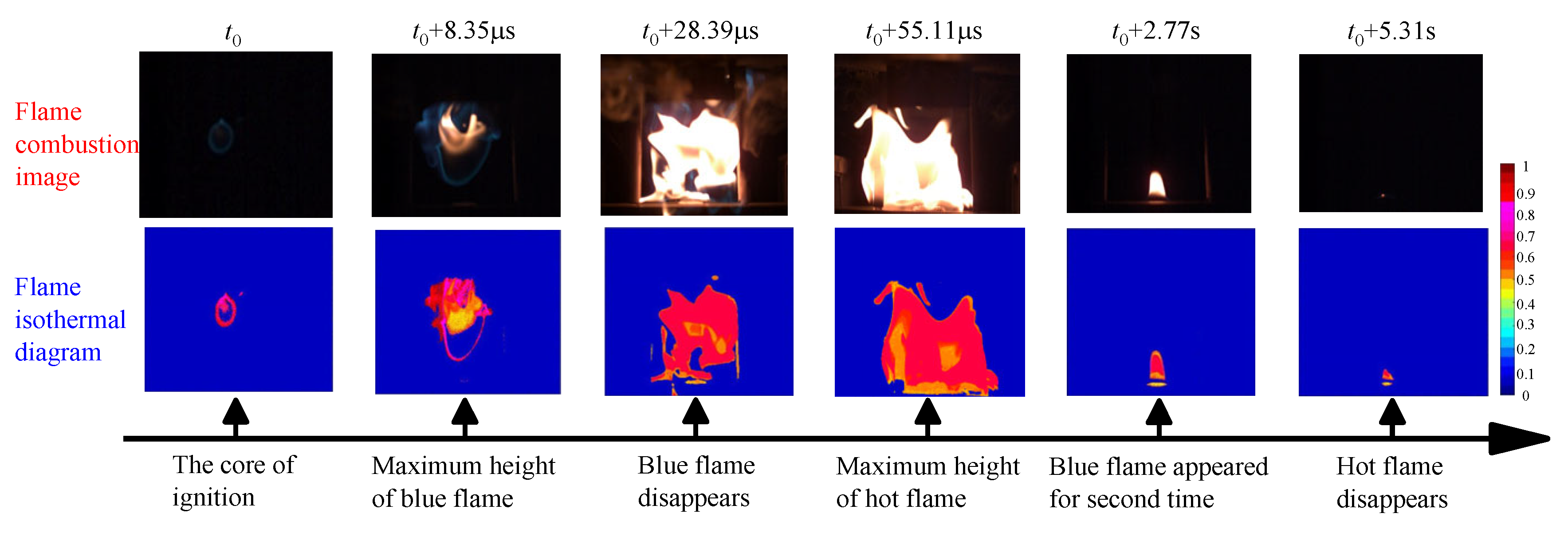
4.2. Flame Development Characteristics
5. Flame Combustion Characteristics
5.1. Flame Height
5.2. Flame Propagation Velocity
5.3. Flame Temperature
6. Conclusions
- The lowest ignition temperature and the highest ignition temperature of the hot surface are significantly negatively correlated with the oil volume. As the oil quantity increases from 0.1 mL to 0.5 mL, the minimum ignition temperature decreases from 370 °C to 312 °C, and the maximum ignition temperature decreases from 415 °C to 355 °C. This indicates an increased risk of ignition on the hot surface with higher oil quantities. The combustion process of LO on the hot surface can be categorized into five stages: formation of the ignition core with blue flame; a dual-structure flame with the blue flame enveloping the hot flame; development of a yellowish-white hot flame; a secondary dual-structure flame with a small blue flame at the base of the hot flame; a final stage with a small area of blue flame.
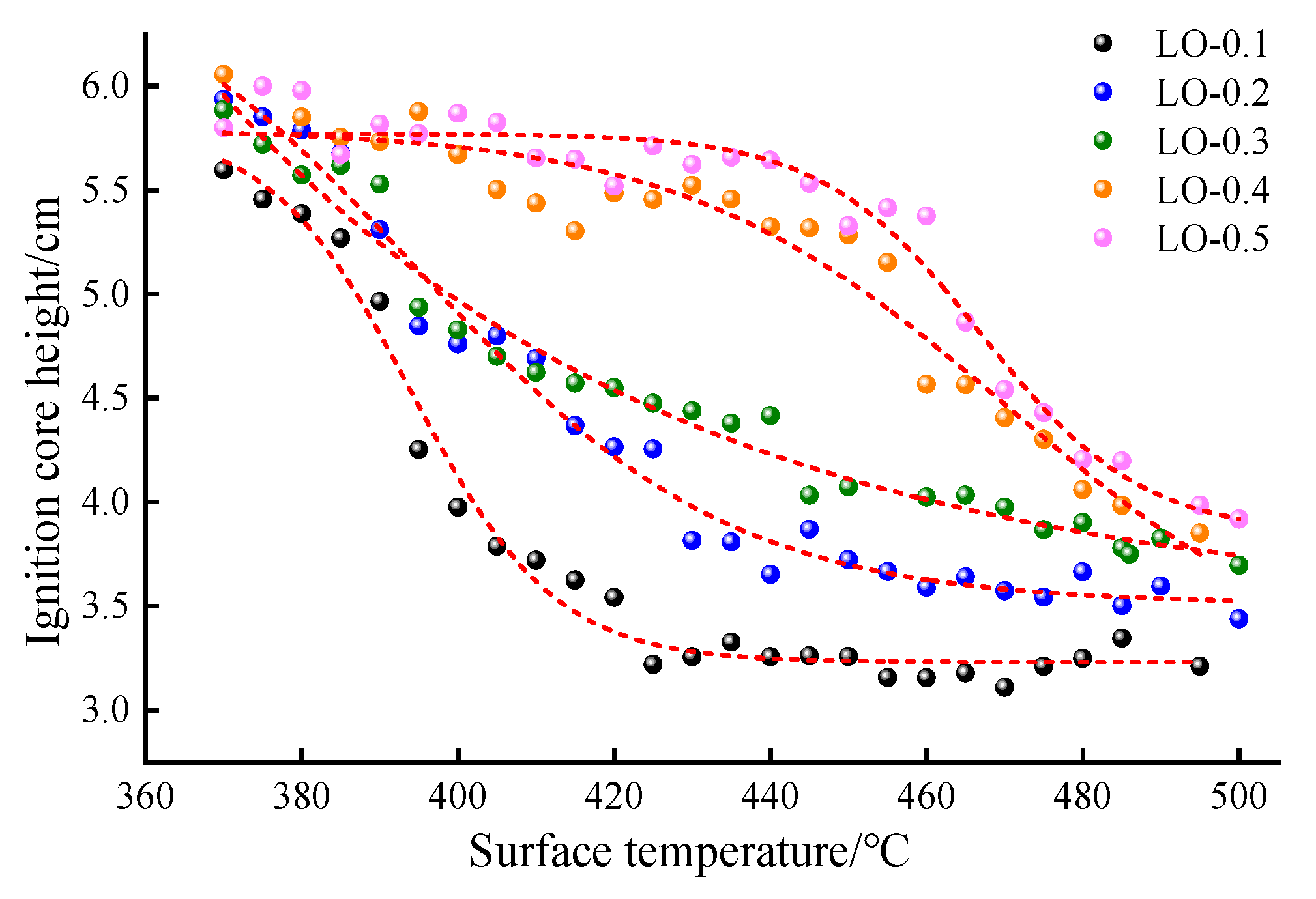
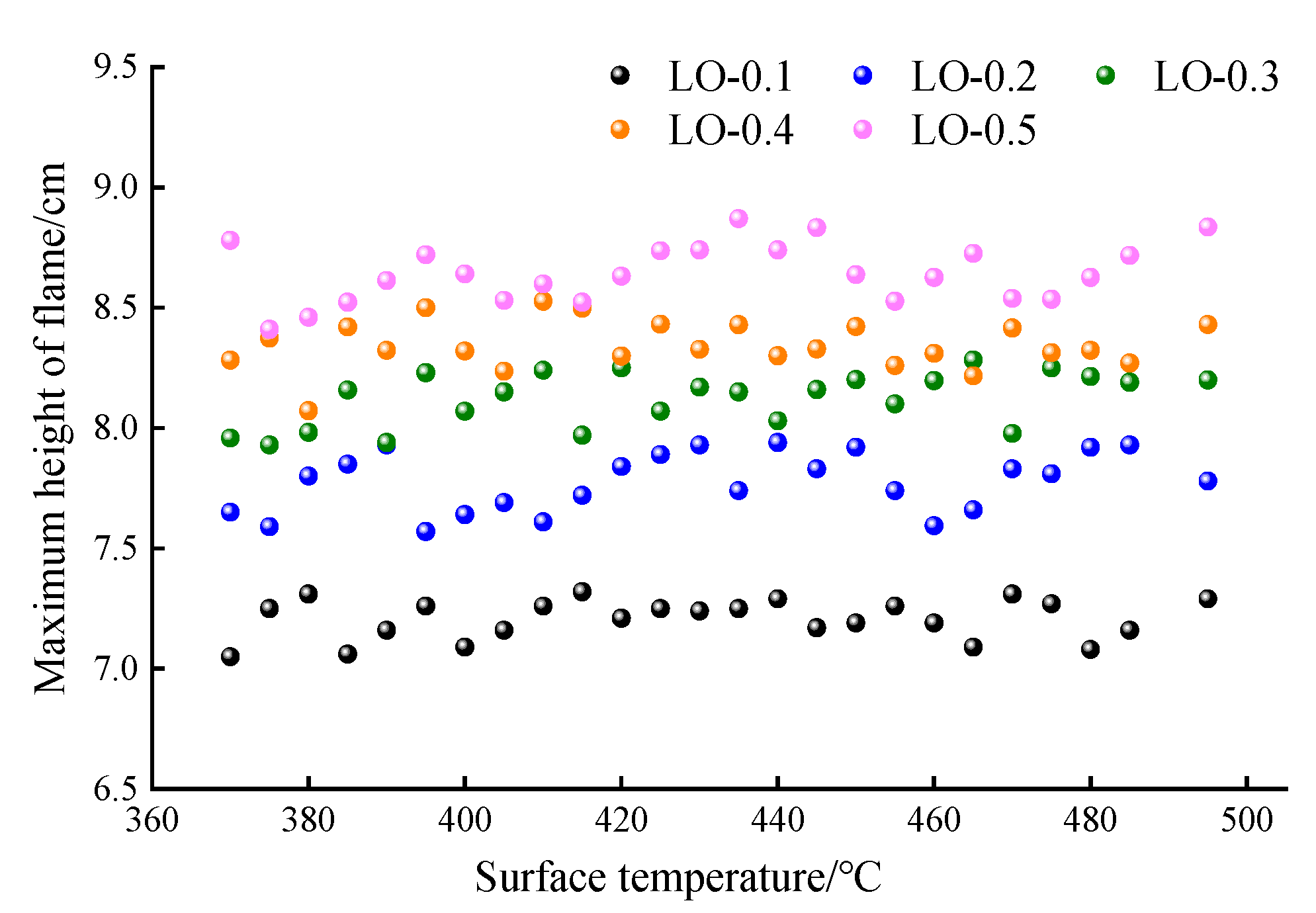
- The ignition core height is positively correlated with the hot surface temperature, and the ignition core height difference in the LO with a different oil volume is small. The maximum height of the hot flame is less affected by the initial hot surface temperature, but it is significantly positively correlated with the oil volume. At the oil volume of 0.2 mL (LO-0.2), the maximum hot flame height is 79.5 mm, close to the 80 mm distance between the oil pipe and the hot surface, posing a direct risk to fuel line safety. When the oil volume exceeds 0.3 mL (LO-0.3), the hot flame height surpasses 80 mm, further elevating the potential risk of automobile fires.
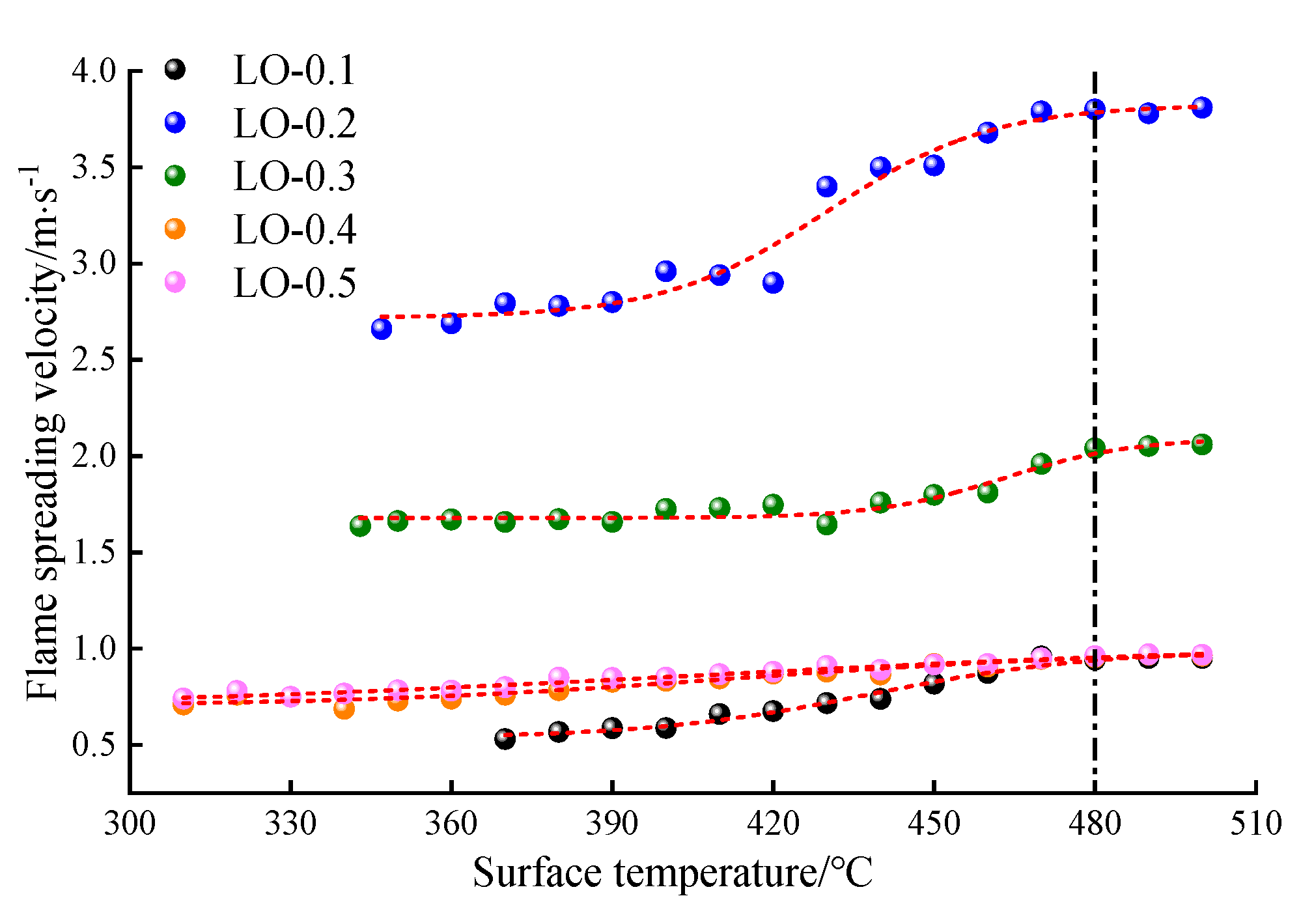
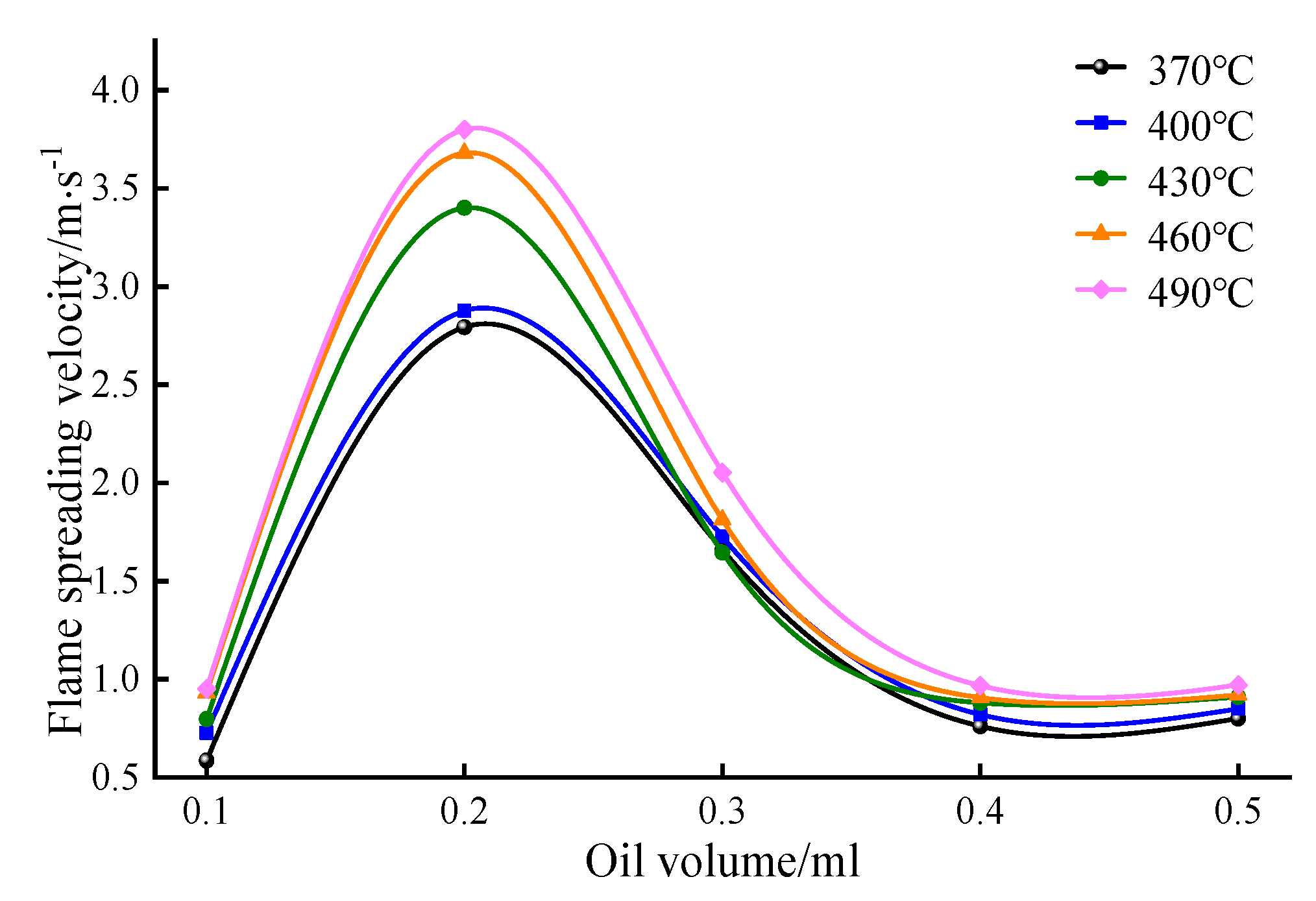
- The flame propagation velocity increases with the increase in the hot surface temperature and adheres to the Boltzmann relationship. The flame propagation velocity is the highest among the tested oil volumes, with an oil volume of 0.2 mL. At this volume, the air around the hot surface is closest to the theoretical air volume mixed with the combustible gas mixture according to chemical equivalence ratios, resulting in a high concentration of activated molecules. This enhances the chemical reaction rate of the lubricating oil and accelerates the flame propagation velocity.
- During the flame combustion, the flame temperature generally shows a trend of increasing first and then decreasing. At the peaks of the blue and hot flame heights, the flame area is the largest, and the average flame temperature is relatively high, indicating greater danger. The average flame temperature increases with the oil volume at the maximum height of the blue flame, although the difference is relatively small. Conversely, the average flame temperature rises at the maximum hot flame height with the increased oil volume. Notably, at the oil volume of 0.2 mL, the increment in the average flame temperature is the largest, intensifying the fire risk in automotive applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deng, J.; Yang, W.; Zhang, Y.; Chen, J.; Li, Y.; Ji, X.; Shu, C. Experimental study on hot surface ignition and flame characteristic parameters of lubricating oil. J. Therm. Anal. Calorim. 2024, 149, 10213–10225. [Google Scholar] [CrossRef]
- Colwell, J.; Reza, A. Hot surface ignition of automotive and aviation fluids. Fire Technol. 2005, 41, 105–123. [Google Scholar] [CrossRef]
- Arndt, S.; Stevens, D.; Arndt, M. The motor vehicle in the post-crash environment, an understanding of ignition properties of spilled fuels. SAE Trans. 1999, 108, 116–128. [Google Scholar]
- Davis, S.; Kelly, S.; Somandepalli, V. Hot surface ignition of performance fuels. Fire Technol. 2010, 46, 363–374. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Lu, S. Ignition process of leaked fuel on horizontal heated surface. Fire Saf. J. 2010, 45, 160–164. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, D.; Fang, Y.; Song, M.; Gong, Z.; Feng, L. Experimental and numerical investigation of the auto-ignition characteristics of cylinder oil droplets under low-speed two-stroke natural gas engines in-cylinder conditions. Fuel 2022, 329, 125498–125512. [Google Scholar] [CrossRef]
- Ebrahim, M.; Elkenani, B.; Ortega, A. Transient surface temperatures upon the impact of a single droplet onto a heated surface in the film evaporation regime. Int. J. Heat Mass Transf. 2022, 186, 122463–122495. [Google Scholar] [CrossRef]
- Lv, X.; Yao, S.; Du, M. Analysis of inerting index when inerting capability of aircraft fuel tank was degraded. J. Aerosp. Power 2023, 38, 1067–1074. [Google Scholar]
- Jeff, C.; Kaushik, B. Steady-state and transient motor vehicle exhaust system temperatures. SAE Int. J. Passeng. Cars-Mech. Syst. 2009, 2, 206–218. [Google Scholar]
- Boeck, L.; Melguizo, G.; Shepherd, J. Hot surface ignition dynamics in premixed hydrogen—Air near the lean flammability limit. Combust. Flame 2019, 210, 467–478. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Z.; Li, R.; Li, K.; Hua, M.; Pan, X.; Wang, S.; Jiang, J. Study on the effect of ignition mode on overpressure of underexpanded hydrogen jet explosion. CIESC J. 2023, 74, 1409–1418. [Google Scholar]
- Tang, W.; Bahrami, D.; Yuan, L.; Thomas, R.; Soles, J. Hot surface ignition of liquid fuels under ventilation. Min. Metall. Explor. 2022, 39, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.; Long, W.; Feng, L.; Chen, L.; Cui, J.; Gong, W. Investigation of evaporation and auto-ignition of isolated lubricating oil droplets in natural gas engine in-cylinder conditions. Fuel 2019, 235, 1172–1183. [Google Scholar] [CrossRef]
- Shaw, A.; Weckman, B. Evaluation of the ignition of diesel fuels on hot surfaces. Fire Technol. 2010, 46, 407–423. [Google Scholar] [CrossRef]
- Babrauskas, V. Ignition of gases, vapors, and liquids by hot surfaces. Fire Technol. 2022, 58, 281–310. [Google Scholar] [CrossRef]
- Orlova, E.; Glushkov, D.; Abedtazehabadi, A.; Belyaev, S.; Feoktistov, D. Influence of the texture configuration of heating surfaces created by laser irradiation on the ignition and combustion characteristics of liquid fuels. Appl. Sci. 2023, 13, 95. [Google Scholar] [CrossRef]
- Psarros, E.; Polykrati, A.; Karagiannopoulos, C.; Bourkas, P. A model for calculating the temperature of aluminium particles ejected from overhead low-voltage lines owing to a short-circuit. Int. J. Wildland Fire 2009, 18, 722–726. [Google Scholar] [CrossRef]
- Feoktistov, D.; Glushkov, D.; Kuznetsov, G.; Nikitin, D.; Orlova, E.; Paushkina, K. Ignition and combustion characteristics of coal-water-oil slurry placed on modified metal surface at mixed heat transfer. Fuel Process. Technol. 2022, 233, 107291–107303. [Google Scholar] [CrossRef]
- Qi, Y.; Fei, S.; Wang, Z. Explosion mechanism of lubricating oil droplets in high-temperature and high-pressure combustion environments. Lubricants 2023, 11, 118. [Google Scholar] [CrossRef]
- Shlegel, N.; Strizhak, P. Regime maps of collisions of fuel oil/water emulsion droplets with solid heated surface. Fuel 2023, 342, 127734–127744. [Google Scholar] [CrossRef]
- Fei, S.; Qi, Y.L.; Liu, W.; Wang, Y.; Wang, Z.; Zhang, H. Combustion modes induced by oil-droplet gas-phase pre-ignition in the chamber under different environmental conditions. Combust. Sci. Technol. 2023, 195, 379–397. [Google Scholar] [CrossRef]
- Tao, C.; Miao, K.; Tang, F.; Qian, Y.; Zhang, Y.; Meng, S. Evaporation and combustion characteristics for a droplet of lubricating oil and gasoline. Int. Commun. Heat Mass Transf. 2021, 127, 105513–105520. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Zhang, Y.; Li, Y.; Tam, W.; Kong, D.; Deng, J. New insights into the ignition characteristics of liquid fuels on hot surfaces based on TG-FTIR. Appl. Energy 2024, 360, 122827. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Deng, J.; Chen, J.; Ji, X.; Wu, H.; Zhao, J. Experimental study on combustion characteristics of electrolyte pool fire. J. Energy Storage 2024, 93, 112214. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, Q. Experimental discussion on fire image recognition based on feature extraction. J. Phys. Conf. Ser. 2021, 2066, 21086–21094. [Google Scholar] [CrossRef]
- De, S.; Bhattacharya, A.; Mukhopadhyay, A.; Sen, S. Early detection of lean blowout in a combustor using symbolic analysis of colour images. Measurement 2021, 186, 110113–110130. [Google Scholar] [CrossRef]
- Chen, J.; He, Y.; Wang, J. Multi-feature fusion based fast video flame detection. Build. Environ. 2010, 45, 1113–1122. [Google Scholar] [CrossRef]
- Hu, A. Color features description and extraction of flame image segmented region of early fire in large space. Adv. Mater. Res. 2013, 706, 1862–1865. [Google Scholar] [CrossRef]
- Xie, K.; Cui, Y.; Wang, C.; Cui, G.; Wang, G.; Qiu, X. Study on threshold selection method of continuous flame images of spray combustion in the low-pressure chamber. Case Stud. Therm. Eng. 2021, 26, 101195–102103. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, L.; Xing, Y. Study on analytical noise propagation in convolutional neural network methods used in computed tomography imaging. Nucl. Sci. Tech. 2022, 33, 116–129. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Y.; Han, Q.; Bai, X. Study on ignition process and flame expansion and propagation characteristics in jet-cooled pilot flameholders using image processing techniques. Aerosp. Sci. Technol. 2022, 129, 107807–107818. [Google Scholar] [CrossRef]
- Patcharaporn, W.; Makiko, T.; Akio, U.; Mohammad, H.; Isao, Y.; Hidetoshi, O. Isolation and characterization of novel strains of pseudomonas aeruginosa and serratia marcescens possessing high efficiency to degrade gasoline, kerosene, diesel oil, and lubricating oil. Curr. Microbiol. 2004, 49, 415–422. [Google Scholar]
- Wang, Z.; Chen, J.; Yu, Y.; Kong, D. Experimental study on the ignition and burning characteristics of liquid fuels on hot surfaces. Process Saf. Environ. Prot. 2023, 176, 725–733. [Google Scholar] [CrossRef]
- Pejman, N.; Ehsan, H.; Mehdi, A.; Pilva, S. Experimental investigation on premixed flame of H2/CO in a slot burner using the Mach-Zehnder interferometry. Opt. Laser. Technol. 2019, 115, 140–148. [Google Scholar]
- Shang, J.; Gan, Z.; Li, J. Effects of pressure on soot formation in laminar coflow kerosene diffusion flames at pressures between 1 and 20 atm. J. Therm. Anal. Calorim. 2024, 149, 4733–4753. [Google Scholar] [CrossRef]
- Tang, Q.; Shi, H.; Uddeen, K.; Sharma, P.; Yao, M.; Turner, J.W.G.; Magnotti, G. Study of engine knocking combustion using simultaneous high-speed shadowgraph and natural flame luminosity imaging. Appl. Therm. Eng. 2023, 235, 121440. [Google Scholar] [CrossRef]
- Seunghyun, J. Effects of CO2 dilution on ignition characteristics in lean premixed H2/Air flames. Int. J. Hydrogen Energy 2024, 84, 265–278. [Google Scholar]
- Zhang, X.; Liu, X.; Huang, W.; Hu, J.; Du, Y.; Ge, D.; Sun, X. Gas-flow-rate inversion based on experiments and simulation of flame combustion characteristics in a drilling blowout. Processes 2024, 12, 766. [Google Scholar] [CrossRef]
- Pezoa, J.; Arias, L. Data-Driven Models for Predicting the Flame Spectral Behavior in Industrial Combustion Processes; Universidad de Concepción: Concepción, Chile, 2012; Volume 8439, pp. 84391A–84391A-10. [Google Scholar]
- Wang, L.; Pan, J.; Wei, H.; Shu, G. Thermal and chemical effects of low-temperature chemistry on flame initiation and propagation. Combust. Flame 2023, 253, 112779. [Google Scholar] [CrossRef]
- Walter, V.; Pascal, S.; Patrick, A.; Silas, W.; Kai, H. Experimental study of lube oil pre-ignition in an optical gas engine test rig. Int. J. Engine Res. 2024, 25, 1037–1049. [Google Scholar]
- Tormos, B.; Garcia-Oliver, J.M.; Carreres, M.; Moreno-Montagud, C.; Dominguez, B.; Cardenas, M.D. Experimental assessment of ignition characteristics of lubricating oil sprays related to low-speed preignition. Int. J. Engine Res. 2022, 23, 1327–1338. [Google Scholar] [CrossRef]
- Shi, Y.X.; Ni, S.J.; Zhao, N.; Wang, W.K.; Cai, Y.X. Effect of aged lubricating oil on the regeneration of diesel particulate filters and ash physical characteristics with non-thermal plasma technology. Int. J. Automot. Technol. 2021, 22, 1189–1200. [Google Scholar]
- Espada, J.J.; Coto, B.; Grieken, R.V.; Moreno, J.M. Simulation of pilotplant extraction experiments to reduce the aromatic content from lubricating oils. Chem. Eng. Process. 2007, 47, 1403–1415. [Google Scholar]
- Sommer, H.T. Ignition studies of fuel droplet streams. Symp. Combust. 1988, 21, 641–646. [Google Scholar] [CrossRef]
- Yehia, O.R.; Reuter, C.B.; Ju, Y. Low-temperature multistage warm diffusion flames. Combust. Flame 2018, 195, 63–74. [Google Scholar] [CrossRef]
- Johnson, A.M.; Roth, A.J.; Moussa, N.A. Hot-Surface Ignition Tests of Aircraft Fluids. Final Report, May 1987–May 1988; Boeing Advanced Systems Co.: Seattle, WA, USA, 1988. [Google Scholar]
- Yamamoto, A.; Oshibe, H.; Nakamura, H.; Tezuka, T.; Hasegawa, S.; Maruta, K. Stabilized three-stage oxidation of gaseous n-heptane/air mixture in a micro flow reactor with a controlled temperature profile. Proc. Combust. Inst. 2011, 33, 3259–3266. [Google Scholar] [CrossRef]
- Zhang, T.; Ju, Y. Structures and propagation speeds of autoignition-assisted premixed n-heptane/air cool and warm flames at elevated temperatures and pressures. Combust. Flame 2020, 211, 8–17. [Google Scholar] [CrossRef]
- Yuan, L. Ignition of hydraulic fluid sprays by open flames and hot surfaces. J. Loss Prev. Process Ind. 2005, 19, 353–361. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Duan, H.; Jia, M.; Zhang, Y. Experimental study on the boiling criterion of the fuel film formed from spray/wall impingement. Exp. Fluids 2019, 60, 179. [Google Scholar] [CrossRef]
- Feoktistov, D.; Glushkov, D.; Kuznetsov, G.; Orlova, E.; Paushkinaet, K. Ignition and combustion enhancement of composite fuel in conditions of droplets dispersion during conductive heating on steel surfaces with different roughness parameters. Fuel 2022, 314, 122745. [Google Scholar] [CrossRef]
- Yi, P.; Long, W.; Feng, L.; Wang, W.; Liu, C. An experimental and numerical study of the evaporation and pyrolysis characteristics of lubricating oil droplets in the natural gas engine conditions. Int. J. Heat Mass Tranf. 2016, 103, 646–660. [Google Scholar] [CrossRef]
- Zhu, F.; Li, K. Numerical Modeling of Heat and Moisture Through Wet Cotton Fabric Using the Method of Chemical Thermodynamic Law Under Simulated Fire. Fire Technol. 2011, 47, 801–819. [Google Scholar] [CrossRef]
- John, G.; Benjamin, L.; Brian, G. Experimental study of high compression ratio spark ignition with ethanol, ethanol–water blends, and methanol. Fuel 2024, 375, 132528. [Google Scholar]
- Zhang, T.; Ju, Y. Propagation speeds and kinetic analysis of premixed hep tane/air cool and warm flames at large ignition. Damköhler numbers. In Proceedings of the 11th US National Combustion Meeting, Pasadena, CA, USA, 24–27 March 2019; pp. 1–4. [Google Scholar]
- Peters, G.P.; Andrew, R.M.; Canadell, J.G.; Friedlingstein, P.; Jackson, R.B.; Korsbakken, J.I.; Le Quéré, C.; Peregon, A. Carbon dioxide emissions continue to grow amidst slowly emerging climate policies. Nat. Clim. Change 2020, 10, 3–6. [Google Scholar] [CrossRef]




| Physical Parameters | Standard | Results |
|---|---|---|
| Kinematic viscosity at 100 °C | ASTM D445 | 13.420 mm2/s |
| Kinematic viscosity at 40 °C | ASTM D445 | 74.190 mm2/s |
| Flash point | ASTM D92 | 234 °C |
| Boiling temperature | ASTM D92 | 232 °C |
| Surface tension | ASTM D971 | 0.03 N/m |
| Dynamic viscosity (−35 °C) | ASTM D5293 | 6125 mpa.s |
| Viscosity index | ASTM D2270 | 180 |
| Density (15 °C) | ASTM D4052 | 0.85 kg/m3 |
| Evaporative loss | ASTM D5800 | 10.16% |
| Zinc content | ASTM D6481 | 0.104% |
| Sulphur content | ASTM D874 | 0.78% |
| Oil Samples | Oil Volume (mL) | Minimum Ignition Temperature Tmin (°C) | Maximum Ignition Temperature Tmax (°C) | ||
|---|---|---|---|---|---|
| Test Value | Average Value | Test Value | Average Value | ||
| LO-0.1-1 | 0.1 | 370 | 370 | 415 | 415 |
| LO-0.1-2 | 368 | 416 | |||
| LO-0.1-3 | 369 | 415 | |||
| LO-0.1-4 | 370 | 414 | |||
| LO-0.1-5 | 369 | 415 | |||
| LO-0.1-6 | 372 | 417 | |||
| LO-0.1-7 | 371 | 416 | |||
| LO-0.1-8 | 370 | 414 | |||
| LO-0.1-9 | 371 | 415 | |||
| LO-0.1-10 | 368 | 416 | |||
| LO-0.2-1 | 0.2 | 347 | 347 | 390 | 390 |
| LO-0.2-2 | 344 | 388 | |||
| LO-0.2-3 | 348 | 389 | |||
| LO-0.2-4 | 346 | 392 | |||
| LO-0.2-5 | 349 | 392 | |||
| LO-0.2-6 | 350 | 389 | |||
| LO-0.2-7 | 347 | 391 | |||
| LO-0.2-8 | 346 | 390 | |||
| LO-0.2-9 | 346 | 389 | |||
| LO-0.2-10 | 348 | 390 | |||
| LO-0.3-1 | 0.3 | 345 | 343 | 385 | 385 |
| LO-0.3-2 | 343 | 384 | |||
| LO-0.3-3 | 343 | 386 | |||
| LO-0.3-4 | 342 | 383 | |||
| LO-0.3-5 | 346 | 388 | |||
| LO-0.3-6 | 345 | 385 | |||
| LO-0.3-7 | 340 | 384 | |||
| LO-0.3-8 | 341 | 383 | |||
| LO-0.3-9 | 343 | 387 | |||
| LO-0.3-10 | 343 | 385 | |||
| LO-0.4-1 | 0.4 | 309 | 310 | 360 | 360 |
| LO-0.4-2 | 310 | 359 | |||
| LO-0.4-3 | 310 | 361 | |||
| LO-0.4-4 | 312 | 361 | |||
| LO-0.4-5 | 308 | 358 | |||
| LO-0.4-6 | 309 | 360 | |||
| LO-0.4-7 | 310 | 360 | |||
| LO-0.4-8 | 310 | 361 | |||
| LO-0.4-9 | 311 | 362 | |||
| LO-0.4-10 | 312 | 358 | |||
| LO-0.5-1 | 0.5 | 311 | 312 | 355 | 355 |
| LO-0.5-2 | 313 | 356 | |||
| LO-0.5-3 | 312 | 356 | |||
| LO-0.5-4 | 311 | 354 | |||
| LO-0.5-5 | 312 | 355 | |||
| LO-0.5-6 | 310 | 353 | |||
| LO-0.5-7 | 313 | 355 | |||
| LO-0.5-8 | 312 | 355 | |||
| LO-0.5-9 | 314 | 356 | |||
| LO-0.5-10 | 312 | 355 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, L.; Cheng, F.; Dong, Y. Ignition Characteristics and Flame Behavior of Automotive Lubricating Oil on Hot Surfaces. Processes 2024, 12, 2522. https://doi.org/10.3390/pr12112522
Bai L, Cheng F, Dong Y. Ignition Characteristics and Flame Behavior of Automotive Lubricating Oil on Hot Surfaces. Processes. 2024; 12(11):2522. https://doi.org/10.3390/pr12112522
Chicago/Turabian StyleBai, Lei, Fangming Cheng, and Yuting Dong. 2024. "Ignition Characteristics and Flame Behavior of Automotive Lubricating Oil on Hot Surfaces" Processes 12, no. 11: 2522. https://doi.org/10.3390/pr12112522
APA StyleBai, L., Cheng, F., & Dong, Y. (2024). Ignition Characteristics and Flame Behavior of Automotive Lubricating Oil on Hot Surfaces. Processes, 12(11), 2522. https://doi.org/10.3390/pr12112522





