Advanced Control Strategies of Membrane Fouling in Wastewater Treatment: A Review
Abstract
:1. Introduction
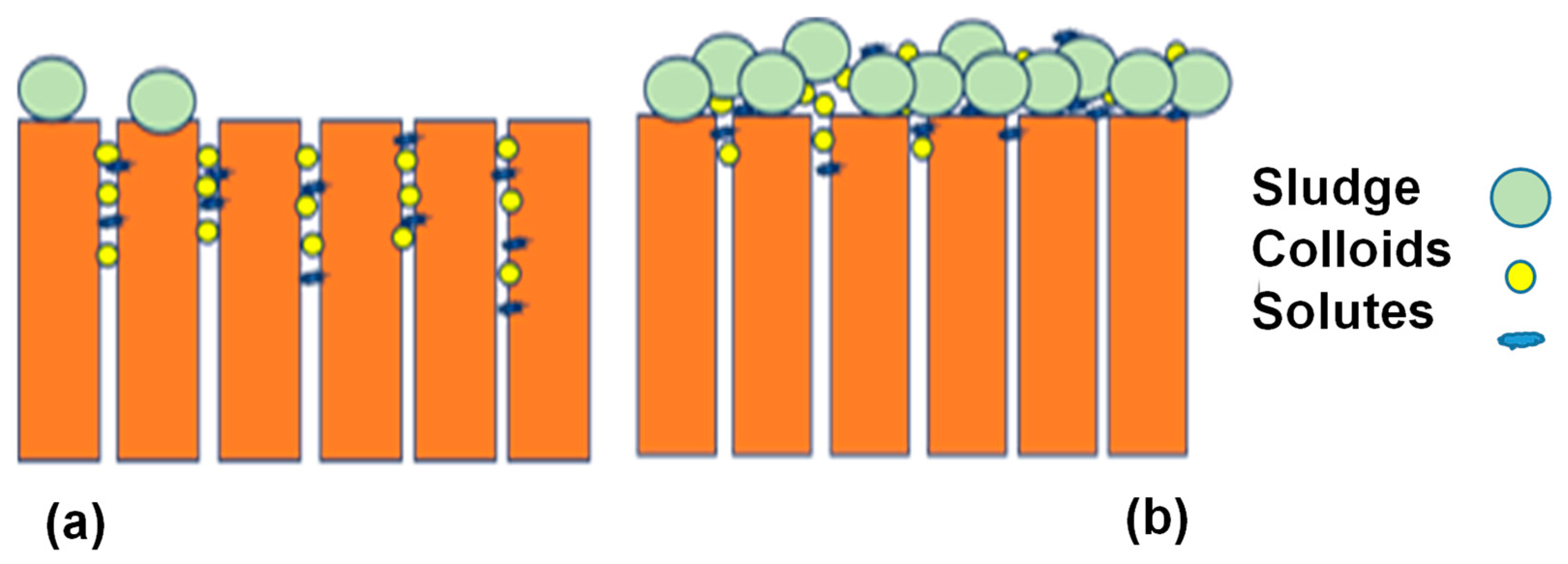
2. Membrane Fouling
2.1. Membrane Fouling Phenomenon
2.2. Classification of Membrane Fouling
2.2.1. Reversible and Irreversible Fouling
2.2.2. Bio Fouling
2.2.3. Organic Fouling
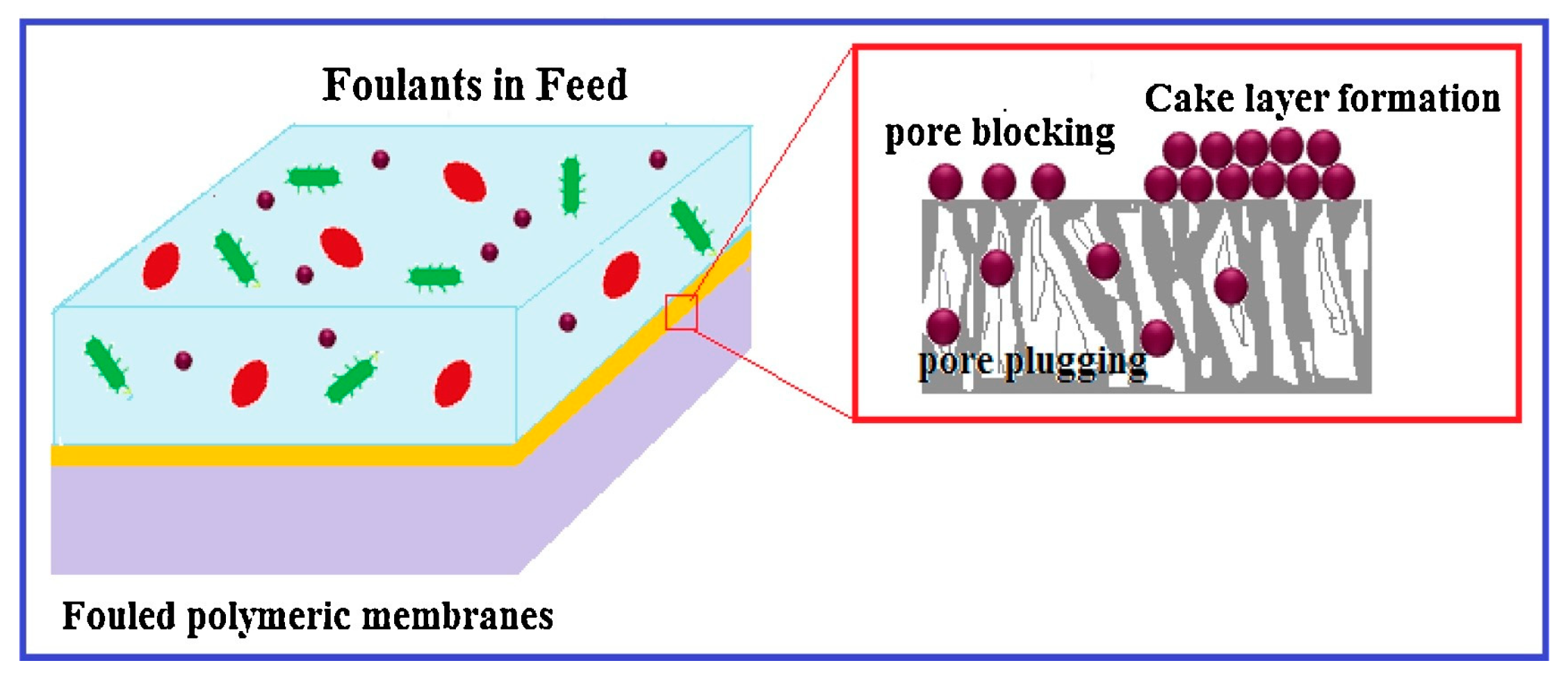
2.2.4. Inorganic Fouling
3. Membrane Fouling Mitigation
3.1. Surface Modification of Membranes
3.2. Physical Surface Modifications
3.3. Chemical Surface Modifications
3.4. Treatment by Plasma
3.5. Fouling-Resistant Coatings
3.5.1. Golden Standard-Linear PEG Brushes Coatings
3.5.2. Bottle Brushes
3.5.3. Nanoparticle Thin Films Coatings
3.6. Novel Membrane Materials
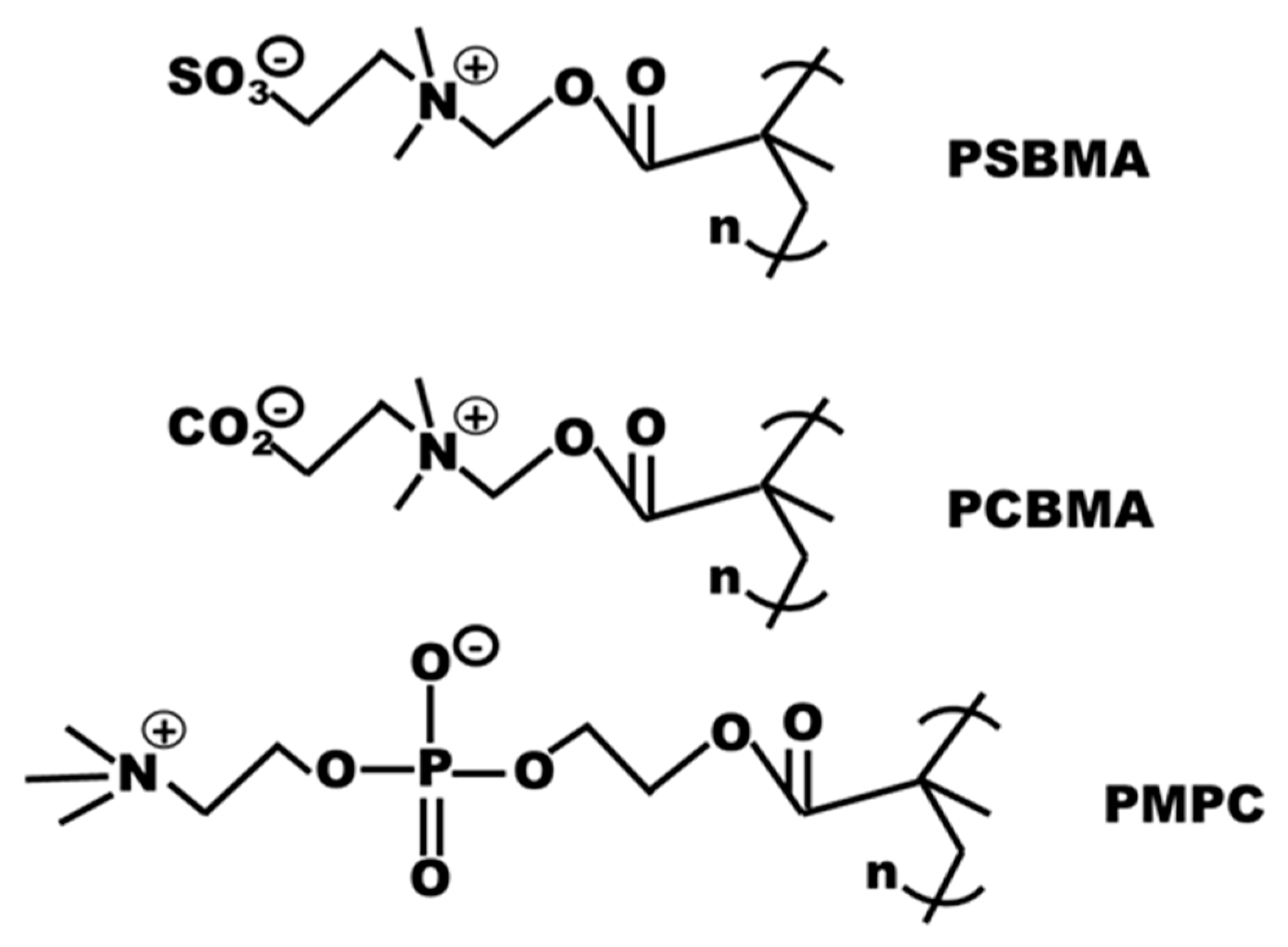
3.7. Zwitterial Materials
3.7.1. Super Hydrophilicity in Zwitterionic Materials
3.7.2. Surface Coating Using Dense Zwitterions
3.7.3. Surface Grafting of Zwitterions
3.7.4. Hydrophilic Surface Modification
3.8. Role of Economic Membrane Technology in Sustainable Water Generation
3.9. Interaction Between Membranes and Emerging Pollutants
3.10. Potential of New Methods for Wastewater Treatment and Their Advantges and Disadvantages
3.11. Future Trends of Membrane
4. Conclusions
5. Future Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, S.; Singh, A.; Sharma, T.; Kaur, R.; Khandelwal, V.; Rawat, K.D.; Pathak, S.; Sharma, M.K.; Singh, J.; Shah, M.P. Applications of ultrafiltration, nanofiltration, and reverse osmosis in pharmaceutical wastewater treatment. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2024; pp. 33–49. [Google Scholar]
- Mohammed, S.; Aburabie, J.; Hashaikeh, R. A review on the potential of cellulose nanomaterials for the development of thin film composite polyamide membranes for water treatment. Chemosphere 2024, 363, 142927. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.; Hughes, R.; Hughes, R. Industrial Membrane Separation Technology; Springer Science & Business Media: New York, NY, USA, 1996. [Google Scholar]
- Zhang, J.; Li, G.; Yuan, X.; Li, P.; Yu, Y.; Yang, W.; Zhao, S. Reduction of Ultrafiltration Membrane Fouling by the Pretreatment Removal of Emerging Pollutants: A Review. Membranes 2023, 13, 77. [Google Scholar] [CrossRef]
- Abu-Zurayk, R.; Alnairat, N.; Khalaf, A.; Ibrahim, A.; Halaweh, G. Cellulose Acetate Membranes: Fouling Types and Antifouling Strategies—A Brief Review. Processes 2023, 11, 489. [Google Scholar] [CrossRef]
- Abulkhair, H.; Moujdin, I.A.; Kaddoura, B.; Khan, M.S. Fouling Mitigation in Membrane Distillation Using Pulsation Flow Technique. Processes 2023, 11, 2759. [Google Scholar] [CrossRef]
- Tomczak, W. Fouling of the Nanofiltration Membrane NF270 Used for Separation of Fermentation Broths: Impact of Feed Pretreatment Process. Processes 2023, 11, 817. [Google Scholar] [CrossRef]
- Russo, F.; Bulzomì, M.; Di Nicolò, E.; Ursino, C.; Figoli, A. Enhanced anti-fouling behavior and performance of pes membrane by uv treatment. Processes 2021, 9, 246. [Google Scholar] [CrossRef]
- Aquino, M.; Santoro, S.; Di Profio, G.; La Russa, M.F.; Limonti, C.; Straface, S.; D’Andrea, G.; Curcio, E.; Siciliano, A. Membrane distillation for separation and recovery of valuable compounds from anaerobic digestates. Sep. Purif. Technol. 2023, 315, 123687. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Drews, A. Membrane fouling in membrane bioreactors—Characterisation, contradictions, cause and cures. J. Membr. Sci. 2010, 363, 1–28. [Google Scholar] [CrossRef]
- Le-Clech, P. Membrane bioreactors and their uses in wastewater treatments. Appl. Microbiol. Biotechnol. 2010, 88, 1253–1260. [Google Scholar] [CrossRef]
- Lin, H.; Gao, W.; Meng, F.; Liao, B.-Q.; Leung, K.-T.; Zhao, L.; Chen, J.; Hong, H. Membrane bioreactors for industrial wastewater treatment: A critical review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 677–740. [Google Scholar] [CrossRef]
- Mutamim, N.S.A.; Noor, Z.Z.; Hassan, M.A.A.; Olsson, G. Application of membrane bioreactor technology in treating high strength industrial wastewater: A performance review. Desalination 2012, 305, 1–11. [Google Scholar] [CrossRef]
- Porcelli, N.; Judd, S. Chemical cleaning of potable water membranes: A review. Sep. Purif. Technol. 2010, 71, 137–143. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Y. Biological control of microbial attachment: A promising alternative for mitigating membrane biofouling. Appl. Microbiol. Biotechnol. 2010, 86, 825–837. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.-H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Lin, H.; Langevin, S.; Gao, W.; Leppard, G. Effects of temperature and dissolved oxygen on sludge properties and their role in bioflocculation and settling. Water Res. 2011, 45, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Ghayeni, S.S.; Madaeni, S.; Fane, A.; Schneider, R. Aspects of microfiltration and reverse osmosis in municipal wastewater reuse. Desalination 1996, 106, 25–29. [Google Scholar] [CrossRef]
- Bennett, A. Membranes in industry: Facilitating reuse of wastewater. Filtr. Sep. 2005, 42, 28–30. [Google Scholar] [CrossRef]
- Maddela, N.R.; Abiodun, A.S.; Zhang, S.; Prasad, R. Biofouling in membrane bioreactors—Mitigation and current status: A review. Appl. Biochem. Biotechnol. 2023, 195, 5643–5668. [Google Scholar] [CrossRef]
- Sullivan, F.; Europe, F.S. Membrane filtration technologies tackle water reuse and purification. Membr. Technol. 2007, 2007, 9–11. [Google Scholar]
- Zheng, W.; Chen, Y.; Xu, X.; Peng, X.; Niu, Y.; Xu, P.; Li, T. Research on the factors influencing nanofiltration membrane fouling and the prediction of membrane fouling. J. Water Process Eng. 2024, 59, 104876. [Google Scholar] [CrossRef]
- Brinck, J.; Jönsson, A.-S.; Jönsson, B.; Lindau, J. Influence of pH on the adsorptive fouling of ultrafiltration membranes by fatty acid. J. Membr. Sci. 2000, 164, 187–194. [Google Scholar] [CrossRef]
- Mänttäri, M.; Puro, L.; Nuortila-Jokinen, J.; Nyström, M. Fouling effects of polysaccharides and humic acid in nanofiltration. J. Membr. Sci. 2000, 165, 1–17. [Google Scholar] [CrossRef]
- Mänttäri, M.; Pihlajamäki, A.; Kaipainen, E.; Nyström, M. Effect of temperature and membrane pre-treatment by pressure on the filtration properties of nanofiltration membranes. Desalination 2002, 145, 81–86. [Google Scholar] [CrossRef]
- Yang, J.; Spanjers, H.; Jeison, D.; Van Lier, J.B. Impact of Na+ on biological wastewater treatment and the potential of anaerobic membrane bioreactors: A review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 2722–2746. [Google Scholar] [CrossRef]
- Simstich, B.; Beimfohr, C.; Horn, H. Lab scale experiments using a submerged MBR under thermophilic aerobic conditions for the treatment of paper mill deinking wastewater. Bioresour. Technol. 2012, 122, 11–16. [Google Scholar] [CrossRef]
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A review on anaerobic membrane bioreactors: Applications, membrane fouling and future perspectives. Desalination 2013, 314, 169–188. [Google Scholar] [CrossRef]
- Qu, X.; Gao, W.; Han, M.; Chen, A.; Liao, B. Effect of hydraulic retention time on sludge properties, cake layer structure, and membrane fouling in a thermophilic submerged aerobic membrane bioreactor. Sep. Sci. Technol. 2013, 48, 1529–1536. [Google Scholar] [CrossRef]
- Lin, H.; Gao, W.; Leung, K.; Liao, B. Characteristics of different fractions of microbial flocs and their role in membrane fouling. Water Sci. Technol. 2011, 63, 262–269. [Google Scholar] [CrossRef]
- Gao, W.J.; Lin, H.; Leung, K.; Liao, B. Influence of elevated pH shocks on the performance of a submerged anaerobic membrane bioreactor. Process Biochem. 2010, 45, 1279–1287. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, W.-Y.; Lee, C.-H. Comparison of the filtration characteristics between attached and suspended growth microorganisms in submerged membrane bioreactor. Water Res. 2001, 35, 2435–2445. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.M.; Hong, P.; Guo, H.; Liu, W.-T. Biofilm formation characteristics of bacterial isolates retrieved from a reverse osmosis membrane. Environ. Sci. Technol. 2005, 39, 7541–7550. [Google Scholar] [CrossRef] [PubMed]
- Gizer, G.; Önal, U.; Ram, M.; Şahiner, N. Biofouling and mitigation methods: A review. Biointerface Res. Appl. Chem. 2023, 13, 185. [Google Scholar]
- Ramesh, A.; Lee, D.; Lai, J. Membrane biofouling by extracellular polymeric substances or soluble microbial products from membrane bioreactor sludge. Appl. Microbiol. Biotechnol. 2007, 74, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Watnick, P.; Kolter, R. Biofilm, city of microbes. J. Bacteriol. 2000, 182, 2675–2679. [Google Scholar] [CrossRef]
- Ivnitsky, H.; Katz, I.; Minz, D.; Shimoni, E.; Chen, Y.; Tarchitzky, J.; Semiat, R.; Dosoretz, C. Characterization of membrane biofouling in nanofiltration processes of wastewater treatment. Desalination 2005, 185, 255–268. [Google Scholar] [CrossRef]
- Neu, T.R.; Lawrence, J.R. In situ characterization of extracellular polymeric substances (EPS) in biofilm systems. Microb. Extracell. Polym. Subst. Charact. Struct. Funct. 1999, 21–47. [Google Scholar]
- Maddah, H.; Chogle, A. Biofouling in reverse osmosis: Phenomena, monitoring, controlling and remediation. Appl. Water Sci. 2017, 7, 2637–2651. [Google Scholar] [CrossRef]
- Yigit, N.; Harman, I.; Civelekoglu, G.; Koseoglu, H.; Cicek, N.; Kitis, M. Membrane fouling in a pilot-scale submerged membrane bioreactor operated under various conditions. Desalination 2008, 231, 124–132. [Google Scholar] [CrossRef]
- Rosenberger, S.; Kraume, M. Filterability of activated sludge in membrane bioreactors. Desalination 2003, 151, 195–200. [Google Scholar] [CrossRef]
- Fallah, N.; Bonakdarpour, B.; Nasernejad, B.; Moghadam, M.A. Long-term operation of submerged membrane bioreactor (MBR) for the treatment of synthetic wastewater containing styrene as volatile organic compound (VOC): Effect of hydraulic retention time (HRT). J. Hazard. Mater. 2010, 178, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Al-Halbouni, D.; Traber, J.; Lyko, S.; Wintgens, T.; Melin, T.; Tacke, D.; Janot, A.; Dott, W.; Hollender, J. Correlation of EPS content in activated sludge at different sludge retention times with membrane fouling phenomena. Water Res. 2008, 42, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Sponza, D.T. Extracellular polymer substances and physicochemical properties of flocs in steady and unsteady-state activated sludge systems. Process Biochem. 2002, 37, 983–998. [Google Scholar] [CrossRef]
- Jin, Y.-L.; Lee, W.-N.; Lee, C.-H.; Chang, I.-S.; Huang, X.; Swaminathan, T. Effect of DO concentration on biofilm structure and membrane filterability in submerged membrane bioreactor. Water Res. 2006, 40, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.-A.; Yeon, K.-M.; Park, J.-S.; Lee, C.-H.; Chun, J.; Lim, D.J. Characterization of biofilm structure and its effect on membrane permeability in MBR for dye wastewater treatment. Water Res. 2006, 40, 45–52. [Google Scholar] [CrossRef]
- Ng, H.; Tan, T.; Ong, S.; Toh, C.; Loo, Z. Effects of solid retention time on the performance of submerged anoxic/oxic membrane bioreactor. Water Sci. Technol. 2006, 53, 7–13. [Google Scholar] [CrossRef]
- Dereli, R.K.; Grelot, A.; Heffernan, B.; van der Zee, F.P.; van Lier, J.B. Implications of changes in solids retention time on long term evolution of sludge filterability in anaerobic membrane bioreactors treating high strength industrial wastewater. Water Res. 2014, 59, 11–22. [Google Scholar] [CrossRef]
- Tian, Y.; Li, H.; Li, L.; Su, X.; Lu, Y.; Zuo, W.; Zhang, J. In-situ integration of microbial fuel cell with hollow-fiber membrane bioreactor for wastewater treatment and membrane fouling mitigation. Biosens. Bioelectron. 2015, 64, 189–195. [Google Scholar] [CrossRef]
- Alturki, A.; McDonald, J.; Khan, S.J.; Hai, F.I.; Price, W.E.; Nghiem, L.D. Performance of a novel osmotic membrane bioreactor (OMBR) system: Flux stability and removal of trace organics. Bioresour. Technol. 2012, 113, 201–206. [Google Scholar] [CrossRef]
- Aslam, M.; McCarty, P.L.; Shin, C.; Bae, J.; Kim, J. Low energy single-staged anaerobic fluidized bed ceramic membrane bioreactor (AFCMBR) for wastewater treatment. Bioresour. Technol. 2017, 240, 33–41. [Google Scholar] [CrossRef]
- Jin, Z.; Meng, F.; Gong, H.; Wang, C.; Wang, K. Improved low-carbon-consuming fouling control in long-term membrane-based sewage pre-concentration: The role of enhanced coagulation process and air backflushing in sustainable sewage treatment. J. Membr. Sci. 2017, 529, 252–262. [Google Scholar] [CrossRef]
- Ayyavoo, J.; Nguyen, T.P.N.; Jun, B.-M.; Kim, I.-C.; Kwon, Y.-N. Protection of polymeric membranes with antifouling surfacing via surface modifications. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 190–201. [Google Scholar] [CrossRef]
- Kim, S.-R.; Lee, K.-B.; Kim, J.-E.; Won, Y.-J.; Yeon, K.-M.; Lee, C.-H.; Lim, D.-J. Macroencapsulation of quorum quenching bacteria by polymeric membrane layer and its application to MBR for biofouling control. J. Membr. Sci. 2015, 473, 109–117. [Google Scholar] [CrossRef]
- Ali, S.M.; Kim, J.E.; Phuntsho, S.; Jang, A.; Choi, J.Y.; Shon, H.K. Forward osmosis system analysis for optimum design and operating conditions. Water Res. 2018, 145, 429–441. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef]
- Son, M.; Yang, W.; Bucs, S.S.; Nava-Ocampo, M.F.; Vrouwenvelder, J.S.; Logan, B.E. Polyelectrolyte-based sacrificial protective layer for fouling control in reverse osmosis desalination. Environ. Sci. Technol. Lett. 2018, 5, 584–590. [Google Scholar] [CrossRef]
- Akbari, A.; Derikvandi, Z.; Rostami, S.M.M. Influence of chitosan coating on the separation performance, morphology and anti-fouling properties of the polyamide nanofiltration membranes. J. Ind. Eng. Chem. 2015, 28, 268–276. [Google Scholar] [CrossRef]
- Lei, J.; Ulbricht, M. Macroinitiator-mediated photoreactive coating of membrane surfaces with antifouling hydrogel layers. J. Membr. Sci. 2014, 455, 207–218. [Google Scholar] [CrossRef]
- Zhang, S.; Qiu, G.; Ting, Y.P.; Chung, T.-S. Silver–PEGylated dendrimer nanocomposite coating for anti-fouling thin film composite membranes for water treatment. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 207–214. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H.; Beygzadeh, M. Novel high flux antifouling nanofiltration membranes for dye removal containing carboxymethyl chitosan coated Fe3O4 nanoparticles. Desalination 2014, 349, 145–154. [Google Scholar] [CrossRef]
- Liu, L.-F.; Yu, S.-C.; Wu, L.-G.; Gao, C.-J. Study on a novel polyamide-urea reverse osmosis composite membrane (ICIC-MPD): II. Analysis of membrane antifouling performance. J. Membr. Sci. 2006, 283, 133–146. [Google Scholar] [CrossRef]
- Bai, L.; Liang, H.; Crittenden, J.; Qu, F.; Ding, A.; Ma, J.; Du, X.; Guo, S.; Li, G. Surface modification of UF membranes with functionalized MWCNTs to control membrane fouling by NOM fractions. J. Membr. Sci. 2015, 492, 400–411. [Google Scholar] [CrossRef]
- Li, Q.; Chen, G.Q.; Liu, L.; Kentish, S.E. Spray assisted layer-by-layer assembled one-bilayer polyelectrolyte reverse osmosis membranes. J. Membr. Sci. 2018, 564, 501–507. [Google Scholar] [CrossRef]
- Halakoo, E.; Feng, X. Layer-by-layer assembly of polyethyleneimine/graphene oxide membranes for desalination of high-salinity water via pervaporation. Sep. Purif. Technol. 2020, 234, 116077. [Google Scholar] [CrossRef]
- Shao, F.; Dong, L.; Dong, H.; Zhang, Q.; Zhao, M.; Yu, L.; Pang, B.; Chen, Y. Graphene oxide modified polyamide reverse osmosis membranes with enhanced chlorine resistance. J. Membr. Sci. 2017, 525, 9–17. [Google Scholar] [CrossRef]
- Nurkhamidah, S.; Devi, B.; Febriansyah, B.; Ramadhani, A.; Nyamiati, R.; Rahmawati, Y.; Chafidz, A. Characteristics of Cellulose Acetate/Polyethylene Glycol membrane with the addition of Graphene Oxide by using surface coating method. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1184, 012002. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Y.; Guo, M.; Pan, G.; Shi, H.; Yao, X.; Liu, Y. Surface modification on thin-film composite reverse osmosis membrane by cation complexation for antifouling. J. Polym. Res. 2019, 26, 68. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Delnavaz, M.; Vatanpour, V. Investigation of raw and oxidized multiwalled carbon nanotubes in fabrication of reverse osmosis polyamide membranes for improvement in desalination and antifouling properties. Desalination 2017, 410, 1–9. [Google Scholar] [CrossRef]
- Isawi, H.; El-Sayed, M.H.; Feng, X.; Shawky, H.; Mottaleb, M.S.A. Surface nanostructuring of thin film composite membranes via grafting polymerization and incorporation of ZnO nanoparticles. Appl. Surf. Sci. 2016, 385, 268–281. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Y.; Pan, G.; Shi, H.; Yan, H.; Xu, J.; Guo, M.; Wang, Z.; Liu, Y. Surface modification of polyamide reverse osmosis membrane with sulfonated polyvinyl alcohol for antifouling. Appl. Surf. Sci. 2017, 419, 177–187. [Google Scholar] [CrossRef]
- Falath, W.; Sabir, A.; Jacob, K.I. Novel reverse osmosis membranes composed of modified PVA/Gum Arabic conjugates: Biofouling mitigation and chlorine resistance enhancement. Carbohydr. Polym. 2017, 155, 28–39. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Flux and salt rejection enhancement of polyvinyl (alcohol) reverse osmosis membranes using nano-zeolite. Desalination 2019, 470, 114104. [Google Scholar] [CrossRef]
- Miyamoto, K.; Ikeshima, D.; Furutani, T.; Xiao, H.; Yonezu, A.; Chen, X. On the surface hydrophilization of a blended polysulfone membrane: Atomic force microscopy measurement and molecular dynamics simulation. Surf. Topogr. Metrol. Prop. 2019, 7, 035003. [Google Scholar] [CrossRef]
- Ragunath, S.; Roy, S.; Mitra, S. Selective hydrophilization of the permeate surface to enhance flux in membrane distillation. Sep. Purif. Technol. 2016, 170, 427–433. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Z.; Zhang, Z.; Wang, J.; Wang, S. Surface modification of commercial aromatic polyamide reverse osmosis membranes by graft polymerization of 3-allyl-5, 5-dimethylhydantoin. J. Membr. Sci. 2010, 351, 222–233. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, K.; Yan, F.; Shi, Y.; Yu, P.; Yu, S.; Li, S.; Gao, C. Enhancing the performance of aromatic polyamide reverse osmosis membrane by surface modification via covalent attachment of polyvinyl alcohol (PVA). J. Membr. Sci. 2016, 501, 209–219. [Google Scholar] [CrossRef]
- Lin, S.; Huang, H.; Zeng, Y.; Zhang, L.; Hou, L.a. Facile surface modification by aldehydes to enhance chlorine resistance of polyamide thin film composite membranes. J. Membr. Sci. 2016, 518, 40–49. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A.; Zarrabi, H.; Gholami, P.; Yekavalangi, M.E. High flux and fouling resistant reverse osmosis membrane modified with plasma treated natural zeolite. Desalination 2017, 411, 89–100. [Google Scholar] [CrossRef]
- Jahangiri, F.; Asadollahi, M.; Mousavi, S.A.; Farhadi, F. Improvement of performance of polyamide reverse osmosis membranes using dielectric barrier discharge plasma treatment as a novel surface modification method. Polym. Eng. Sci. 2019, 59, E468–E475. [Google Scholar] [CrossRef]
- Hirsch, U.; Ruehl, M.; Teuscher, N.; Heilmann, A. Antifouling coatings via plasma polymerization and atom transfer radical polymerization on thin film composite membranes for reverse osmosis. Appl. Surf. Sci. 2018, 436, 207–216. [Google Scholar] [CrossRef]
- Ankoliya, D.; Mehta, B.; Raval, H. Advances in surface modification techniques of reverse osmosis membrane over the years. Sep. Sci. Technol. 2019, 54, 293–310. [Google Scholar] [CrossRef]
- Choi, J.; Choi, Y.; Ju, J.; Park, Y.; Lee, S. Inorganic fouling mitigation using the baffle system in direct contact membrane distillation (DCMD). Desalination 2020, 496, 114714. [Google Scholar] [CrossRef]
- Lee, W.J.; Ng, Z.C.; Hubadillah, S.K.; Goh, P.S.; Lau, W.J.; Othman, M.; Ismail, A.F.; Hilal, N. Fouling mitigation in forward osmosis and membrane distillation for desalination. Desalination 2020, 480, 114338. [Google Scholar] [CrossRef]
- Seman, M.A.; Khayet, M.; Hilal, N. Development of antifouling properties and performance of nanofiltration membranes modified by interfacial polymerisation. Desalination 2011, 273, 36–47. [Google Scholar] [CrossRef]
- Zhang, H.; Lamb, R.; Lewis, J. Engineering nanoscale roughness on hydrophobic surface—Preliminary assessment of fouling behaviour. Sci. Technol. Adv. Mater. 2005, 6, 236–239. [Google Scholar] [CrossRef]
- Abid, H.S.; Johnson, D.J.; Hashaikeh, R.; Hilal, N. A review of efforts to reduce membrane fouling by control of feed spacer characteristics. Desalination 2017, 420, 384–402. [Google Scholar] [CrossRef]
- Gong, B.; Yang, H.; Wu, S.; Xiong, G.; Yan, J.; Cen, K.; Bo, Z.; Ostrikov, K. Graphene array-based anti-fouling solar vapour gap membrane distillation with high energy efficiency. Nano-Micro Lett. 2019, 11, 51. [Google Scholar] [CrossRef]
- Hou, D.; Ding, C.; Li, K.; Lin, D.; Wang, D.; Wang, J. A novel dual-layer composite membrane with underwater-superoleophobic/hydrophobic asymmetric wettability for robust oil-fouling resistance in membrane distillation desalination. Desalination 2018, 428, 240–249. [Google Scholar] [CrossRef]
- Francis, L.; Ghaffour, N.; Amy, G. Fabrication and characterization of functionally graded poly (vinylidine fluoride)-silver nanocomposite hollow fibers for sustainable water recovery. Sci. Adv. Mater. 2014, 6, 2659–2665. [Google Scholar] [CrossRef]
- Liao, Y.; Zheng, G.; Huang, J.J.; Tian, M.; Wang, R. Development of robust and superhydrophobic membranes to mitigate membrane scaling and fouling in membrane distillation. J. Membr. Sci. 2020, 601, 117962. [Google Scholar] [CrossRef]
- Magin, C.M.; Cooper, S.P.; Brennan, A.B. Non-toxic antifouling strategies. Mater. Today 2010, 13, 36–44. [Google Scholar] [CrossRef]
- Yang, W.J.; Neoh, K.-G.; Kang, E.-T.; Teo, S.L.-M.; Rittschof, D. Polymer brush coatings for combating marine biofouling. Prog. Polym. Sci. 2014, 39, 1017–1042. [Google Scholar] [CrossRef]
- Luo, M.-L.; Zhao, J.-Q.; Tang, W.; Pu, C.-S. Hydrophilic modification of poly (ether sulfone) ultrafiltration membrane surface by self-assembly of TiO2 nanoparticles. Appl. Surf. Sci. 2005, 249, 76–84. [Google Scholar] [CrossRef]
- Basheer, C.; Suresh, V.; Renu, R.; Lee, H.K. Development and application of polymer-coated hollow fiber membrane microextraction to the determination of organochlorine pesticides in water. J. Chromatogr. A 2004, 1033, 213–220. [Google Scholar] [CrossRef]
- Zhen, H.; Jang, S.M.; Teo, W.; Li, K. Modified silicone–PVDF composite hollow-fiber membrane preparation and its application in VOC separation. J. Appl. Polym. Sci. 2006, 99, 2497–2503. [Google Scholar] [CrossRef]
- Yeow, M.; Field, R.; Li, K.; Teo, W. Preparation of divinyl-PDMS/PVDF composite hollow fibre membranes for BTX removal. J. Membr. Sci. 2002, 203, 137–143. [Google Scholar] [CrossRef]
- Song, L.; Li, B.; Sirkar, K.K.; Gilron, J.L. Direct contact membrane distillation-based desalination: Novel membranes, devices, larger-scale studies, and a model. Ind. Eng. Chem. Res. 2007, 46, 2307–2323. [Google Scholar] [CrossRef]
- Greene, G.W.; Martin, L.L.; Tabor, R.F.; Michalczyk, A.; Ackland, L.M.; Horn, R. Lubricin: A versatile, biological anti-adhesive with properties comparable to polyethylene glycol. Biomaterials 2015, 53, 127–136. [Google Scholar] [CrossRef]
- Lee, J.H.; Kopecek, J.; Andrade, J.D. Protein-resistant surfaces prepared by PEO-containing block copolymer surfactants. J. Biomed. Mater. Res. 1989, 23, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Prime, K.L.; Whitesides, G.M. Self-assembled organic monolayers: Model systems for studying adsorption of proteins at aurfaces. Science 1991, 252, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Wazawa, T.; Ishizuka-Katsura, Y.; Nishikawa, S.; Iwane, A.H.; Aoyama, S. Grafting of poly (ethylene glycol) onto poly (acrylic acid)-coated glass for a protein-resistant surface. Anal. Chem. 2006, 78, 2549–2556. [Google Scholar] [CrossRef] [PubMed]
- Hui, N.; Sun, X.; Niu, S.; Luo, X. PEGylated polyaniline nanofibers: Antifouling and conducting biomaterial for electrochemical DNA sensing. ACS Appl. Mater. Interfaces 2017, 9, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Wang, H.; Hu, J.; Wang, X. Formation of antifouling functional coating from deposition of a zwitterionic-co-nonionic polymer via “grafting to” approach. J. Saudi Chem. Soc. 2019, 23, 1080–1089. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Xie, Q.; Pan, J.; Ma, C.; Zhang, G. Dynamic surface antifouling: Mechanism and systems. Soft Matter 2019, 15, 1087–1107. [Google Scholar] [CrossRef]
- Kalasin, S.; Letteri, R.; Emrick, T.; Santore, M. Adsorbed polyzwitterion copolymer layers designed for protein repellency and interfacial retention. Langmuir 2017, 33, 13708–13717. [Google Scholar] [CrossRef]
- Ma, H.; Hyun, J.; Stiller, P.; Chilkoti, A. “Non-fouling” oligo (ethylene glycol)-functionalized polymer brushes synthesized by surface-initiated atom transfer radical polymerization. Adv. Mater. 2004, 16, 338–341. [Google Scholar] [CrossRef]
- Mitra, I.; Li, X.; Pesek, S.L.; Makarenko, B.; Lokitz, B.S.; Uhrig, D.; Ankner, J.F.; Verduzco, R.; Stein, G.E. Thin film phase behavior of bottlebrush/linear polymer blends. Macromolecules 2014, 47, 5269–5276. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Y.; Zhang, S.; Liu, W.; Wu, Z.; Chen, H. Protein-resistant properties of poly (N-vinylpyrrolidone)-modified gold surfaces: The advantage of bottle-brushes over linear brushes. Colloids Surf. B Biointerfaces 2019, 177, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, D.; Sheng, X.; Zhao, B.; Chilkoti, A. Protein-resistant polymer coatings on silicon oxide by surface-initiated atom transfer radical polymerization. Langmuir 2006, 22, 3751–3756. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, C.; Bai, L.; Liu, S.; Tan, L.; Wang, Y. Antifouling property of monothiol-terminated bottle-brush poly (methylacrylic acid)-graft-poly (2-methyl-2-oxazoline) copolymer on gold surfaces. J. Mater. Chem. B 2015, 3, 1921–1930. [Google Scholar] [CrossRef]
- Morgese, G.; Verbraeken, B.; Ramakrishna, S.N.; Gombert, Y.; Cavalli, E.; Rosenboom, J.G.; Zenobi-Wong, M.; Spencer, N.D.; Hoogenboom, R.; Benetti, E.M. Chemical design of non-ionic polymer brushes as biointerfaces: Poly (2-oxazine) s outperform both poly (2-oxazoline) s and PEG. Angew. Chem. Int. Ed. 2018, 57, 11667–11672. [Google Scholar] [CrossRef]
- Benetti, E.M.; Divandari, M.; Ramakrishna, S.N.; Morgese, G.; Yan, W.; Trachsel, L. Loops and cycles at surfaces: The unique properties of topological polymer brushes. Chem.–A Eur. J. 2017, 23, 12433–12442. [Google Scholar] [CrossRef] [PubMed]
- Knowles, B.R.; Wagner, P.; Maclaughlin, S.; Higgins, M.J.; Molino, P.J. Silica nanoparticles functionalized with zwitterionic sulfobetaine siloxane for application as a versatile antifouling coating system. ACS Appl. Mater. Interfaces 2017, 9, 18584–18594. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J. Nanoparticles in reverse osmosis membranes for desalination: A state of the art review. Desalination 2020, 475, 114171. [Google Scholar] [CrossRef]
- Vatanpour, V.; Zoqi, N. Surface modification of commercial seawater reverse osmosis membranes by grafting of hydrophilic monomer blended with carboxylated multiwalled carbon nanotubes. Appl. Surf. Sci. 2017, 396, 1478–1489. [Google Scholar] [CrossRef]
- Li, K.; Lee, B.; Kim, Y. High performance reverse osmosis membrane with carbon nanotube support layer. J. Membr. Sci. 2019, 592, 117358. [Google Scholar] [CrossRef]
- Ma, R.; Ji, Y.-L.; Guo, Y.-S.; Mi, Y.-F.; An, Q.-F.; Gao, C.-J. Fabrication of antifouling reverse osmosis membranes by incorporating zwitterionic colloids nanoparticles for brackish water desalination. Desalination 2017, 416, 35–44. [Google Scholar] [CrossRef]
- Yang, Z.; Saeki, D.; Matsuyama, H. Zwitterionic polymer modification of polyamide reverse-osmosis membranes via surface amination and atom transfer radical polymerization for anti-biofouling. J. Membr. Sci. 2018, 550, 332–339. [Google Scholar] [CrossRef]
- Mahdavi, H.; Rahimi, A. Zwitterion functionalized graphene oxide/polyamide thin film nanocomposite membrane: Towards improved anti-fouling performance for reverse osmosis. Desalination 2018, 433, 94–107. [Google Scholar] [CrossRef]
- Armendariz Ontiveros, M.; Quintero, Y.; Llanquilef, A.; Morel, M.; Argentel Martínez, L.; García García, A.; Garcia, A. Anti-biofouling and desalination properties of thin film composite reverse osmosis membranes modified with copper and iron nanoparticles. Materials 2019, 12, 2081. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Al-Ghouti, M.A.; Zouari, N. Functionalization of reverse osmosis membrane with graphene oxide to reduce both membrane scaling and biofouling. Carbon 2020, 166, 374–387. [Google Scholar] [CrossRef]
- Gu, G.; Yang, X.; Li, Y.; Guo, J.; Huang, J.; Nxumalo, E.N.; Mamba, B.B.; Shao, L. Advanced zwitterionic polymeric membranes for diverse applications beyond antifouling. Sep. Purif. Technol. 2024, 356, 129848. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, R.; Song, D.; Sun, G.; Yu, J.; Liu, Q.; Liu, J.; Zhu, J.; Liu, P.; Wang, J. Silicone-modified polyurea-interpenetrating polymer network fouling release coatings with excellent wear resistance property tailored to regulations. J. Colloid Interface Sci. 2024, 653, 971–980. [Google Scholar] [CrossRef]
- Shah, S.; Liu, J.; Ng, S.; Luo, S.; Guo, R.; Cheng, C.; Lin, H. Transport properties of small molecules in zwitterionic polymers. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1924–1934. [Google Scholar] [CrossRef]
- Wu, J.; Lin, W.; Wang, Z.; Chen, S.; Chang, Y. Investigation of the hydration of nonfouling material poly (sulfobetaine methacrylate) by low-field nuclear magnetic resonance. Langmuir 2012, 28, 7436–7441. [Google Scholar] [CrossRef]
- Ni, L.; Meng, J.; Geise, G.M.; Zhang, Y.; Zhou, J. Water and salt transport properties of zwitterionic polymers film. J. Membr. Sci. 2015, 491, 73–81. [Google Scholar] [CrossRef]
- Choi, H.; Jung, Y.; Han, S.; Tak, T.; Kwon, Y.-N. Surface modification of SWRO membranes using hydroxyl poly (oxyethylene) methacrylate and zwitterionic carboxylated polyethyleneimine. J. Membr. Sci. 2015, 486, 97–105. [Google Scholar] [CrossRef]
- Bengani-Lutz, P.; Sadeghi, I.; Lounder, S.J.; Panzer, M.J.; Asatekin, A. High flux membranes with ultrathin zwitterionic copolymer selective layers with ∼1 nm pores using an ionic liquid cosolvent. ACS Appl. Polym. Mater. 2019, 1, 1954–1959. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Ozaydin-Ince, G.; Wong, S.Y.; Gleason, K.K. Surface-tethered zwitterionic ultrathin antifouling coatings on reverse osmosis membranes by initiated chemical vapor deposition. Chem. Mater. 2011, 23, 1263–1272. [Google Scholar] [CrossRef]
- Yang, R.; Moni, P.; Gleason, K.K. Ultrathin zwitterionic coatings for roughness-independent underwater superoleophobicity and gravity-driven oil–water separation. Adv. Mater. Interfaces 2015, 2, 1400489. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Shahkaramipour, N.; Tran, T.N.; Ramanan, S.; Lin, H. Membranes with surface-enhanced antifouling properties for water purification. Membranes 2017, 7, 13. [Google Scholar] [CrossRef]
- Chang, C.C.; Kolewe, K.W.; Li, Y.; Kosif, I.; Freeman, B.D.; Carter, K.R.; Schiffman, J.D.; Emrick, T. Underwater superoleophobic surfaces prepared from polymer zwitterion/dopamine composite coatings. Adv. Mater. Interfaces 2016, 3, 1500521. [Google Scholar] [CrossRef]
- Chang, Y.; Chang, Y.; Higuchi, A.; Shih, Y.-J.; Li, P.-T.; Chen, W.-Y.; Tsai, E.-M.; Hsiue, G.-H. Bioadhesive control of plasma proteins and blood cells from umbilical cord blood onto the interface grafted with zwitterionic polymer brushes. Langmuir 2012, 28, 4309–4317. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.-R.; Shin, H.-S.; Yang, F.; Zhou, Z. Recent advances in membrane bioreactors: Configuration development, pollutant elimination, and sludge reduction. Environ. Eng. Sci. 2012, 29, 139–160. [Google Scholar] [CrossRef]
- Zuthi, M.; Ngo, H.; Guo, W.; Zhang, J.; Liang, S. A review towards finding a simplified approach for modelling the kinetics of the soluble microbial products (SMP) in an integrated mathematical model of membrane bioreactor (MBR). Int. Biodeterior. Biodegrad. 2013, 85, 466–473. [Google Scholar] [CrossRef]
- Yoon, Y.; Hwang, Y.; Kwon, M.; Jung, Y.; Hwang, T.-M.; Kang, J.-W. Application of O3 and O3/H2O2 as post-treatment processes for color removal in swine wastewater from a membrane filtration system. J. Ind. Eng. Chem. 2014, 20, 2801–2805. [Google Scholar] [CrossRef]
- Logan, B.E.; Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 2012, 488, 313–319. [Google Scholar] [CrossRef]
- Ghaffour, N. The challenge of capacity-building strategies and perspectives for desalination for sustainable water use in MENA. Desalination Water Treat. 2009, 5, 48–53. [Google Scholar] [CrossRef]
- Li, D.; Yan, Y.; Wang, H. Recent advances in polymer and polymer composite membranes for reverse and forward osmosis processes. Prog. Polym. Sci. 2016, 61, 104–155. [Google Scholar] [CrossRef]
- Attia, H.; Alexander, S.; Wright, C.J.; Hilal, N. Superhydrophobic electrospun membrane for heavy metals removal by air gap membrane distillation (AGMD). Desalination 2017, 420, 318–329. [Google Scholar] [CrossRef]
- Gong, W.; Bai, L.; Liang, H. Membrane-based technologies for removing emerging contaminants in urban water systems: Limitations, successes, and future improvements. Desalination 2024, 590, 117974. [Google Scholar] [CrossRef]
- Libutti, A.; Gatta, G.; Gagliardi, A.; Vergine, P.; Pollice, A.; Beneduce, L.; Disciglio, G.; Tarantino, E. Agro-industrial wastewater reuse for irrigation of a vegetable crop succession under Mediterranean conditions. Agric. Water Manag. 2018, 196, 1–14. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Van Lier, J.; Van der Zee, F.; Frijters, C.; Ersahin, M. Celebrating 40 years anaerobic sludge bed reactors for industrial wastewater treatment. Rev. Environ. Sci. Bio/Technol. 2015, 14, 681–702. [Google Scholar] [CrossRef]
- Niwa, T.; Yamashita, T.; Mitsumizo, M.; Takahashi, M.; Hatamoto, M.; Yamaguchi, T.; Kekre, K.A.; Lin, L.L.; Tao, G.; Seah, H. Pilot-scale test of industrial wastewater treatment by UASB and MBR using a ceramic flat sheet membrane for water reuse. J. Water Reuse Desalination 2018, 8, 490–496. [Google Scholar] [CrossRef]
- Dulov, A.; Dulova, N.; Trapido, M. Combined physicochemical treatment of textile and mixed industrial wastewater. Ozone Sci. Eng. 2011, 33, 285–293. [Google Scholar] [CrossRef]
- Canizares, P.; Paz, R.; Lobato, J.; Sáez, C.; Rodrigo, M. Electrochemical treatment of the effluent of a fine chemical manufacturing plant. J. Hazard. Mater. 2006, 138, 173–181. [Google Scholar] [CrossRef]
- Boricha, A.G.; Murthy, Z. Preparation of N, O-carboxymethyl chitosan/cellulose acetate blend nanofiltration membrane and testing its performance in treating industrial wastewater. Chem. Eng. J. 2010, 157, 393–400. [Google Scholar] [CrossRef]
- Bodalo-Santoyo, A.; Gomez-Gomez, E.; Maximo-Martin, M.; Hidalgo-Montesinos, A. Spiral-wound membrane reverse osmosis and the treatment of industrial effluents. Desalination 2004, 160, 151–158. [Google Scholar] [CrossRef]
- Mbakop, S.; Nthunya, L.N.; Onyango, M.S. Recent advances in the synthesis of nanocellulose functionalized–hybrid membranes and application in water quality improvement. Processes 2021, 9, 611. [Google Scholar] [CrossRef]
- Sakthivel, T.; Rajendran, A.; Chang, J.W. Advancements in piezoelectric membrane technology: Fundamentals and future outlook. Sep. Purif. Technol. 2024, 435, 127021. [Google Scholar] [CrossRef]
- Zhu, L.-J.; Song, H.-M.; Wang, G.; Zeng, Z.-X.; Zhao, C.-T.; Xue, Q.-J.; Guo, X.-P. Microstructures and performances of pegylated polysulfone membranes from an in situ synthesized solution via vapor induced phase separation approach. J. Colloid Interface Sci. 2018, 515, 152–159. [Google Scholar] [CrossRef]
- Geleta, T.A.; Maggay, I.V.; Chang, Y.; Venault, A. Recent advances on the fabrication of antifouling phase-inversion membranes by physical blending modification method. Membranes 2023, 13, 58. [Google Scholar] [CrossRef]



| Microorganism | Examples |
|---|---|
| Bacteria | Corynebacterium, Bacillus, Mycobacterium, Flavobacterium, Pseudomonas, Cytophaga, Moraxella, Aeromonas, Lactobacillus, Serratia, and Micrococcus. |
| Fungi | Trichoderma, Mucor, Fusarium, Penicillium, and Aspergillus |
| Factors Affecting Fouling | Effects of Fouling | References |
|---|---|---|
| MLSS | With an increase in MLSS concentration, EPS and SMP both have higher protein and carbohydrate contents. | [41] |
| OLR or F/M ratio | Low sludge filterability and filtration index are caused by the formation of EPS and SMP when the F/M or OLR ratio is high. | [42] |
| Dissolved oxygen concentration | Excessive aeration breaks up sludge flocs and raises SMP concentrations, causing poor filterability. | [17] |
| HRT | Reduced HRT causes the production of EPS by bacterial cells, which raises SMP and results in sludge deflocculation. Additionally, increased filamentous bacterial growth and the formation of enormous, atypical flocs are caused by reduced HRT. On the other hand, excessive HRT leads to the accumulation of foulants. | [43] |
| Temperature | Increased temperature results in the degradation of biomass, an increase in SMP and turbidity, and a reduction in the protein content of EPS. In contrast, filamentous bacteria grow at low temperatures, increasing the SMP they create in the mixed liquid. | [44] |
| Nutrients | Protein concentrations in EPS rise due to nitrogen deficiency resulting in negatively charged floc surfaces. Phosphorus deficit in activated sludge causes flocs to have a reduced surface charge, lower protein content in EPS, and to increase. | [45] |
| Salinity | The physical and biochemical characteristics of activated sludge are significantly affected by higher salt concentrations, which result in higher concentrations of SMP and EPS and poorer membrane permeability. | [39] |
| Cations | Anions | ||||
|---|---|---|---|---|---|
| Al3+ | F− | OH− | CO32− | SO42− | PO43− |
| AlF3 | Al(OH)3 | - | - | AlPO4 | |
| Ca2+ | CaF2 | Ca(OH)2 | CaCO3 | CaSO4 | Ca3(PO4)2 |
| Fe3+ | FeF3 | Fe(OH)3 | - | - | FePO4 |
| Mg2+ | MgF2 | Mg(OH)2 | MgCO3 | MgSO4 | Mg3(PO4)2 |
| Surface Coatings | Base Polymers | References |
|---|---|---|
| Chitosan coatings | Polyamide membrane | [60] |
| Macro initiators photo reactor coatings from PEG based hydrogel | Polyamide on PSF membrane | [61] |
| Silver pegylated dendrimer nanocomposite coatings | TFC membrane | [62] |
| PDMS/PMMA copolymers | PES membranes | [63] |
| Zwitterionic coating | PDMS coatings | [64] |
| Ylene glycol diacrylate | TFC-HR, flat sheet Koch | [65] |
| Method | Modifier | Test Conditions | Permeate Flux (Lm−2 h−1) | Salt Rejection (%) | References |
|---|---|---|---|---|---|
| Surface coating | PDDA and PSS | 2000 ppm NaCl solution at 4.1 MPa | 15.5 | 99 | [59] |
| LbL surface coating | Pluronic F127 amphiphilic triblock copolymer | 2 gL−1 NaCl solution at 4 MPa | 30 | 94 | [66] |
| LbL surface coating | PEI and GO | 200 g L−1 NaCl at 65 °C | 8.4 kg m−2 h−1 | 99.99 | [67] |
| Surface coating | SPVA | 2000 ppm NaCl solution at 1.55 MPa | 42.6 | 99.18 | [74] |
| Surface coating | Pluronic F127 and Gum arabic | 2000 gL−1 NaCl solution at 55.2 bar | - | 98 | [75] |
| Slip casting | Nano zeolite-Y | 25,000 mgL−1 NaCl solution at 25 bar | 5.1 | 99.52 | [76] |
| Hydrophilization treatment | PVP | - | - | - | [77] |
| Hydrophilization treatment | Chromic acid | 60 °C | 61 | - | [78] |
| Free radical grafting | ADMH | 200 PPM NaCl solution at 1.5 Mpa and 25 °C | 184.5 | 95.8 | [79] |
| Free radical grafting | ZnO NPs | 200 mgL−1 NaCl solution at 15 bar and 25 °C | 35 | 97 | [73] |
| Chemical coupling | PVA | 500 mg L−1 NaCl solution at 5 bar and 25 °C | 27 | 98.46 | [80] |
| Chemical coupling | Aldehydes | 2000 ppm NaCl solution at 1.6 MPa and 25 °C | 37.5 | 98.6 | [81] |
| Glow discharge plasma treatment | Clinoptilolite | 16,000 ppm NaCl solution at 1.5 MPa and 25 °C | - | 97.12 | [82] |
| Dielectric barrier discharge plasma treatment | - | - | - | - | [83] |
| Plasma polymerization and si-ATRP | HEMA, MPC, and SBMA | - | 6042 | 99 | [84] |
| Membrane | Coated Material | Application | References |
|---|---|---|---|
| Polyether sulphone | TiO2 nano-particles | Ultra-Filtration | [97] |
| Poly-propylene | Polyhydroxylated | Fltration | [98] |
| PVDF composite membrane | Polydimethylsiloxane | Separation of VOCs | [99] |
| Utem/P84 co-polyamide | Al2O3 | Gas separation | [100] |
| Polypropylene | Fluorosilicone | Polydimethylsiloxane | [101] |
| Modifier | Test Conditions | Permeate Flux | Salt Rejection % | References |
|---|---|---|---|---|
| Carboxylated CNf | 500 mgL−1 BSA, 200 mg L−1 Nacl solution at pressure of 15 bar with temperature of 25 °C | - | 94 | [120] |
| CNf | 200 mgL−1 NaCl solution at pressure of 15 bar with temperature of 25 °C | 25.9 | 96 | [72] |
| Zwitterionic diamine monomer N, amino-ethyl piperazine | Nacl solution of 2000 ppm at pressure of 1.5 Mpa with temperature of 25 °C | 54.5 | 98.3 | [121] |
| Zwitterionic colloid nano-particles | Nacl solution of 2000 ppm at pressure of 1.5 Mpa with temperature of 25 °C | 37.3 | 96.5 | [122] |
| Zwitterionic Polymer | 0.85 wt% solution at pressure of 1.5 Mpa with temperature of 30 °C | 50.48 | 96.9 | [123] |
| GO zinc oxide | 200 mgL−1 Nacl solution at the pressure of 20 bar with temperature of 25 °C | 31.42 | 96.3 | [124] |
| Cu and Fe nano particles | 100 mgL−1 Nacl solution at pressure of 300 psi with temperature of 25 °C | 3 (Cu NP) and 8.4 Fe NP | 74.36 (Cu NP) and 92.6 (Fe NP) | [125] |
| Graphene oxide | 800 mgL−1 CaCl2 and Na2SO4 at 25 °C and pressure of 20 bar | - | 98 | [126] |
| Treatment Technology | Advantages | Disadvantages | References |
|---|---|---|---|
| Chemical coagulation | Ferric chloride percoagulation is one of the possible pretreatment step for chemical oxidation causes the lower toxicity and higher samples biodegradability. | Organic compounds are are not degraded through this technology. | [153] |
| Electrocoagulation process | In a small treatment facility, the high treatment particulate removal effifiency is achived. It is the rapid process in eliminating colloidal, suspended and charged particles. | It is not good efficient for removal of persistent organic compounds. | [154] |
| Chemical precipitation | Due to high metal selectivity, potential removal efficiency and ease of its use, chemical precipitation is considered as a superior technique. | It is the more expensive due to the use of chelating agents and unable to reduce concentrations at acceptable limit. | [155] |
| Advanced oxidation processes | Wastewater biodegrability and toxicity is improved, organic pollutants are completely miniralized into H2O, CO2 and inorganic ions. | Large concentrations of FeSO4 and H2O2 are needed during Fenton treatment. | [153] |
| Membrane process | This method is much more robust in treating wastewater. It separates high concentrated metals and other valuable chemicals without changing their state. This is also promising technology in separating heavy metals. | Damage of membrane affects on the cost and serious membrane fouling issue which requires frequent membrane cleaning. | [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallah, N.B.; Shah, A.A.; Pirzada, A.M.; Ali, I.; Khan, M.I.; Jatoi, A.S.; Ullman, J.L.; Mahar, R.B. Advanced Control Strategies of Membrane Fouling in Wastewater Treatment: A Review. Processes 2024, 12, 2681. https://doi.org/10.3390/pr12122681
Mallah NB, Shah AA, Pirzada AM, Ali I, Khan MI, Jatoi AS, Ullman JL, Mahar RB. Advanced Control Strategies of Membrane Fouling in Wastewater Treatment: A Review. Processes. 2024; 12(12):2681. https://doi.org/10.3390/pr12122681
Chicago/Turabian StyleMallah, Nabi Bakhsh, Ayaz Ali Shah, Abdul Majeed Pirzada, Imran Ali, Mohammad Ilyas Khan, Abdul Sattar Jatoi, Jeffrey L. Ullman, and Rasool Bux Mahar. 2024. "Advanced Control Strategies of Membrane Fouling in Wastewater Treatment: A Review" Processes 12, no. 12: 2681. https://doi.org/10.3390/pr12122681
APA StyleMallah, N. B., Shah, A. A., Pirzada, A. M., Ali, I., Khan, M. I., Jatoi, A. S., Ullman, J. L., & Mahar, R. B. (2024). Advanced Control Strategies of Membrane Fouling in Wastewater Treatment: A Review. Processes, 12(12), 2681. https://doi.org/10.3390/pr12122681









