Effect of Froth on the Interaction Between Coal Particles and Cake Structures in the Dewatering Process of Clean Coal
Abstract
1. Introduction
2. Materials and Methods
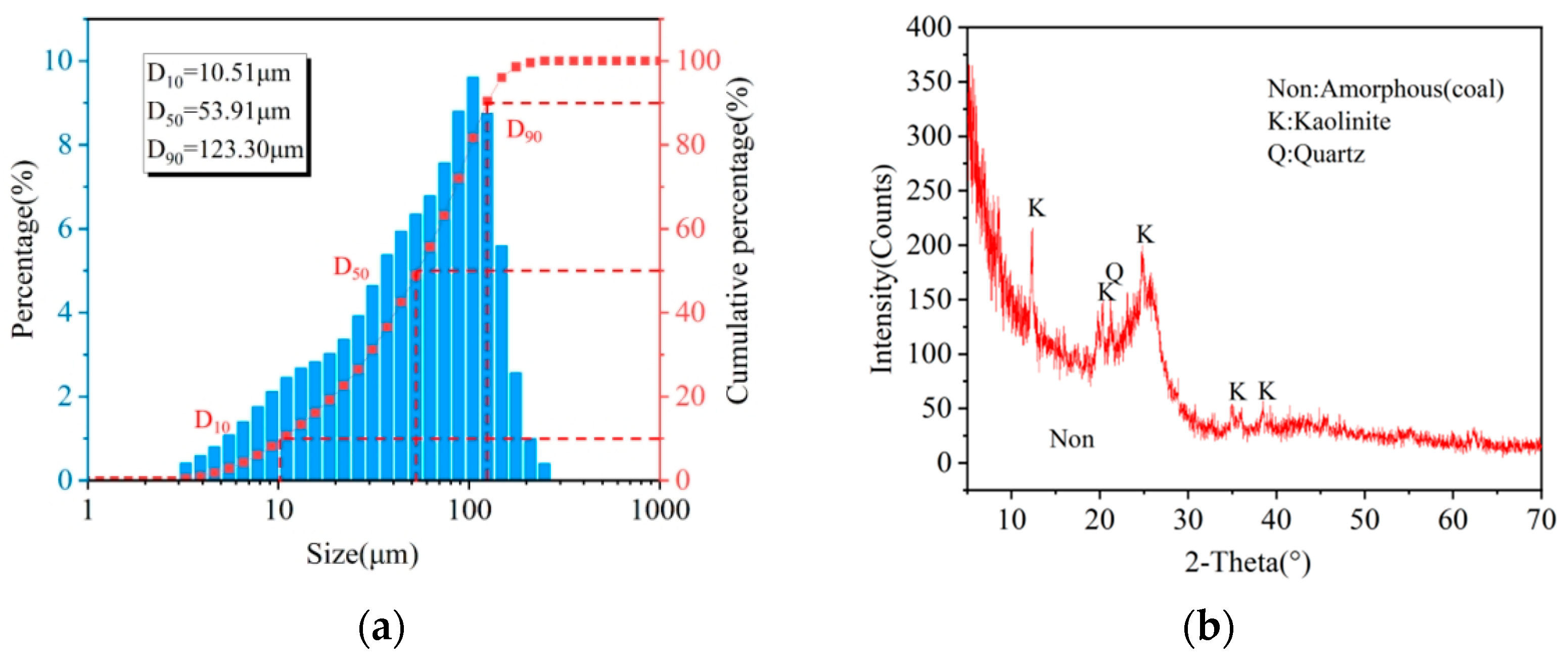
2.1. Materials and Reagents
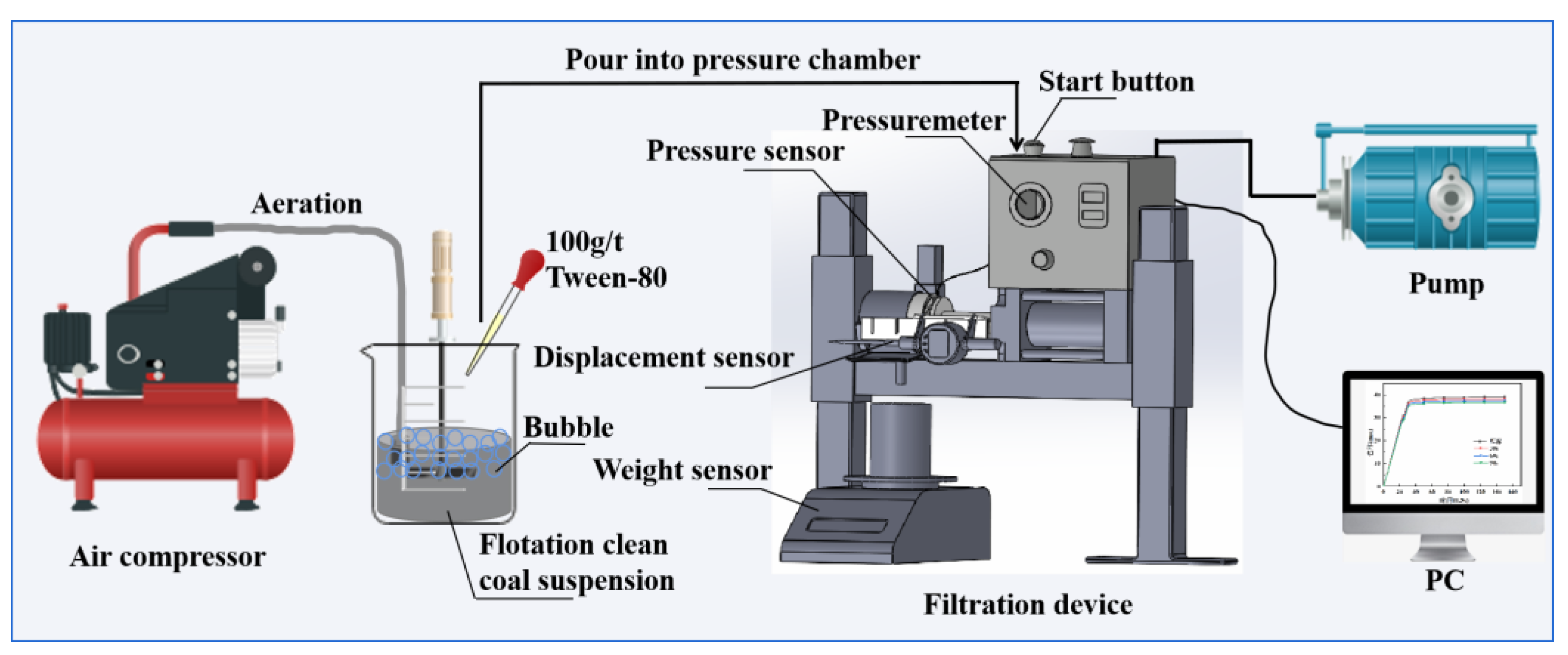
2.2. Filtration Tests
2.3. Rheological Measurement
2.4. Flocculation Particle Size Measurement
2.5. SEM Measurement
2.6. Analysis of Filter Cake Structure
3. Results
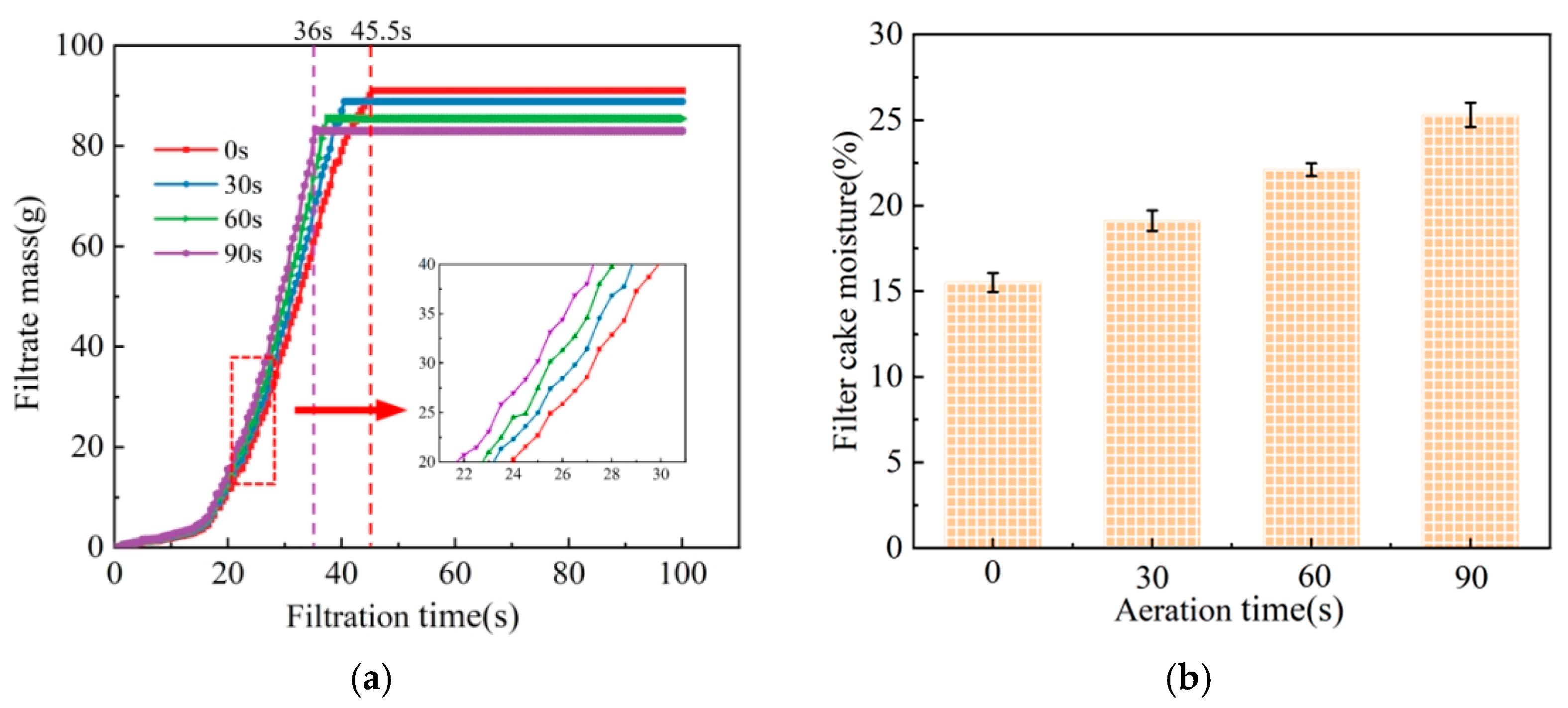
3.1. Dewatering Tests
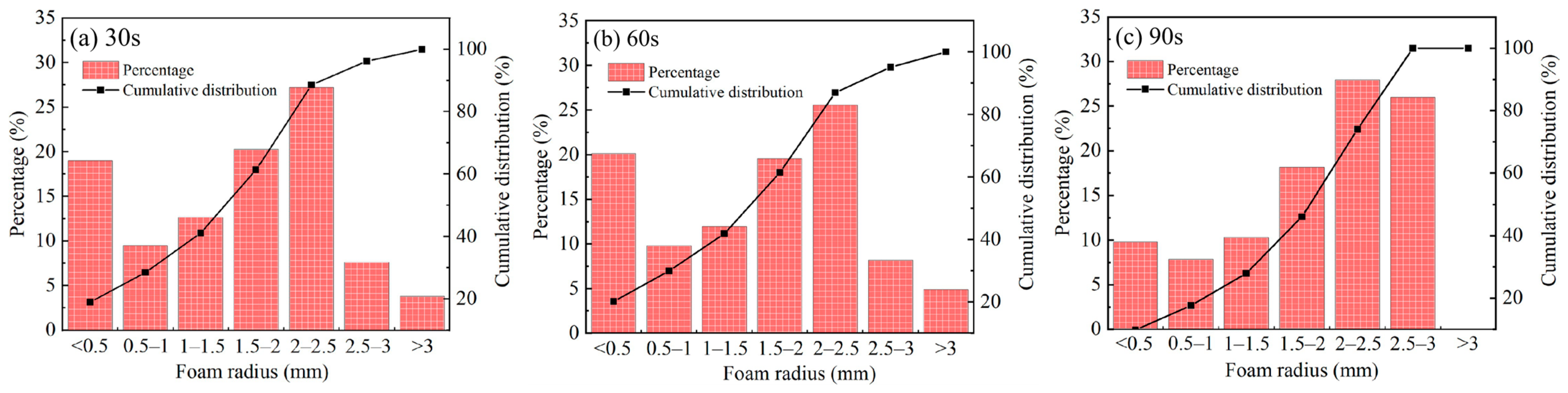
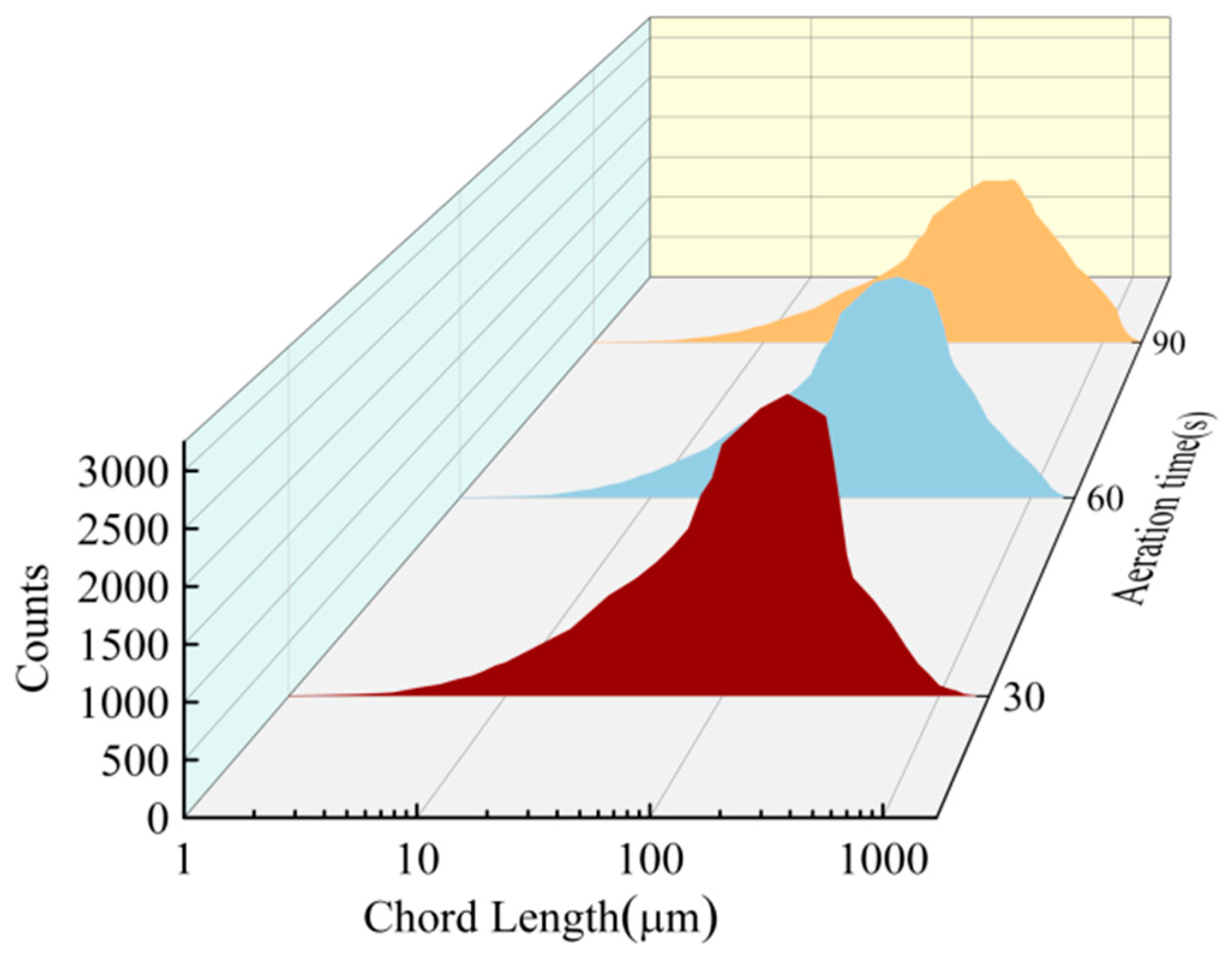
3.2. Bubble Size Measurement
3.3. Rheological Properties
3.4. Floc Size Measurement
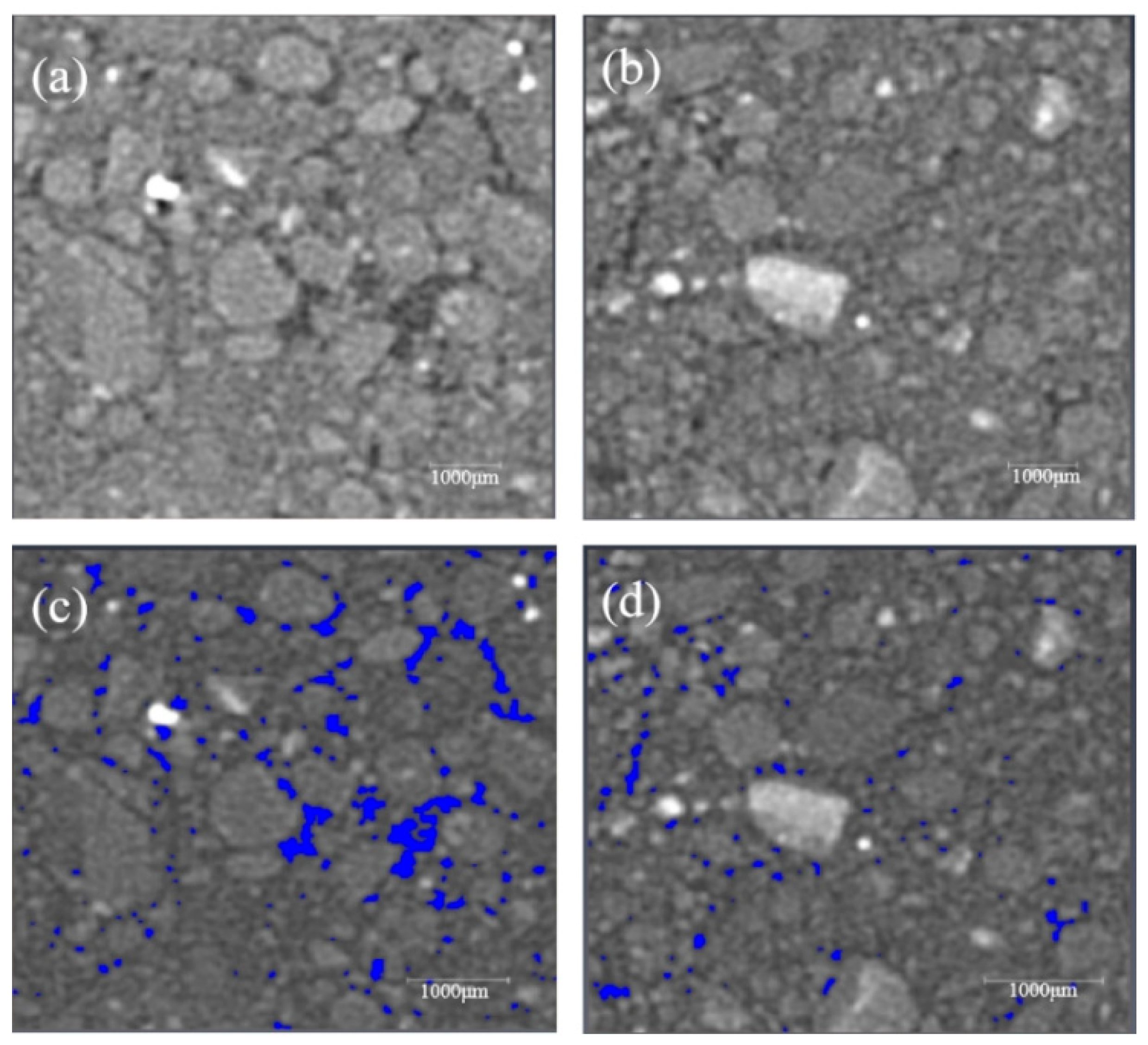
3.5. Particle Aggregation Morphology
3.6. Microstructural Analysis of Filter Cake
3.6.1. CT Scanning and Image Threshold Segmentation
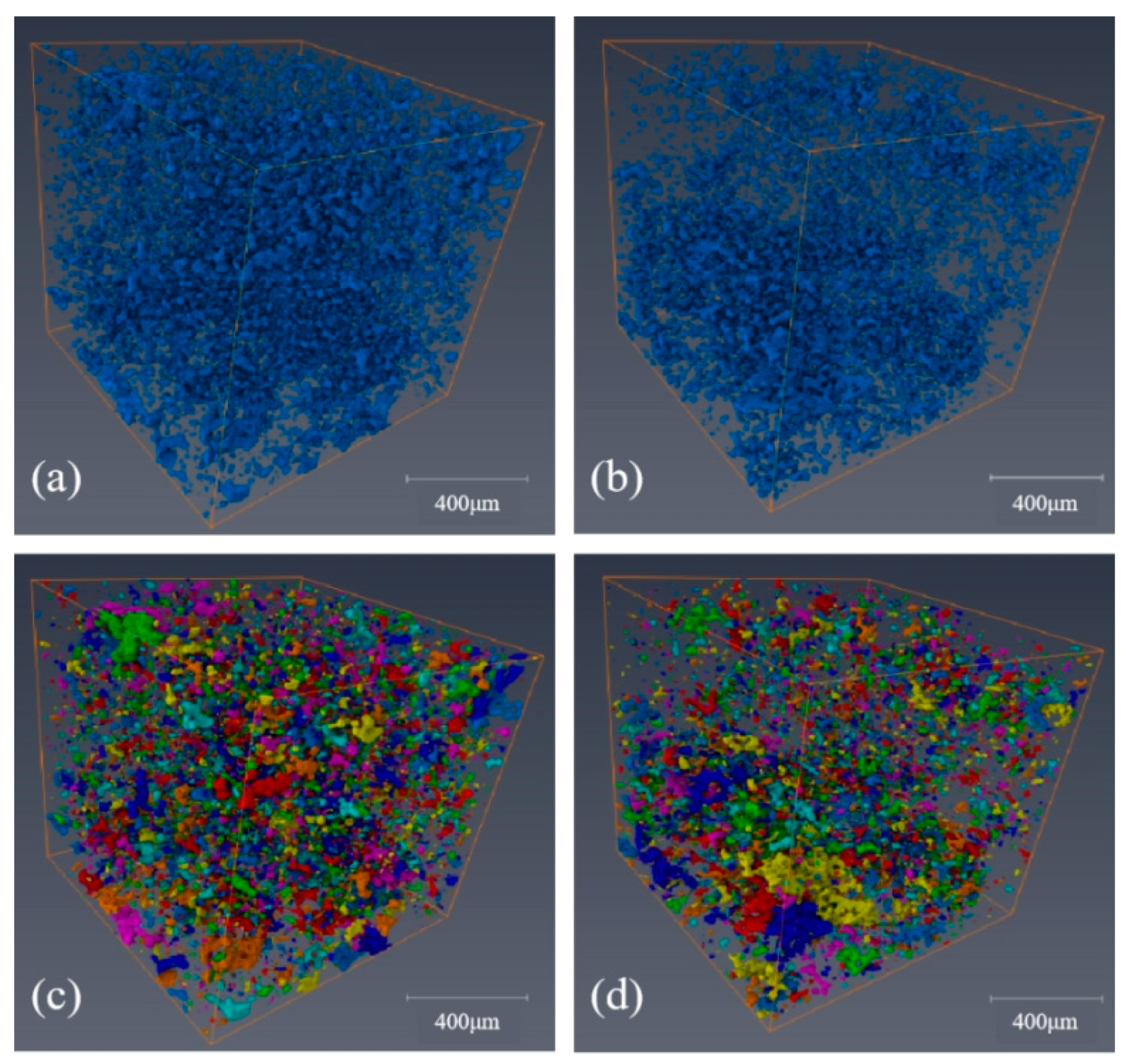
3.6.2. Porosity and Pore Size Distribution
3.6.3. Quantitative Characterization of Pore–Throat Networks
4. Discussion
5. Conclusions
- The number of bubbles gradually increased with the increase in aeration time. When the aeration times were 30 s, 60 s, and 90 s, the corresponding numbers of bubbles were 158, 184, and 204, respectively. The longer the aeration time was, the more uniform the bubble size distribution. In the pressure filtration test, the presence of froth led to the increase in the moisture in the filter cake and a reduction in the dewatering time. When the aeration time reached 90 s, the moisture content of the filter cake reached 25.3%. This demonstrates that the interaction between coal particles in the froth was enhanced as a result of the dynamic shear value of the suspension, combined with an increased floc size and network “wall” structure, which led to a dense microstructure of the filter cake.
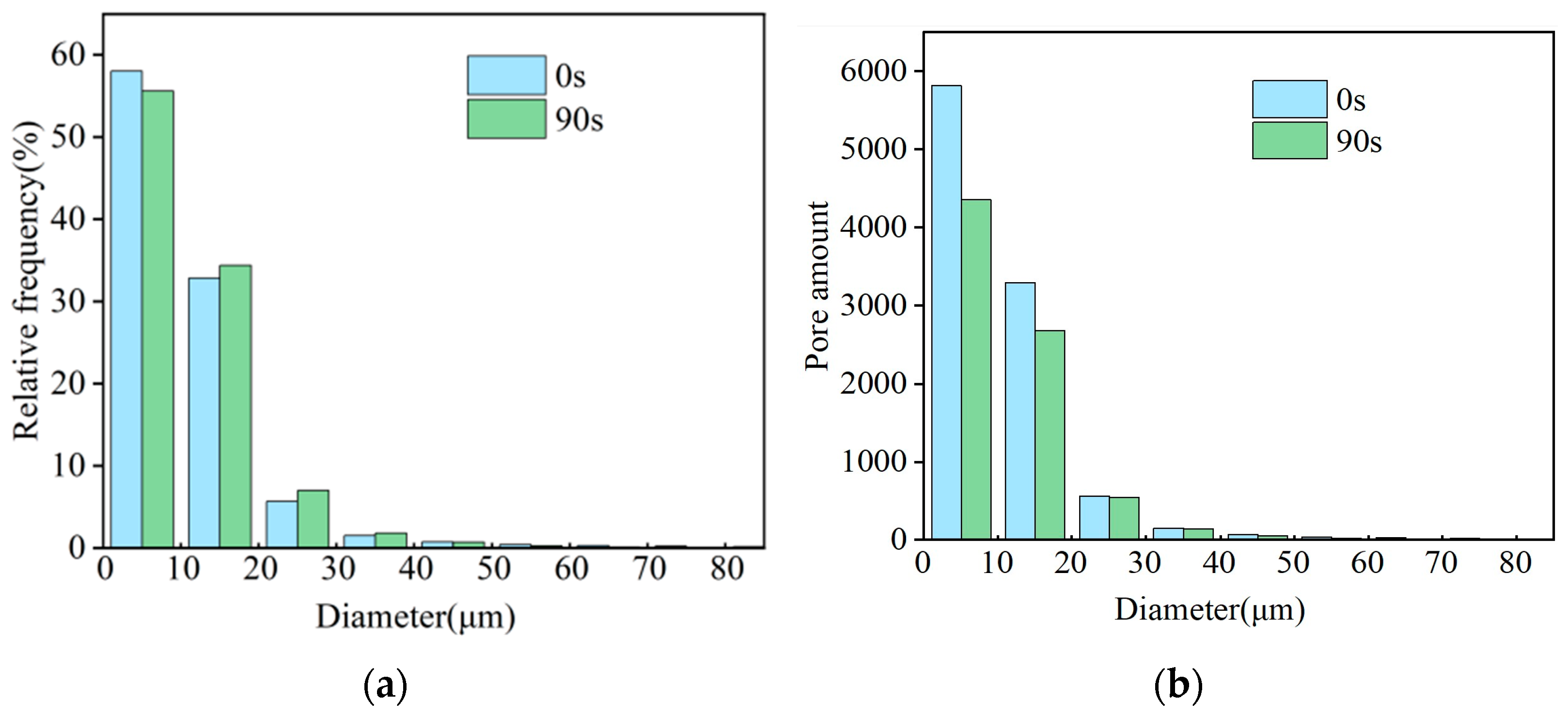
- The characterization result of the pore structure for the filter cake showed that when the aeration time was 90 s, the porosity of the filter cake decreased to 2.05% due to the particles aggregating under the strong capillary force at the gas–liquid interface. With the increase in aeration time, the total number of pores decreased from 10030 to 7833, which reduced the path for water discharge significantly. The presence of bubbles reduced the throat radius in the filter cake to 1.32 μm, and the number of throat passages was reduced to one third of the original. The content of blind holes in the clean coal filter cake with froth was higher, and the maximum value of the coordination number in the filter cake was only 6, resulting in poor connectivity of the pore network, thus limiting the discharge of water.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, Z.; Zhang, Y.; Wang, S.; Zhao, B. Effect of plasma-activated water on the settling characteristics of ultrafine kaolinite. Process Saf. Environ. Prot. 2024, 192, 613–620. [Google Scholar] [CrossRef]
- Xia, Y.; Xing, Y.; Gui, X.; Cao, Y. Interaction between hydrocarbon oil and hydrophilic mineral surfaces: A chemical force microscopy and molecular dynamics simulation study. Fuel 2022, 323, 124402. [Google Scholar] [CrossRef]
- Guo, F.; Chen, L.; Li, Y.; Zhu, Y.; Jia, W.; Guo, Y.; Guo, S.; Zhang, Y.; Wu, J. Review on the attribute cognition and carbon-ash-water separation of coal gasification fine slag. Sep. Purif. Technol. 2023, 320, 124121. [Google Scholar] [CrossRef]
- Doi, A.; Nguyen, T.A.H.; Nguyen, N.N.; Nguyen, C.V.; Raji, F.; Nguyen, A.V. Enhancing shear strength and handleability of dewatered clay-rich coal tailings for dry-stacking. J. Environ. Manag. 2023, 344, 118488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xing, Y.; Chang, G.; Yang, Z.; Gui, X. Enhanced lignite flotation using interfacial nanobubbles based on temperature difference method. Fuel 2021, 293, 120313. [Google Scholar] [CrossRef]
- Liu, D.; Wang, W.; Tu, Y.; Ren, G.; Yan, S.; Liu, H.; He, H. Flotation specificity of coal gasification fine slag based on release analysis. J. Clean. Prod. 2022, 363, 132426. [Google Scholar] [CrossRef]
- Niu, C.; Xia, W.; Li, Y.; Bu, X.; Wang, Y.; Xie, G. Insight into the low-rank coal flotation using amino acid surfactant as a promoter. Fuel 2022, 307, 121810. [Google Scholar] [CrossRef]
- Ping, A.; Peng, Y.; Xie, G.; Wang, M.; Xia, W. Enhancement of dewatering performance of vitrinite-rich and inertinite-rich coals by surfactants: Experiment and simulation. Fuel 2022, 318, 123571. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J. Settling characteristics analysis of coal slime water based on original hardness. J. China Coal Soc. 2014, 39, 757–763. [Google Scholar]
- Ejtemaei, M.; Ramli, S.; Osborne, D.; Nguyen, A.V. Synergistic effects of surfactant-flocculant mixtures on ultrafine coal dewatering and their linkage with interfacial chemistry. J. Clean. Prod. 2019, 232, 953–965. [Google Scholar] [CrossRef]
- Chen, R.; Fan, Y.; Dong, X.; Ma, X.; Feng, Z.; Chang, M.; Li, N. Impact of pH on interaction between the polymeric flocculant and ultrafine coal with atomic force microscopy (AFM). Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126698. [Google Scholar] [CrossRef]
- Guo, F.; Liu, H.; Zhao, X.; Guo, Y.; Guo, Z.; Miao, Z.; Zhang, Y.; Li, J.; Wu, J. Insights on water temporal-spatial migration laws of coal gasification fine slag filter cake during water removal process and its enlightenment for efficient dewatering. Fuel 2021, 292, 120274. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, B.; Tian, C.; Zhang, Y.; Sheng, Q.; Cheng, M.; Zhang, N.; Li, Z.; Liu, D. Plasma pretreatment to enhance the sedimentation of ultrafine kaolinite particles: Experiments and mechanisms. J. Water Process Eng. 2024, 59, 105012. [Google Scholar] [CrossRef]
- You, X.; He, M.; Zhu, X.; Wei, H.; Cao, X.; Wang, P.; Li, L. Influence of surfactant for improving dewatering of brown coal: A comparative experimental and MD simulation study. Sep. Purif. Technol. 2019, 210, 473–478. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, X.; Ding, J.; Zhao, K. Synergistic improvement of the fine size coal dewatering performance by using binary surfactant mixtures. Fuel 2024, 357, 130044. [Google Scholar] [CrossRef]
- Hennemann, M.; Gastl, M.; Becker, T. Influence of particle size uniformity on the filter cake resistance of physically and chemically modified fine particles. Sep. Purif. Technol. 2021, 272, 118966. [Google Scholar] [CrossRef]
- Kinnarinen, T.; Tuunila, R.; Häkkinen, A. Reduction of the width of particle size distribution to improve pressure filtration properties of slurries. Miner. Eng. 2017, 102, 68–74. [Google Scholar] [CrossRef]
- Ma, X.; Fan, Y.; Dong, X.; Chen, R.; Li, H.; Sun, D.; Yao, S. Impact of clay minerals on the dewatering of coal slurry: An experimental and molecular-simulation study. Minerals 2018, 8, 400. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Dao, V.H.; Cameron, N.R.; Saito, K. Synthesis, properties and performance of organic polymers employed in flocculation applications. Polym. Chem. 2016, 7, 11–25. [Google Scholar] [CrossRef]
- Reis, L.G.; Oliveira, R.S.; Palhares, T.N.; Spinelli, L.S.; Lucas, E.F.; Vedoy, D.R.; Asare, E.; Soares, J.B. Using acrylamide/propylene oxide copolymers to dewater and densify mature fine tailings. Miner. Eng. 2016, 95, 29–39. [Google Scholar] [CrossRef]
- Sun, X.; Ma, X.; Fan, Y.; Dong, X.; Chang, M.; Feng, Z.; Peng, D. Insight into filter cake characteristics of fine coal tailings assisted by CPAM and α-HH during pressure filtration. Sep. Purif. Technol. 2023, 326, 124822. [Google Scholar] [CrossRef]
- Zeng, H.; Tang, H.; Sun, W.; Wang, L. Deep dewatering of bauxite residue via the synergy of surfactant, coagulant, and flocculant: Effect of surfactants on dewatering and settling properties. Sep. Purif. Technol. 2022, 302, 122110. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Peng, Y. Deaeration of stable coal froth by surfactants to modify the interfacial properties. Fuel 2021, 298, 120839. [Google Scholar] [CrossRef]
- Cheng, Y.; Min, F.; Li, H.; Chen, J.; Fu, X. Effect of reagent interaction on froth stability of coal flotation. Fuel 2022, 318, 123417. [Google Scholar] [CrossRef]
- Xu, M.; Xing, Y.; Jin, W.; Li, M.; Cao, Y.; Gui, X. Effect of diesel on the froth stability and its antifoam mechanism in fine coal flotation used MIBC as the frother. Powder Technol. 2020, 364, 183–188. [Google Scholar] [CrossRef]
- Ata, S. Phenomena in the froth phase of flotation—A review. Int. J. Miner. Process. 2012, 102–103, 1–12. [Google Scholar] [CrossRef]
- Arriagada, S.; Acuña, C.; Vera, M. New technology to improve the recovery of fine particles in froth flotation based on using hydrophobized glass bubbles. Miner. Eng. 2020, 156, 106364. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Peng, Y. Effects of froth properties on dewatering of flotation products—A critical review. Miner. Eng. 2020, 155, 106477. [Google Scholar] [CrossRef]
- Wei, Y.C.; Peng, Y. Effect of froth stability on dewatering of coal flotation concentrates. Miner. Process. Extr. Metall. 2015, 124, 167–174. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Nicholson, T.; Peng, Y. The effect of froth on the dewatering of coals—An oscillatory rheology study. Fuel 2018, 222, 362–369. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Nicholson, T.; Peng, Y. The effect of saline water on the settling of coal slurry and coal froth. Powder Technol. 2019, 344, 161–168. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, K.; Ni, C.; Cao, H.; Jesse, T.; Xie, G.; Tan, Z.; Yu, H. Ash determination of coal flotation concentrate by analyzing froth image using a novel hybrid model based on deep learning algorithms and attention mechanism. Energy 2022, 260, 125027. [Google Scholar] [CrossRef]
- Wen, Z.; Zhou, C.; Pan, J.; Nie, T.; Jia, R.; Yang, F. Froth image feature engineering-based prediction method for concentrate ash content of coal flotation. Miner. Eng. 2021, 170, 107023. [Google Scholar] [CrossRef]
- Kakwani, R.M.; Gala, H.B.; Chiang, S.H.; Klinzing, G.E.; Tierney, J.W. Dewatering of Fine Coal-Micrographic Analysis of Filter Cake Structure. Powder Technol. 1985, 41, 239–250. [Google Scholar] [CrossRef]
- Chi, S.-M.; Klinzing, G.E.; Chang, S.-H.; Wen, W.W. Effect of Entrapped Air Bubbles on Fine Coal Dewatering via Filtration. Powder Technol. 1985, 45, 25–34. [Google Scholar] [CrossRef]
- Chase, G.G.; Willis, M.S. Flow Resistance in Filter Cakes Due to Air. Sep. Sci. Technol. 1991, 26, 117–126. [Google Scholar] [CrossRef]
- Xu, G.; Fan, M.; Liu, A.; Yang, H. Role of Tweens in improved centrifuging of coarse clean coal. J. China Coal Soc. 2018, 43, 284–289. [Google Scholar]
- Lu, H.; Bian, Y.; Wang, Z.; Guo, X.; Liu, H.; Cao, J.; Qu, K. Characterization of non-Newtonian rheological behaviors of powders. Powder Technol. 2023, 417, 118281. [Google Scholar] [CrossRef]
- Feng, Z.; Dong, X.; Fan, Y.; Li, H.; Dong, Y.; Ma, X.; Chen, R. Use of X-ray microtomography to quantitatively characterize the pore structure of three-dimensional filter cakes. Miner. Eng. 2020, 152, 106275. [Google Scholar] [CrossRef]
- Férec, J.; Bertevas, E.; Khoo, B.C.; Ausias, G.; Phan-Thien, N. A rheological constitutive model for semiconcentrated rod suspensions in Bingham fluids. Phys. Fluids 2017, 29, 073103. [Google Scholar] [CrossRef]
- Chen, G.; Li, C.; Ruan, Z.; Bürger, R.; Gao, Y.; Hou, H. Structural evolution of bed drainage channels under the shear effect of the whole process of tailings thickening. Miner. Eng. 2023, 203, 108364. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, J.; Zhou, H. Evaluating the permeability properties of green bed in iron ore sintering using high resolution X-Ray computed tomography and orthogonal array tests. Powder Technol. 2020, 375, 360–368. [Google Scholar] [CrossRef]
- Dao, Q.D.; Nguyen, T.V.; Nguyen, D.M.A.T.; Nguyen, N.N.; Nguyen, C.V.; Nguyen, A.V. Application of X-ray computed tomography scanning in describing partition curves of dense medium cyclones. Miner. Eng. 2023, 201, 108164. [Google Scholar] [CrossRef]
- Yang, A.; Liao, Y.; An, M.; Cao, Y.; Yang, Z.; Ren, H.; Su, H.; Zou, Q.; Chen, L. Effect of surfactant and flocculant on low-rank coal slime filtration: From filter cake characteristics point of view. Fuel 2022, 327, 125136. [Google Scholar] [CrossRef]
- Blunt, M.J.; Bijeljic, B.; Dong, H.; Gharbi, O.; Iglauer, S.; Mostaghimi, P.; Paluszny, A.; Pentland, C. Pore-scale imaging and modelling. Adv. Water Resour. 2013, 51, 197–216. [Google Scholar] [CrossRef]
- Xiong, Q.; Baychev, T.G.; Jivkov, A.P. Review of pore network modelling of porous media: Experimental characterisations, network constructions and applications to reactive transport. J. Contam. Hydrol. 2016, 192, 101–117. [Google Scholar]
- Feng, Z.; Fan, Y.; Dong, X.; Ma, X.; Chen, R. Permeability estimation in filter cake based on X-ray microtomography and Lattice Boltzmann method. Sep. Purif. Technol. 2021, 275, 119114. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, L. Synergistic mechanism of CTAB and nonionic polyacrylamide on pore structure of kaolinite filter cake. Sep. Purif. Technol. 2023, 324, 124575. [Google Scholar] [CrossRef]


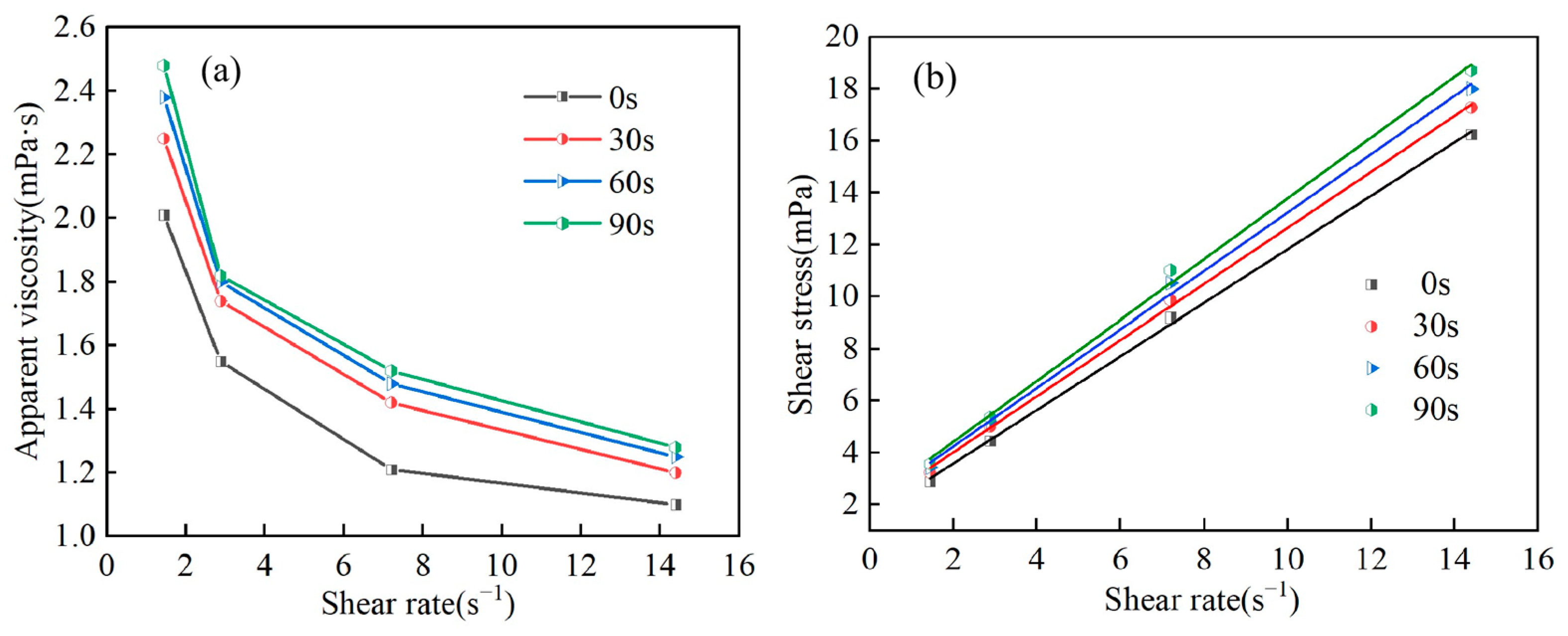


| Time (s) | Bingham Model Fitting Equation | R2 | τ0 (mPa) | Hp (mPa·s) |
|---|---|---|---|---|
| 0 | τ = 1.5295 + 1.0293γ | 0.9999 | 1.5295 | 1.0293 |
| 30 | τ = 1.8597 + 1.0785γ | 0.9989 | 1.8597 | 1.0785 |
| 60 | τ = 1.9981 + 1.1238γ | 0.9975 | 1.9981 | 1.1238 |
| 90 | τ = 2.0824 + 1.1703γ | 0.9991 | 2.0824 | 1.1703 |
| Sample | Pore Volume (μm3) | Total Volume (μm3) | Porosity (%) |
|---|---|---|---|
| 0 s | 4.64 × 107 | 1.16 × 109 | 4.02 |
| 90 s | 2.33 × 107 | 1.14 × 109 | 2.05 |
| Sample | Pore Radii (μm) | Throat Radii (μm) | Number | |||||
|---|---|---|---|---|---|---|---|---|
| Max | Min | Ave | Max | Min | Ave | Pore | Throat | |
| 0 s | 78.50 | 2.60 | 5.83 | 34.93 | 1.44 | 13.57 | 10030 | 171 |
| 90 s | 77.51 | 2.59 | 5.76 | 27.63 | 1.32 | 12.76 | 11135 | 54 |
| Coordination Number | 0 s (%) | 90 s (%) |
|---|---|---|
| 0 | 98.784 | 99.623 |
| 1 | 0.338 | 0.126 |
| 2 | 0.361 | 0.089 |
| 3 | 0.238 | 0.063 |
| 4 | 0.150 | 0.036 |
| 5 | 0.039 | 0.055 |
| 6 | 0.012 | 0.008 |
| 7 | 0.029 | – |
| 8 | 0 | – |
| 9 | 0.019 | – |
| ≥10 | 0.030 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Dong, X.; Feng, Z.; Fan, Y.; Ma, X. Effect of Froth on the Interaction Between Coal Particles and Cake Structures in the Dewatering Process of Clean Coal. Processes 2024, 12, 2738. https://doi.org/10.3390/pr12122738
Chen R, Dong X, Feng Z, Fan Y, Ma X. Effect of Froth on the Interaction Between Coal Particles and Cake Structures in the Dewatering Process of Clean Coal. Processes. 2024; 12(12):2738. https://doi.org/10.3390/pr12122738
Chicago/Turabian StyleChen, Ruxia, Xianshu Dong, Zeyu Feng, Yuping Fan, and Xiaomin Ma. 2024. "Effect of Froth on the Interaction Between Coal Particles and Cake Structures in the Dewatering Process of Clean Coal" Processes 12, no. 12: 2738. https://doi.org/10.3390/pr12122738
APA StyleChen, R., Dong, X., Feng, Z., Fan, Y., & Ma, X. (2024). Effect of Froth on the Interaction Between Coal Particles and Cake Structures in the Dewatering Process of Clean Coal. Processes, 12(12), 2738. https://doi.org/10.3390/pr12122738







