Methane Adsorption Energy Variation Affected by Industrial Components in Deep and Thick Coal Reservoirs
Abstract
:1. Introduction
2. Sample Collection and Experimental Methods
2.1. Experimental Methods
- -
- Low-temperature carbon dioxide adsorption test (LTCO2 GA)
- -
- High-temperature and high-pressure isothermal adsorption test (HTHP)
2.2. Calculation of SFE
3. Results and Discussion
3.1. Coal Quality and Coal Facies Classification
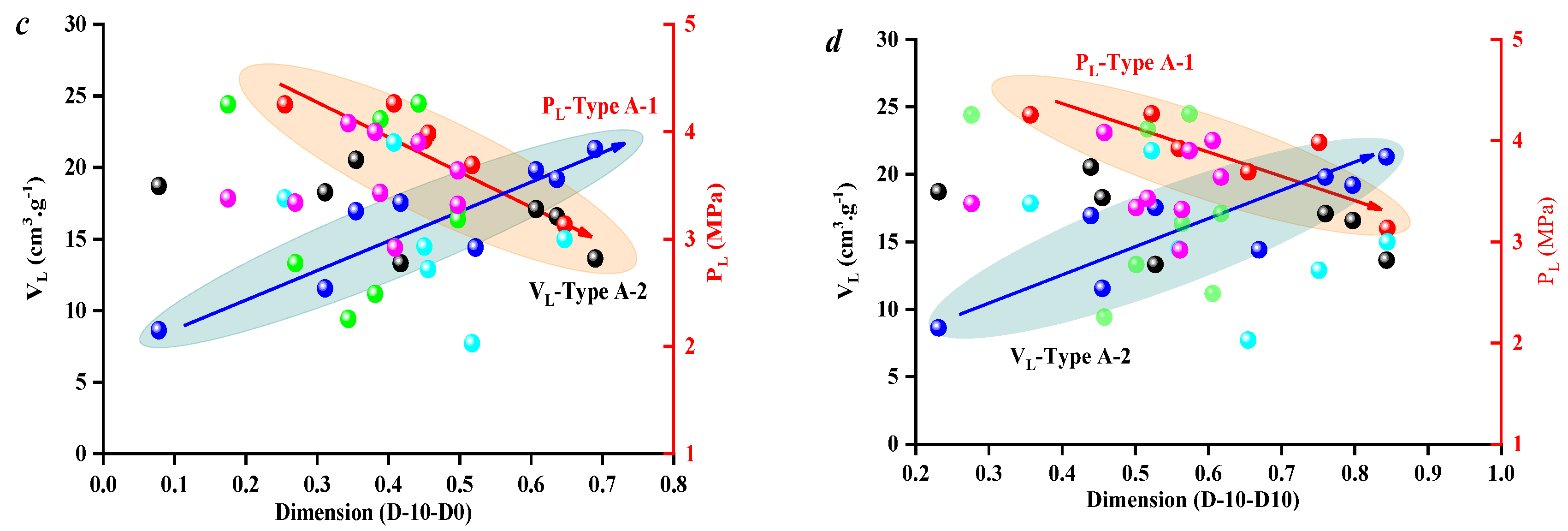
3.2. Effect of Coal Facies and Coal Quality on Methane Adsorption Ability
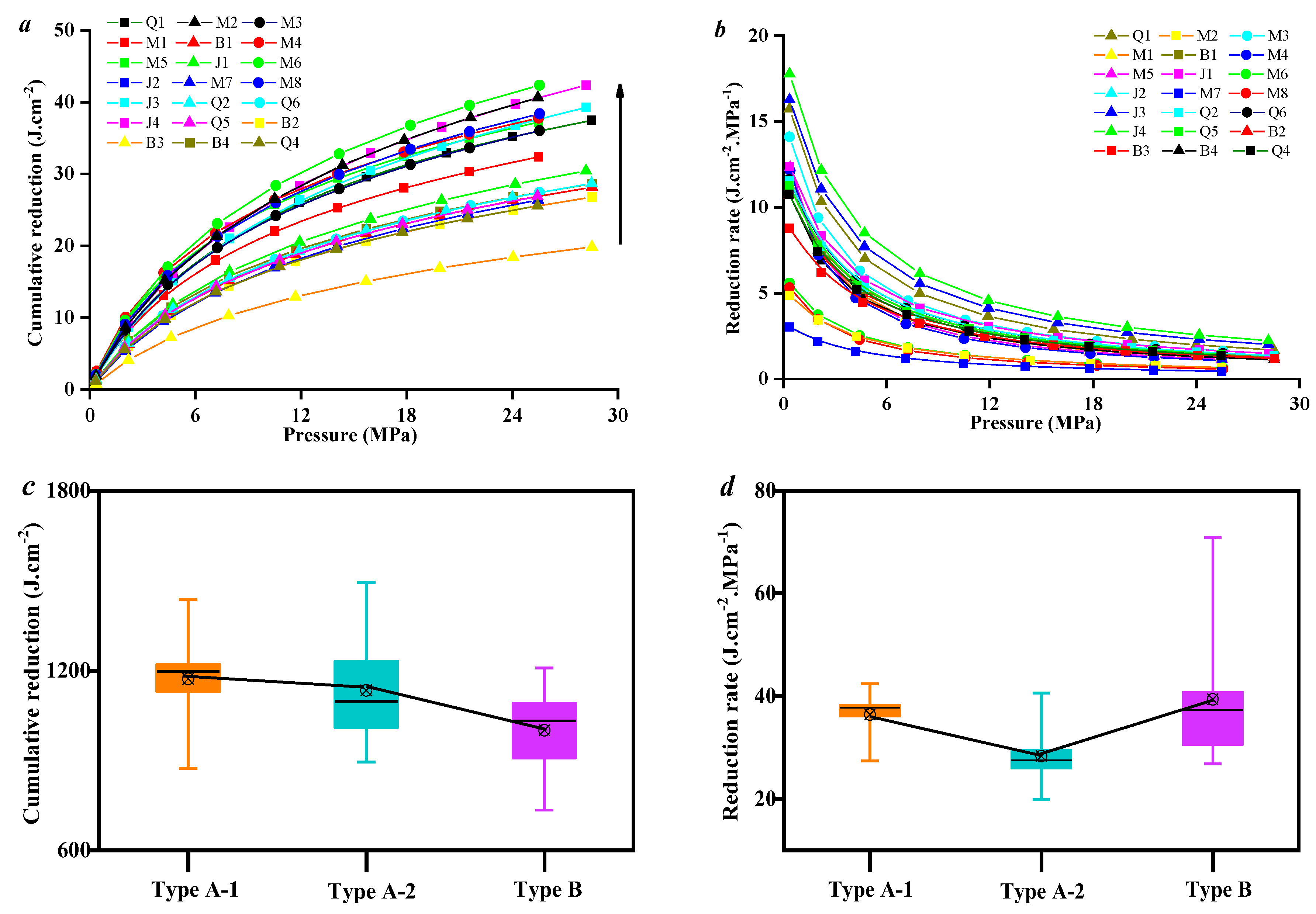
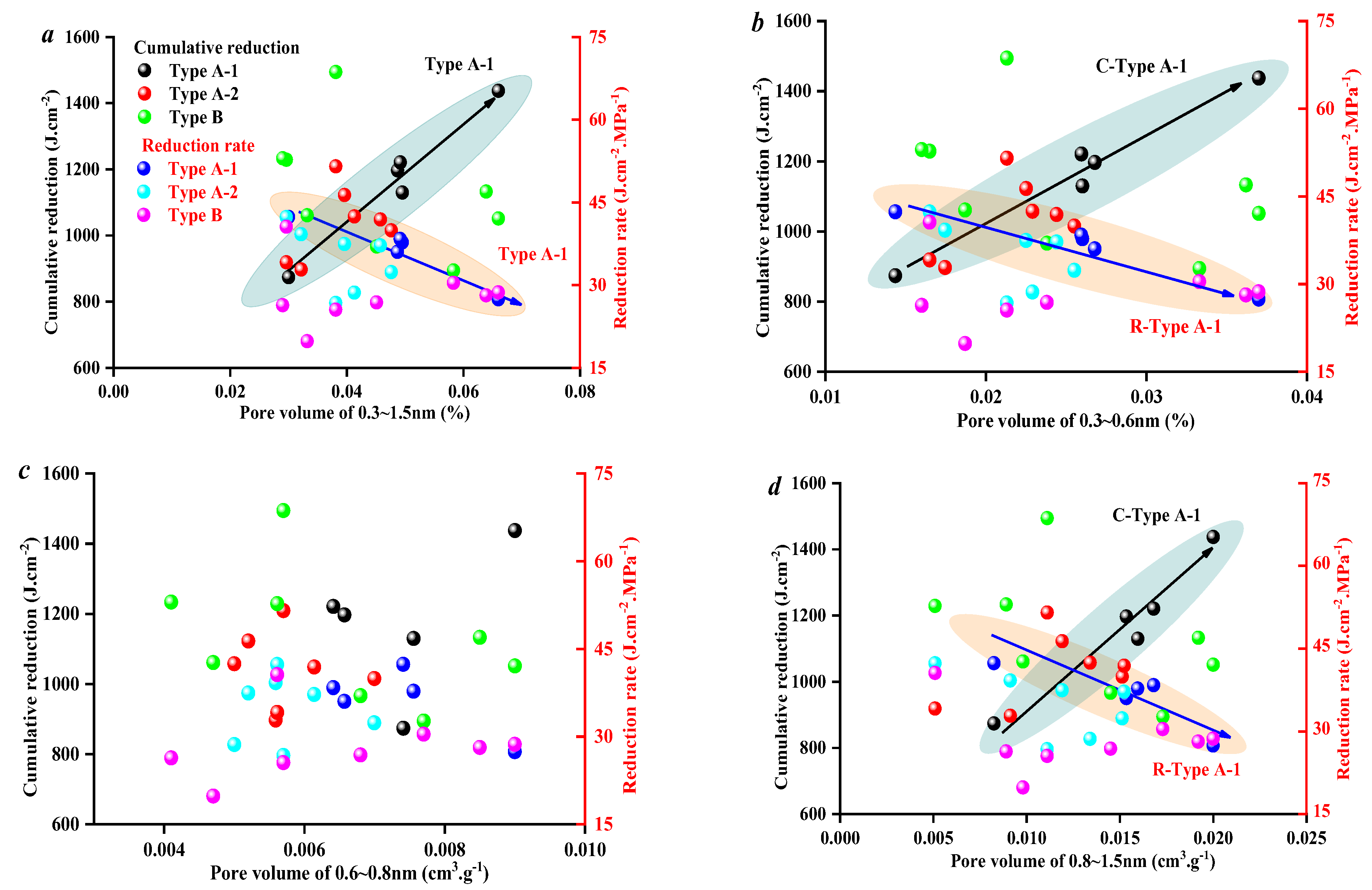
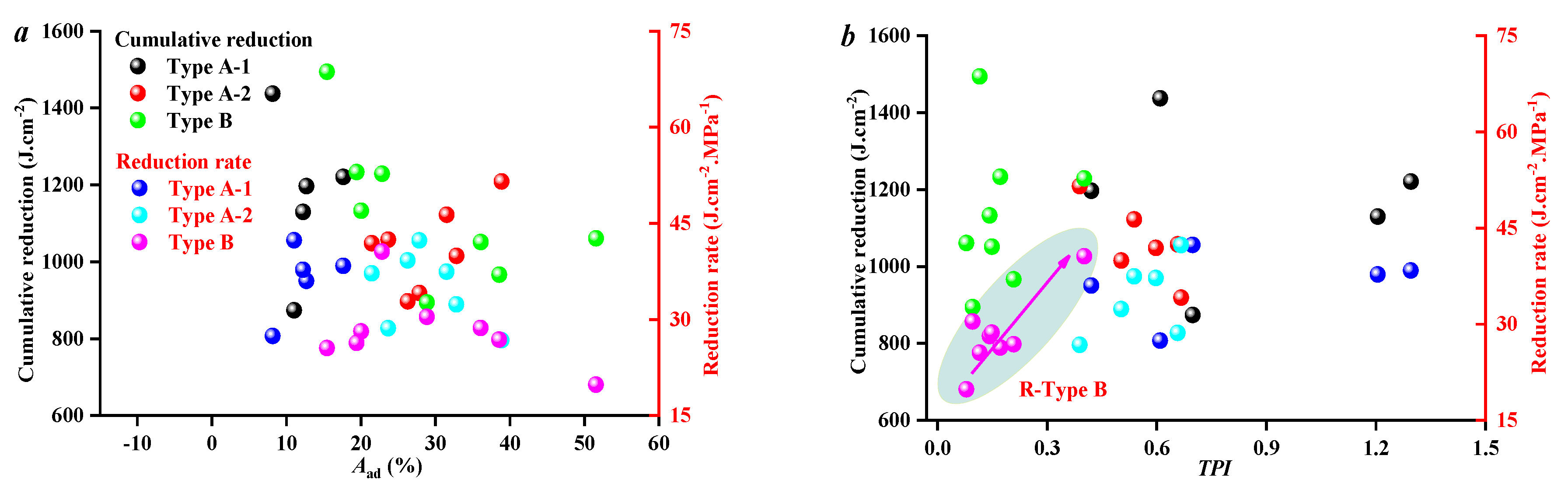
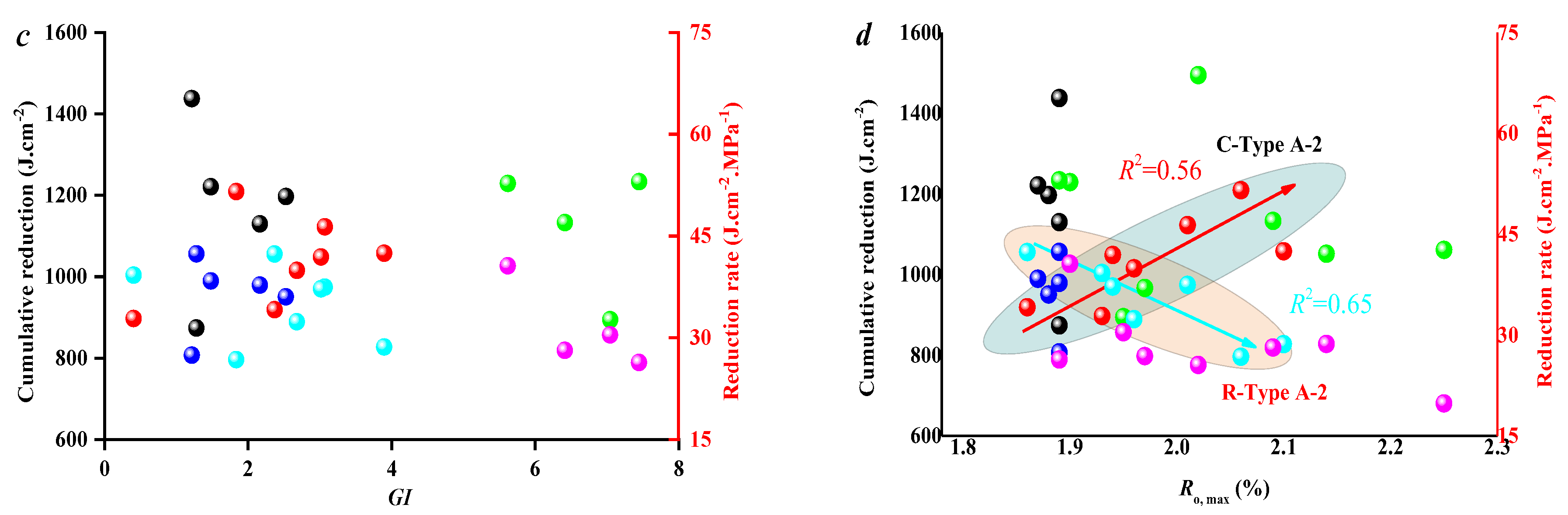
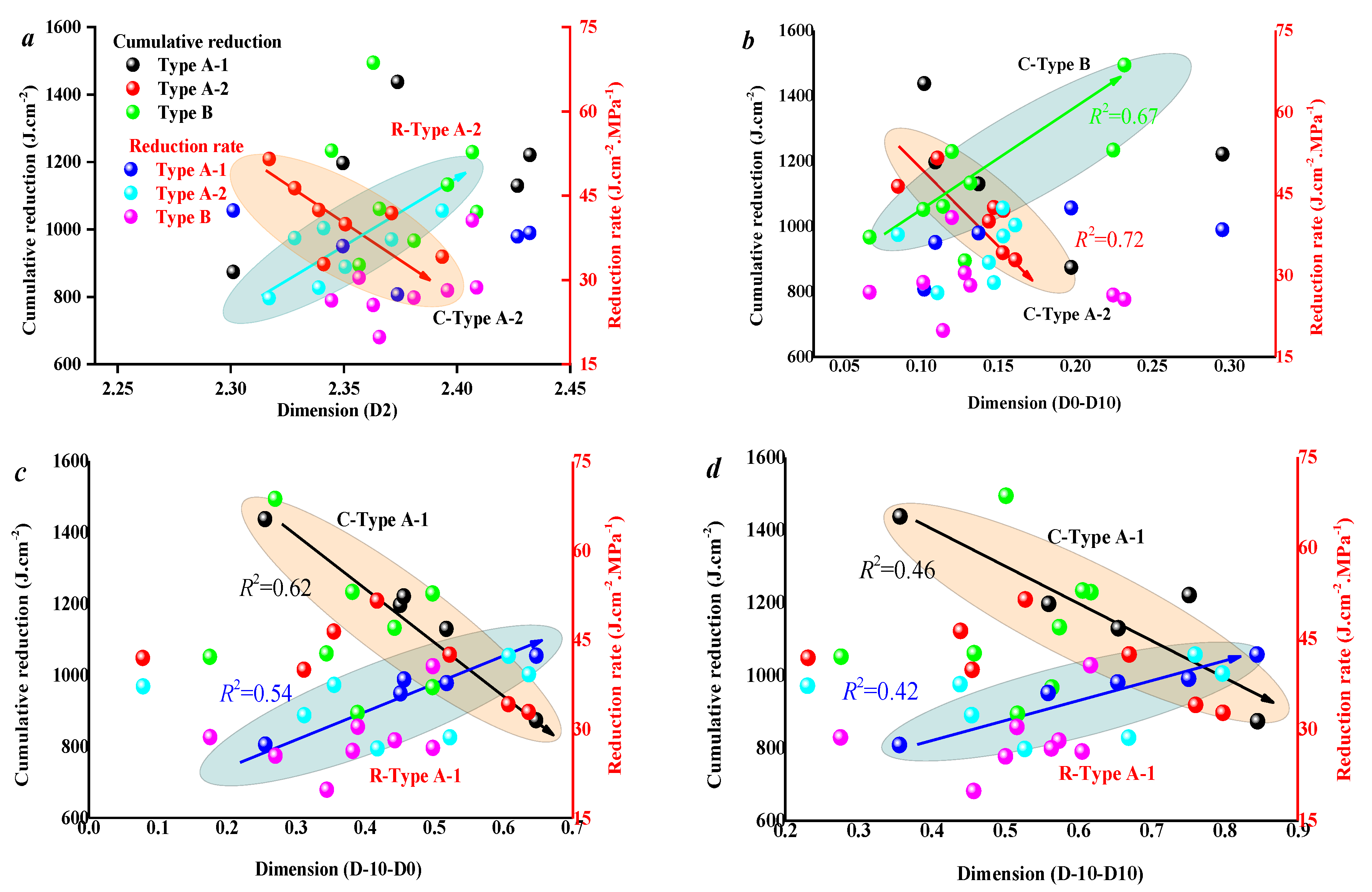
3.3. Effect of Coal Facies and Coal Quality on SFE Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Term | Full name |
| GI | Gel index |
| TPI | Plant Structure Preservation Index |
| GWI | Groundwater flow index |
| VL | Langmuir volume |
| PL | Langmuir pressure |
| CBM | Coalbed methane |
| SSA | Specific surface area |
| Aad | Yield contents |
| CSFE | Cumulative surface free energy |
| MSFE | Maximum surface free energy |
References
- Tonnsen, R.R.; Miskimins, J. Simulation of deep-coalbed-methane permeability and production assuming variable pore-volume compressibility. J. Can. Pet. Technol. 2011, 50, 23–31. [Google Scholar] [CrossRef]
- Xu, F.; Hou, W.; Xiong, X.; Xu, B.; Wu, P.; Wang, H.; Feng, K.; Yun, J.; Li, S.; Zhang, L.; et al. The status and development strategy of coalbed methane industry in China. Pet. Explor. Dev. 2023, 50, 765–783. [Google Scholar] [CrossRef]
- Hao, D.; Zhang, L.; Li, M.; Tu, S.; Zhang, C.; Bai, Q.; Wang, C. Experimental study of the moisture content influence on CH4 adsorption and deformation characteristics of cylindrical bituminous coal core. Adsorpt. Sci. Technol. 2018, 36, 1512–1537. [Google Scholar] [CrossRef]
- Huang, R.; Lei, Q.; Chen, J.; Weng, D.; Wang, X.; Liang, H. Gas content prediction model of water-sensitive shale based on gas–water miscible competitive adsorption. Pet. Sci. Technol. 2022, 42, 1841–1863. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, D.; Guo, C.; Yang, F.; Mu, T.; Gao, Z. An Experimental Study on the Conductivity Changes in Coal during Methane Adsorption-Desorption and their Influencing Factors. Acta Geol. Sin.-Engl. Ed. 2019, 93, 704–717. [Google Scholar] [CrossRef]
- Cheng, Y.; Pan, Z. Reservoir properties of Chinses tectonic coal: A review. Fuel 2020, 260, 116350. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, C.; Zhao, C.; Zhang, T.; Lu, G.; Zou, M. Effects of nano-pore and macromolecule structure of coal samples on energy parameters variation during methane adsorption under different temperature and pressure. Fuel 2021, 289, 119804. [Google Scholar] [CrossRef]
- Qin, L.; Wang, P.; Li, S.; Lin, H.; Wang, R.; Wang, P.; Ma, C. Gas adsorption capacity changes in coals of different ranks after liquid nitrogen freezing. Fuel 2021, 292, 120404. [Google Scholar] [CrossRef]
- Ye, J.; Tao, S.; Zhao, S.; Li, S.; Chen, S.; Cui, Y. Characteristics of methane adsorption/desorption heat and energy with respect to coal rank. J. Nat. Gas Sci. Eng. 2022, 99, 104445. [Google Scholar] [CrossRef]
- Sun, F.; Liu, D.; Cai, Y.; Qiu, Y. Coal rank-pressure coupling control mechanism on gas adsorption/desorption in coalbed methane reservoirs. Energy 2023, 270, 126849. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Zhao, W.; Wang, L. Intrinsic relationship between Langmuir sorption volume and pressure for coal: Experimental and thermodynamic modeling study. Fuel 2019, 241, 105–117. [Google Scholar] [CrossRef]
- Niu, Q.; Pan, J.; Jin, Y.; Wang, H.; Li, M.; Ji, Z.; Wang, K.; Wang, Z. Fractal study of adsorption-pores in pulverized coals with various metamorphism degrees using N2 adsorption, X-ray scattering and image analysis methods. J. Pet. Sci. Eng. 2019, 176, 584–593. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, J.; Liang, X.; Wang, S.; Ma, Z. Ecological risk assessment of soil and water loss by thermal enhanced methane recovery: Numerical study using two-phase flow simulation. J. Clean. Prod. 2022, 334, 130183. [Google Scholar] [CrossRef]
- Li, Z.; Ren, T.; Li, X.; Qiao, M.; Yang, X.; Tan, L.; Nie, B. Multi-scale pore fractal characteristics of differently ranked coal and its impact on gas adsorption. Int. J. Min. Sci. Technol. 2023, 33, 389–401. [Google Scholar] [CrossRef]
- Li, T.; Wu, C. Coal facies characteristics and its control on methane content in South Yanchuan Block, Southeast Ordos Basin, China. Int. J. Green Energy Coal Geol. 2017, 14, 63–74. [Google Scholar] [CrossRef]
- Zou, J.; Rezaee, M.R.; Xie, Q.; You, L.; Liu, K.; Saeedi, A. Investigation of moisture effect on methane adsorption capacity of shale samples. Fuel 2018, 232, 323–332. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Cheng, Y.-P.; Li, H.-R.; Wang, L.; Jin, K.; Dong, J. Impact of inherent moisture on the methane adsorption characteristics of coals with various degrees of metamorphism. J. Nat. Gas Sci. Eng. 2018, 55, 312–320. [Google Scholar] [CrossRef]
- Yan, J.; Meng, Z.; Li, G. Diffusion characteristics of methane in various rank coals and the control mechanism. Fuel 2021, 283, 118959. [Google Scholar] [CrossRef]
- Ocampo-Pérez, R.; Rivera-Utrilla, J.; Gómez-Pacheco, C.; Sánchez-Polo, M.; López-Peñalver, J. Kinetic study of tetracycline adsorption on sludge-derived adsorbents in aqueous phase. Chem. Eng. J. 2012, 213, 88–96. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, H.; Tang, D.; Mathews, J.P.; Li, S.; Tao, S. A comparative evaluation of coal specific surface area by CO2 and N2 adsorption and its influence on CH4 adsorption capacity at different pore sizes. Fuel 2016, 183, 420–431. [Google Scholar] [CrossRef]
- Fu, H.; Tang, D.; Xu, T.; Xu, H.; Tao, S.; Li, S.; Yin, Z.; Chen, B.; Zhang, C.; Wang, L. Characteristics of pore structure and fractal dimension of low-rank coal: A case study of Lower Jurassic Xishanyao coal in the southern Junggar Basin, NW China. Fuel 2017, 193, 254–264. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.; Yuan, L. Impact of coal composition and pore structure on gas adsorption: A study based on a synchrotron radiation facility. Greenh. Gases Sci. Technol. 2020, 10, 116–129. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, S.; Li, G. Adsorption capacity, adsorption potential and surface free energy of different structure high rank coals. J. Pet. Sci. Eng. 2016, 146, 856–865. [Google Scholar] [CrossRef]
- Gao, C.; Liu, D.; Vandeginste, V.; Cai, Y.; Sun, F. Thermodynamic energy change and occurrence mechanism of multiple fluids in coal reservoirs. Energy 2023, 283, 129089. [Google Scholar] [CrossRef]
- Zhang, L.; Aziz, N.; Ren, T.X.; Wang, Z. Influence of Temperature on Coal Sorption Characteristics and the Theory of Coal Surface Free Energy. Procedia Eng. 2011, 26, 1430–1439. [Google Scholar] [CrossRef]
- Lu, G.; Wei, C.; Wang, J.; Zhang, J.; Quan, F.; Tamehe, L.S. Variation of surface free energy in the process of methane adsorption in the nanopores of tectonically deformed coals: A Case Study of Middle-Rank Tectonically Deformed Coals in the Huaibei Coalfield. Energy Fuels 2019, 33, 7155–7165. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, C.; Luo, J.; Lu, G.; Quan, F.; Zheng, K.; Peng, Y. Volume and Surface Distribution Heterogeneity of Nano-pore in Coal Samples by CO2 and N2 Adsorption Experiments. Acta Geol. Sin.-Engl. 2020, 94, 1662–1675. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Vandeginste, V.; Miao, H.; Guo, Y.; Ji, Y.; Liu, P.; Peng, Y. Effect of litho-facies on nano-pore structure of continental shale in shuinan formation of Jiaolai Basin. Geoenergy Sci. Eng. 2023, 228, 212020. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, S.; Tang, D.; Pan, Z.; Yang, F. The characteristics of coal reservoir pores and coal facies in Liulin district, Hedong coal field of China. Int. J. Coal Geol. 2010, 81, 117–127. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, K.; Ju, Y.; Fan, L.; Cao, H.; Yang, Y.; Shu, L.; Feng, Z.; Cui, R.; Guo, X.; et al. Quantification of the asynchronous gas diffusivity in macro-/micropores using a Nelder-Mead simplex algorithm and its application on predicting desorption-based indexes. Fuel 2023, 332, 126149. [Google Scholar] [CrossRef]
- GB/T 19222-2003; Sampling of Coal Petrology. Standardization Administration of China: Beijing, China, 2003.
- GB/T 6948-1998; Microscopical Determination of the Reflectance of Vitrinite in Coal. Standardization Administration of China: Beijing, China, 1998.
- GB/T 212-2001; Proximate Analysis of Coal. Standardization Administration of China: Beijing, China, 2001.
- Pan, J.; Wang, K.; Hou, Q.; Niu, Q.; Wang, H.; Ji, Z. Micro-pores and fractures of coals analyzed by field emission scanning electron microscopy and fractal theory. Fuel 2016, 164, 277–285. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, H.; Wang, H.; Liu, D.; Liu, X. Research on the controlling effect of coal facies on pore structure—Deeply buried coal seam 8 # in Yulin Area, Ordos Basin for Example. Coal Sci. Technol. 2023, 52, 142–154. (In Chinese) [Google Scholar] [CrossRef]
- GB/19560-2008; Experimental Method for High Pressure Isothermal Adsorption of Coal. Standardization Administration of China: Beijing, China, 2008.
- Mosher, K.; He, J.; Liu, Y.; Rupp, E.; Wilcox, J. Molecular simulation of methane adsorption in micro- and mesoporous carbons with applications to coal and gas shale systems. Int. J. Coal Geol. 2013, 109–110, 36–44. [Google Scholar] [CrossRef]
- Mukherjee, M.; Misra, S. A review of experimental research on Enhanced Coal Bed Methane (ECBM) recovery via CO2 sequestration. Earth-Sci. Rev. 2018, 179, 392–410. [Google Scholar] [CrossRef]
- Feng, Z.-C.; Wang, C.; Zhou, D.; Zhao, D. Variation law of adsorption heat of methane and coal with inhomogeneous potential well. Adsorpt. Sci. Technol. 2018, 36, 1538–1549. [Google Scholar] [CrossRef]
- Mahmood, N.; Khan, M.; Khan, A. Purification, Surface Modification of Coal Ash Silica and lts Potential Application in Rubber Composites. J. Appl. Polym. Sci. 2010, 117, 1493–1501. [Google Scholar] [CrossRef]
- Lopanov, A.N.; Fanina, E.A.; Tikhomirova, K.V. Calculation of the Free Surface Energy of Coals from the Donets Basin and Graphites. Solid Fuel Chem. 2018, 52, 15–20. [Google Scholar] [CrossRef]
- Hazrin-Chong, N.H.; Das, T.; Manefield, M. Surface physico-chemistry governing microbial cell attachment and biofilm formation on coal. Int. J. Coal Geol. 2021, 236, 103671. [Google Scholar] [CrossRef]
- Pugi, L.; Cocci, M.; Frillici, F.S.; Berzi, L.; Mariottoni, E.; Sacchetto, M. Modeling of an underwater drilling platform for very near shore applications. Proc. Inst. Mech. Eng. Part M J. Eng. Marit. Environ. 2023, 237, 263–272. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Hao, F.; Liu, M.; Mitri, H.S. Constitutive model for methane desorption and diffusion based on pore structure differences between soft and hard coal. Int. J. Min. Sci. Technol. 2017, 27, 937–944. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, G.; Lin, J.; Barakos, G.; Chang, P. Fractal Evolution Characteristics on the Three-Dimensional Fractures in Coal Induced by CO2 Phase Transition Fracturing. Fractal Fract. 2024, 8, 273. [Google Scholar] [CrossRef]
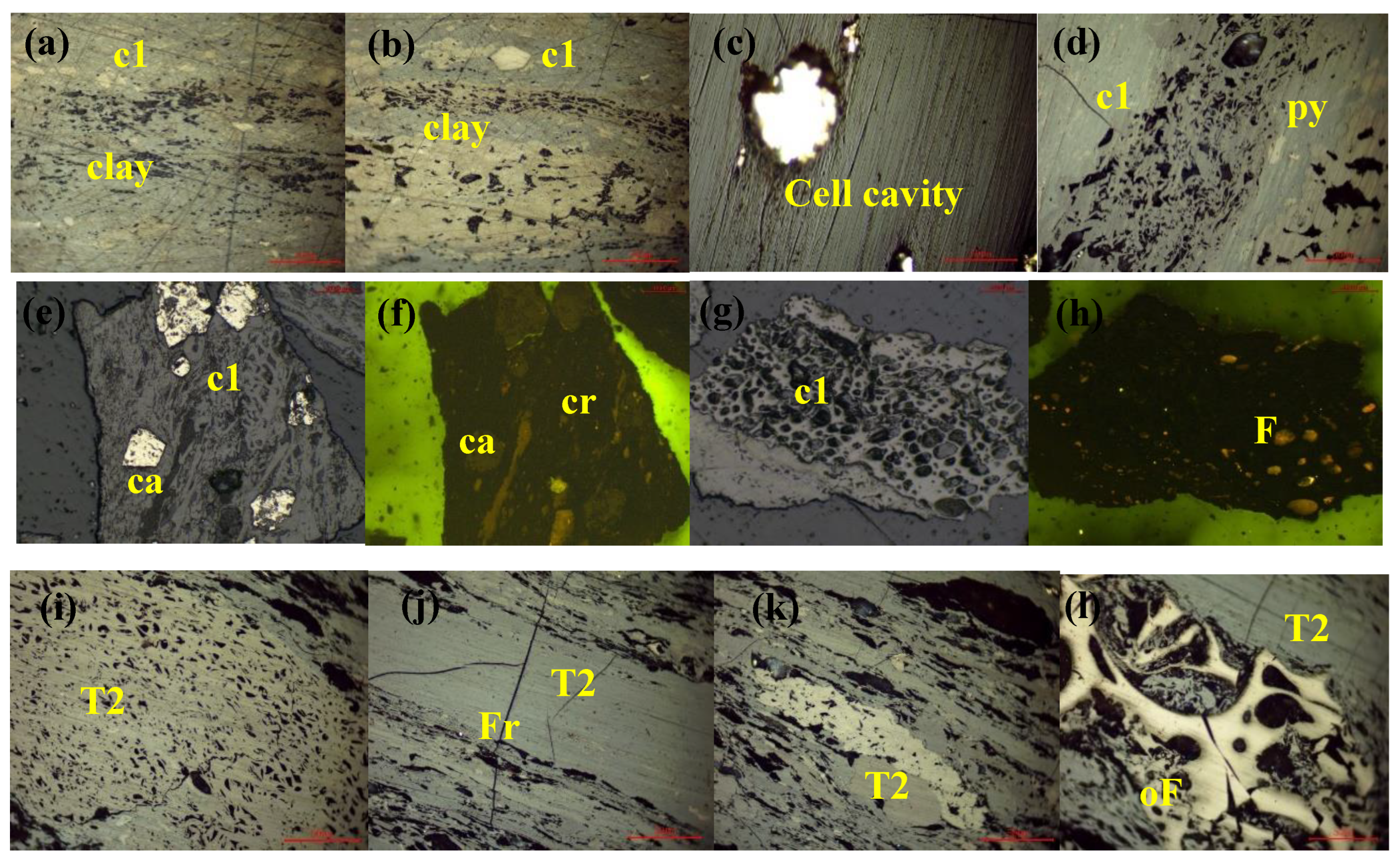
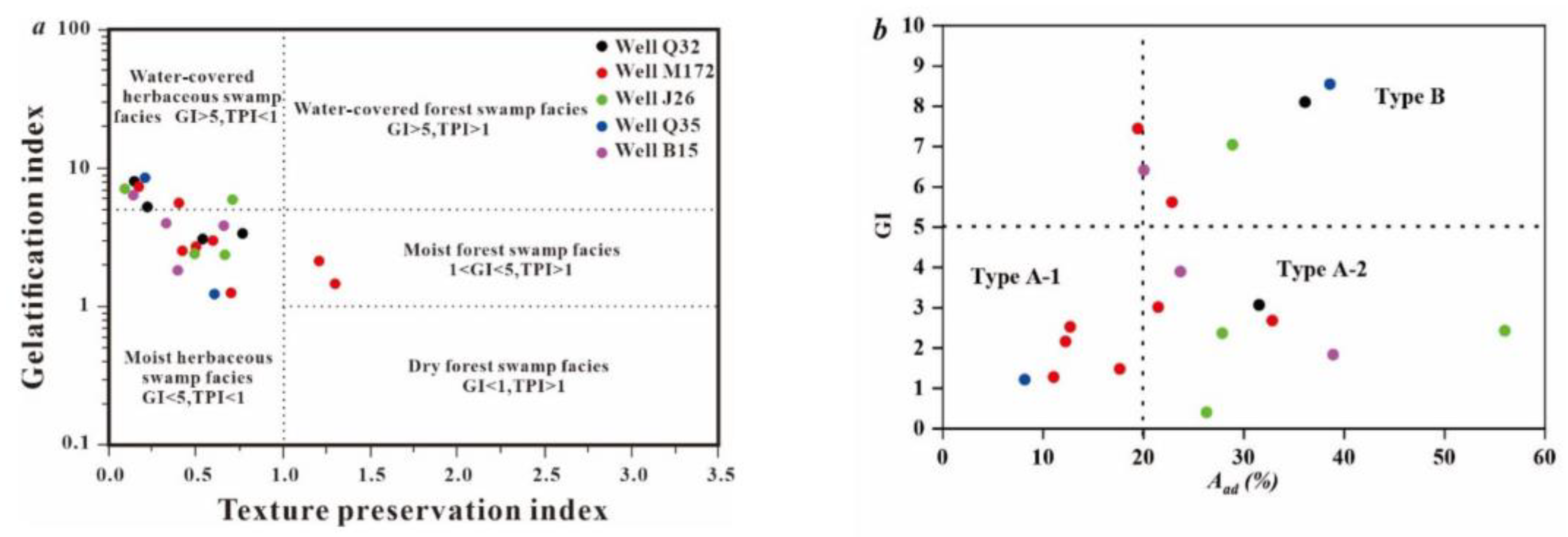
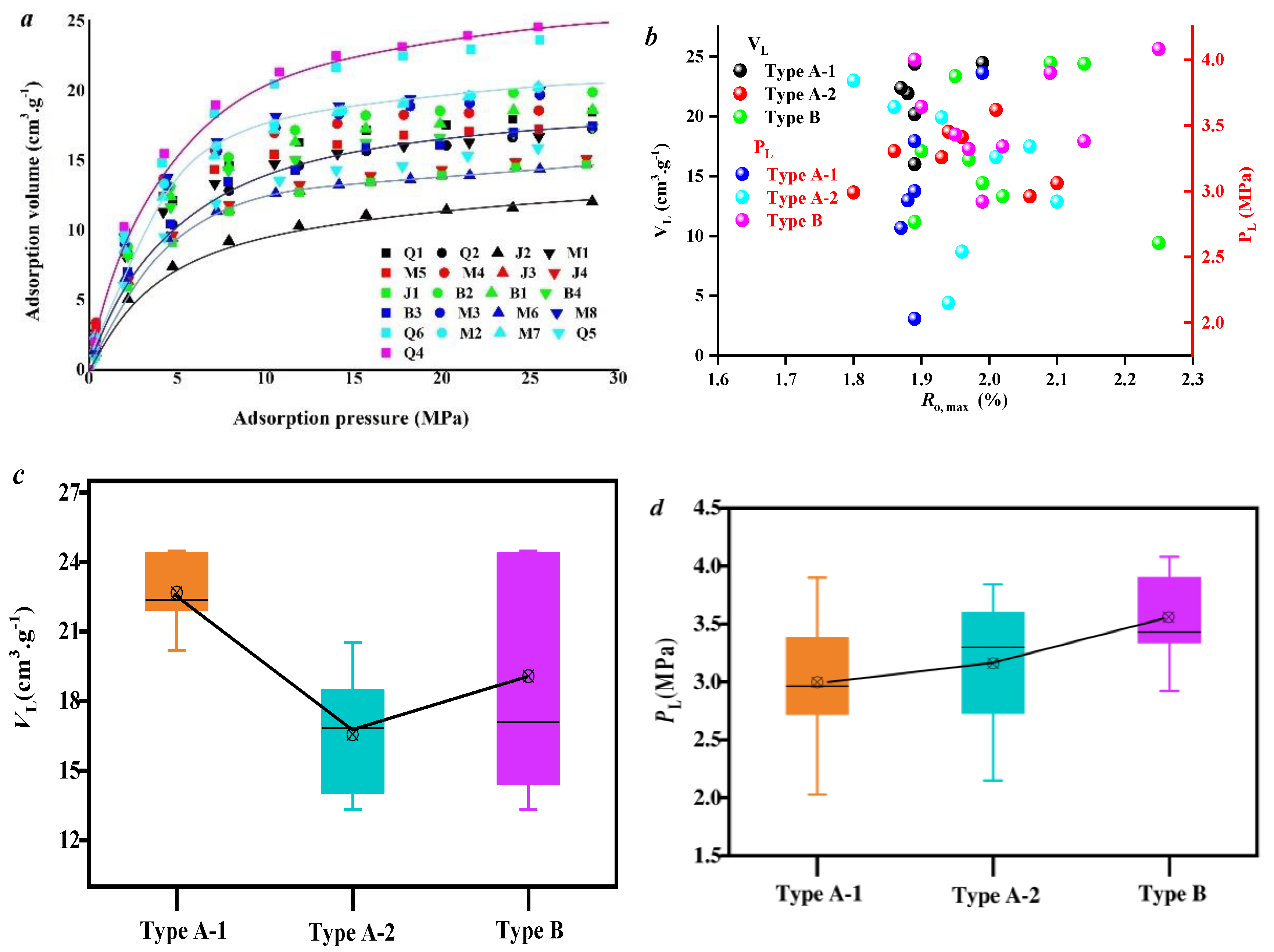
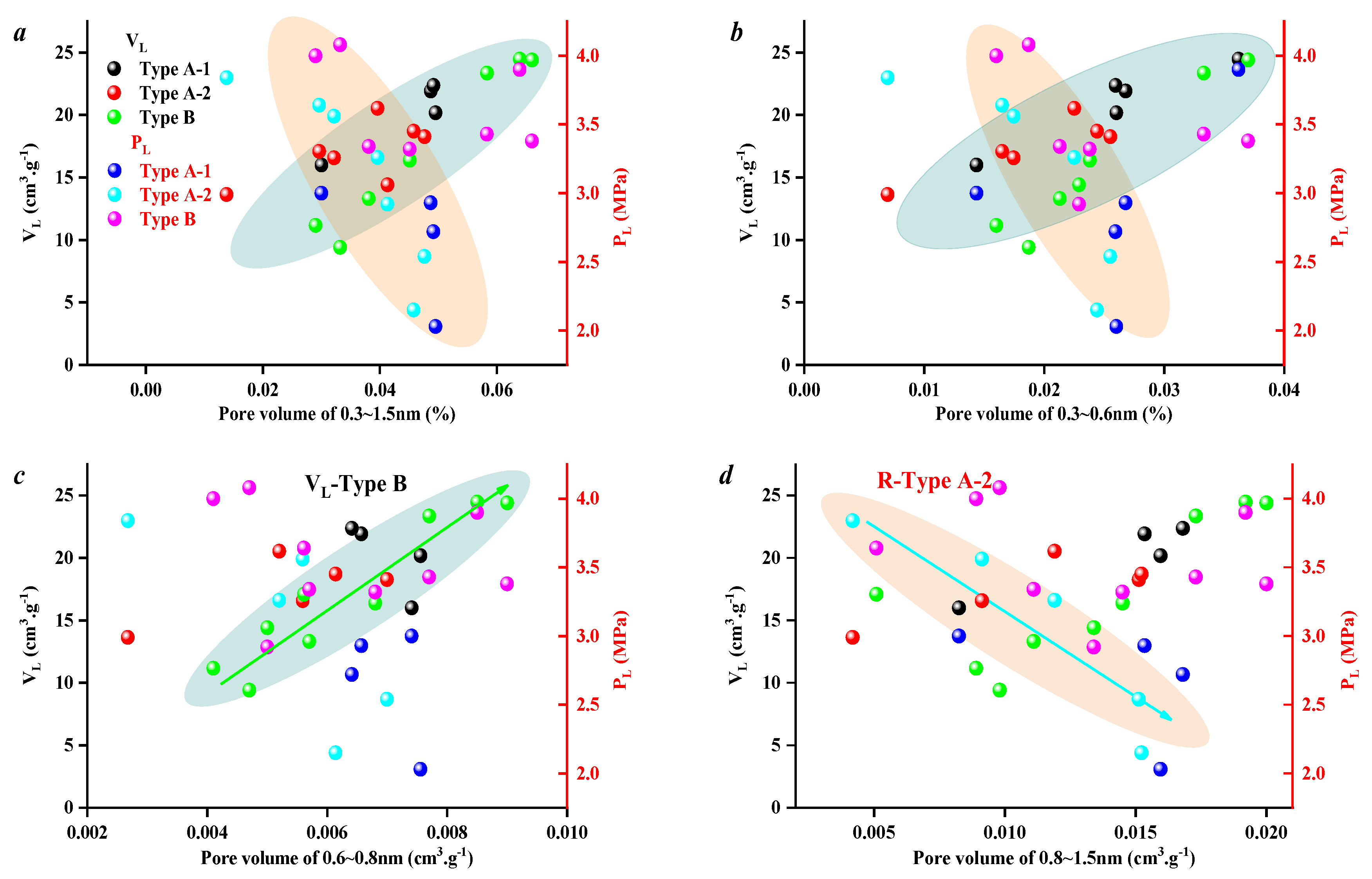



| Well No. | Sample No. | Ro,max (%) | Vitrinite (%) | Exinite (%) | Inertinite (%) | Mad (%) | Aad (%) | Vdaf (%) | FCdaf (%) |
|---|---|---|---|---|---|---|---|---|---|
| Q-1 | Q1 | 2.01 | 74.81 | 0 | 25.13 | 0.6 | 31.51 | 8.97 | 58.92 |
| Q2 | 2.14 | 88.27 | 0 | 11.72 | 0.56 | 36.09 | 9.33 | 54.02 | |
| M | M1 | 1.96 | 59.26 | 2.36 | 38.38 | 0.48 | 32.83 | 15.57 | 51.12 |
| M2 | 1.9 | 47.22 | 32.29 | 20.49 | 0.65 | 22.84 | 12.75 | 63.76 | |
| M3 | 1.88 | 50.52 | 17.53 | 31.96 | 0.98 | 12.69 | 12.16 | 74.17 | |
| M4 | 1.89 | 52.86 | 2.02 | 45.12 | 0.65 | 12.23 | 11.95 | 75.17 | |
| M5 | 1.94 | 58.11 | 1.69 | 40.2 | 0.79 | 21.46 | 11.81 | 65.94 | |
| M6 | 1.89 | 33.45 | 16.72 | 49.83 | 1.09 | 11.04 | 11.54 | 76.33 | |
| M7 | 1.89 | 59.72 | 5.56 | 34.72 | 0.79 | 19.43 | 11.96 | 67.82 | |
| M8 | 1.87 | 41.45 | 17.09 | 41.45 | 0.72 | 17.63 | 13.13 | 68.52 | |
| J | J1 | 1.95 | 59.44 | 8.74 | 31.82 | 0.65 | 28.86 | 12.21 | 58.28 |
| J2 | 1.8 | 47.79 | 20.35 | 31.86 | 0.72 | 55.99 | / | / | |
| J3 | 1.93 | 28.62 | 0.67 | 70.71 | 0.73 | 26.28 | 12.7 | 60.29 | |
| J4 | 1.86 | 48.58 | 14.18 | 37.23 | 0.68 | 27.85 | 12.53 | 58.94 | |
| Q-2 | Q3 | 1.99 | 97.71 | 2.29 | 0 | 0.88 | 10.39 | 8.21 | 80.52 |
| Q4 | 2.02 | 91.79 | 1.93 | 6.28 | 0.74 | 15.45 | 8.71 | 75.1 | |
| Q5 | 1.97 | 86.03 | 2.79 | 11.17 | 0.35 | 38.57 | 21.59 | 39.49 | |
| Q6 | 1.89 | 47.77 | 1.62 | 50.61 | 0.76 | 8.16 | 8.03 | 83.05 | |
| B | B1 | 2.09 | 86.34 | 0.00 | 13.73 | 0.63 | 20.04 | 9.14 | 70.19 |
| B2 | 2.06 | 62.57 | 0.00 | 37.37 | 0.45 | 38.89 | 9.68 | 50.98 | |
| B3 | 2.25 | 96.93 | 0.00 | 3.19 | 0.5 | 51.57 | / | / | |
| B4 | 2.1 | 79.21 | 0.00 | 20.74 | 0.3 | 23.67 | 12.1 | 63.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Wang, K.; Yan, B.; Qin, Z.; He, S.; Quan, F.; Vandeginste, V. Methane Adsorption Energy Variation Affected by Industrial Components in Deep and Thick Coal Reservoirs. Processes 2024, 12, 2780. https://doi.org/10.3390/pr12122780
Zhou X, Wang K, Yan B, Qin Z, He S, Quan F, Vandeginste V. Methane Adsorption Energy Variation Affected by Industrial Components in Deep and Thick Coal Reservoirs. Processes. 2024; 12(12):2780. https://doi.org/10.3390/pr12122780
Chicago/Turabian StyleZhou, Xiaogang, Kai Wang, Baozhen Yan, Zhengyuan Qin, Shi He, Fangkai Quan, and Veerle Vandeginste. 2024. "Methane Adsorption Energy Variation Affected by Industrial Components in Deep and Thick Coal Reservoirs" Processes 12, no. 12: 2780. https://doi.org/10.3390/pr12122780
APA StyleZhou, X., Wang, K., Yan, B., Qin, Z., He, S., Quan, F., & Vandeginste, V. (2024). Methane Adsorption Energy Variation Affected by Industrial Components in Deep and Thick Coal Reservoirs. Processes, 12(12), 2780. https://doi.org/10.3390/pr12122780








