Heteroatom-Doped Carbon-Based Catalysts Synthesized through a “Cook-Off” Process for Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Experimental
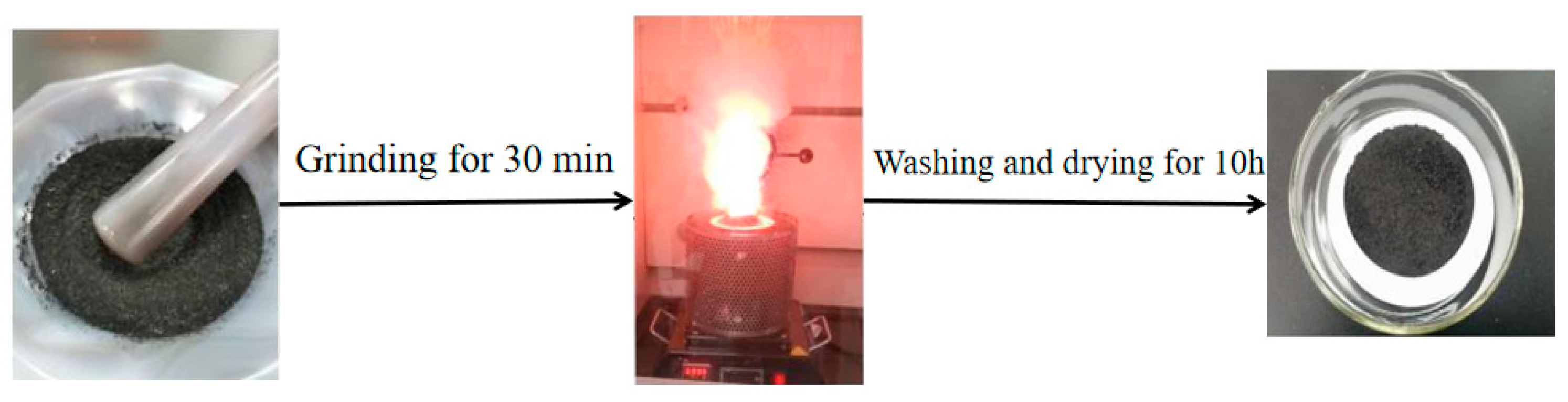
2.1. Preparation
2.2. Characterizations
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Characterizations of Heteroatom-Doped Carbon-Based Catalysts
3.2. Catalytic Performance
3.3. Promotion Mechanism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Luo, Z.; Tang, Y.; Deng, W. Analysis of the Impact of Lateral Conduit and Metal Wire Mesh on Methane Explosions. Processes 2023, 11, 3446. [Google Scholar] [CrossRef]
- Yao, Z.; Yan, T.; Hu, M. Comparison of undergraduate chemical engineering curricula between China and America Universities based on statistical analysis. Educ. Chem. Eng. 2022, 38, 55–59. [Google Scholar] [CrossRef]
- Fu, G.; Tang, Y.; Lee, J.-M. Recent Advances in Carbon-Based Bifunctional Oxygen Electrocatalysts for Zn-Air Batteries. ChemElectroChem 2018, 5, 1424–1434. [Google Scholar] [CrossRef]
- Li, W.; Hu, Z.-Y.; Zhang, Z.; Wei, P.; Zhang, J.; Pu, Z.; Zhu, J.; He, D.; Mu, S.; Van Tendeloo, G. Nano-single crystal coalesced PtCu nanospheres as robust bifunctional catalyst for hydrogen evolution and oxygen reduction reactions. J. Catal. 2019, 375, 164–170. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- Li, Y.; Tong, Y.; Peng, F. Metal-free carbocatalysis for electrochemical oxygen reduction reaction: Activity origin and mechanism. J. Energy Chem. 2020, 48, 308–321. [Google Scholar] [CrossRef]
- Qu, K.; Zheng, Y.; Dai, S.; Qiao, S.Z. Graphene oxide-polydopamine derived N, S-codoped carbon nanosheets as superior bifunctional electrocatalysts for oxygen reduction and evolution. Nano Energy 2016, 19, 373–381. [Google Scholar] [CrossRef]
- Shi, Q.; Lei, Y.; Wang, Y.; Wang, H.; Jiang, L.; Yuan, H.; Fang, D.; Wang, B.; Wu, N.; Gou, Y. B, N-codoped 3D micro-/mesoporous carbon nanofibers web as efficient metal-free catalysts for oxygen reduction. Curr. Appl. Phys. 2015, 15, 1606–1614. [Google Scholar] [CrossRef]
- Bayatsarmadi, B.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Soft-Templating Synthesis of N-Doped Mesoporous Carbon Nanospheres for Enhanced Oxygen Reduction Reaction. Chem. Asian J. 2015, 10, 1546–1553. [Google Scholar] [CrossRef]
- Yao, Z.; Li, L.; Liu, X.; Hui, K.N.; Shi, L.; Zhou, F.; Hu, M.; Hui, K.S. Mechanistic insights into NO-H2 reaction over Pt/boron-doped graphene catalyst. J. Hazard. Mater. 2021, 406, 124327. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Z.; Zhang, R.; Hu, M.; Shi, L.; Yao, Z. Recent Progress of Non-Pt Catalysts for Oxygen Reduction Reaction in Fuel Cells. Processes 2023, 11, 361. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, S.; Zhuang, H.; Lu, B.; Li, C.; Wang, Y.; Wang, G. Fluorine-doped carbon: A metal-free electrocatalyst for oxygen reduction to peroxide. Electrochim. Acta 2022, 420, 140460. [Google Scholar] [CrossRef]
- Sibul, R.; Kibena-Põldsepp, E.; Mäeorg, U.; Merisalu, M.; Kikas, A.; Kisand, V.; Treshchalov, A.; Sammelselg, V.; Tammeveski, K. Sulphur and nitrogen co-doped graphene-based electrocatalysts for oxygen reduction reaction in alkaline medium. Electrochem. Commun. 2019, 109, 106603. [Google Scholar] [CrossRef]
- Wei, P.; Li, X.; He, Z.; Sun, X.; Liang, Q.; Wang, Z.; Fang, C.; Li, Q.; Yang, H.; Han, J.; et al. Porous N, B co-doped carbon nanotubes as efficient metal-free electrocatalysts for ORR and Zn-air batteries. Chem. Eng. J. 2021, 422, 130134. [Google Scholar] [CrossRef]
- Nam, D.; Kim, J. Fe, P, N, S multidoping porous graphene material as a Bifunctional OER/ORR electrocatalytic activity for enhancing rechargeable Zn-air batteries. Ionics 2022, 28, 4719–4728. [Google Scholar] [CrossRef]
- Li, G.-L.; Cheng, G.-C.; Chen, W.-W.; Liu, C.-D.; Yuan, L.-F.; Yang, B.-B.; Hao, C. N/S/B-doped graphitized carbon encased Fe species as a highly active and durable catalyst towards oxygen reduction reaction. J. Colloid Interface Sci. 2018, 514, 108–116. [Google Scholar] [CrossRef]
- Li, B.; Xiang, T.; Shao, Y.; Lv, F.; Cheng, C.; Zhang, J.; Zhu, Q.; Zhang, Y.; Yang, J. Secondary-Heteroatom-Doping-Derived Synthesis of N, S Co-Doped Graphene Nanoribbons for Enhanced Oxygen Reduction Activity. Nanomaterials 2022, 12, 3306. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, R.; Zhao, S.; Ma, W.; Zhang, X.; Song, Y.; Ma, C.; Shi, J. B, N, F tri-doped lignin-derived carbon nanofibers as an efficient metal-free bifunctional electrocatalyst for ORR and OER in rechargeable liquid/solid-state Zn-air batteries. Appl. Surf. Sci. 2022, 598, 153891. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Du, Y.; Qing, M.; Yu, F.; Tian, Z.Q.; Shen, P.K. Highly active N, S co-doped hierarchical porous carbon nanospheres from green and template-free method for super capacitors and oxygen reduction reaction. Electrochim. Acta 2019, 318, 272–280. [Google Scholar] [CrossRef]
- Nallayagari, A.R.; Sgreccia, E.; Pasquini, L.; Vacandio, F.; Kaciulis, S.; Di Vona, M.L.; Knauth, P. Catalytic electrodes for the oxygen reduction reaction based on co-doped (B-N, Si-N, S-N) carbon quantum dots and anion exchange ionomer. Electrochim. Acta 2022, 427, 140861. [Google Scholar] [CrossRef]
- Park, J.-E.; Jang, Y.J.; Kim, Y.J.; Song, M.-S.; Yoon, S.; Kim, D.H.; Kim, S.-J. Sulfur-doped graphene as a potential alternative metal-free electrocatalyst and Pt-catalyst supporting material for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2014, 16, 103–109. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Multifunctional Carbon-Based Metal-Free Electrocatalysts for Simultaneous Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution. Adv. Mater. 2017, 29, 1604942. [Google Scholar] [CrossRef]
- Oh, T.; Kim, K.; Kim, J. Controllable active sites and facile synthesis of cobalt nanoparticle embedded in nitrogen and sulfur co-doped carbon nanotubes as efficient bifunctional electrocatalysts for oxygen reduction and evolution reactions. J. Energy Chem. 2019, 38, 60–67. [Google Scholar] [CrossRef]
- Sadegh Hassani, S.; Samiee, L.; Rashidi, A.; Ganjali, M.R. Comparative study of various preparation methods of metal-free N and S Co-doped porous graphene as an ORR catalyst in alkaline solution. J. Chem. Sci. 2022, 134, 27. [Google Scholar] [CrossRef]
- Hu, M.; Yao, Z.; Wang, X. Graphene-Based Nanomaterials for Catalysis. Ind. Eng. Chem. Res. 2017, 56, 3477–3502. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Li, R.; Sun, X.; Désilets, S.; Abou-Rachid, H.; Jaidann, M.; Lussier, L.-S. Structural and morphological control of aligned nitrogen-doped carbon nanotubes. Carbon 2010, 48, 1498–1507. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, J.-S.; Chen, X.-B.; Xiong, H.-M. Heteroatom-doped carbon dots based catalysts for oxygen reduction reactions. J. Colloid Interface Sci. 2019, 537, 716–724. [Google Scholar] [CrossRef]
- Gutru, R.; Turtayeva, Z.; Xu, F.; Maranzana, G.; Thimmappa, R.; Mamlouk, M.; Desforges, A.; Vigolo, B. Recent progress in heteroatom doped carbon based electrocatalysts for oxygen reduction reaction in anion exchange membrane fuel cells. Int. J. Hydrogen Energy 2023, 48, 3593–3631. [Google Scholar] [CrossRef]
- Feng, X.; Bai, Y.; Liu, M.; Li, Y.; Yang, H.; Wang, X.; Wu, C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci. 2021, 14, 2036–2089. [Google Scholar] [CrossRef]
- Yang, H.; Yang, G.; Qiao, Z.; Bao, H.; Zhang, S.; Li, X.; Liu, Y. Facile Deflagration Synthesis of Hollow Carbon Nanospheres with Efficient Performance for Solar Water Evaporation. ACS Appl. Mater. Interfaces 2020, 12, 35193–35200. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Hu, M.; Iqbal, Z.; Wang, X. N8− Polynitrogen Stabilized on Boron-Doped Graphene as Metal-Free Electrocatalysts for Oxygen Reduction Reaction. ACS Catal. 2020, 10, 160–167. [Google Scholar] [CrossRef]
- Han, Y.; Yan, D.; Ma, Z.; Wang, Q.; Wang, X.; Li, Y.; Sun, G. Lignin-derived sulfonate base metal-free N, S co-doped carbon microspheres doped with different nitrogen sources as catalysts for oxygen reduction reactions. Int. J. Biol. Macromol. 2023, 244, 125363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, X.; Xing, L.; Liu, J.; Kong, A.; Shan, Y. Copper-assisted thermal conversion of microporous covalent melamine-boroxine frameworks to hollow B, N-codoped carbon capsules as bifunctional metal-free electrode materials. Electrochim. Acta 2019, 298, 210–218. [Google Scholar] [CrossRef]
- Pan, F.; Duan, Y.; Liang, A.; Zhang, J.; Li, Y. Facile Integration of Hierarchical Pores and N,P-Codoping in Carbon Networks Enables Efficient Oxygen Reduction Reaction. Electrochim. Acta 2017, 238, 375–383. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Chen, X.; Peng, Y.; Song, Y.; Lv, C.; Liu, H.; Sun, J.; Yuan, D.; Li, X.; et al. Defect-Rich Nitrogen Doped Co3O4/C Porous Nanocubes Enable High-Efficiency Bifunctional Oxygen Electrocatalysis. Adv. Funct. Mater. 2019, 29, 1902875. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Z.; Meng, N.; Mccarthy, D.T.; Deletic, A.; Pan, J.H.; Zhang, X. Highly dispersed TiO2 nanocrystals and carbon dots on reduced graphene oxide: Ternary nanocomposites for accelerated photocatalytic water disinfection. Appl. Catal. B Environ. 2017, 202, 33–41. [Google Scholar] [CrossRef]
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-Based Metal-Free ORR Electrocatalysts for Fuel Cells: Past, Present, and Future. Adv. Mater. 2019, 31, 1804799. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.; Gao, Y.; Xiao, Z.; Zhang, J.; Wang, Q.; Zhang, X.; Wang, L. Doping carbon nanotubes with N, S, and B for electrocatalytic oxygen reduction: A systematic investigation on single, double, and triple doped modes. Catal. Sci. Technol. 2017, 7, 4007–4016. [Google Scholar] [CrossRef]
- Dong, F.; Cai, Y.; Liu, C.; Liu, J.; Qiao, J. Heteroatom (B, N and P) doped porous graphene foams for efficient oxygen reduction reaction electrocatalysis. Int. J. Hydrogen Energy 2018, 43, 12661–12670. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Y.; Wei, Z.; Wang, L.; Zhuo, M.; Zhang, J.; Han, X.; Ma, J. Ternary doped porous carbon nanofibers with excellent ORR and OER performance for zinc–air batteries. J. Mater. Chem. A 2018, 6, 10918–10925. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Origin of the Electrocatalytic Oxygen Reduction Activity of Graphene-Based Catalysts: A Roadmap to Achieve the Best Performance. J. Am. Chem. Soc. 2014, 136, 4394–4403. [Google Scholar] [CrossRef]
- Jin, J.; Pan, F.; Jiang, L.; Fu, X.; Liang, A.; Wei, Z.; Zhang, J.; Sun, G. Catalyst-Free Synthesis of Crumpled Boron and Nitrogen Co-Doped Graphite Layers with Tunable Bond Structure for Oxygen Reduction Reaction. ACS Nano 2014, 8, 3313–3321. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ren, B.; Jin, Q.; Cui, H.; Wang, C. Nitrogen-doped, oxygen-functionalized, edge- and defect-rich vertically aligned graphene for highly enhanced oxygen evolution reaction. J. Mater. Chem. A 2018, 6, 2176–2183. [Google Scholar] [CrossRef]
- Liu, T.; Guo, Y.-F.; Yan, Y.-M.; Wang, F.; Deng, C.; Rooney, D.; Sun, K.-N. CoO nanoparticles embedded in three-dimensional nitrogen/sulfur co-doped carbon nanofiber networks as a bifunctional catalyst for oxygen reduction/evolution reactions. Carbon 2016, 106, 84–92. [Google Scholar] [CrossRef]
- Qu, K.; Zheng, Y.; Jiao, Y.; Zhang, X.; Dai, S.; Qiao, S.-Z. Polydopamine-Inspired, Dual Heteroatom-Doped Carbon Nanotubes for Highly Efficient Overall Water Splitting. Adv. Energy Mater. 2017, 7, 1602068. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.-H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef]
- Patel, M.A.; Luo, F.; Savaram, K.; Kucheryavy, P.; Xie, Q.; Flach, C.; Mendelsohn, R.; Garfunkel, E.; Lockard, J.V.; He, H. P and S dual-doped graphitic porous carbon for aerobic oxidation reactions: Enhanced catalytic activity and catalytic sites. Carbon 2017, 114, 383–392. [Google Scholar] [CrossRef]
- You, C.; Liao, S.; Li, H.; Hou, S.; Peng, H.; Zeng, X.; Liu, F.; Zheng, R.; Fu, Z.; Li, Y. Uniform nitrogen and sulfur co-doped carbon nanospheres as catalysts for the oxygen reduction reaction. Carbon 2014, 69, 294–301. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, Y.; Wang, H.; Zhu, C.; Gao, J.; Wu, D.; Huang, H.; Liu, Y.; Kang, Z. A nitrogen and boron co-doped metal-free carbon electrocatalyst for an efficient oxygen reduction reaction. Inorg. Chem. Front. 2018, 5, 2985–2991. [Google Scholar] [CrossRef]
- Shin, D.; Jeong, B.; Mun, B.S.; Jeon, H.; Shin, H.-J.; Baik, J.; Lee, J. On the Origin of Electrocatalytic Oxygen Reduction Reaction on Electrospun Nitrogen-Carbon Species. J. Phys. Chem. C 2013, 117, 11619–11624. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, H.; Zhang, C.; Zhang, J.; Mu, S. Dual active nitrogen doped hierarchical porous hollow carbon nanospheres as an oxygen reduction electrocatalyst for zinc-air batteries. Nanoscale 2017, 9, 13257–13263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Selch, D.; Xie, Z.; Roberts, C.; Cooper, H.; Chen, G. Object-based benthic habitat mapping in the Florida Keys from hyperspectral imagery. Estuar. Coast. Shelf Sci. 2013, 134, 88–97. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Yuan, W.; Shen, Y.; Xie, A. B, N Co-Doped Three-Dimensional Carbon Aerogels with Excellent Electrochemical Performance for the Oxygen Reduction Reaction. Chem. A Eur. J. 2019, 25, 2877–2883. [Google Scholar] [CrossRef]
- Kodithuwakku, U.S.; Wanninayake, N.; Thomas, M.P.; Guiton, B.S.; Kim, D.Y. Unlocking efficiency in oxygen reduction reaction: Synergistic edge dopants of nitrogen and boron in carbon nano onions. Electrochim. Acta 2023, 471, 143365. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.-Q.; Tao, Y.-R.; Zhu, S.-N.; Zhang, Y.-X.; Wu, X.-C. Flowerlike Ag-Supported Ce-Doped Mn3O4 Nanosheet Heterostructure for a Highly Efficient Oxygen Reduction Reaction: Roles of Metal Oxides in Ag Surface States. ACS Catal. 2019, 9, 3498–3510. [Google Scholar] [CrossRef]
- Jin, H.; Huang, H.; He, Y.; Feng, X.; Wang, S.; Dai, L.; Wang, J. Graphene Quantum Dots Supported by Graphene Nanoribbons with Ultrahigh Electrocatalytic Performance for Oxygen Reduction. J. Am. Chem. Soc. 2015, 137, 7588–7591. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-Doped Graphene Quantum Dots with Oxygen-Rich Functional Groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wang, K.; Song, S.; Tsiakaras, P. 3D interconnected hierarchically porous N-doped carbon with NH3 activation for efficient oxygen reduction reaction. Appl. Catal. B Environ. 2017, 210, 57–66. [Google Scholar] [CrossRef]
- Liu, J.-N.; Li, B.-Q.; Zhao, C.-X.; Yu, J.; Zhang, Q. A Composite Bifunctional Oxygen Electrocatalyst for High-Performance Rechargeable Zinc-Air Batteries. ChemSusChem 2020, 13, 1529–1536. [Google Scholar] [CrossRef]
- Cao, S.; Shang, W.; Li, G.-L.; Lu, Z.-F.; Wang, X.; Yan, Y.; Hao, C.; Wang, S.; Sun, G. Defect-rich and metal-free N, S co-doped 3D interconnected mesoporous carbon material as an advanced electrocatalyst towards oxygen reduction reaction. Carbon 2021, 184, 127–135. [Google Scholar] [CrossRef]
- Duraisamy, V.; Senthil Kumar, S.M. N and P dual heteroatom doped mesoporous hollow carbon as an efficient oxygen reduction reaction catalyst in alkaline electrolyte. Int. J. Hydrogen Energy 2022, 47, 17992–18006. [Google Scholar] [CrossRef]
- Kim, M.; Firestein, K.L.; Fernando, J.F.S.; Xu, X.; Lim, H.; Golberg, D.V.; Na, J.; Kim, J.; Nara, H.; Tang, J.; et al. Strategic design of Fe and N co-doped hierarchically porous carbon as superior ORR catalyst: From the perspective of nanoarchitectonics. Chem. Sci. 2022, 13, 10836–10845. [Google Scholar] [CrossRef]
- Zhang, W.; Pu, W.; Qu, Y.; Yang, H.; Liu, Y. Facile synthesis of ultrathin S-N co-doped carbon nanosheet as ORR electrocatalysts for application in sustainable zinc-air battery. Electrochim. Acta 2023, 462, 142800. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Li, W.; Hou, Y. Noble metal-free catalysts for oxygen reduction reaction. Sci. China Chem. 2017, 60, 1494–1507. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, L. Carbon nanomaterials as metal-free catalysts in next generation fuel cells. Nano Energy 2012, 1, 514–517. [Google Scholar] [CrossRef]
- Zhao, L.; Sui, X.-L.; Li, J.-Z.; Zhang, J.-J.; Zhang, L.-M.; Huang, G.-S.; Wang, Z.-B. Supramolecular assembly promoted synthesis of three-dimensional nitrogen doped graphene frameworks as efficient electrocatalyst for oxygen reduction reaction and methanol electrooxidation. Appl. Catal. B Environ. 2018, 231, 224–233. [Google Scholar] [CrossRef]
- Meng, Y.; Voiry, D.; Goswami, A.; Zou, X.; Huang, X.; Chhowalla, M.; Liu, Z.; Asefa, T. N-, O-, and S-Tridoped Nanoporous Carbons as Selective Catalysts for Oxygen Reduction and Alcohol Oxidation Reactions. J. Am. Chem. Soc. 2014, 136, 13554–13557. [Google Scholar] [CrossRef]
- Chang, F.; Su, P.; Guharoy, U.; Ye, R.; Ma, Y.; Zheng, H.; Jia, Y.; Liu, J. Edge-enriched N, S co-doped hierarchical porous carbon for oxygen reduction reaction. Chin. Chem. Lett. 2023, 34, 107462. [Google Scholar] [CrossRef]
- Palm, I.; Kibena-Põldsepp, E.; Mooste, M.; Kozlova, J.; Käärik, M.; Kikas, A.; Treshchalov, A.; Leis, J.; Kisand, V.; Tamm, A.; et al. Nitrogen and sulphur co-doped carbon-based composites as electrocatalysts for the anion-exchange membrane fuel cell cathode. Int. J. Hydrogen Energy 2024, 55, 805–814. [Google Scholar] [CrossRef]







| Catalysts | Mass Loading (mg cm−2) | Eonset (V) | E1/2 (V) | Current Densities (mA cm−2) |
|---|---|---|---|---|
| CS | 0.414 | −0.199 | −0.465 | −3.94 |
| NCS | 0.414 | −0.203 | −0.491 | −4.71 |
| NBCS | 0.414 | −0.148 | −0.393 | −4.98 |
| Pt/C | 0.204 | 0 | −0.151 | −5.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Liu, Q.; Wan, M.; Yao, Z.; Hu, M. Heteroatom-Doped Carbon-Based Catalysts Synthesized through a “Cook-Off” Process for Oxygen Reduction Reaction. Processes 2024, 12, 264. https://doi.org/10.3390/pr12020264
Zhang R, Liu Q, Wan M, Yao Z, Hu M. Heteroatom-Doped Carbon-Based Catalysts Synthesized through a “Cook-Off” Process for Oxygen Reduction Reaction. Processes. 2024; 12(2):264. https://doi.org/10.3390/pr12020264
Chicago/Turabian StyleZhang, Ruiquan, Qiongyu Liu, Ming Wan, Zhenhua Yao, and Maocong Hu. 2024. "Heteroatom-Doped Carbon-Based Catalysts Synthesized through a “Cook-Off” Process for Oxygen Reduction Reaction" Processes 12, no. 2: 264. https://doi.org/10.3390/pr12020264





