Optimization of Ternary Activator for Enhancing Mechanical Properties of Carbonized Cementitious Material Based on Circulating Fluidized Bed Fly Ash
Abstract
:1. Introduction
2. Experiment
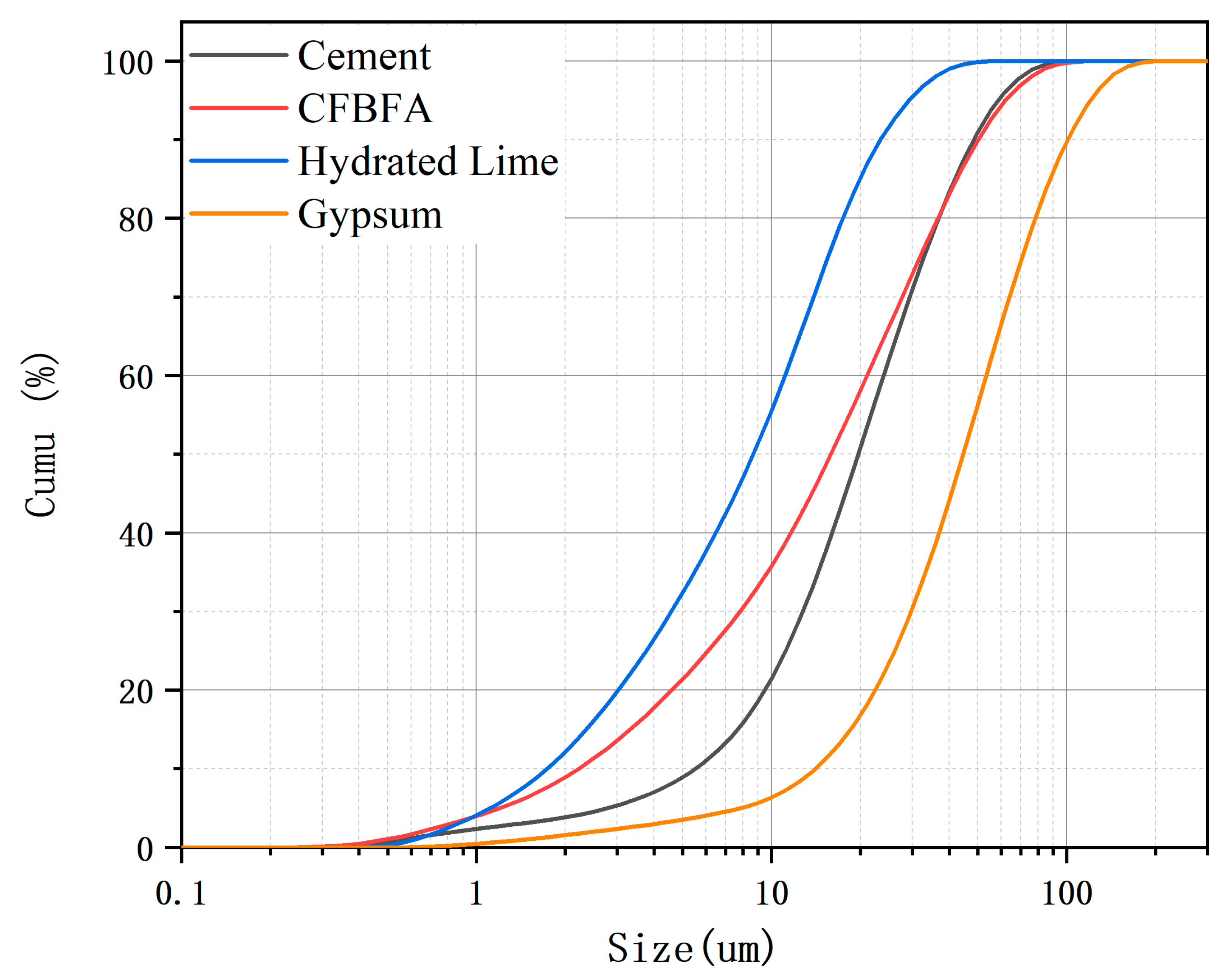
2.1. Materials
2.2. Design of Experiments
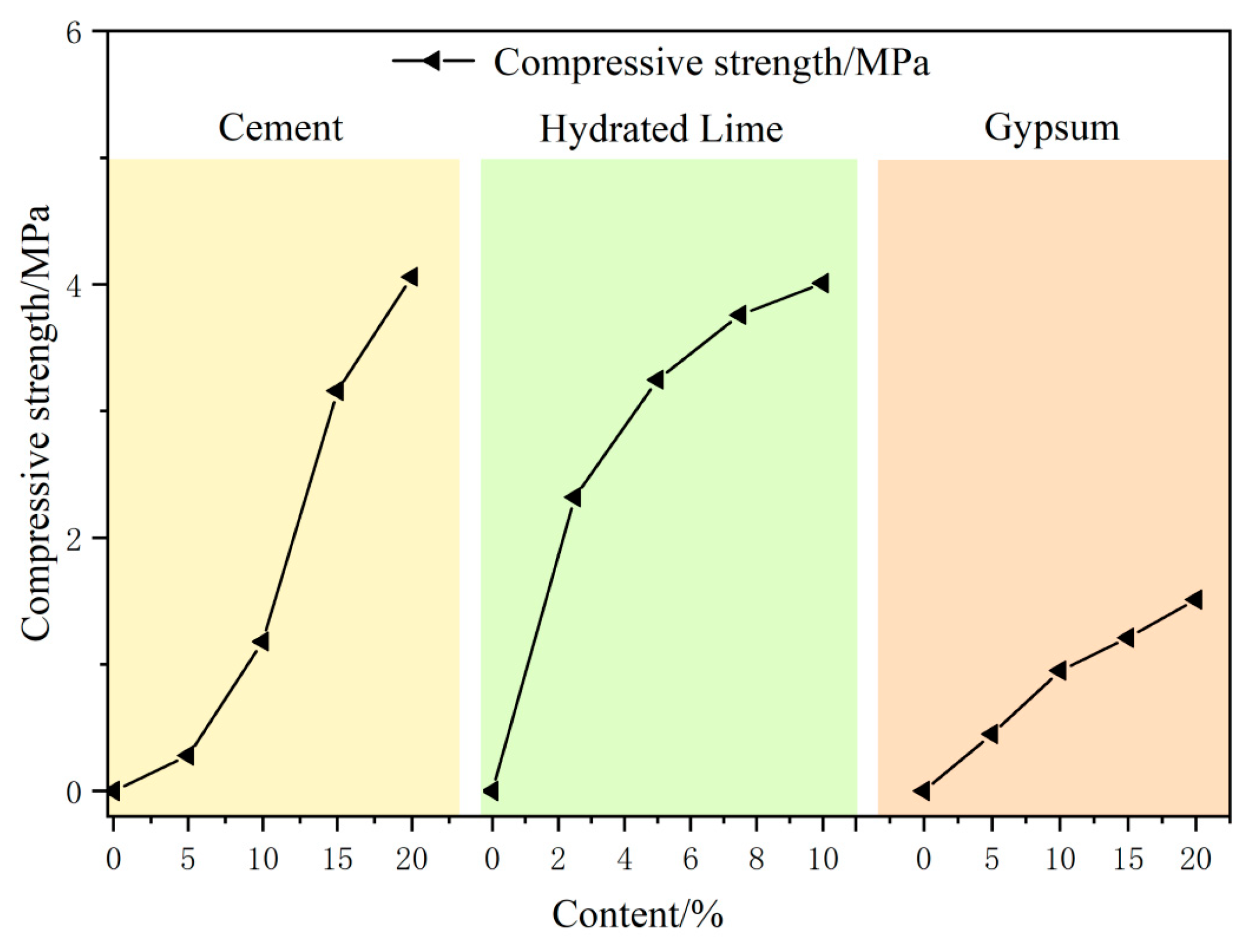
2.2.1. Single Factor Variable Experiment
2.2.2. Multi-Factor Variable Experiment
2.2.3. Mechanism Analysis
3. Analyses
3.1. Single-Factor Analyses
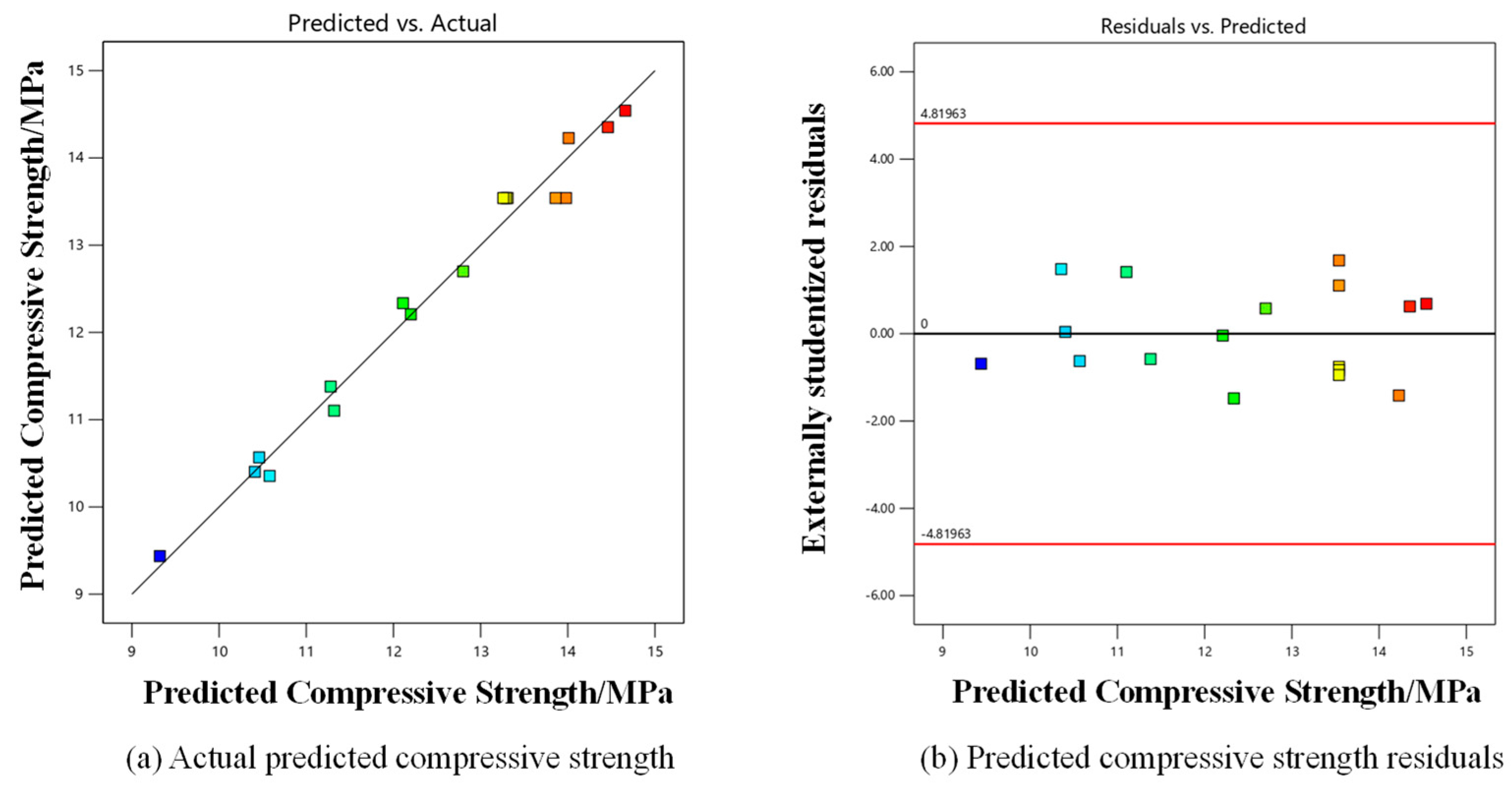
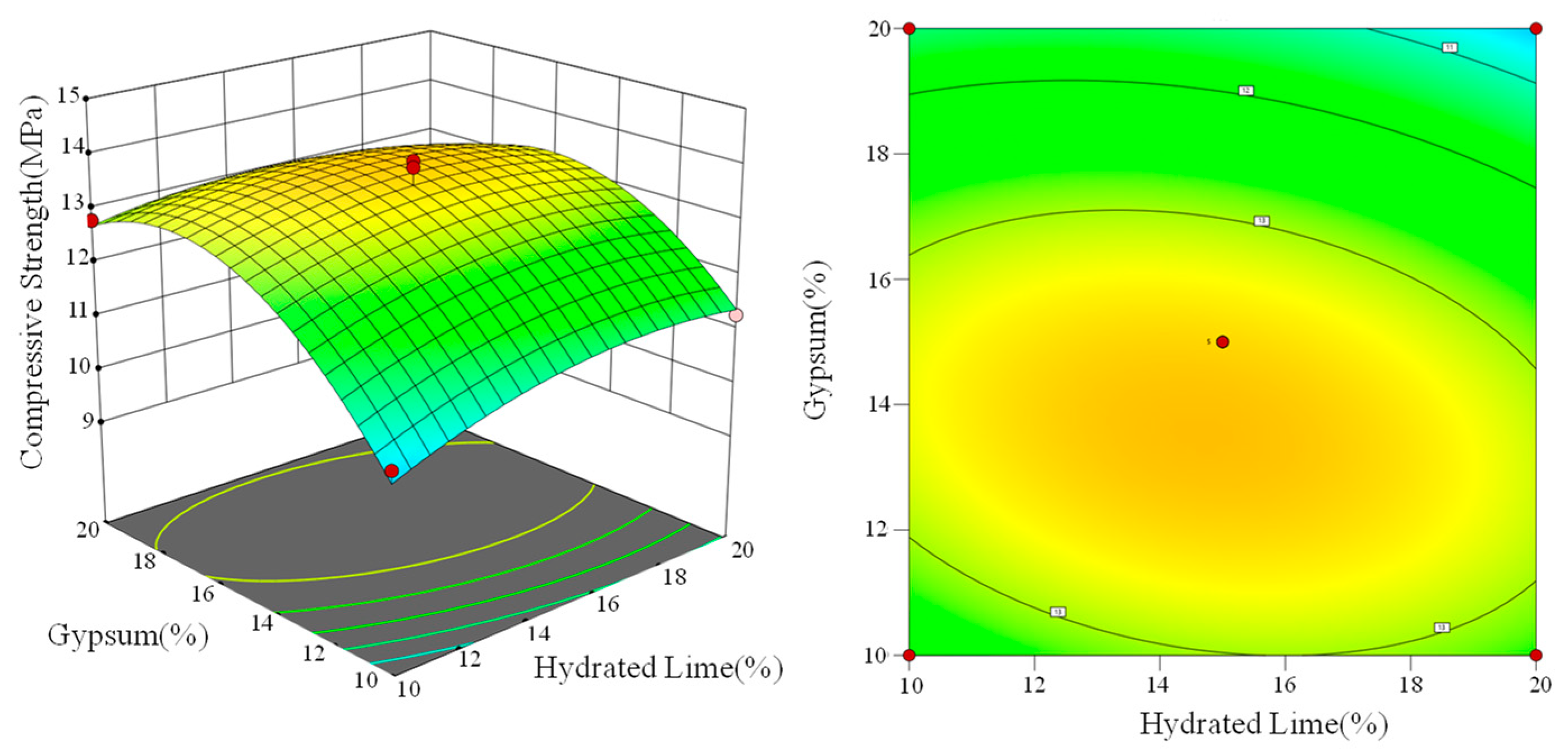
3.2. Modeling by BBD-RSM
3.3. Analysising
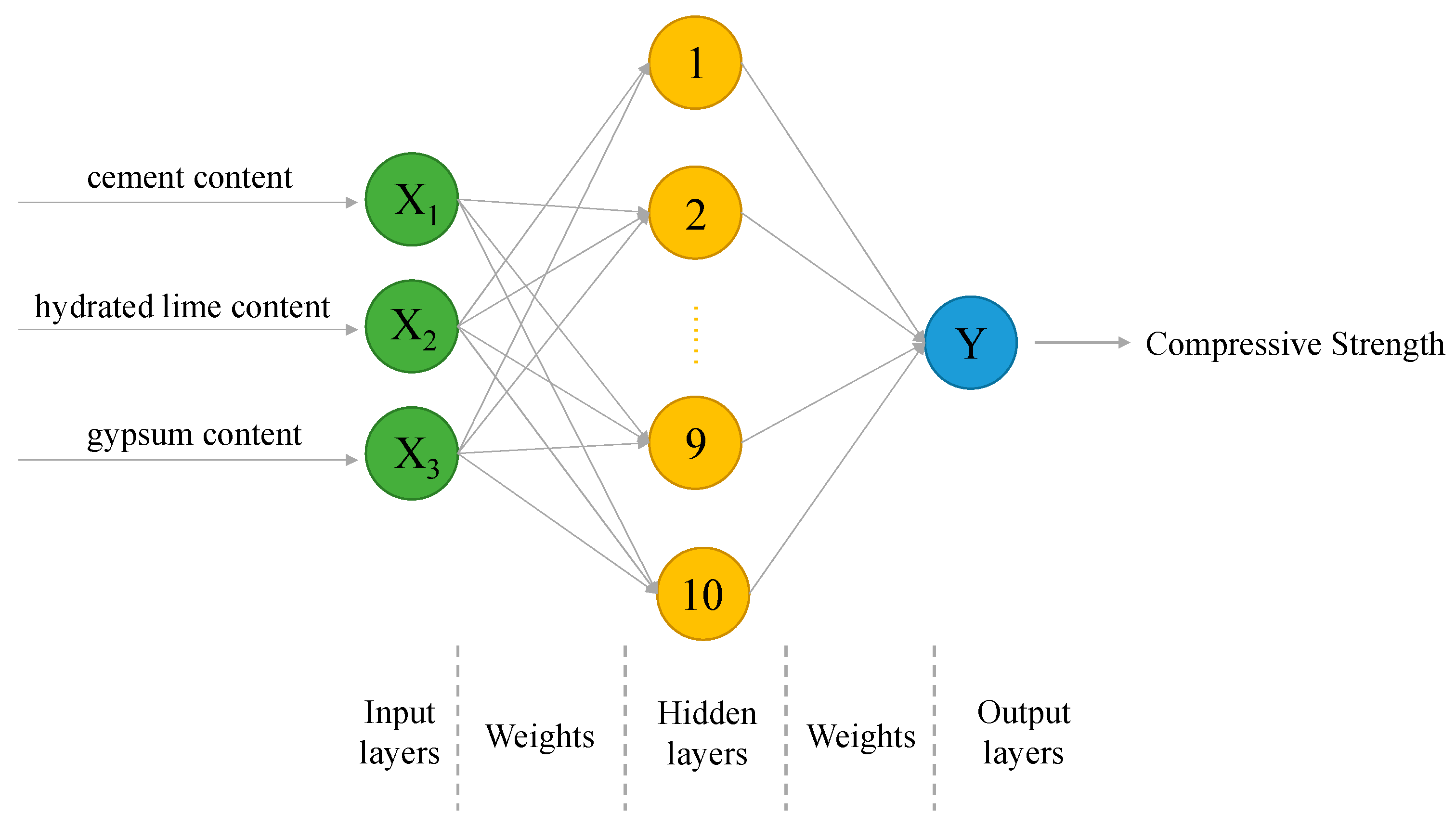
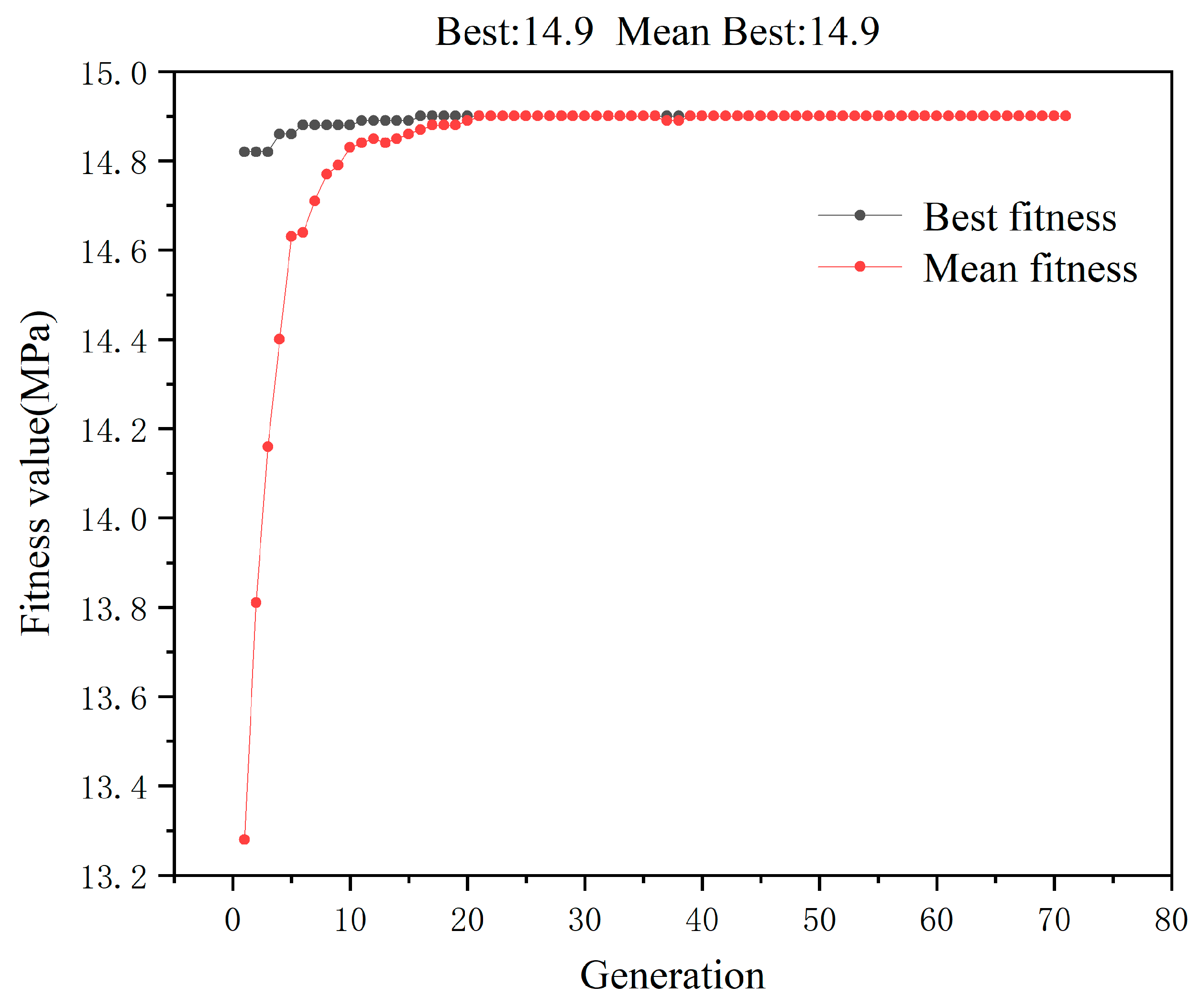
3.4. Modeling by GA-ANN
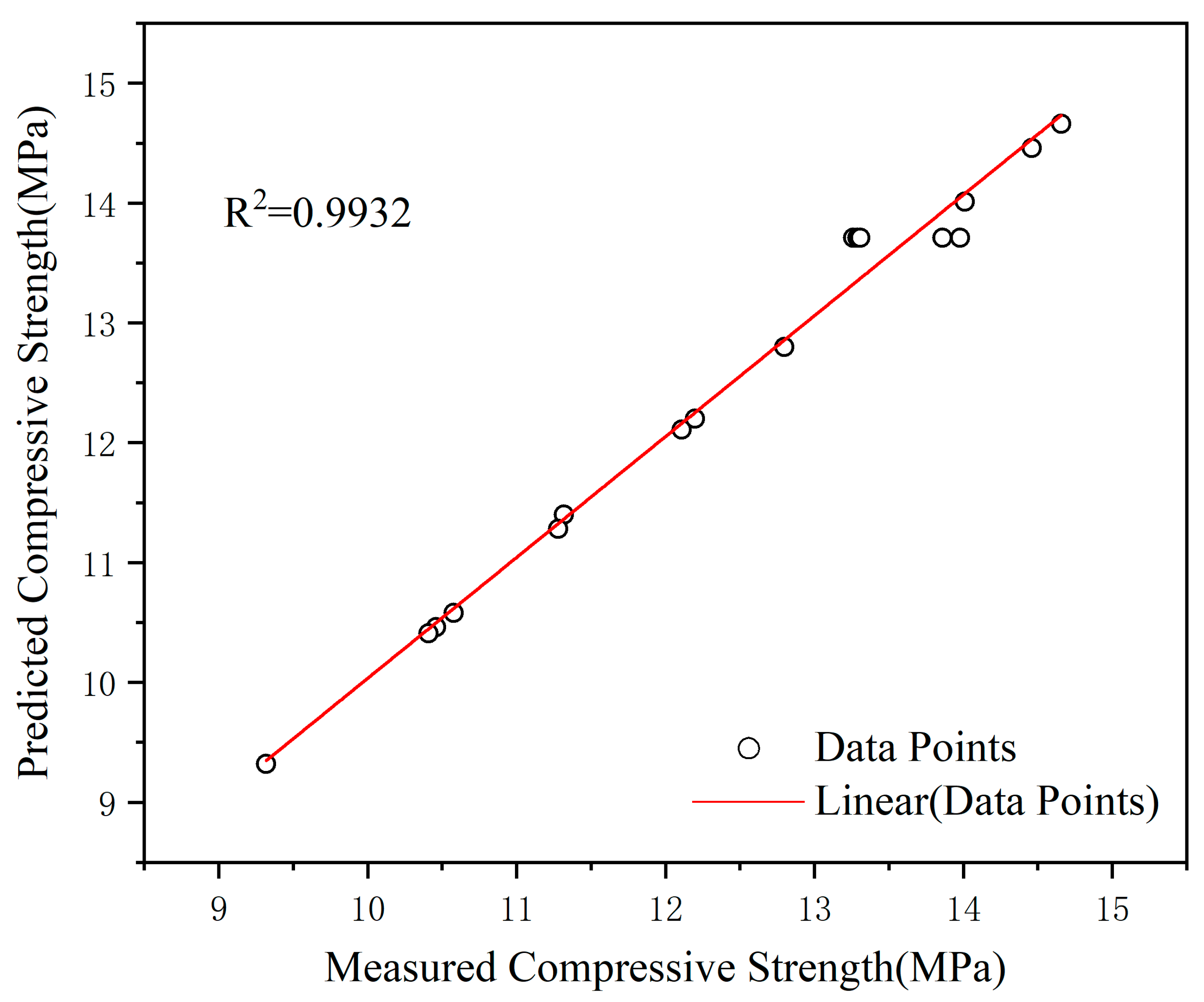
3.5. Comparative Analysis of BBD-RSM and GA-ANN Techniques
3.6. Internal Mechanism Analysis
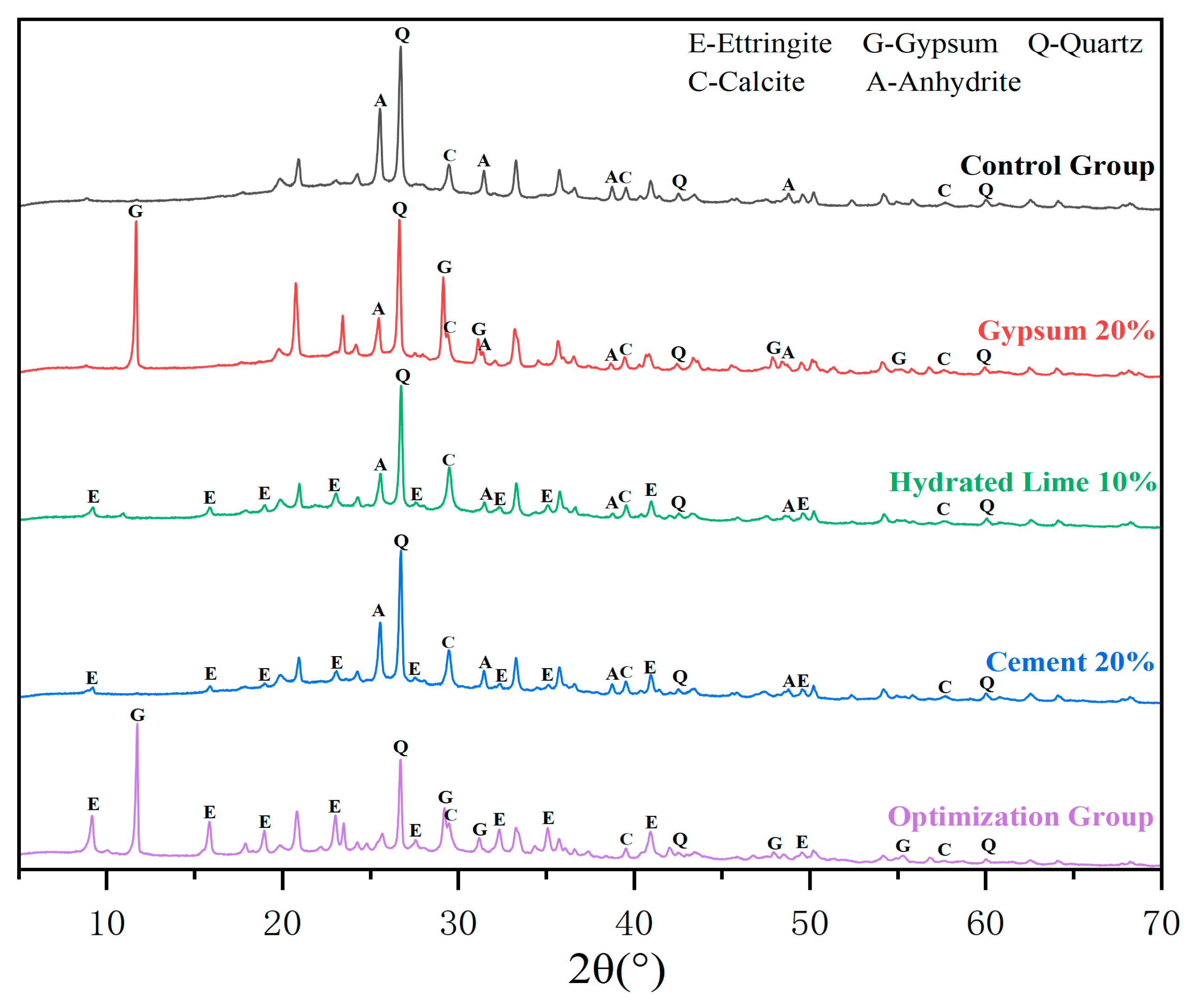
3.6.1. XRD Analysis
3.6.2. TG-DTG Analysis
3.6.3. FTIR Analysis
3.6.4. Microstructural Analysis
4. Conclusions
- BBD-RSM and GA-ANN can be employed for statistical modeling and optimization of the compressive strength of composite gravels. These two numerical analysis methods have their own advantages. ANN predicts more accurately than RSM, with R2 of 0.9932 and 0.9820, respectively.
- The RSM model can be applied to describe the interaction effect between the pairwise activators. CFBFA activated only by cement and lime produced less ettringite. CFBFA activated only by cement and gypsum cannot be fully activated. CFBFA activated only by hydrated lime and gypsum lacks binders. Binary activator is not as effective as the ternary activator.
- The utilization of XRD and FTIR characterization techniques revealed that the combined action of the three activators resulted in the generation of abundant hydration products. Cement undergoes pozzolanic reaction with CFBFA. The alkalinity of cement is not enough to activate large amounts of CFBFA. Hydrated lime is introduced to increase the alkalinity, effectively supplementing the required Ca(OH)2 for pozzolanic reactions. Gypsum provides SO42− to form ettringite. The ternary activator synergistically influences the overall hydration and carbonation transformations in the cementitious system, contributing to its improved performance and properties.
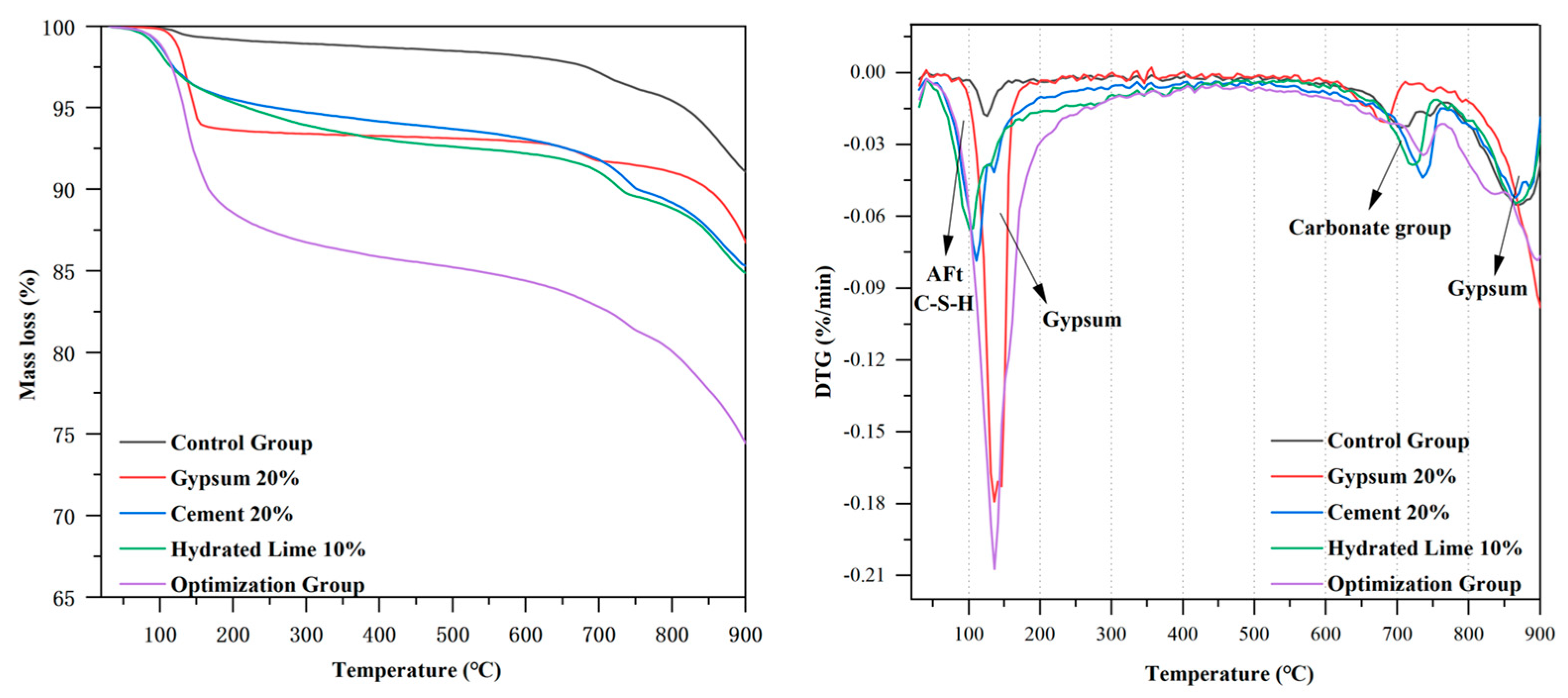
- The utilization of TG-DTG characterization techniques revealed that the CO2 curing process resulted in the generation of significant amounts of calcite, improving the mechanical properties of the material. The optimization group generated the most hydration products and carbonation products.
- The application of SEM characterization techniques unveiled that the synergistic action of the ternary activator led to a strong activation of CFBFA, resulting in the needle and rod-shaped ettringite intertwined with a substantial quantity of flocculent C-S-H gel. The existence of this interwoven structure makes the microstructure compact and the pore structure optimized. These characterization findings are consistent with the experimental results of uniaxial unconfined compressive strength.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jang, J.G.; Park, S.-M.; Chung, S.; Ahn, J.-W.; Kim, H.-K. Utilization of circulating fluidized bed combustion ash in producing controlled low-strength materials with cement or sodium carbonate as activator. Constr. Build. Mater. 2018, 159, 642–651. [Google Scholar] [CrossRef]
- Cheng, A.; Hsu, H.-M.; Chao, S.-J. Properties of concrete incorporating bed ash from circulating fluidized bed combustion and ground granulates blast-furnace slag. J. Wuhan Univ. Technol. Sci. Ed. 2011, 26, 348–354. [Google Scholar] [CrossRef]
- Chen, X.; Yan, Y.; Liu, Y.; Hu, Z. Utilization of circulating fluidized bed fly ash for the preparation of foam concrete. Constr. Build. Mater. 2014, 54, 137–146. [Google Scholar] [CrossRef]
- Li, X.-G.; Chen, Q.-B.; Huang, K.-Z.; Ma, B.-G.; Wu, B. Cementitious properties and hydration mechanism of circulating fluidized bed combustion (CFBC) desulfurization ashes. Constr. Build. Mater. 2012, 36, 182–187. [Google Scholar] [CrossRef]
- Nguyen, H.-A.; Chang, T.-P.; Shih, J.-Y.; Chen, C.-T.; Nguyen, T.-D. Influence of circulating fluidized bed combustion (CFBC) fly ash on properties of modified high volume low calcium fly ash (HVFA) cement paste. Constr. Build. Mater. 2015, 91, 208–215. [Google Scholar] [CrossRef]
- Park, S.M.; Lee, N.K.; Lee, H.K. Circulating fluidized bed combustion ash as controlled low-strength material (CLSM) by alkaline activation. Constr. Build. Mater. 2017, 156, 728–738. [Google Scholar] [CrossRef]
- Koukouzas, N.; Ketikidis, C.; Itskos, G.; Spiliotis, X.; Karayannis, V.; Papapolymerou, G. Synthesis of CFB-Coal Fly Ash Clay Bricks and Their Characterisation. Waste Biomass Valorization 2011, 2, 87–94. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.; Yang, J.; Zhou, T.; Zhang, H. Effects of particle size on microstructure and mechanical strength of a fly ash based ceramic membrane. Ceram. Int. 2023, 49, 15655–15664. [Google Scholar] [CrossRef]
- Qin, J.; Cui, C.; Cui, X.; Hussain, A.; Yang, C. Preparation and characterization of ceramsite from lime mud and coal fly ash. Constr. Build. Mater. 2015, 95, 10–17. [Google Scholar] [CrossRef]
- Jia, G.; Wang, Y.; Yang, F.; Ma, Z. Preparation of CFB fly ash/sewage sludge ceramsite and the morphological transformation and release properties of sulfur. Constr. Build. Mater. 2023, 373, 130864. [Google Scholar] [CrossRef]
- Xu, F.; Liu, W.; Bu, S.; Zhang, L.; Fang, J.; Yu, Z.; Xu, W.; Ding, H.; Huo, G. Manufacturing non-sintered ceramsite from incinerated municipal solid waste ash (IMSWA): Production and performance. Process. Saf. Environ. Prot. 2022, 163, 116–130. [Google Scholar] [CrossRef]
- Li, D.; Sun, R.; Wang, D.; Ren, C.; Fang, K. Study on the pozzolanic activity of ultrafine circulating fluidized-bed fly ash prepared by jet mill. Fuel 2021, 291, 120220. [Google Scholar] [CrossRef]
- Yang, J.; Hu, H.; He, X.; Su, Y.; Wang, Y.; Tan, H.; Pan, H. Effect of steam curing on compressive strength and microstructure of high volume ultrafine fly ash cement mortar. Constr. Build. Mater. 2021, 266, 120894. [Google Scholar] [CrossRef]
- Zhang, G.; Peng, G.-F.; Zuo, X.-Y.; Niu, X.-J.; Ding, H. Adding hydrated lime for improving microstructure and mechanical properties of mortar for ultra-high performance concrete. Cem. Concr. Res. 2023, 167, 107130. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Hou, Y.; Wu, Z. Optimization of mechanical properties and water absorption behavior of building gypsum by ternary matrix mixture. Constr. Build. Mater. 2022, 350, 128910. [Google Scholar] [CrossRef]
- Illikainen, M.; Tanskanen, P.; Kinnunen, P.; Körkkö, M.; Peltosaari, O.; Wigren, V.; Österbacka, J.; Talling, B.; Niinimäki, J. Reactivity and self-hardening of fly ash from the fluidized bed combustion of wood and peat. Fuel 2014, 135, 69–75. [Google Scholar] [CrossRef]
- Watanabe, A.; Furukawa, H.; Miyamoto, S.; Minagawa, H. Non-destructive chemical analysis of water and chlorine content in cement paste using near-infrared spectroscopy. Constr. Build. Mater. 2019, 196, 95–104. [Google Scholar] [CrossRef]
- Iribarne, J.; Iribarne, A.; Blondin, J.; Anthony, E. Hydration of combustion ashes—A chemical and physical study. Fuel 2001, 80, 773–784. [Google Scholar] [CrossRef]
- Moghal, A.A.B.; Rehman, A.U.; Vydehi, K.V.; Umer, U. Sustainable Perspective of Low-Lime Stabilized Fly Ashes for Geotechnical Applications: PROMETHEE-Based Optimization Approach. Sustainability 2020, 12, 6649. [Google Scholar] [CrossRef]
- Kursuncu, B.; Gencel, O.; Bayraktar, O.Y.; Shi, J.; Nematzadeh, M.; Kaplan, G. Optimization of foam concrete characteristics using response surface methodology and artificial neural networks. Constr. Build. Mater. 2022, 337, 127575. [Google Scholar] [CrossRef]
- Lv, S.; Yuan, J.; Peng, X.; Cabrera, M.B.; Guo, S.; Luo, X.; Gao, J. Performance and optimization of bio-oil/Buton rock asphalt composite modified asphalt. Constr. Build. Mater. 2020, 264, 120235. [Google Scholar] [CrossRef]
- Hacene, S.M.A.B.; Ghomari, F.; Schoefs, F.; Khelidj, A. Probabilistic Modelling of Compressive Strength of Concrete Using Response Surface Methodology and Neural Networks. Arab. J. Sci. Eng. 2014, 39, 4451–4460. [Google Scholar] [CrossRef]
- Junyan, L.; Qingju, T.; Xun, L.; Yang, W. Research on the quantitative analysis of subsurface defects for non-destructive testing by lock-in thermography. NDT E Int. 2012, 45, 104–110. [Google Scholar] [CrossRef]
- Getahun, M.A.; Shitote, S.M.; Abiero Gariy, Z.C. Artificial neural network based modelling approach for strength prediction of concrete incorporating agricultural and construction wastes. Constr. Build. Mater. 2018, 190, 517–525. [Google Scholar] [CrossRef]
- Kittinaraporn, W.; Tuprakay, S.; Prasittisopin, L. Effective Modeling for Construction Activities of Recycled Aggregate Concrete Using Artificial Neural Network. J. Constr. Eng. Manag. 2022, 148, 04021206. [Google Scholar] [CrossRef]
- Guo, H.; Shi, C.; Guan, X.; Zhu, J.; Ding, Y.; Ling, T.-C.; Zhang, H.; Wang, Y. Durability of recycled aggregate concrete—A review. Cem. Concr. Compos. 2018, 89, 251–259. [Google Scholar] [CrossRef]
- Shi, C.; He, F.; Wu, Y. Effect of pre-conditioning on CO2 curing of lightweight concrete blocks mixtures. Constr. Build. Mater. 2012, 26, 257–267. [Google Scholar] [CrossRef]
- Lu, B.; Shi, C.; Cao, Z.; Guo, M.; Zheng, J. Effect of carbonated coarse recycled concrete aggregate on the properties and microstructure of recycled concrete. J. Clean. Prod. 2019, 233, 421–428. [Google Scholar] [CrossRef]
- Johannesson, B.; Utgenannt, P. Microstructural changes caused by carbonation of cement mortar. Cem. Concr. Res. 2001, 31, 925–931. [Google Scholar] [CrossRef]
- Monkman, S.; Kenward, P.A.; Dipple, G.; MacDonald, M.; Raudsepp, M. Activation of cement hydration with carbon dioxide. J. Sustain. Cem. Mater. 2018, 7, 160–181. [Google Scholar] [CrossRef]
- Zhan, B.J.; Poon, C.S.; Shi, C.J. Materials characteristics affecting CO2 curing of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2016, 67, 50–59. [Google Scholar] [CrossRef]
- Wu, T.; Chi, M.; Huang, R. Characteristics of CFBC fly ash and properties of cement-based composites with CFBC fly ash and coal-fired fly ash. Constr. Build. Mater. 2014, 66, 172–180. [Google Scholar] [CrossRef]
- Neto, A.A.M.; Cincotto, M.A.; Repette, W. Mechanical properties, drying and autogenous shrinkage of blast furnace slag activated with hydrated lime and gypsum. Cem. Concr. Compos. 2010, 32, 312–318. [Google Scholar] [CrossRef]
- Adesanya, E.; Aladejare, A.; Adediran, A.; Lawal, A.; Illikainen, M. Predicting shrinkage of alkali-activated blast furnace-fly ash mortars using artificial neural network (ANN). Cem. Concr. Compos. 2021, 124, 104265. [Google Scholar] [CrossRef]
- Rachmatullah, M.I.C.; Santoso, J.; Surendro, K. Determining the number of hidden layer and hidden neuron of neural network for wind speed prediction. PeerJ Comput. Sci. 2021, 7, e724. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Nguyen, D.T.; Le, G.T.; Tomul, F.; Lima, E.C.; Woo, S.H.; Sarmah, A.K.; Nguyen, H.Q.; Nguyen, P.T.; Nguyen, D.D.; et al. Adsorption mechanism of hexavalent chromium onto layered double hydroxides-based adsorbents: A systematic in-depth review. J. Hazard. Mater. 2019, 373, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Geiker, M.; Justnes, H.; Lauten, R.; De Weerdt, K. The effect of calcium lignosulfonate on ettringite formation in cement paste. Cem. Concr. Res. 2018, 107, 188–205. [Google Scholar] [CrossRef]
- Takahashi, H.; Ogawa, S.; Shibata, M.; Kuranaga, M.; Watanabe, S.; Mishiba, K.; Kawabata, Y. Expansion behavior of cement pastes containing additives due to delayed ettringite formation. In Bridge Maintenance, Safety, Management, Life-Cycle Sustainability and Innovations; Yokota, H., Frangopol, D.M., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 2659–2663. [Google Scholar] [CrossRef]
- Bertos, M.F.; Simons, S.J.R.; Hills, C.D.; Carey, P.J. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, 112, 193–205. [Google Scholar] [CrossRef]
- Chang, J.; Xiong, C.; Zhang, Y.; Wang, D. Foaming characteristics and microstructure of aerated steel slag block prepared by accelerated carbonation. Constr. Build. Mater. 2019, 209, 222–233. [Google Scholar] [CrossRef]
- Paul, K.T.; Satpathy, S.; Manna, I.; Chakraborty, K.; Nando, G. Preparation and Characterization of Nano structured Materials from Fly Ash: A Waste from Thermal Power Stations, by High Energy Ball Milling. Nanoscale Res. Lett. 2007, 2, 397–404. [Google Scholar] [CrossRef]
- Patil, A.G.; Shanmugharaj, A.; Anandhan, S. Interparticle interactions and lacunarity of mechano-chemically activated fly ash. Powder Technol. 2015, 272, 241–249. [Google Scholar] [CrossRef]
- Tian, X.; Rao, F.; León-Patiño, C.A.; Song, S. Effects of aluminum on the expansion and microstructure of alkali-activated MSWI fly ash-based pastes. Chemosphere 2020, 240, 124986. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Jang, J.G. Acid and sulfate resistance of seawater based alkali activated fly ash: A sustainable and durable approach. Constr. Build. Mater. 2021, 281, 122601. [Google Scholar] [CrossRef]
- Lodeiro, I.G.; Macphee, D.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalis on fresh C–S–H gels. FTIR analysis. Cem. Concr. Res. 2009, 39, 147–153. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Glasser, F. Alkali binding in cement pastes: Part I. The C-S-H phase. Cem. Concr. Res. 1999, 29, 1893–1903. [Google Scholar] [CrossRef]
- Li, N.; Farzadnia, N.; Shi, C. Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem. Concr. Res. 2017, 100, 214–226. [Google Scholar] [CrossRef]
- Thomas, S.; Meise-Gresch, K.; Müller-Warmuth, W.; Odler, I. MAS NMR Studies of Partially Carbonated Portland Cement and Tricalcium Silicate Pastes. J. Am. Ceram. Soc. 1993, 76, 1998–2004. [Google Scholar] [CrossRef]
- Wang, X.; Ni, W.; Li, J.; Zhang, S.; Hitch, M.; Pascual, R. Carbonation of steel slag and gypsum for building materials and associated reaction mechanisms. Cem. Concr. Res. 2019, 125, 105893. [Google Scholar] [CrossRef]




| Material | SO3 | CaO | SiO2 | Al2O3 | Fe2O3 | MgO |
|---|---|---|---|---|---|---|
| CFBFA | 3.967 | 5.762 | 40.886 | 34.704 | 8.053 | 2.567 |
| Cement | 4.029 | 53.678 | 21.245 | 7.298 | 4.591 | 6.037 |
| Hydrated Lime | 0.647 | 93.886 | 0.928 | 0.956 | - | 2.511 |
| Gypsum | 51.549 | 43.205 | - | 0.794 | - | 3.925 |
| Run | Real Variable Level | Compressive Strength/MPa | ||||||
|---|---|---|---|---|---|---|---|---|
| Cement/% | Hydrated Lime/% | Gypsum/% | Actual | RSM | Error | ANN | Error | |
| 1 | 15 | 5 | 10 | 12.11 | 12.34 | 0.0186 | 12.11 | 0 |
| 2 | 15 | 7.5 | 15 | 13.98 | 13.54 | 0.0315 | 13.71 | 0.2677 |
| 3 | 15 | 7.5 | 15 | 13.86 | 13.54 | 0.0231 | 13.71 | 0.1477 |
| 4 | 20 | 7.5 | 20 | 12.2 | 12.21 | 0.0006 | 12.20 | 0 |
| 5 | 15 | 7.5 | 15 | 13.26 | 13.54 | 0.0211 | 13.71 | 0.4523 |
| 6 | 15 | 10 | 20 | 10.58 | 10.36 | 0.0213 | 10.58 | 0 |
| 7 | 10 | 7.5 | 20 | 9.32 | 9.44 | 0.0126 | 9.32 | 0 |
| 8 | 10 | 5 | 15 | 11.32 | 11.10 | 0.0192 | 11.40 | 0.0768 |
| 9 | 15 | 5 | 20 | 11.28 | 11.38 | 0.0089 | 11.28 | 0 |
| 10 | 20 | 7.5 | 10 | 14.66 | 14.54 | 0.0080 | 14.66 | 0 |
| 11 | 20 | 5 | 15 | 14.46 | 14.35 | 0.0074 | 14.46 | 0 |
| 12 | 10 | 10 | 15 | 10.46 | 10.57 | 0.0103 | 10.46 | 0 |
| 13 | 20 | 10 | 15 | 14.01 | 14.23 | 0.0155 | 14.01 | 0 |
| 14 | 15 | 10 | 10 | 12.8 | 12.70 | 0.0078 | 12.80 | 0 |
| 15 | 15 | 7.5 | 15 | 13.29 | 13.54 | 0.0188 | 13.71 | 0.4223 |
| 16 | 15 | 7.5 | 15 | 13.31 | 13.54 | 0.0173 | 13.71 | 0.4023 |
| 17 | 10 | 7.5 | 10 | 10.41 | 10.40 | 0.0007 | 10.41 | 0 |
| Variable | Statistical Analysis | ||||
|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F-Value | p-Value | |
| Model | 41.39 | 9 | 4.60 | 42.55 | <0.0001 |
| X1 (Cement) | 23.87 | 1 | 23.87 | 220.91 | <0.0001 |
| X2 (Hydrated Lime) | 0.22 | 1 | 0.22 | 2.02 | 0.1987 |
| X3 (Gypsum) | 5.45 | 1 | 5.45 | 50.38 | 0.0002 |
| X1 X2 | 0.04 | 1 | 0.04 | 0.39 | 0.5527 |
| X1 X3 | 0.47 | 1 | 0.47 | 4.34 | 0.0757 |
| X2 X3 | 0.48 | 1 | 0.48 | 4.47 | 0.0723 |
| X12 | 1.10 | 1 | 1.10 | 10.18 | 0.0153 |
| X22 | 0.92 | 1 | 0.92 | 8.47 | 0.0227 |
| X32 | 8.03 | 1 | 8.03 | 74.33 | <0.0001 |
| Residual | 0.76 | 7 | 0.11 | ||
| Lack of fit | 0.27 | 3 | 0.09 | 0.73 | 0.5874 |
| Pure error | 0.49 | 4 | 0.12 | ||
| Cor Total | 42.14 | 16 | |||
| Variables | BBD-RSM | GA-ANN | ||
|---|---|---|---|---|
| Predicted Contents | Experimental Contents | Predicted Contents | Experimental Contents | |
| Cement | 19.94 | 20 | 20 | 20 |
| Hydrated Lime | 7.748 | 7.7 | 5.648 | 5.6 |
| Gypsum | 13.118 | 13 | 12.817 | 12.8 |
| Compressive Strength | 14.992 | 14.59 | 14.9 | 14.72 |
| Relative Error (%) | 2.76% | 1.22% | ||
| R2 | 0.9820 | 0.9932 | ||
| RMSE | 0.21 | 0.19 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, N.; Ma, S.; Wang, N.; Feng, Y.; Liu, Y.; Ren, K.; Bai, S. Optimization of Ternary Activator for Enhancing Mechanical Properties of Carbonized Cementitious Material Based on Circulating Fluidized Bed Fly Ash. Processes 2024, 12, 289. https://doi.org/10.3390/pr12020289
Xu N, Ma S, Wang N, Feng Y, Liu Y, Ren K, Bai S. Optimization of Ternary Activator for Enhancing Mechanical Properties of Carbonized Cementitious Material Based on Circulating Fluidized Bed Fly Ash. Processes. 2024; 12(2):289. https://doi.org/10.3390/pr12020289
Chicago/Turabian StyleXu, Nuo, Suxia Ma, Nana Wang, Yuchuan Feng, Yunqi Liu, Ke Ren, and Shanshui Bai. 2024. "Optimization of Ternary Activator for Enhancing Mechanical Properties of Carbonized Cementitious Material Based on Circulating Fluidized Bed Fly Ash" Processes 12, no. 2: 289. https://doi.org/10.3390/pr12020289
APA StyleXu, N., Ma, S., Wang, N., Feng, Y., Liu, Y., Ren, K., & Bai, S. (2024). Optimization of Ternary Activator for Enhancing Mechanical Properties of Carbonized Cementitious Material Based on Circulating Fluidized Bed Fly Ash. Processes, 12(2), 289. https://doi.org/10.3390/pr12020289







