Migration and Transformation of Heavy Metal and Its Fate in Intertidal Sediments: A Review
Abstract
:1. Introduction
2. The Distribution Characteristics of Heavy Metals in Intertidal Sediments
2.1. The Heavy Metal Contents of Intertidal Sediments
2.2. The Fractions of Heavy Metal in Intertidal Sediments
3. Migration and Transformation of Heavy Metals in Intertidal Sediments
3.1. The Migration and Transformation Behavior of Heavy Metals in Intertidal Sediments and Its Influencing Factors
3.1.1. Properties of the Sediment
3.1.2. Physicochemical Conditions in the Aquatic Environment
3.1.3. Disturbance Effects
3.2. Mechanisms of the Migration and Transformation of Heavy Metals in the Intertidal Zone
4. Fate of Heavy Metals in Intertidal Sediments
4.1. Bioaccumulation of Heavy Metals by the Organisms in Intertidal Sediments
4.2. Heavy Metal Remediation of Intertidal Sediments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, X.; Zhou, F.; Chen, C.-T.A. Pollution Status of the Bohai Sea: An Overview of the Environmental Quality Assessment Related Trace Metals. Environ. Int. 2014, 62, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Mao, L.; Jia, Y.; Gu, Z.; Shi, W.; Chen, L.; Ye, H. Distribution and Risk Assessment of Trace Metals in Sediments from Yangtze River Estuary and Hangzhou Bay, China. Environ. Sci. Pollut. Res. 2018, 25, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.; Wang, H.; Han, B.; Rao, Y.; Gong, H.; Zhang, W.; Gu, Y.; Fan, Z.; Wang, S.; Huang, H. Coastal Sediment Heavy Metal(Loid) Pollution under Multifaceted Anthropogenic Stress: Insights Based on Geochemical Baselines and Source-Related Risks. Chemosphere 2023, 339, 139653. [Google Scholar] [CrossRef] [PubMed]
- Geng, N.; Xia, Y.; Lu, D.; Bai, Y.; Zhao, Y.; Wang, H.; Ren, L.; Xu, C.; Hua, E.; Sun, G.; et al. The Bacterial Community Structure in Epiphytic Biofilm on Submerged Macrophyte Potamogetom crispus L. and Its Contribution to Heavy Metal Accumulation in an Urban Industrial Area in Hangzhou. J. Hazard. Mater. 2022, 430, 128455. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Pan, B.; Han, X.; Lu, Y.; Wang, X. Toxicity Risks Associated with Trace Metals Call for Conservation of Threatened Fish Species in Heavily Sediment-Laden Yellow River. J. Hazard. Mater. 2023, 448, 130928. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-S.; Huang, J.; Zeng, D.-L.; Peng, J.-S.; Zhang, G.-B.; Ma, H.-L.; Guan, Y.; Yi, H.-Y.; Fu, Y.-L.; Han, B.; et al. A Defensin-like Protein Drives Cadmium Efflux and Allocation in Rice. Nat. Commun. 2018, 9, 645. [Google Scholar] [CrossRef]
- Lu, Y.; Jenkins, A.; Ferrier, R.C.; Bailey, M.; Gordon, I.J.; Song, S.; Huang, J.; Jia, S.; Zhang, F.; Liu, X.; et al. Addressing China’s Grand Challenge of Achieving Food Security While Ensuring Environmental Sustainability. Sci. Adv. 2015, 1, e1400039. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, X.; Song, J.; Chen, C.-T.A.; Chu, J. Colloidal Toxic Trace Metals in Urban Riverine and Estuarine Waters of Yantai City, Southern Coast of North Yellow Sea. Sci. Total Environ. 2020, 717, 135265. [Google Scholar] [CrossRef]
- Liu, J.-J.; Diao, Z.-H.; Xu, X.-R.; Xie, Q. Effects of Dissolved Oxygen, Salinity, Nitrogen and Phosphorus on the Release of Heavy Metals from Coastal Sediments. Sci. Total Environ. 2019, 666, 894–901. [Google Scholar] [CrossRef]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M.G. Trace Metal Behaviour in Estuarine and Riverine Floodplain Soils and Sediments: A Review. Sci. Total Environ. 2009, 407, 3972–3985. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, E.; Xia, P.; Feng, A.; Chi, Y.; Sun, Y. Distribution and Pollution Assessment of Heavy Metals in the Intertidal Zone Environments of Typical Sea Areas in China. Mar. Pollut. Bull. 2019, 138, 397–406. [Google Scholar] [CrossRef]
- Jia, Z.; Li, S.; Liu, Q.; Jiang, F.; Hu, J. Distribution and Partitioning of Heavy Metals in Water and Sediments of a Typical Estuary (Modaomen, South China): The Effect of Water Density Stratification Associated with Salinity. Environ. Pollut. 2021, 287, 117277. [Google Scholar] [CrossRef]
- Jeong, H.; Ra, K. Pollution and Ecological Risk Assessments for Heavy Metals in Coastal, River, and Road-Deposited Sediments from Apia City in Upolu Island, Samoa. Mar. Pollut. Bull. 2023, 188, 114596. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-G.; Kim, Y.-B.; Kim, R.-H.; Hyon, T.-S. Spatial Distribution, Origin and Contamination Assessment of Heavy Metals in Surface Sediments from Jangsong Tidal Flat, Kangryong River Estuary, DPR Korea. Mar. Pollut. Bull. 2021, 168, 112414. [Google Scholar] [CrossRef] [PubMed]
- Strady, E.; Dinh, Q.T.; Némery, J.; Nguyen, T.N.; Guédron, S.; Nguyen, N.S.; Denis, H.; Nguyen, P.D. Spatial Variation and Risk Assessment of Trace Metals in Water and Sediment of the Mekong Delta. Chemosphere 2017, 179, 367–378. [Google Scholar] [CrossRef]
- Nour, H.E.; Alshehri, F.; Sahour, H.; El-Sorogy, A.S.; Tawfik, M. Assessment of Heavy Metal Contamination and Health Risk in the Coastal Sediments of Suez Bay, Gulf of Suez, Egypt. J. Afr. Earth Sci. 2022, 195, 104663. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Carr, R.S.; Calder, F.D.; Long, E.R.; Ingersoll, C.G. Development and Evaluation of Sediment Quality Guidelines for Florida Coastal Waters. Ecotoxicology 1996, 5, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bing, H.; Wu, Y.; Zhou, J.; Sun, H.; Wang, J.; Wang, X. The Spatial and Vertical Distribution of Heavy Metal Contamination in Sediments of the Three Gorges Reservoir Determined by Anti-Seasonal Flow Regulation. Sci. Total Environ. 2019, 664, 79–88. [Google Scholar] [CrossRef]
- Chai, X.; Wei, N.; Ren, S.; Mu, Q. Analysis of Surface Sediment Environment Division and Heavy Metal Pollution Character in Hangzhou Bay and Its Adjacent Areas. Mar. Sci. 2019, 8, 29–35. [Google Scholar] [CrossRef]
- Maity, S.; Sahu, S.K.; Pandit, G.G. Determination of Heavy Metals and Their Distribution In Different Size Fractionated Sediment Samples Using Different Analytical Techniques. Soil Sediment Contam. Int. J. 2016, 25, 332–345. [Google Scholar] [CrossRef]
- Dou, Y.; Li, J.; Zhao, J.; Hu, B.; Yang, S. Distribution, Enrichment and Source of Heavy Metals in Surface Sediments of the Eastern Beibu Bay, South China Sea. Mar. Pollut. Bull. 2013, 67, 137–145. [Google Scholar] [CrossRef]
- Zheng, Q.; Tu, S.; Hou, J.; Ni, C.; Wang, M.; Ren, L.; Wang, M.; Cao, M.; Xiong, S.; Tan, W. Insights into the Underlying Mechanisms of Stability Working for As(III) Removal by Fe-Mn Binary Oxide as a Highly Efficient Adsorbent. Water Res. 2021, 203, 117558. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Shen, H. Review of Accumulation Features Study of Heavy Metal in Sediment of Tidal Flat. Adv. Earth Sci. 2002, 1, 69–77. [Google Scholar] [CrossRef]
- Liao, J.; Feng, H.; Yan, S.; Cui, X.; Tang, S.; Liu, X. Comprehensive Distribution Characteristics and Risks of Heavy Metals in Typical Intertidal Zones and Their Relationship with Urban Economic Indicators. Ecol. Indic. 2023, 148, 110112. [Google Scholar] [CrossRef]
- Han, S.; Zhang, Y.; Masunaga, S.; Zhou, S.; Naito, W. Relating Metal Bioavailability to Risk Assessment for Aquatic Species: Daliao River Watershed, China. Environ. Pollut. 2014, 189, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.S.; Ayoko, G.A.; Egodawatta, P.; Goonetilleke, A. Adsorption-Desorption Behavior of Heavy Metals in Aquatic Environments: Influence of Sediment, Water and Metal Ionic Properties. J. Hazard. Mater. 2022, 421, 126743. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Mu, Q.; She, Y.; Wang, H. Speciation Distribution and Ecological Risk of Heavy Metal in the Intertidal Sediments of Zhoushan Fishing Ground. Environ. Pollut. Control 2022, 44, 1054–1060, 1067. [Google Scholar] [CrossRef]
- Gu, Y.-G. Heavy Metal Fractionation and Ecological Risk Implications in the Intertidal Surface Sediments of Zhelin Bay, South China. Mar. Pollut. Bull. 2018, 129, 905–912. [Google Scholar] [CrossRef]
- Madurapperuma, W.S.; Kumaragamage, D. Evaluation of Ammonium Bicarbonate–Diethylene Triamine Penta Acetic Acid as a Multinutrient Extractant for Acidic Lowland Rice Soils. Commun. Soil Sci. Plant Anal. 2008, 39, 1773–1790. [Google Scholar] [CrossRef]
- Gu, Y.-G.; Wang, X.-N.; Wang, Z.-H.; Jordan, R.W.; Jiang, S.-J. Rare Earth Elements in Sediments from a Representative Chinese Mariculture Bay: Characterization, DGT-Based Bioaccessibility, and Probabilistic Ecological Risk. Environ. Pollut. 2023, 335, 122338. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, R.; Sheng, G.D.; Pan, L.; Lian, E.; Su, N.; Tang, X.; Yang, S.; Yin, D. Geochemical Controls on the Distribution and Bioavailability of Heavy Metals in Sediments from Yangtze River to the East China Sea: Assessed by Sequential Extraction versus Diffusive Gradients in Thin-Films (DGT) Technique. J. Hazard. Mater. 2023, 452, 131253. [Google Scholar] [CrossRef]
- Gu, Y.-G.; Wang, Y.-S.; Jordan, R.W.; Su, H.; Jiang, S.-J. Probabilistic Ecotoxicological Risk Assessment of Heavy Metal and Rare Earth Element Mixtures in Aquatic Biota Using the DGT Technique in Coastal Sediments. Chemosphere 2023, 329, 138592. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-G. Risk Assessment of Eight Metals and Their Mixtures to Aquatic Biota in Sediments with Diffusive Gradients in Thin Films (DGT): A Case Study in Pearl River Intertidal Zone. Environ. Sci. Eur. 2021, 33, 122. [Google Scholar] [CrossRef]
- Oomen, A.G.; Hack, A.; Minekus, M.; Zeijdner, E.; Cornelis, C.; Schoeters, G.; Verstraete, W.; Van De Wiele, T.; Wragg, J.; Rompelberg, C.J.M.; et al. Comparison of Five In Vitro Digestion Models to Study the Bioaccessibility of Soil Contaminants. Environ. Sci. Technol. 2002, 36, 3326–3334. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzifard, M.; Moore, F.; Sharifi, R. The Influence of Physicochemical Parameters on Bioavailability and Bioaccessibility of Heavy Metals in Sediments of the Intertidal Zone of Asaluyeh Region, Persian Gulf, Iran. Geochemistry 2019, 79, 178–187. [Google Scholar] [CrossRef]
- Fang, H.; Huang, L.; Wang, J.; He, G.; Reible, D. Environmental Assessment of Heavy Metal Transport and Transformation in the Hangzhou Bay, China. J. Hazard. Mater. 2016, 302, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Debnath, A.; Singh, P.K.; Chandra Sharma, Y. Metallic Contamination of Global River Sediments and Latest Developments for Their Remediation. J. Environ. Manag. 2021, 298, 113378. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Li, Y.; Wang, L.; Ahmed, M.K.; Chen, K.; Wu, J.; Xu, Y.; Lin, Y.; Xiao, X.; Chen, B.; et al. Distribution and Assessment of Heavy Metals in Suspended Particles in the Sundarban Mangrove River, Bangladesh. Mar. Pollut. Bull. 2022, 181, 113856. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Wang, X.; Zhang, Y.; Zhang, M.; Wang, K.; Xie, P.; Ji, H. The Optimum pH and Eh for Simultaneously Minimizing Bioavailable Cadmium and Arsenic Contents in Soils under the Organic Fertilizer Application. Sci. Total Environ. 2020, 711, 135229. [Google Scholar] [CrossRef]
- Geng, N.; Wu, Y.; Zhang, M.; Tsang, D.C.W.; Rinklebe, J.; Xia, Y.; Lu, D.; Zhu, L.; Palansooriya, K.N.; Kim, K.-H.; et al. Bioaccumulation of Potentially Toxic Elements by Submerged Plants and Biofilms: A Critical Review. Environ. Int. 2019, 131, 105015. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, F.; Huang, Y.; Tang, J. Variability of Dissolved Organic Matter in Two Coastal Wetlands along the Changjiang River Estuary: Responses to Tidal Cycles, Seasons, and Degradation Processes. Sci. Total Environ. 2022, 807, 150993. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, L.; Jia, H. Molecular Dynamics Study of the Exchange Processes of Heavy Metals into Montmorillonite: Characterization of Hydrated Edge Surfaces and Dynamic Exchange Mechanism. Appl. Geochem. 2023, 150, 105587. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, S.; Liu, D.; Yu, Z.; Zhang, L.; Cui, J.; Xie, K.; Li, T.; Fu, C. Mobility and Potential Risk of Sediment-Associated Heavy Metal Fractions under Continuous Drought-Rewetting Cycles. Sci. Total Environ. 2018, 625, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Qu, B.; Yuan, H.; Song, J.; Li, W. Heavy Metal Mobility in Contaminated Sediments under Seawater Acidification. Mar. Pollut. Bull. 2023, 192, 115062. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.R.; Do, N.T.; Panizzo, V.N.; Taylor, S.; Watts, M.; Hamilton, E.; McGowan, S.; Trinh, D.A.; Leng, M.J.; Salgado, J. In Flux: Annual Transport and Deposition of Suspended Heavy Metals and Trace Elements in the Urbanised, Tropical Red River Delta, Vietnam. Water Res. 2022, 224, 119053. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Geng, N.; Wang, C.; Qian, J.; Hou, J.; Qi, N. Evaluating the Impact of Long Term Hydrodynamic Conditions on the Release of Metals from Contaminated Sediments in Taihu Lake, China. J. Environ. Inform. 2016, 27, 62–71. [Google Scholar] [CrossRef]
- Calmano, W.; Förstner, U.; Hong, J. Mobilization and Scavenging of Heavy Metals Following Resuspension of Anoxic Sediments from the Elbe River; American Chemical Society: Washington, DC, USA, 1994; ISBN 978-0-8412-2772-9. [Google Scholar]
- De Jonge, M.; Teuchies, J.; Meire, P.; Blust, R.; Bervoets, L. The Impact of Increased Oxygen Conditions on Metal-Contaminated Sediments Part I: Effects on Redox Status, Sediment Geochemistry and Metal Bioavailability. Water Res. 2012, 46, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Campana, O.; Blasco, J.; Simpson, S.L. Demonstrating the Appropriateness of Developing Sediment Quality Guidelines Based on Sediment Geochemical Properties. Environ. Sci. Technol. 2013, 47, 7483–7489. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Liu, S.; Hu, J.; He, Y.; Zhou, H.; Zhang, X. Profiling of Microbial Community during in Situ Remediation of Volatile Sulfide Compounds in River Sediment with Nitrate by High Throughput Sequencing. Int. Biodeterior. Biodegrad. 2013, 85, 429–437. [Google Scholar] [CrossRef]
- Ko, F.-C.; Sanford, L.P.; Baker, J.E. Internal Recycling of Particle Reactive Organic Chemicals in the Chesapeake Bay Water Column. Mar. Chem. 2003, 81, 163–176. [Google Scholar] [CrossRef]
- Ritvo, G.; Kochba, M.; Avnimelech, Y. The Effects of Common Carp Bioturbation on Fishpond Bottom Soil. Aquaculture 2004, 242, 345–356. [Google Scholar] [CrossRef]
- Bao, T.; Wang, P.; Hu, B.; Wang, X.; Qian, J. Mobilization of Colloids during Sediment Resuspension and Its Effect on the Release of Heavy Metals and Dissolved Organic Matter. Sci. Total Environ. 2023, 861, 160678. [Google Scholar] [CrossRef]
- Saeedi, M.; Li, L.Y.; Karbassi, A.R.; Zanjani, A.J. Sorbed Metals Fractionation and Risk Assessment of Release in River Sediment and Particulate Matter. Environ. Monit. Assess. 2013, 185, 1737–1754. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, L.; Bao, R.; Hu, R.; Jiang, S.; Zhu, Y.; Song, Y. Hydrodynamically–Driven Distribution and Remobilization of Heavy Metals in Surface Sediments around the Coastal Area of Shandong Peninsula, China. Sci. Total Environ. 2023, 857, 159286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jia, Z.; Liu, G.; Li, S.; Hu, J. Assessment of Heavy Metals Remobilization and Release Risks at the Sediment-Water Interface in Estuarine Environment. Mar. Pollut. Bull. 2023, 187, 114517. [Google Scholar] [CrossRef]
- Nasnodkar, M.R.; Nayak, G.N.; Bhangle, P.P.; Tiwari, A.K. Spring-Neap Tides Influence on Bioavailability of Metals and Bioaccumulation in Edible Biota of the Zuari (Tropical) Estuary. Environ. Monit. Assess. 2021, 193, 167. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Yuan, C.; Zheng, J.; Liu, Y. Distributions, Contamination Level and Ecological Risk of Heavy Metals in Surface Sediments from Intertidal Zone of the Sanmen Bay, East China. J. Sea Res. 2022, 190, 102302. [Google Scholar] [CrossRef]
- Kalnejais, L.H.; Martin, W.R.; Signell, R.P.; Bothner, M.H. Role of Sediment Resuspension in the Remobilization of Particulate-Phase Metals from Coastal Sediments. Environ. Sci. Technol. 2007, 41, 2282–2288. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.D. Empirical Evidence of the Importance of Sediment Resuspension in Lakes. Hydrobiologia 1994, 284, 5–12. [Google Scholar] [CrossRef]
- Roberts, D.A. Causes and Ecological Effects of Resuspended Contaminated Sediments (RCS) in Marine Environments. Environ. Int. 2012, 40, 230–243. [Google Scholar] [CrossRef]
- Geng, N.; Bai, Y.; Pan, S. Research on Heavy Metal Release with Suspended Sediment in Taihu Lake under Hydrodynamic Condition. Environ. Sci. Pollut. Res. 2022, 29, 28588–28597. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sheng, Y.; Wang, W.; Li, C.; Zhao, G. Remediation and Its Biological Responses of Cd Contaminated Sediments Using Biochar and Minerals with Nanoscale Zero-Valent Iron Loading. Sci. Total Environ. 2020, 713, 136650. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhuang, W.-E.; Yang, L. Critical Evaluation of the Interaction between Fluorescent Dissolved Organic Matter and Pb(II) under Variable Environmental Conditions. Chemosphere 2022, 307, 135875. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Zhang, L.; Zhang, P.; Wang, F.; Wang, J.; Chen, N.; Li, Z.; Pan, F.; Lu, Z.; Li, H. Tidal Exchange of Dissolved Metal(Loid)s and Organic Matters across the Sediment–Water Interface in a Salt Marsh-Mangrove Ecotone. J. Hydrol. 2023, 622, 129665. [Google Scholar] [CrossRef]
- Harmesa; Wahyudi, A.J.; Lestari; Taufiqurrahman, E. Variability of Trace Metals in Coastal and Estuary: Distribution, Profile, and Drivers. Mar. Pollut. Bull. 2022, 174, 113173. [Google Scholar] [CrossRef]
- Chinnadurai, S.; Elavarasan, K.; Geethalakshmi, V.; Kripa, V.; Mohamed, K.S. Temperature, Salinity and Body-Size Influences Depuration of Heavy Metals in Commercially Important Edible Bivalve Molluscs of India. Chemosphere 2022, 307, 135879. [Google Scholar] [CrossRef]
- Chai, M.; Li, R.; Gong, Y.; Shen, X.; Yu, L. Bioaccessibility-Corrected Health Risk of Heavy Metal Exposure via Shellfish Consumption in Coastal Region of China. Environ. Pollut. 2021, 273, 116529. [Google Scholar] [CrossRef]
- Vinothkannan, A.; Charles, P.E.; Rajaram, R. Consumption of Metal-Contaminated Shellfish from the Cuddalore Coast in Southeastern India Poses a Hazard to Public Health. Mar. Pollut. Bull. 2022, 181, 113827. [Google Scholar] [CrossRef]
- Hao, Z.; Chen, L.; Wang, C.; Zou, X.; Zheng, F.; Feng, W.; Zhang, D.; Peng, L. Heavy Metal Distribution and Bioaccumulation Ability in Marine Organisms from Coastal Regions of Hainan and Zhoushan, China. Chemosphere 2019, 226, 340–350. [Google Scholar] [CrossRef]
- Puspitasari, R.; Takarina, N.D.; Soesilo, T.E.B.; Agustina, H. Potential Risks of Heavy Metals in Green Mussels (Perna Viridis) Harvested from Cilincing and Kamal Muara, Jakarta Bay, Indonesia to Human Health. Mar. Pollut. Bull. 2023, 189, 114754. [Google Scholar] [CrossRef] [PubMed]
- Ratheesh Kumar, M.; Anoop Krishnan, K.; Vimexen, V. Effect of Trace Metal Contamination in Sediments on the Bioaccumulation of Bivalve Meretrix Meretrix. Mar. Pollut. Bull. 2022, 176, 113422. [Google Scholar] [CrossRef] [PubMed]
- Pandion, K.; Khalith, S.B.M.; Ravindran, B.; Chandrasekaran, M.; Rajagopal, R.; Alfarhan, A.; Chang, S.W.; Ayyamperumal, R.; Mukherjee, A.; Arunachalam, K.D. Potential Health Risk Caused by Heavy Metal Associated with Seafood Consumption around Coastal Area. Environ. Pollut. 2022, 294, 118553. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Chen, S.; Li, Z.; Liu, P.; Xu, C.; Yang, X. Assessment of Heavy Metals in Water, Sediment and Shellfish Organisms in Typical Areas of the Yangtze River Estuary, China. Mar. Pollut. Bull. 2020, 151, 110864. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Ding, Z.; Li, R.; Shi, Z. Kinetics of Cadmium (Cd), Nickel (Ni), and Lead (Pb) Release from Fulvic Acid: Role of Re-Association Reactions and Quantitative Models. Sci. Total Environ. 2022, 843, 156996. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Tan, Q.-G. Applications of Dynamic Models in Predicting the Bioaccumulation, Transport and Toxicity of Trace Metals in Aquatic Organisms. Environ. Pollut. 2019, 252, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kuang, W.; Xu, J.; Chen, J.; Sun, X.; Lin, C.; Lin, H. Distribution, Source and Risk Assessment of Heavy Metals in the Seawater, Sediments, and Organisms of the Daya Bay, China. Mar. Pollut. Bull. 2022, 174, 113297. [Google Scholar] [CrossRef] [PubMed]
- Geng, N.; Wang, P.-F.; Chao, W.; Jun, H.; Jin, Q.; Miao, L.-Z. Mechanisms of Cadmium Accumulation (Adsorption and Absorption) by the Freshwater Bivalve Corbicula Fluminea under Hydrodynamic Conditions. Environ. Pollut. 2016, 212, 550–558. [Google Scholar] [CrossRef]
- Lin, Z.; Fan, X.; Huang, J.; Chen, R.; Tan, Q.-G. Intertidal Mussels Do Not Stop Metal Bioaccumulation Even When out of Water: Cadmium Toxicokinetics in Xenostrobus Atratus under Influences of Simulated Tidal Exposure. Environ. Pollut. 2020, 261, 114192. [Google Scholar] [CrossRef]
- Jeong, W.-G.; Cho, S.-M.; Lee, S.-J. Physiochemical Characteristics and Heavy Metal in the Surface Sediments of Marine Shellfish Farming Waters in Anjung Bay, Korea. Korean J. Malacol. 2014, 30, 421–428. [Google Scholar] [CrossRef]
- Du, R.; Jia, X.; Lin, Y.; Bi, E.; Jian, L. Heavy Metal Enrichment Characteristics and Influencing Factors of Marine Shellfish: Research Progres. Chin. Agric. Sci. Bull. 2019, 11, 155–159. [Google Scholar]
- Hu, J.; Liu, J.; Li, J.; Lv, X.; Yu, L.; Wu, K.; Yang, Y. Metal Contamination, Bioaccumulation, ROS Generation, and Epigenotoxicity Influences on Zebrafish Exposed to River Water Polluted by Mining Activities. J. Hazard. Mater. 2021, 405, 124150. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.-F.; Wang, W.-X. Comparison of the Bioavailability of Cr and Fe Bound with Natural Colloids of Different Origins and Sizes to Two Marine Bivalves. Mar. Biol. 2002, 141, 915–924. [Google Scholar] [CrossRef]
- Labianca, C.; De Gisi, S.; Todaro, F.; Notarnicola, M.; Bortone, I. A Review of the In-Situ Capping Amendments and Modeling Approaches for the Remediation of Contaminated Marine Sediments. Sci. Total Environ. 2022, 806, 151257. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, S.; Zhang, C.; Zeng, G.; Tan, X.; Song, B.; Zhang, P.; Yang, H.; Li, M.; Chen, Q. Application of Biochar for the Remediation of Polluted Sediments. J. Hazard. Mater. 2021, 404, 124052. [Google Scholar] [CrossRef] [PubMed]
- Wikström, J.; Bonaglia, S.; Rämö, R.; Renman, G.; Walve, J.; Hedberg, J.; Gunnarsson, J.S. Sediment Remediation with New Composite Sorbent Amendments to Sequester Phosphorus, Organic Contaminants, and Metals. Environ. Sci. Technol. 2021, 55, 11937–11947. [Google Scholar] [CrossRef]
- Haghnazar, H.; Sabbagh, K.; Johannesson, K.H.; Pourakbar, M.; Aghayani, E. Phytoremediation Capability of Typha latifolia L. to Uptake Sediment Toxic Elements in the Largest Coastal Wetland of the Persian Gulf. Mar. Pollut. Bull. 2023, 188, 114699. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Ning, X.; Li, Z. Research Progress and Hotspots on Microbial Remediation of Heavy Metal-Contaminated Soil: A Systematic Review and Future Perspectives. Environ. Sci. Pollut. Res. 2023, 30, 118192–118212. [Google Scholar] [CrossRef]
- Huang, X.-N.; Min, D.; Liu, D.-F.; Cheng, L.; Qian, C.; Li, W.-W.; Yu, H.-Q. Formation Mechanism of Organo-Chromium (III) Complexes from Bioreduction of Chromium (VI) by Aeromonas Hydrophila. Environ. Int. 2019, 129, 86–94. [Google Scholar] [CrossRef]
- Haghnazar, H.; Hudson-Edwards, K.A.; Kumar, V.; Pourakbar, M.; Mahdavianpour, M.; Aghayani, E. Potentially Toxic Elements Contamination in Surface Sediment and Indigenous Aquatic Macrophytes of the Bahmanshir River, Iran: Appraisal of Phytoremediation Capability. Chemosphere 2021, 285, 131446. [Google Scholar] [CrossRef]

| Location | Cu | Pb | Zn | Cd | Cr | Ni | As | Hg |
|---|---|---|---|---|---|---|---|---|
| Bohai Sea [11] | 2.67–30.1 | 10.7–33.6 | 16.9–117 | 0.01–0.26 | 5.98–99.9 | - | - | - |
| Yellow Sea [11] | 1.36–35.0 | 13.8–33.6 | 1.92–98.8 | 0.01–0.51 | 0.00–147 | - | - | - |
| East China Sea [2,11] | 3.39–133 | 48.2–181 | 48.2–181 | 0.05–0.39 | 62.0–121 | - | - | 0.23–0.76 |
| South China Sea [11,12] | 0.25–61.0 | 1.02–176 | 0.78–191 | 0.00–1.63 | 0.17–102 | 0.62–8.97 | 0.63–2.60 | - |
| Apia, Samoa [13] | 29 | 7.4 | 98.5 | 0.13 | 368 | 161 | 3.6 | 0.026 |
| Kangryong River estuary, Korea [14] | 17 | - | 68.3 | - | 15.2 | 15.5 | - | - |
| Mekong Delta, Vietnam [15] | 0.60–3.60 | 0.01–0.80 | 4.90–72.0 | 0.010–0.024 | 0.01–0.31 | 0.44–0.73 | 0.80–2.60 | - |
| Suez Bay, Egypt [16] | 0.23–7.53 | 0.74–6.92 | 0.78–15.6 | 0.10–0.97 | 1.75–19.5 | 0.78–10.9 | - | 0.11–0.89 |
| TEL [17] | 18.7 | 30.2 | 124 | 0.68 | 52.3 | 15.9 | 7.24 | 0.13 |
| PEL [17] | 108 | 112 | 271 | 4.21 | 160 | 42.8 | 41.6 | 0.7 |
| Species | Cu | Pb | Zn | Cd | Cr | Hg | Location |
|---|---|---|---|---|---|---|---|
| Bivalves [68] | 0.15–12.64 | 0.02–0.05 | 2.50–27.6 | 0.01–1.32 | 0.12–0.18 | Shenzhen, Zhoushan, Qingdao, and Dandong, China. | |
| Crabs [69] | 8.59–49.7 | 0.19–1.25 | 15.4–50.7 | 0.26–3.31 | Cuddalore coast, India | ||
| Bivalves [69] | 3.93–24.7 | 0.00–3.44 | 34.1–39.5 | 1.08–3.00 | |||
| Crabs [70] | 3.2 | 0.06 | 17.8 | 0.03 | 0.23 | 0.012 | Hainan, China |
| Crabs [70] | 4.4 | 0.23 | 9.1 | 0.19 | 0.72 | 0.028 | Zhoushan, China |
| Bivalves [71] | 0.00–0.96 | 0.01–0.08 | Jakarta Bay, Indonesia | ||||
| Bivalves [72] | 298 | 18.7 | 176 | 1.03 | 420 | 0.06 | Netravathi estuary, India |
| Fishes [73] | 5.67–7.41 | 0.10–0.12 | 9.98–12.0 | 0.12–0.14 | 0.02–0.11 | 0.08–0.11 | Bay of Bengal, India |
| WHO (1989) | 1 | 2 | 100 | 50 | 30 | 0.3 | |
| USEPA (2000) | 2 | 4 | 120 | 8 | 120 | 0.5 |
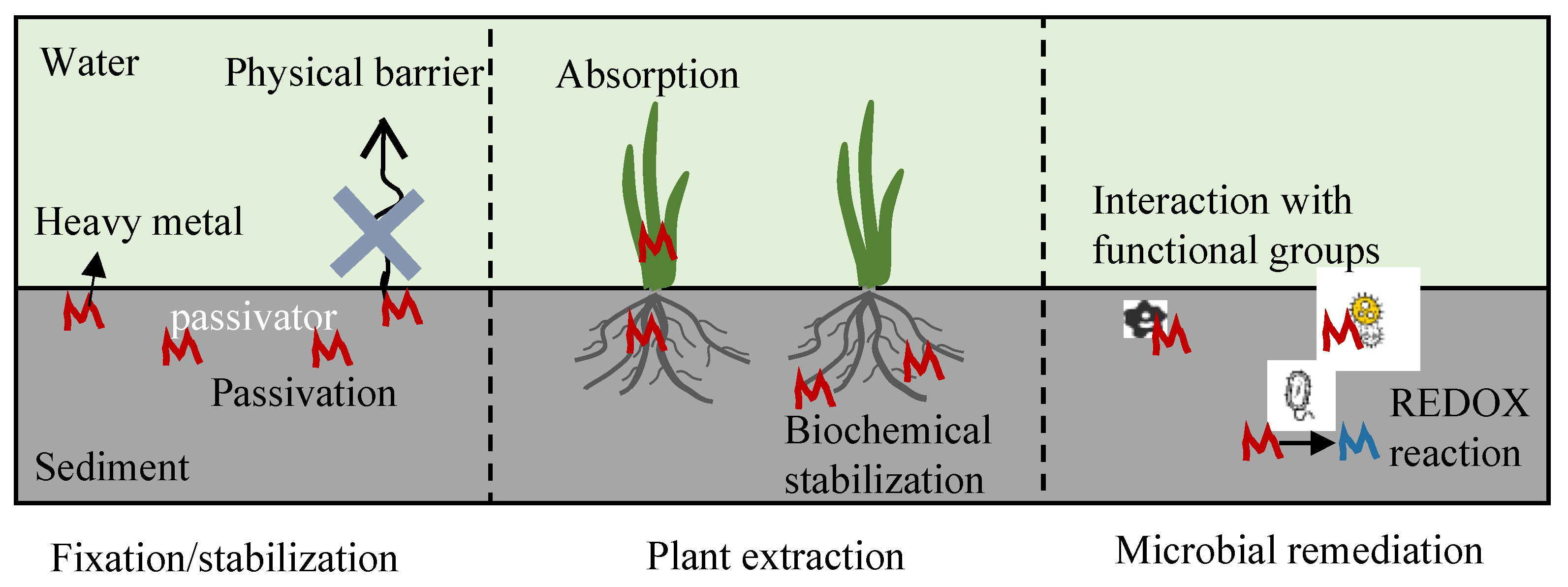
| Method | Approach | Advantages | Disadvantages |
|---|---|---|---|
| Fixation/ stabilization [85,86] | Physical barriers to reduce the heavy metal resuspension with the sediment; immobilization and passivation of heavy metal by physicochemical interactions with the passivators | Significantly changes the morphology of heavy metals and reduces the bioavailability of heavy metals | Aging of the passivators; environmental risk |
| Plant extraction [87,90] | Absorption by plant tissues; biochemical action of root exudates and rhizosphere microbes | Effective accumulation of heavy metals by hyperenriched plants; heavy metals can be transferred out of the sediment by harvesting | Requires a long period; limited plant species; the plants need management |
| Microbial remediation [88,89] | Adsorption, dissolution and precipitation, redox, and other biochemical effects | Wide variety and number of microorganisms that can alter the morphology of heavy metals | Species invasion; sensitivity and limitations of microbial growth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, N.; Xia, Y.; Li, D.; Bai, F.; Xu, C. Migration and Transformation of Heavy Metal and Its Fate in Intertidal Sediments: A Review. Processes 2024, 12, 311. https://doi.org/10.3390/pr12020311
Geng N, Xia Y, Li D, Bai F, Xu C. Migration and Transformation of Heavy Metal and Its Fate in Intertidal Sediments: A Review. Processes. 2024; 12(2):311. https://doi.org/10.3390/pr12020311
Chicago/Turabian StyleGeng, Nan, Yinfeng Xia, Dongfeng Li, Fuqing Bai, and Cundong Xu. 2024. "Migration and Transformation of Heavy Metal and Its Fate in Intertidal Sediments: A Review" Processes 12, no. 2: 311. https://doi.org/10.3390/pr12020311






