Current Status and Economic Analysis of Green Hydrogen Energy Industry Chain
Abstract
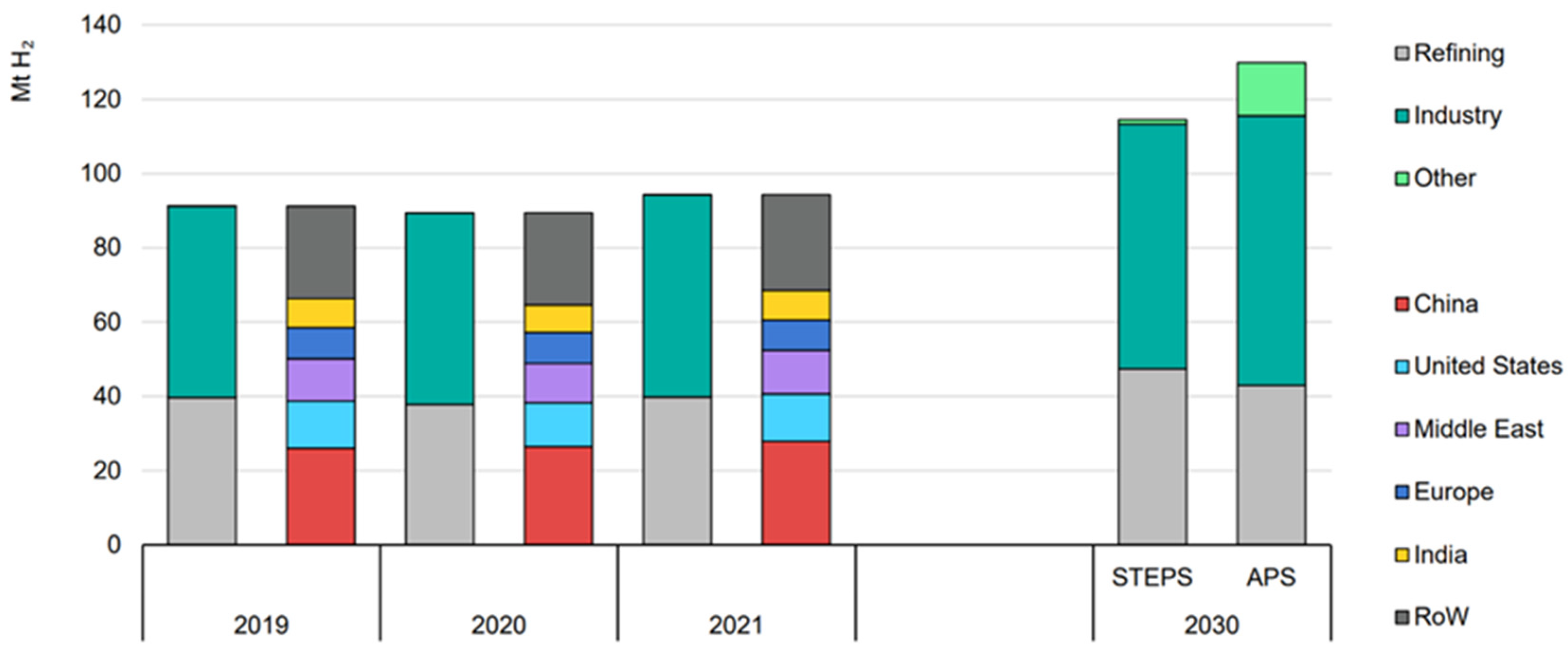
:1. Introduction
2. Hydrogen Production
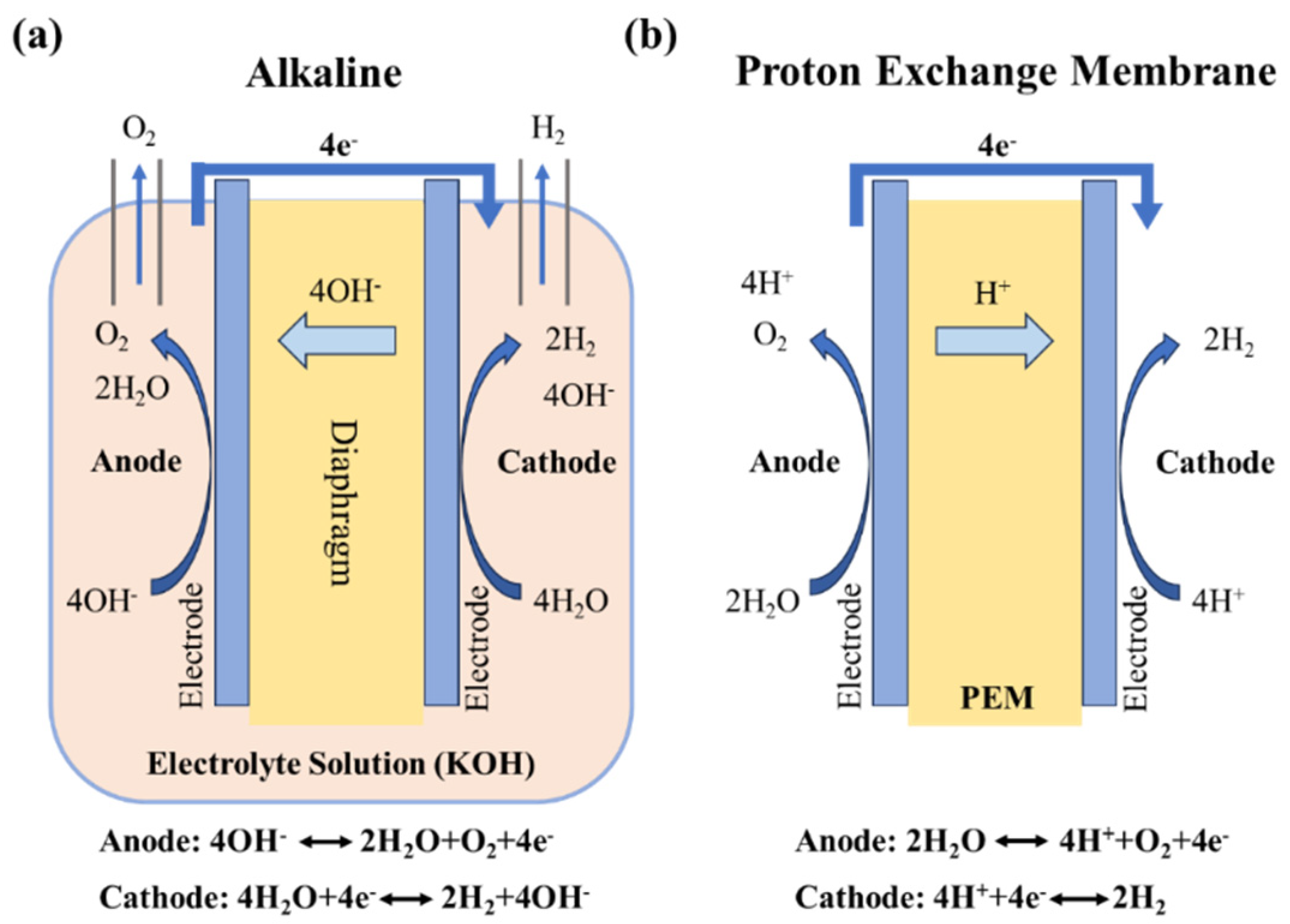
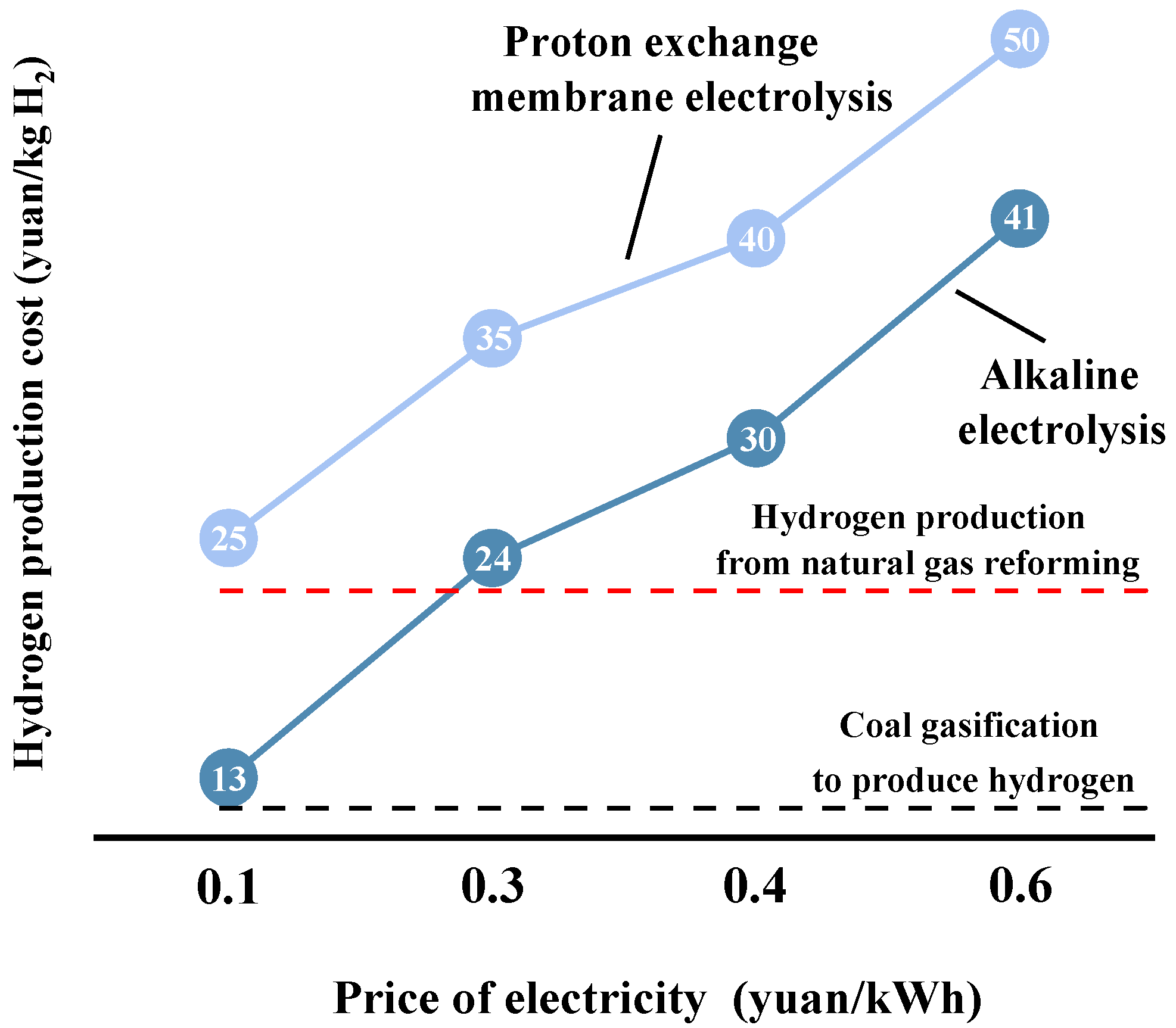
2.1. Alkaline Water Electrolysis for Hydrogen Production
2.2. Proton Exchange Membrane Water Electrolysis for Hydrogen Production
3. Hydrogen Storage and Transportation
3.1. High-Pressure Gaseous Hydrogen Storage
3.2. Low Temperature Liquid Hydrogen Storage
3.3. Organic Liquid Hydrogen Storage
3.4. Solid-State Hydrogen Storage
3.5. Hydrogen Safety
4. Hydrogen Applications
4.1. Transportation
4.2. Industrial Engineering
4.2.1. Synthesis Ammonia
4.2.2. Methanol Synthesis
4.3. Energy Storage
4.4. Power-to-Gas
4.5. Microgrid
5. Summary and Outlook
5.1. Conclusions
5.2. Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Acronyms and Parameters | Full Title |
| AWE | Alkaline water electrolysis |
| PEM | Proton exchange membrane |
| CNY | China Yuan |
| HPC | Hydrogen production cost |
| UEC | Unit electricity consumption |
| AD | Annual depreciation |
| AF | Annual freight |
| TAHP | Total annual hydrogen production |
| UWC | Unit water consumption |
| WP | Water price |
| HER | Hydrogen evolution reaction |
| SOEC | Solid oxide electrolysis cell |
| AEM | Anion exchange membrane |
| LFL | Lower flammable limit |
| UFT | Upper flammable limit |
| HE | Hydrogen embrittlement |
| P2G | Power to Gas |
| CCHP | Combined cooling heating and power |
| CHP | Combined heating and power |
References
- International Energy Agency. Global Hydrogen Review 2022 [R/OL]. Available online: https://www.iea.org/reports/global-hydrogen-review-2022 (accessed on 1 September 2022).
- Zhang, W. The Progress and Economic Analysis of Hydrogen Production Technology. Pet. Petrochem. Today 2022, 30, 31–36. [Google Scholar]
- Germany Pledges to Invest 4 Billion Euros in Green Energy Projects in Africa [R/OL]. Available online: https://apnews.com/article/germany-g20-compact-africa-green-energy-investment-dfd9e54ee108758bc22a8ce8e842a910 (accessed on 21 November 2023).
- Jiang, H.; Xu, X.; Liu, Z.; Zhang, R.; Hu, X. Energy transition and hydrogen development prospects in Saudi Arabia. Energy Storage Sci. Technol. 2022, 11, 2354–2365. [Google Scholar]
- Li, S.; Shi, Y.; Cai, N. Progress in hydrogen production from fossil fuels and renewable energy sources for the green energy revolution. J. Tsinghua Univ. (Sci. Technol.) 2022, 62, 655–662. [Google Scholar]
- Zou, C.; Li, J.; Zhang, X.; Jin, X.; Xiong, B.; Yu, H.; Liu, X.; Wang, S.; Li, Y.; Zhang, L.; et al. Industrial status, technological progress, challenges and prospects of hydrogen energy. Nat. Gas Ind. 2022, 42, 1–20. [Google Scholar] [CrossRef]
- Han, L. Nanoporous Metal-Based Hybrid Electrodes as Highly Efficient Electrocatalysts for Alkaline Hydrogen Evolution Reaction. Ph.D. Thesis, Jilin University, Changchun, China, 2020. [Google Scholar]
- Zhang, L. Study on the Electrocatalytic Hydrogen Evolution Performance of Transition Metal Dichalcogenides and Phosphides Heterojunctions. Ph.D. Thesis, Changchun University of Technology, Changchun, China, 2023. [Google Scholar]
- Liu, W.; Wan, Y.M.; Xiong, Y.L.; Tao, Z.J.; Zhu, Y.B. Key Technology of Water Electrolysis and Levelized Cost of Hydrogen Analysis under Carbon Neutral Vision. Trans. China Electrotech. Soc. 2022, 37, 2888–2896. [Google Scholar]
- Song, Z. Development of Polysulfone Membrane for Alkaline Water Electrolyzer. Master’s. Thesis, Hunan University, Changsha, China, 2018. [Google Scholar]
- Johan, C.E.; Anders, A.F.; Kasper, T.T.; Gastón, O.L. Affordable Green Hydrogen from Alkaline Water Electrolysis: Key Research Needs from an Industrial Perspective. ACS Energy Lett. 2023, 8, 1502–1509. [Google Scholar]
- Anh, L.H.; Sivakumar, B.; Aaron, H.; George, T.; Atheer, A.; Klaudia, W.; Chong, L.; Gerhard, F.S.; Gordon, G.W. High-performing catalysts for energy-efficient commercial alkaline water electrolysis. Sustain. Energy Fuels 2023, 7, 31–60. [Google Scholar]
- CNY, T.; Wan, Z.; Wang, J.; Zhang, D.; Jiang, D. The Day-Ahead Output Plan of Hydrogen Production System Considering the Start-Stop Characteristics of Electrolytic Cell. Electr. Power 2022, 55, 101–109. [Google Scholar]
- Soochow Securities Research Institute. Hydrogen Energy Series Research II: Industry Chain Economy Measurement and Cost Reduction Outlook. [R/OL]. Available online: https://pdf.dfcfw.com/pdf/H3_AP202205081564384333_1.pdf?1652088458000.pdf (accessed on 8 May 2022).
- Zhang, X.; Wang, K.; Fan, X.; Zheng, L. Cost analysis on hydrogen production via water electrolysis. Mod. Chem. Ind. 2021, 41, 7–11. [Google Scholar]
- Mostafa, E. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar]
- Shiva, K.S.; Aleksey, N.; Himabindu, V.; Hankwon, L. Experimental and simulation of PEM water electrolyser with Pd/PN-CNPs electrodes for hydrogen evolution reaction: Performance assessment and validation. Appl. Energy 2023, 348, 121565. [Google Scholar]
- Mehrnaz, M.N.; Pouria, A.; Ehsan, H. Comparative optimization study of three novel integrated hydrogen production systems with SOEC, PEM, and alkaline electrolyzer. Fuel 2023, 336, 126835. [Google Scholar]
- Babar, P.T.; Pawar, B.S.; Lokhande, A.C.; Gang, M.G.; Jang, J.S.; Suryawanshi, M.P.; Pawar, S.M.; Kim, J.H. Annealing temperature dependent catalytic water oxidation activity of iron oxyhydroxide thin films. J. Energy Chem. 2017, 26, 757–761. [Google Scholar] [CrossRef]
- Bishnupad, M.; Piyali, B.; Bikash, K.J. An overview on advances in design and development of materials for electrochemical generation of hydrogen and oxygen. Mater. Today Energy 2022, 23, 100902. [Google Scholar]
- Niu, S.; Fang, Y.; Rao, D.; Liang, G.; Li, S.; Cai, J.; Liu, B.; Li, J.; Wang, G. Reversing the Nucleophilicity of Active Sites in CoP2 Enables Exceptional Hydrogen Evolution Catalysis. Small 2022, 18, 2106870. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, L.; Bian, Q.; Yu, F.; Li, D.; Zeng, J.; Zhang, L.; Wang, H.; Tang, D.; Zhou, H.; et al. Engineering In-Plane Nickel Phosphide Heterointerfaces with Interfacial sp H-P Hybridization for Highly Efficient and Durable Hydrogen Evolution at 2 A cm−2. Small 2022, 18, 2105642. [Google Scholar] [CrossRef]
- Sun, L.; Cao, M.; Jing, Z.; Cheng, Z.; Zheng, D.; Xu, H.; Zhou, Q.; Lin, J. 1 T-Phase Enriched P doped WS2 nanosphere for highly efficient electrochemical hydrogen evolution reaction. Chem. Eng. J. 2022, 429, 132187. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Li, Y.; CNY, S.; Huang, G.; Li, X.; Li, N. Activation engineering on metallic 1T-MoS2 by constructing In-plane heterostructure for efficient hydrogen generation. Appl. Catal. B Environ. 2022, 300, 120696. [Google Scholar] [CrossRef]
- Chen, H.; Hu, M.; Jing, P.; Liu, B.; Gao, R.; Zhang, J. Constructing heterostructure of CeO2/WS2 to enhance catalytic activity and stability toward hydrogen generation. J. Power Sources 2022, 521, 230948. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, W.; Shen, K.; Zhang, Q.; Zhao, R.; Xiang, H.; Wu, J.; Li, X.; Yang, N. Electrochemically Reconstructed Cu-FeOOH/Fe3O4 Catalyst for Efficient Hydrogen Evolution in Alkaline Media. Adv. Energy Mater. 2022, 12, 2200077. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, F.; Zhang, Y.; Tian, M.; Lin, H.; Guo, W.; Qu, F. Constructing Heteroatom-Doped Transition-Metal Sulfide Heterostructures for Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2023, 6, 6348–6356. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, Q.; Li, J.; Xia, Y.; Li, H.; Li, Y. Engineering Isolated S Vacancies over 2D MoS2 Basal Planes for Catalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2022, 5, 3521–3530. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Si, H.; Zhang, Q.; Wu, J.; Gao, L.; Wei, X.; Sun, Y.; Liao, Q.; Zhang, Z.; et al. Single-Atom Vacancy Defect to Trigger High-Efficiency Hydrogen Evolution of MoS2. J. Am. Chem. Soc. 2020, 142, 4298–4308. [Google Scholar] [CrossRef]
- Fu, J.; Ali, R.; Mu, C.; Liu, Y.; Mahmood, N.; Lau, W.; Jian, X. Large-scale preparation of 2D VSe2 through a defect-engineering approach for efficient hydrogen evolution reaction. Chem. Eng. J. 2021, 411, 128494. [Google Scholar] [CrossRef]
- Joyner, J.; Oliveira, E.F.; Yamaguchi, H.; Kato, K.; Vinod, S.; Galvao, D.S.; Salpekar, D.; Roy, S.; Martinez, U.; Tiwary, C.S.; et al. Graphene Supported MoS2 Structures with High Defect Density for an Efficient HER Electrocatalysts. ACS Appl. Mater. Interfaces 2020, 12, 12629–12638. [Google Scholar] [CrossRef]
- Kang, M.; Lin, C.; Yang, H.; Guo, Y.; Liu, L.; Xue, T.; Liu, Y.; Gong, Y.; Zhao, Z.; Zhai, T.; et al. Proximity Enhanced Hydrogen Evolution Reactivity of Substitutional Doped Monolayer WS2. ACS Appl. Mater. Interfaces 2021, 13, 19406–19413. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Hu, Y.; Zhou, X.; Zhang, M.; Jia, X.; Yang, Y.; Lin, B.; Chen, G. Highly dispersed Pt nanoparticles on 2D MoS2 nanosheets for efficient and stable hydrogen evolution reaction. J. Mater. Chem. A 2022, 10, 5273–5279. [Google Scholar] [CrossRef]
- Rui, Y.; Zhang, S.; Shi, X.; Zhang, X.; Wang, R.; Li, X. Chemically Activating Tungsten Disulfide via Structural and Electronic Engineering Strategy for Upgrading the Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2021, 13, 49793–49801. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, J.; Chen, X.; Huang, M.; Cai, S.; Han, J.; Li, J. Self-supported bimetallic phosphides with artificial heterointerfaces for enhanced electrochemical water splitting. Appl. Catal. B Environ. 2022, 304, 120914. [Google Scholar] [CrossRef]
- Sarkar, B.; Parui, A.; Das, D.; Singh, A.K.; Nanda, K.K. Interfacial Electron Transfer Strategy to Improve the Hydrogen Evolution Catalysis of CrP Heterostructure. Small 2022, 18, 2106139. [Google Scholar] [CrossRef]
- Zhang, Y.; Hui, Z.; Zhou, H.; Zai, S.; Wen, Z.; Li, J.; Yang, C.; Jiang, Q. Ga doping enables superior alkaline hydrogen evolution reaction performances of CoP. Chem. Eng. J. 2022, 429, 132012. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, S.; Chen, J.; Zhou, Y.; Zhao, P.; Dai, R.; Zhou, W.; Yang, P.; Zhang, H.; Chen, A. Interface engineering of Fe2P@CoMnP4 heterostructured nanoarrays for efficient and stable overall water splitting. J. Colloid Interface Sci. 2023, 633, 897–906. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Lysova, A.A.; Voropaeva, D.Y.; Yaroslavtsev, A.B. Approaches to the Modification of Perfluorosulfonic Acid Membranes. Membranes 2023, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Bai, J.; Ding, Y.; Wen, J.; Li, X. Development status and prospect of hydrogen production technology by renewable energy and its main equipment. J. TaiCNY Univ. Technol. 2023. Available online: https://link.cnki.net/urlid/14.1220.N.20231222.1128.002 (accessed on 22 December 2023).
- IRENA. Green Hydrogen Cost Reduction: Scaling up Electrolysers to Meet the 1.5 °C Climate Goal; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020. [Google Scholar]
- Hu, D. SIEMENS Energy Power-to-X Solution; 2021 Renewable Energy Hydrogen Forum: Shanghai, China, 2021. [Google Scholar]
- Wang, P. Study on Economy of Green Hydrogen Industry Based on Levelized Cost. Green Pet. Petrochem. 2023, 8, 1–9. [Google Scholar]
- Zhao, X.; Li, G.; Sun, X.; Song, J.; Liang, D.; Xu, G.; Deng, Z. Key Technology and Application Progress of Hydrogen Production by Electrolysis Under Peaking Carbon Dioxide Emissions and Carbon Neutrality Targets. J. Glob. Energy Interconnect. 2021, 4, 436–446. [Google Scholar]
- Gul, E.; Baldinelli, G.; Farooqui, A.; Bartocci, P.; Shamim, T. AEM-electrolyzer based hydrogen integrated renewable energy system optimisation model for distributed communities. Energy Convers. Manag. 2023, 285, 117025. [Google Scholar] [CrossRef]
- Zainal, B.S.; Ker, P.J.; Mohamed, H.; Ong, H.C.; Fattah, I.M.R.; Rahman, S.M.A.; Nghiem, L.D.; Mahlia, T.M.I. Recent advancement and assessment of green hydrogen production technologies. Renew. Sustain. Energy Rev. 2024, 189, 113941. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, H.; Zhang, S. Overview of electrolyser and hydrogen production power supply from industrial perspective. Int. J. Hydrogen Energy 2024, 49, 1048–1059. [Google Scholar] [CrossRef]
- Wolf, S.E.; Winterhalder, F.E.; Vibhu, V.; Haart, L.G.J.; Guillon, O.; Eichel, R.; Menzler, N.H. Solid oxide electrolysis cells-current material development and industrial application. J. Mater. Chem. A 2023, 11, 17977–18028. [Google Scholar] [CrossRef]
- Meng, X.; Gu, A.; Zeng, J.; Chen, M.; Zhou, J.; Liu, B.; Mao, Z. Advantages and challenges of China’s participation in international hydrogen trade. Int. J. Hydrogen Energy 2024, 52, 1356–1368. [Google Scholar] [CrossRef]
- Gao, J.; Mi, Y.; Zhou, Y.; Zhou, H.; Xu, Q. Recent developments in new hydrogen storage materials. Chem. Ind. Eng. Prog. 2021, 40, 2962–2971. [Google Scholar]
- Yin, Z.; Ma, Q.; Hao, J.; Yang, G.; Lei, Y. Key Technologies and Prospect Analysis of Hydrogen Energy Storage and Transportation. Liaoning Chem. Ind. 2021, 50, 1480–1482+1487. [Google Scholar] [CrossRef]
- Xue, J.; Yu, P.; Zhang, Y.; Liu, H.; Che, D. Review on advances of dehydrogenation reactor for hydrogen storage system using liquid organic hydrogen carrier. Therm. Power Gener. 2022, 51, 1–10. [Google Scholar]
- Ibrahim, A.; Paskevicius, M.; Buckley, C.E. Chemical compression and transport of hydrogen using sodium borohydride. Sustain. Energy Fuels 2023, 7, 1196–1203. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, J. Hydrogen Storage Technologies and Materials; Chemical Industry Publishers: Beijing, China, 2018. [Google Scholar]
- Wang, X.; Li, B.; Jin, X.; Han, B.; Shu, C. Ultimate pressure-bearing capacity of Type III onboard high-pressure hydrogen storage tanks under typical accident scenarios. J. Energy Storage 2023, 63, 107135. [Google Scholar] [CrossRef]
- Bosu, S.; Rajamohan, N. Recent advancements in hydrogen storage—Comparative review on methods, operating conditions and challenges. Int. J. Hydrogen Energy 2024, 52, 352–370. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Guo, Y. The significance of hydrogen energy development and the current status of hydrogen storage technology. Chem. Eng. Equip. 2023, 312, 205–206. [Google Scholar]
- Zhang, Z.; Xie, H. Hydrogen Energy Utilization—Status and Analysis of Liquid Hydrogen Production, Storage and Transportation Technologies. Renew. Energy 2023, 1–8. [Google Scholar] [CrossRef]
- Song, B. Current Status and Trends of Foreign Hydrogen Storage Technology. CFHI Technol. 2023, 212, 61–63. [Google Scholar]
- Ma, X. T Techno-Economic Analysis of Liquid Organic Hydrogen Carrier and Pt/Al2O3 Catalytic Dehydrogenation Performance Study. Ph.D. Thesis, East China University of Science and Technology, Shanghai, China, 2021. [Google Scholar]
- Ma, X.; Li, Z.; Xiao, Z.; Li, P. Techno-economic analysis and comparison of liquid organic hydrogen carrier system. Mod. Chem. Ind. 2022, 42, 202–205+210. [Google Scholar]
- Si, W.; Yu, D. How Low Can the Cost of Hydrogen Go? Storage and Transportation [R/OL]. Available online: https://www.sohu.com/a/334833486_354900 (accessed on 19 August 2019).
- Song, P.; Hou, J.; Mu, X.; Wang, X. Screening and application scenarios of liquid organic hydrogen carrier systems. Nat. Gas Chem. Ind. 2021, 46, 1–5+33. [Google Scholar]
- Muduli, R.C.; Kale, P. Silicon nanostructures for solid-state hydrogen storage: A review. Int. J. Hydrogen Energy 2023, 48, 1401–1439. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, L.; Ye, J.; Wu, Y.; Guo, X.; Li, Z.; Li, H. Research progress of solid-state hydrogen storage technology. Acta Energiae Solaris Sin. 2022, 43, 345–354. [Google Scholar]
- Mohassel, R.; Shabani-Nooshabadi, M.; Salavati-Niasari, M. Effect of g-C3N4 amount on green synthesized GdFeO3/g-C3N4 nanocomposites as promising compounds for solid-state hydrogen storage. Int. J. Hydrogen Energy 2023, 48, 6586–6596. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, L.; Song, M.; Wu, F.; Wang, J.; Zhao, H.; Li, H. Interfacial engineering of nickel/vanadium based two-dimensional layered double hydroxide for solid-state hydrogen storage in MgH2. Int. J. Hydrogen Energy 2023, 48, 9390–9400. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Lu, X.; Wu, F.; Sun, X.; Zhao, H.; Li, Q. Surprising cocktail effect in high entropy alloys on catalyzing magnesium hydride for solid-state hydrogen storage. Chem. Eng. J. 2023, 465, 142766. [Google Scholar] [CrossRef]
- DT New Energy. Research and Development of Hydrogen Storage and Transportation Technology and Cost Analysis. [R/OL]. Available online: https://mp.weixin.qq.com/s?__biz=MzU5MzY1MzQ2NA==&mid=2247504183&idx=1&sn=ebed31bd93aeab3d59988d1741d8ebb7&chksm=fe0faa80c97823965e9f2bc60f751a8a5562697c36ed96914adbedc7cf42d818e16a2fbfed3c (accessed on 30 April 2022).
- Ding, L.; Tang, T.; Wang, Y. Research progress and development trend of hydrogen storage and transportation technology. Nat. Gas Chem. Ind. 2022, 47, 35–40. [Google Scholar]
- Abohamzeh, E.; Salehi, F.; Sheikholeslami, M.; Abbassi, R.; Khan, F. Review of hydrogen safety during storage, transmission, and applications processes. J. Loss Prev. Process Ind. 2021, 72, 104569. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Effect of hydrogen and absence of passive layer on corrosive properties of aluminum alloys. Materials 2020, 13, 1580. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Influence of hydrogen on shortening the operational reliability time of aluminum alloy construction. Instal 2013, 12, 32–34. [Google Scholar]
- Shahverdian, M.H.; Sohani, A.; Pedram, M.Z.; Sayyaadi, H. An optimal strategy for application of photovoltaic-wind turbine with PEMEC-PEMFC hydrogen storage system based on techno-economic, environmental, and availability indicators. J. Clean. Prod. 2023, 384, 135499. [Google Scholar] [CrossRef]
- Tollefson, J. Hydrogen vehicles: Fuel of the future? Nature 2010, 464, 1262. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, N.; Cao, T.; Hu, M.; Jiang, N. Application of Hydrogen Energy in Transportation and Cost Analysis of Fuel Cell Vehicles. Green Pet. Petrochem. 2022, 7, 1–5+30. [Google Scholar]
- Chang, H.; Wan, Z.; Zheng, Y.; Chen, X.; Shu, S.; Tu, Z.; Chan, S. Energy analysis of a hybrid PEMFC–solar energy residential micro-CCHP system combined with an organic Rankine cycle and vapor compression cycle. Energy Convers Manag. 2017, 142, 374–384. [Google Scholar] [CrossRef]
- What in the World Is Happening with Hydrogen? [R/OL]. Available online: https://www.energy.gov/sites/default/files/2021-09/h2-shot-summit-plenary-global-context.pdf (accessed on 25 August 2021).
- Le, T.T.; Sharma, P.; Bora, B.J.; Tran, V.D.; Truong, T.H.; Le, H.C.; Nguyen, P.Q.P. Fueling the future: A comprehensive review of hydrogen energy systems and their challenges. Int. J. Hydrogen Energy 2024, 54, 791–816. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, Y.; Zhen, X.; Liu, D. Numerical comparative analysis on performance and emission characteristics of methanol/hydrogen, ethanol/hydrogen and butanol/hydrogen blends fuels under lean burn conditions in SI engine. Fuel 2022, 313, 123012. [Google Scholar] [CrossRef]
- Sami, M.M.E.A.; Carlos, R.P.B.; Gabriel, L.R.S.; Renan, S.L.; Edvaldo, S.C.; Paulo, E.V.M. Analysis of performance parameters of an ethanol fueled spark ignition engine operating with hydrogen enrichment. Int. J. Hydrogen Energy 2020, 45, 5588–5606. [Google Scholar]
- Kim, S.; Kim, J. Feasibility assessment of hydrogen-rich syngas spark-ignition engine for heavy-duty long-distance vehicle application. Energy Convers. Manag. 2022, 252, 115048. [Google Scholar] [CrossRef]
- Xu, S.; Yu, B. Current Development and Prospect of Hydrogen Energy Technology in China. J. Beijing Inst. Technol. 2021, 23, 1–12. [Google Scholar]
- Wang, J.; Hu, C. Analysis of the development status and trend of China’s hydrogen fuel cell special-purpose vehicles. Spec. Purp. Veh. 2021, 287, 51–55. [Google Scholar]
- Special Research Report on Solid State Hydrogen Storage. [R/OL]. Available online: https://finance.sina.com.cn/esg/2023-04-07/doc-imypnmqt2018912.shtml (accessed on 7 April 2023).
- Ramachandran, R.; Menon, R.K. An overview of industrial uses of hydrogen. Int. J. Hydrogen Energy 1998, 23, 593–598. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, G.; Yang, Y.; Yan, D. The Analysis and Optimization solution of Hydrogen consumption in Polysilicon Production. World Nonferrous Met. 2017, 2, 32–33. [Google Scholar]
- Humphreys, J.; Lan, R.; Tao, S. Development and Recent Progress on Ammonia Synthesis Catalysts for Haber–Bosch Process. Adv. Energy Sustain. Res. 2021, 2, 2000043. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.C. Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel fuel. Fuel 2011, 90, 87–97. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A.; Bowen, P.J. Study on premixed combustion characteristics of co-firing ammonia/methane fuels. Energy 2017, 140, 125–135. [Google Scholar] [CrossRef]
- Chen, R.; Zheng, S.; Lin, Z.; Liu, Y.; Wang, Z. Performance Study of Direct Ammonia Fuel Cell Based on PtIr/C Anode Electrocatalyst. Acta Chim. Sin. 2021, 79, 11286–11292. [Google Scholar] [CrossRef]
- Li, J. Status and Trends of Green Power-Green Hydrogen-Green Ammonia Integration; Progress Report on Carbon Neutrality in China; Social Science Literature Publishing House: Beijing, China, 2022; pp. 110–122. [Google Scholar]
- Orange Club. New Trends in the Hydrogen Industry: Will the Ammonia Era Arrive? [EB/OL]. Available online: https://www.ofweek.com/hydro-gen/2022-01/ART-180827-8420-30547720_2.html (accessed on 26 January 2022).
- Xu, G.; Xue, X.; Zhang, Z.; Wu, Z.; Liang, S.; Chen, H.; Lei, J. A New Carbon Neutral Energy Technology Route Based on Electrolytic Water to Hydrogen and Methanol Synthesis. Proc. CSEE 2023, 43, 191–201. [Google Scholar]
- Chen, J. “Liquid Sunshine Methanol” could be the mainstay of emission reductions- Interview with Li Can, Academician of Chinese Academy of Sciences, Researcher of Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Director of National Laboratory of Clean Energy (Preparation). China Petrochem. Ind. Obs. 2020, 275, 12–14. [Google Scholar]
- Battaglia, P.; Buffo, G.; Ferrero, D.; Santarelli, M.; Lanzini, A. Methanol synthesis through CO2 capture and hydrogenation: Thermal integration, energy performance and techno-economic assessment. J. CO2 Util. 2021, 44, 101407. [Google Scholar] [CrossRef]
- Pengxiang, S.O.; Bo, Z.H.; Cenyu, Y.A.; Le, W.A.; Yi, J.I.; Shihui, Y.A. An assessment of the use of fuel chemicals synthesized from captured carbon dioxide for renewable electricity storage. Energy Storage Sci. Technol. 2016, 5, 78–84. [Google Scholar]
- Hydrogen Energy Committee of China Energy Conservation Association. Green Methanol-One of the Most Important Downstream Applications of Green Hydrogen. [EB/OL]. Available online: http://www.heic.org.cn/newshow.asp?id=786 (accessed on 12 July 2022).
- Ji, L.; Zhao, Y.; Wang, F.; Song, J.; Li, L.; Song, X.; Pan, Y. Current situation of hydrogen energy technology and hydrogen energy storage applied in power generation. Met. Funct. Mater. 2019, 26, 23–31. [Google Scholar]
- Xu, C.; Liu, J. Hydrogen Energy Storage in China’s New-Type Power System: Application Value, Challenges, and Prospects. Chin. Acad. Eng. 2022, 24, 89–99. [Google Scholar] [CrossRef]
- Mehrjerdi, H.; Iqbal, A.; Rakhshani, E.; Torres, J.R. Daily-seasonal operation in net-zero energy building powered by hybrid renewable energies and hydrogen storage systems. Energy Convers Manag. 2019, 201, 112156. [Google Scholar] [CrossRef]
- Audi, A.G. Audi e-Gas-Project Life Cycle Assessment. [EB/OL]. Available online: https://www.audi.com/en/company/sustainability/downloads-and-contact/documents-and-policies.html (accessed on 3 February 2014).
- Jiang, D.; Jia, Y.; Lu, Q.; Hong, B.; Shen, R.; Zhang, Y. Application Prospect of Hydrogen Energy in Integrated Energy Systems. Electr. Power 2020, 53, 135–142. [Google Scholar]
- Zhang, H. Key Technologies and Development Prospect of Hydrogen Energy Storage System. J. Shandong Electr. Power Coll. 2021, 24, 8–12. [Google Scholar]
- Liu, C.; Xu, Y.; Zhang, J.; Hu, S.; Yue, F.; Ding, J.; Chen, H. Research progress in economic study of energy storage. Energy Storage Sci. Technol. 2017, 6, 1084–1093. [Google Scholar]
- Unicorn Intelligence Center. In-Depth Report on Electrochemical Energy Storage. [R/OL]. Available online: https://xueqiu.com/1048969405/194802324 (accessed on 19 August 2021).
- Yang, J.; Xie, L.; Song, X.; Li, J.; Zhang, P.; Bian, Y. Collaborative Operation Method of Carbon Capture-P2G Based on Renewable Energy. New Energy 2022, 50, 70–78. [Google Scholar]
- Valerie, E.; Tesfaldet, G. A Review of Projected Power-to-Gas Deployment Scenarios. Energies 2018, 11, 1824. [Google Scholar]
- Zakeri, B.; Syri, S. Electrical energy storage systems: A comparative life cycle cost analysis. Renew. Sustain. Energy Rev. 2015, 42, 569–596. [Google Scholar] [CrossRef]
- Xue, Y. Power to Gas Technology of Renewable Energy and Its Life Cycle Assessment. Master’s Thesis, Southeast University, Nanjing, China, 2021. [Google Scholar]
- Tichler, R.; Lehner, M.; Horst, S.; Koppe, M. Power-to-Gas: Technology and Business Models; Springer International Publishing: Cham, Switzerland, 2014. [Google Scholar]
- Pei, Y. Optimal Operation of Combined Cooling, Heating and Power Micro-Energy Grid with Hydrogen Energy. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2020. [Google Scholar]
- Shao, Z.; Yi, B. Developing Trend and Present Status of Hydrogen Energy and Fuel Cell Development. Bull. Chin. Acad. Sci. 2019, 34, 469–477. [Google Scholar]
- Hua, B.; Gong, J. Techno-economic Analysis on Combined Cold Heat and Power & Dsiteibuten Energy System. Nat. Gas Ind. 2007, 7, 118–120+146. [Google Scholar]
| Catalyst | Electrolyte | Overpotential [mV@mA cm−2] | Tafel Slope [mV dec−1] | Strategy | Ref. |
|---|---|---|---|---|---|
| CoS2@1T-MoS2 | 0.5 M H2SO4 | 72@10 | 45 | Heterostructure | [24] |
| CeO2/WS2 | 0.5 M H2SO4 | 128@10 | 60 | Heterostructure | [25] |
| Cu-FeOOH/Fe3O4 | 1.0 M KOH | 129@100 | 11 | Heterostructure | [26] |
| HAs@MoS2/Ce2S3 | 0.5 M H2SO4 | 147@10 | 47 | Heterostructure/Doping | [27] |
| Se-MoS2 | 0.5 M H2SO4 | 100@10 | 49 | Doping/Vacancy | [28] |
| MoS2-60s | 0.5 M H2SO4 | 131@10 | 48 | Vacancy | [29] |
| VSe2-1.8 | 0.5 M H2SO4 | 160@10 | 85 | Vacancy | [30] |
| 1rGO-2MoS2 | 0.5 M H2SO4 | 197@10 | 41 | Carbon-based | [31] |
| Fe-WS2 | 0.5 M H2SO4 | 195@10 | 81 | Doping | [32] |
| E-MoS2-Pt-r | 0.5 M H2SO4 | 38@10 | 29 | Nano-composite | [33] |
| Pd-WS2/W3O | 0.5 M H2SO4 | 54@10 | 70 | Heterostructure/Doping | [34] |
| 1 T-WS2|P-5 | 0.5 M H2SO4 | 125@10 | 73.73 | Doping | [23] |
| Ni2P-Ru2P/NF | 1.0 M KOH | 101@10 | 41.5 | Heterostructure | [35] |
| CrP/NPC | 0.5 M H2SO4 | 34@10 | 39 | Carbon-based | [36] |
| Ga-CoP NSs | 1.0 M KOH | 44@10 | 62 | Doping | [37] |
| NiP2/Ni5P4 | 0.5 M H2SO4 | 30@10 | 30.2 | Heterostructure | [22] |
| Fe2P@CoMnP4 | 1.0 M KOH | 53@10 | 50.6 | Heterostructure | [38] |
| Ru-SA/Pv-CoP2 | 1.0 M KOH | 17@10 | 29 | Doping/Vacancy | [21] |
| Main Technical and Economic Indicators | AWE for Hydrogen Production | PEM for Hydrogen Production | ||
|---|---|---|---|---|
| Current Level | Future Target | Current Level | Future Target | |
| Current density/(A∙cm−2) | 0.2–0.4 | <0.8 | 1–2 | 1.5–3 |
| System efficiency/% | 62–82 | 67–87 | 74–87 | 82–93 |
| Electricity consumption for hydrogen production/(kWh·m−3) | 4.5–6.5 | 4.3–5.7 | 4.5–5.5 | 4.1–4.8 |
| System Power Rating/MW | 150 | -- | 10–20 | 100 |
| System life/a | 20–30 | 30 | 10–20 | 20–30 |
| investors/ (dollars kW−1) | 850–1500 | 800 | 2000–3000 | 800–1300 |
| Hydrogen Storage and Transportation | Transport Vehicle | Specificities | Stresses (MPa) | Hydrogen-Carrying Capacity (kg/Vehicle) | Volumetric Hydrogen Storage Density (kg/m3) | Economic Distance (km) |
|---|---|---|---|---|---|---|
| High-pressure gaseous | trailer | smaller scale and shorter transportation distances | 20 | 300–400 | 14.5 | ≤150 |
| pipeline transport | large-scale hydrogen use with many application areas | 1–4 | - | 3.2 | ≥500 | |
| low-temperature liquid | liquid hydrogen tanker | long-distance transportation and high costs | 0.6 | 7000 | 64 | ≥200 |
| organic liquid | tanker | limited by cost and technical issues | atmospheric | 2000 | 40–50 | ≥200 |
| solid-state | trucks | high hydrogen storage density, high transportation capacity and technical difficulties | 4 | 300–400 | 50 | ≤150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Zheng, W.; Wei, Y.; Yan, Z. Current Status and Economic Analysis of Green Hydrogen Energy Industry Chain. Processes 2024, 12, 315. https://doi.org/10.3390/pr12020315
Yan X, Zheng W, Wei Y, Yan Z. Current Status and Economic Analysis of Green Hydrogen Energy Industry Chain. Processes. 2024; 12(2):315. https://doi.org/10.3390/pr12020315
Chicago/Turabian StyleYan, Xinrong, Wenguang Zheng, Yajuan Wei, and Zhaoqian Yan. 2024. "Current Status and Economic Analysis of Green Hydrogen Energy Industry Chain" Processes 12, no. 2: 315. https://doi.org/10.3390/pr12020315
APA StyleYan, X., Zheng, W., Wei, Y., & Yan, Z. (2024). Current Status and Economic Analysis of Green Hydrogen Energy Industry Chain. Processes, 12(2), 315. https://doi.org/10.3390/pr12020315






