Study on Drying of Seaweeds and Importance of Glass Transition and Stabilization
Abstract
:1. Introduction
- Stable mild temperature and humidity in the drying process. Of course, higher temperatures will reduce the drying time, but at the same time they will reduce the polysaccharides, and decompose components.
2. Materials and Methods
2.1. Material
2.2. Experiment Description
- Air temperature changes: 10, 25 and 38 °C.
- Different layers: 1, 2 and 3 layers. In the drying chamber on shelves in the same direction as the air flow.
- Relative humidity was at 16.0 ± 4.0% for the drying air.
- Velocity of drying medium: 1.5 ± 0.5 m s−1.
- Drying was terminated when moisture content reached the range 20.0~10.0% d.b.
2.3. Drying Behaviour
2.4. Sorption and Desorption Properties with Temperature
2.5. DSC Analysis
2.6. Glass Transition
2.7. Unfreezable Water
2.8. Data Analysis—Statistics
3. Results and Discussion
3.1. Chemical Composition of Seaweeds
3.2. Effect of Pretreatment with Drying
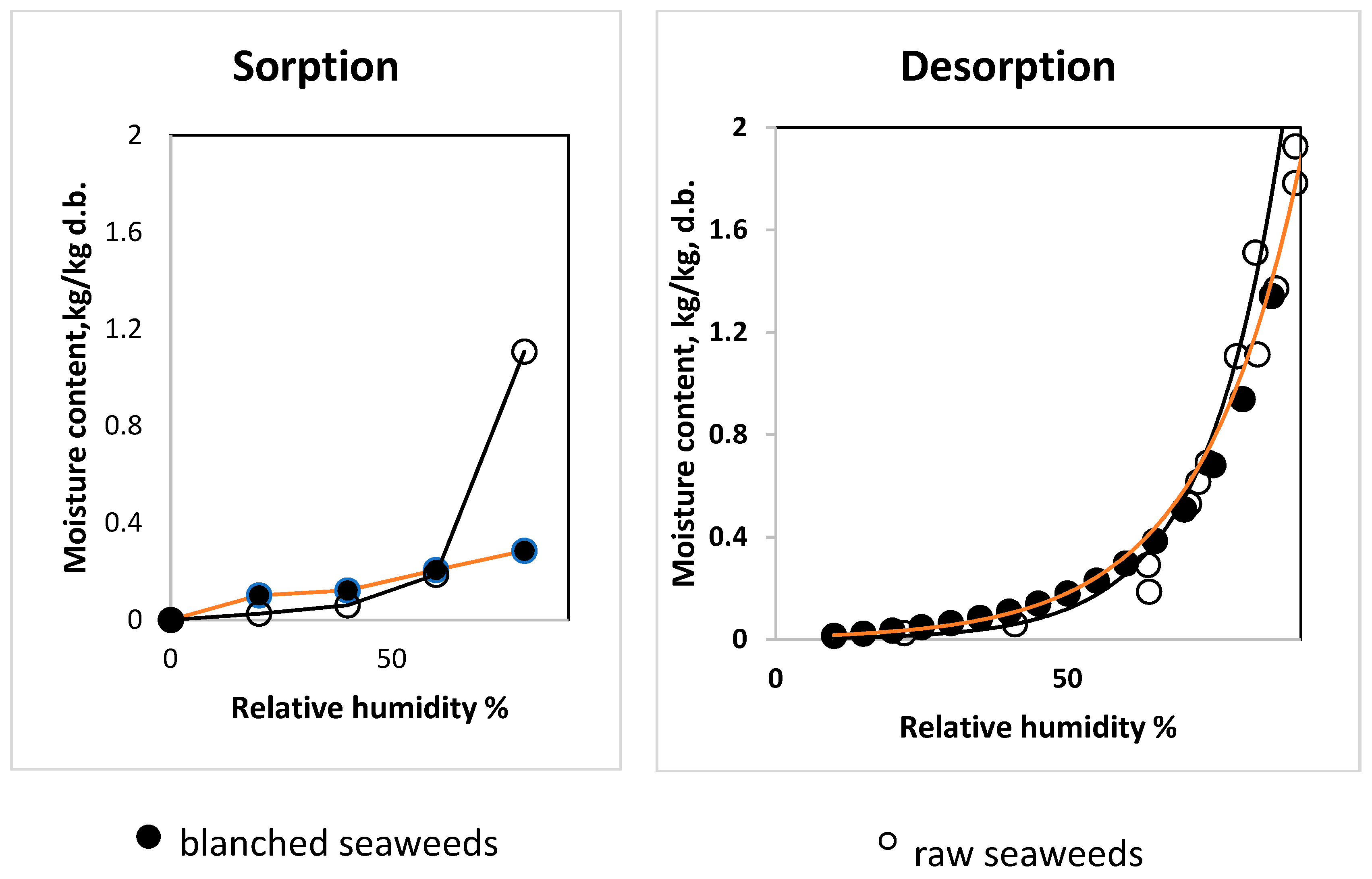
3.3. Influence of Blanching on Sorption–Desorption Characteristics of Saccharina latissimi
3.4. Shrinkage Analysis of Samples during Dewatering
3.5. Drying Kinetics of Seaweeds
3.6. Thermal Transitions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| a | empirical coefficient |
| aw | water activity, - |
| b | thickness of seaweed blade, mm |
| C* | chrominance (CIE L*C*h* color scale) |
| c | empirical coefficient |
| D | coefficient of diffusivity, m2 s−1 |
| d.b. | dry basis, kg kg−1 |
| DSC | differential scanning calorimeter |
| h | half-thickness of seaweed layers, m |
| h* | hue angle (CIE L*C*h* color scale), ° |
| k | drying constant, min−1 |
| MR | moisture ratio, - |
| RH | relative humidity of air, % |
| Y | ratio of MR to volume of sample m−3 |
| E | melting energy, kJ |
| I | mass of ice, kg |
| L | latent heat, kJ kg−1 |
| T | temperature, °C |
| w.b. | wet basis |
| x | mass fraction kg kg−1 |
| Subscript | |
| b | bound water |
| f | freezing |
| l | lipid |
| m | melting |
| p | protein |
| w | water |
References
- FAO. The State of World Fisheries and Aquaculture: Opportunities and Challenges; FAO: Roma, Italy, 2014. [Google Scholar]
- Chopin, T. Seaweed aquaculture provides diversified products, key ecosystem functions Part II. Recent evolution of sea weed industry. Glob. Aquac. Advocate 2012, 14, 24–27. [Google Scholar]
- Radulovich, R. Massive freshwater gains from producing food at sea. Water Policy 2011, 13, 547. [Google Scholar] [CrossRef]
- Forster, J.; Radulovich, R. Chapter 11—Seaweed and food security A2—Tiwari, Brijesh K. In Seaweed Sustainability; Troy, D.J., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 289–313. [Google Scholar]
- Phang, H.-K.; Chu, C.-M.; Kumaresan, S.; Rahman, M.M.; Yasir, S.M. Preliminary study of seaweed drying under a shade and in a natural draft solar dryer. Int. J. Sci. Eng. 2015, 8, 10–14. [Google Scholar] [CrossRef]
- Lemus, R.A.; Pérez, M.; Andrés, A.; Roco, T.; Tello, C.M.; Vega, A. Kinetic study of dehydration and desorption isotherms of red alga Gracilaria. LWT-Food Sci. Technol. 2008, 41, 1592–1599. [Google Scholar] [CrossRef]
- Fudholi, A.; Othman, M.Y.; Ruslan, M.H.; Yahya, M.; Zaharim, A.; Sopian, K. Design and testing of solar dryer for drying kinetics of seaweed in Malaysia. In Proceedings of the 4th WSEAS International Conference on Energy and Development-Environment-Biomedicine, Corfu Island, Greece, 22–25 July 2010. [Google Scholar]
- Chan, J.C.C.; Cheung, P.C.K.; Ang, P.O. Comparative Studies on the Effect of Three Drying Methods on the Nutritional Composition of Seaweed Sargassum hemiphyllum (Turn.) C. Ag. J. Agric. Food Chem. 1997, 45, 3056–3059. [Google Scholar] [CrossRef]
- Gupta, S.; Cox, S.; Abu-Ghannam, N. Effect of different drying temperatures on the moisture and phytochemical constituents of edible Irish brown seaweed. LWT-Food Sci. Technol. 2011, 44, 1266–1272. [Google Scholar] [CrossRef]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Chapter 6—Seaweed proteins, peptides, and amino acids A2—Tiwari, Brijesh K. In Seaweed Sustainability; Troy, D.J., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 125–140. [Google Scholar]
- Ignat IEikevik, T.M.; Petrova, I.; Michael, B. Investigation of influence of pre-treatment and low temperature on drying kinetics, sorption isotherms, shrinkage and color of Brown seaweeds (Saccharina latissima). In Proceedings of the International Drying Sysmposium, Valencia, Spain, 11–14 September 2018. [Google Scholar]
- Lewis, W.K. The rate of drying of solid materials. Ind. Eng. Chem. 1921, 13, 427–432. [Google Scholar] [CrossRef]
- Crank, J. Mathematics of Diffusion; Clarendn Press: Oxford, UK, 1975. [Google Scholar]
- Arévalo-Pinedo, A.; Murr, F.E.X. Kinetics of vacuum drying of pumpkin (Cucurbita maxima): Modeling with shrink age. J. Food Eng. 2006, 76, 562–567. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists, 19th ed.; AOAC: Arlington, VA, USA, 2012. [Google Scholar]
- Tolstorebrov, I.; Eikevik, T.M.; Bantle, M. Thermal phase transitions and mechanical characterization of Atlantic cod muscles at low and ultra-low temperatures. J. Food Eng. 2014, 128, 111–118. [Google Scholar] [CrossRef]
- Riedel, L. Chemie Ingenieur Technik; Wiley: Hoboken, NJ, USA, 1954; Volume 26, ISSN 0009-286X. [Google Scholar]
- Fleurence, J. Seaweed proteins. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Sappati, P.K.; Nayak, B.; van Walsum, G.P. Effect of glass transition on the shrinkage of sugar kelp (Saccharina latissima) during hot air convective drying. J. Food Eng. 2017, 210, 50–61. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Tønder, T. A Study of the Seasonal Variation in Biochemical Composition of Saccharina latissima. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2014. Available online: http://hdl.handle.net/11250/245494 (accessed on 24 December 2023).
- Hallerud, C.B. Pigment Composition of Macroalgae from a Norwegian Kelp Forest. Master’s Thesis, Norwegian Uniwersity of Science and Technology, Trondheim, Norway, 2014. Available online: http://hdl.handle.net/11250/238838 (accessed on 24 December 2023).
- Fung, C.A.Y. The Fucoxanthin Content and Antioxidant Properties of Undaria Pinnatifida from Marlborough Sound, New Zealand. Master’s Thesis, Auckland University of Technology, Auckland, New Zealand, 2012. [Google Scholar]
- Mise, T.; Ueda, M.; Yasumoto, T. Production of fucoxanthin-rich powder from Cladosiphon okamuranus. Adv. J. Food Sci. Technol. 2011, 3, 73–76. [Google Scholar]
- Moreira, R.; Chenlo, F.; Sineiro, J.; Arufe, S.; Sexto, S. Water sorption isotherms and air drying kinetics of Fucus vesiculosus brown seaweed. J. Food Process. Preserv. 2017, 41, e12997. [Google Scholar] [CrossRef]
- Lioutas, T.S.; Bechtel, P.J.; Steinberg, M.P. Desorption and adsorption isotherms of meat-salt mixtures. J. Agric. Food Chem. 1984, 32, 1382–1385. [Google Scholar] [CrossRef]
- Xiao, Q.; Tong, Q. Thermodynamic properties of moisture sorption in pullulan-sodiumalginate based edible films. Food Res. Int. 2013, 54, 1605–1612. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Alginate–calcium films: Water vapor permeability and mechanical properties as affected by plasticizer and relative humidity. LWT-Food Sci. Technol. 2008, 41, 359–366. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashim, N.; Janius, R.B.; Nawi, N.M.; Abdan, K. Modeling the thin-layer drying of fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 599–618. [Google Scholar] [CrossRef]
- Figueroa, Y.; Guevara, M.; Perez, A.; Cova, A.; Sandoval, A.J.; Muller, A.J. Effect of sugar addition on glass transition temperatures of cassava starch with low to intermediate moisture contents. Carbohydr. Polym. 2016, 146, 231–237. [Google Scholar] [CrossRef]
- Gaikwad, A.N.; Wood, E.R.; Ngai, T.; Lodge, T.P. Two calorimetric glass transitions in miscible blends containing Poly(ethylene oxide). Macromolecules 2008, 41, 2502–2508. [Google Scholar] [CrossRef]
- Izutsu, K.; Yomota, C.; Aoyagi, N. Inhibition of mannitol crystallization in frozen solutions by sodium phosphates and citrates. Chem. Pharm. Bull. 2007, 55, 565–570. [Google Scholar] [CrossRef]
- Tolstorebrov, I.; Eikevik, T.M.; Bantle, M. A DSC study of phase transition in muscle and oil of the main commercial fish species from the North-Atlantic. Food Res. Int. 2014, 55, 303–310. [Google Scholar] [CrossRef]
- Chen, C.S. Effective molecular weight of aqueous solutions and liquid foods calculated from the freezing point depression. J. Food Sci. 1986, 51, 1537–1539. [Google Scholar] [CrossRef]
- Gordon, M.; Taylor, J.S. Ideal copolymers and the second-order transitions of synthetic rubbers in non-crystalline copolymers. J. Appl. Chem. 1952, 2, 493–500. [Google Scholar] [CrossRef]
- Telang, C.; Suryanarayanan, R.; Yu, L. Crystallization of D-mannitol in binary mixtures with NaCl: Phase diagram and polymorphism. Pharm. Res. 2003, 20, 1939–1945. [Google Scholar] [CrossRef]




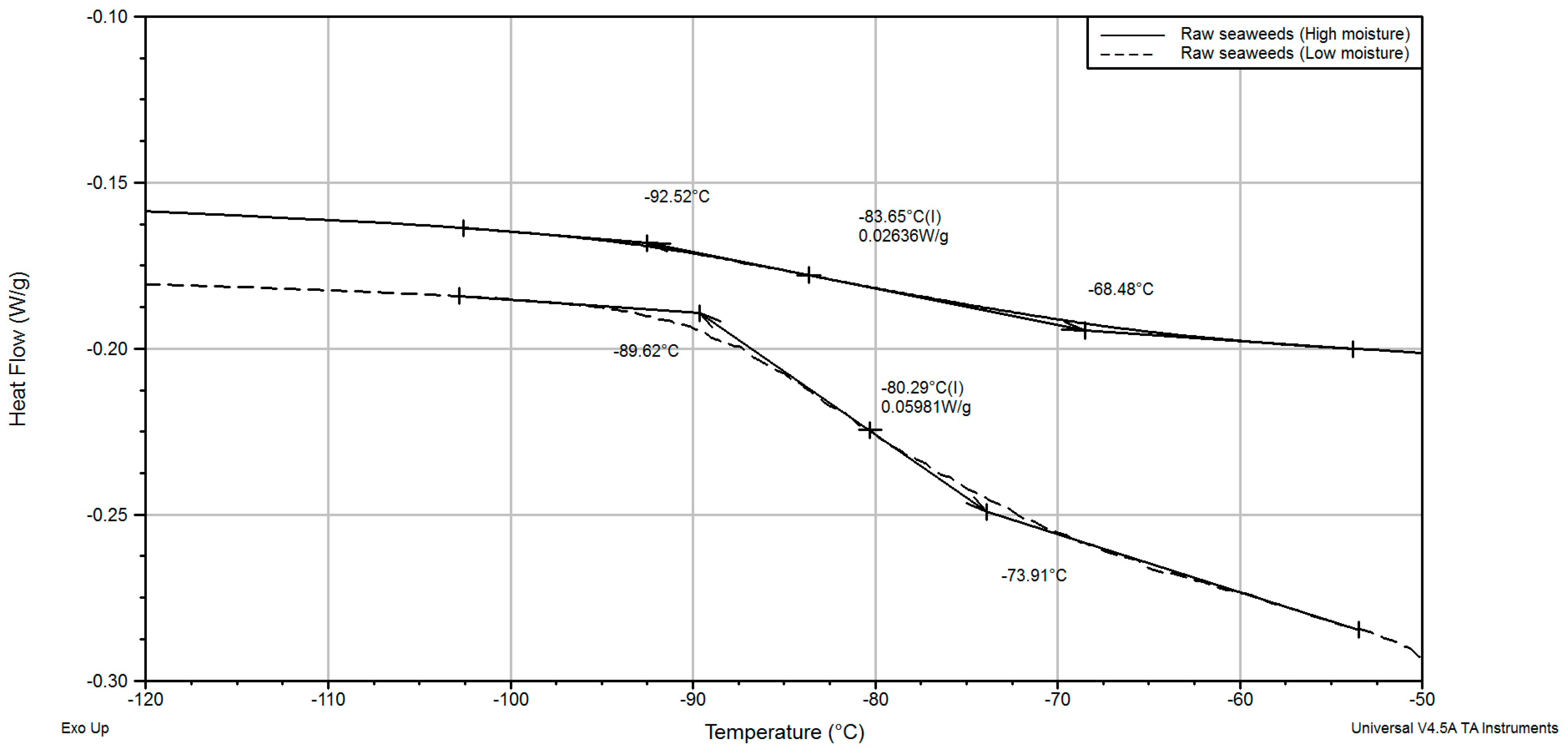
| Sample Type | Moisture Contnet, kg kg−1 d.b. | Glass Transition, Inflection Point, °C | Melting of Mannitol, °C | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Low moisture content | ||||||
| Raw seaweeds, dried at 105.0 °C | 0.003 | 35.44 | n.d. | 120.45 | n.d. | - |
| Raw seaweeds, dried at 105.0 °C | 0.023 | 47.31 | n.d. | 118.56 | n.d. | - |
| Raw seaweeds, dried at 38.0 °C | 0.039 | 20.38 | 52.14 | 96.98 | 139.91 | - |
| Raw seaweeds, dried at 38.0 °C | 0.060 | 5.56 | 55.32 | 88.83 | 135.25 | - |
| Vacuum freeze-dried mucus | 0.061 | −5.85 | n.d. | 96.02 | 148.02 | 152.99 |
| Vacuum freeze-dried mucus | 0.065 | −7.29 | n.d. | 96.99 | 139.75 | 166.13 |
| Vacuum freeze-dried mucus | 0.075 | −7.99 | n.d. | 92.62 | 138.88 | 167.92 |
| Low moisture content, equilibrated in climate chamber | ||||||
| Raw seaweeds, at 10.0 °C | 0.079 | 2.82 | 46.07 | 92.92 | Out of range | |
| Blanched seaweeds, at 10.0 °C | 0.086 | n.d. | 48.65 | n.d. | 135.74 | 165 |
| Raw seaweeds, at 10.0 °C | 0.107 | −0.93 | 49.32 | 83.16 | 138.93 | 156 |
| Raw seaweeds, at 25.0 °C | 0.109 | 1.52 | 53.43 | 85.66 | 136.53 | 167 |
| Raw seaweeds, at 10.0 °C | 0.117 | 0.00 | 38.6 | 80.38 | 136.49 | 151.98 |
| Raw seaweeds, at 10.0 °C | 0.136 | −0.65 | 49.02 | 79.52 | 136.79 | 153.75 |
| Raw seaweeds, at 10.0 °C | 0.15 | −30.41 | 52.43 | Out of range | ||
| Blanched seaweeds, at 10.0 °C | 0.15 | n.d. | 47.59 | n.d. | 124.93 | 144.46 |
| Raw seaweeds, at 25.0 °C | 0.165 | −26.74 | 65.32 | Out of range | ||
| Raw seaweeds, at 25.0 °C | 0.168 | −30.61 | 61.62 | 80.56 | 132.33 | - |
| Blanched seaweeds, dried at 25.0 °C | 0.171 | n.d. | 50.5 | n.d. | 120.84 | 141.96 |
| Blanched seaweeds, dried at 25.0 °C | 0.174 | n.d. | 50.9 | n.d. | 122.48 | 140.9 |
| Raw seaweeds, dried at 38.0 °C | 0.334 | −78.22 | 38.9 | Out of range | ||
| High-moisture-content seaweeds | ||||||
| Raw seaweeds, dried at 38.0 °C | 0.874 | −80.54 | −60.01 | Out of range | ||
| Raw seaweeds, dried at 38.0 °C | 0.917 | −80.32 | −60.64 | |||
| Raw seaweeds, at 25.0 °C | 5.541 | −83.65 | −61.91 | |||
| Raw seaweeds, at 25.0 °C | 7.398 | −84.61 | −61.89 | |||
| Raw seaweeds | 7.418 | −80.98 | −58.08 | |||
| Raw seaweeds | 7.812 | −82.52 | −61.94 | |||
| Raw seaweeds | 7.828 | −82.55 | −57.99 | |||
| Raw seaweeds | 8.144 | −85.33 | −63.27 | |||
| Raw seaweeds | 8.336 | −83.56 | −62.48 | |||
| Raw seaweeds | 8.707 | −84.75 | −61.53 | |||
| Seaweed mucus | 11.902 | −72.39 | −55.41 | |||
| Seaweed mucus | 12.318 | −76.41 | −56.59 | |||
| Equation | Statistical Analysis | Coefficients | |||
|---|---|---|---|---|---|
| F-Ratio | Prob(F) | R2 | R | B | |
| Clausius–Clapeyron | |||||
| Raw seaweeds | 292.57 | 0 | 0.97 | 6.61 × 10−5 | 19.59 × 10−2 |
| Blanched seaweeds | 229.5 | 0.00011 | 0.98 | 1.26 × 10−5 | 1.82 × 10−2 |
| Gordon–Taylor | |||||
| Raw seaweeds | 185.27 | 0 | 0.94 | K | Tgi·s |
| 9.58 | 151.76 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolstorebrov, I.; Senadeera, W.; Eikevik, T.M.; Bantle, M.; Sæther, M.; Petrova, I. Study on Drying of Seaweeds and Importance of Glass Transition and Stabilization. Processes 2024, 12, 373. https://doi.org/10.3390/pr12020373
Tolstorebrov I, Senadeera W, Eikevik TM, Bantle M, Sæther M, Petrova I. Study on Drying of Seaweeds and Importance of Glass Transition and Stabilization. Processes. 2024; 12(2):373. https://doi.org/10.3390/pr12020373
Chicago/Turabian StyleTolstorebrov, Ignat, Wijitha Senadeera, Trygve Magne Eikevik, Michael Bantle, Maren Sæther, and Inna Petrova. 2024. "Study on Drying of Seaweeds and Importance of Glass Transition and Stabilization" Processes 12, no. 2: 373. https://doi.org/10.3390/pr12020373
APA StyleTolstorebrov, I., Senadeera, W., Eikevik, T. M., Bantle, M., Sæther, M., & Petrova, I. (2024). Study on Drying of Seaweeds and Importance of Glass Transition and Stabilization. Processes, 12(2), 373. https://doi.org/10.3390/pr12020373







