Numerical Simulation of Hydrogen–Coal Blending Combustion in a 660 MW Tangential Boiler
Abstract
:1. Introduction
2. Simulation Model and Meshing
3. Numerical Models and Simulation Cases
4. Results and Discussion
4.1. Verification of Simulation Results
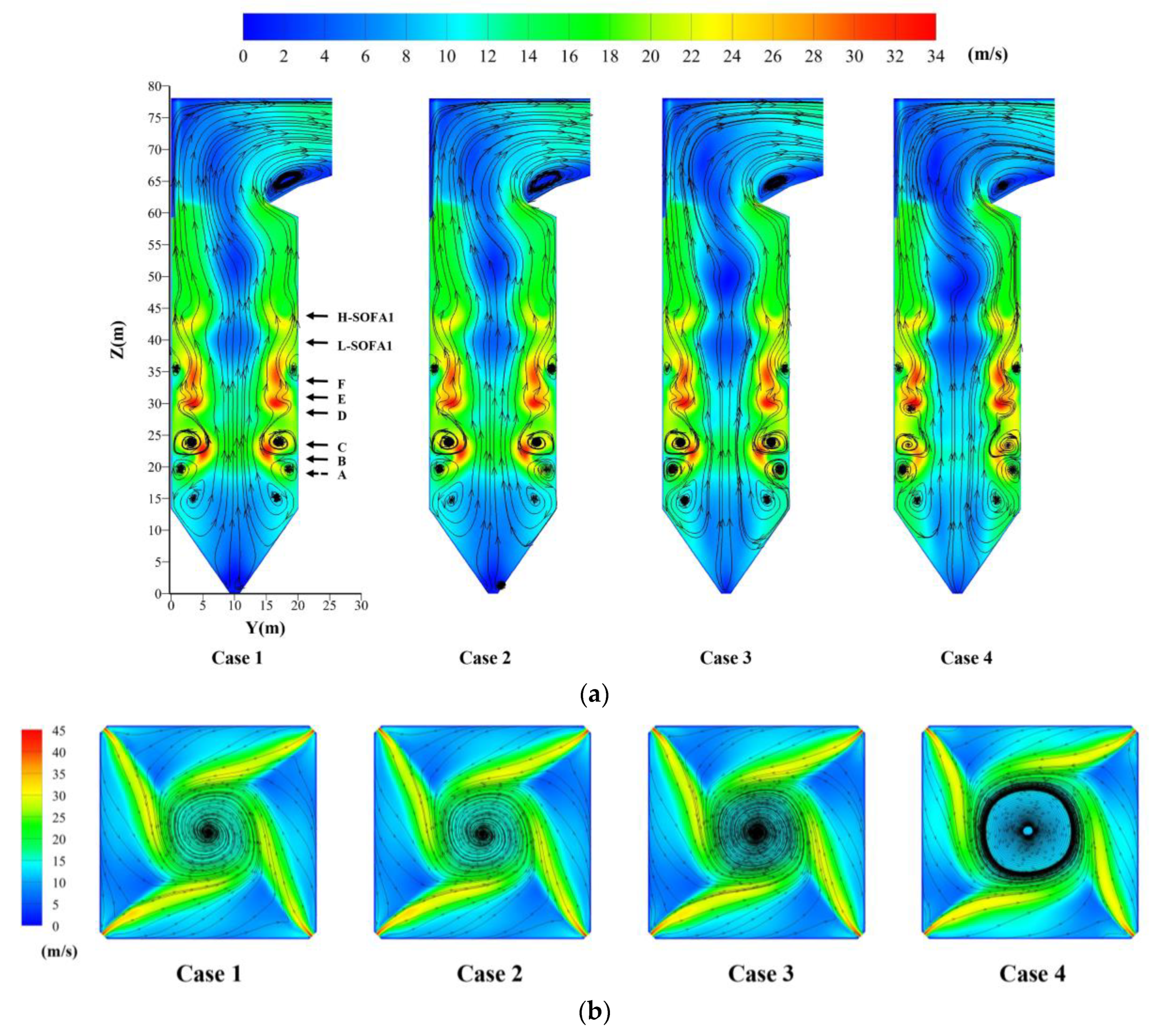
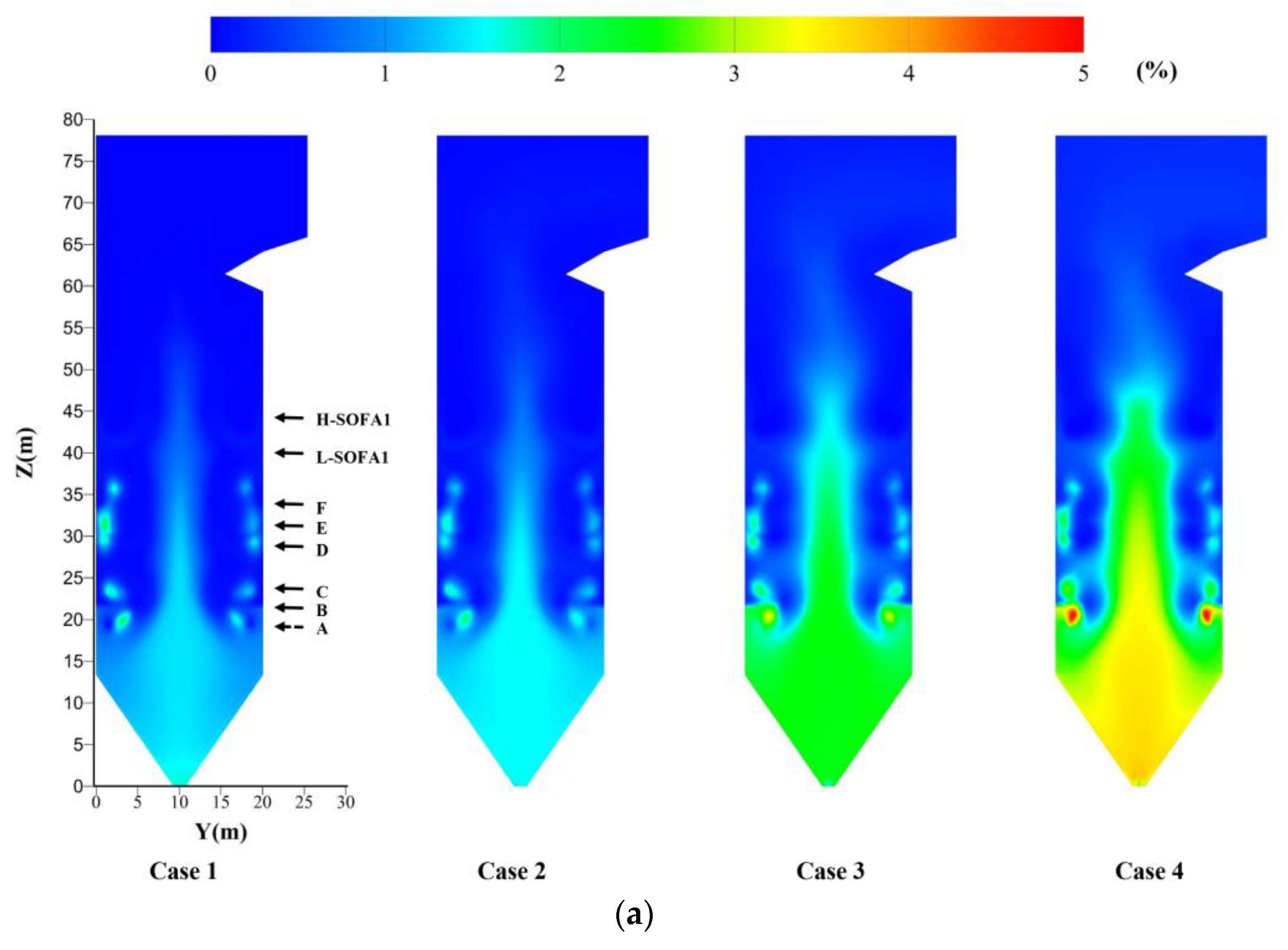
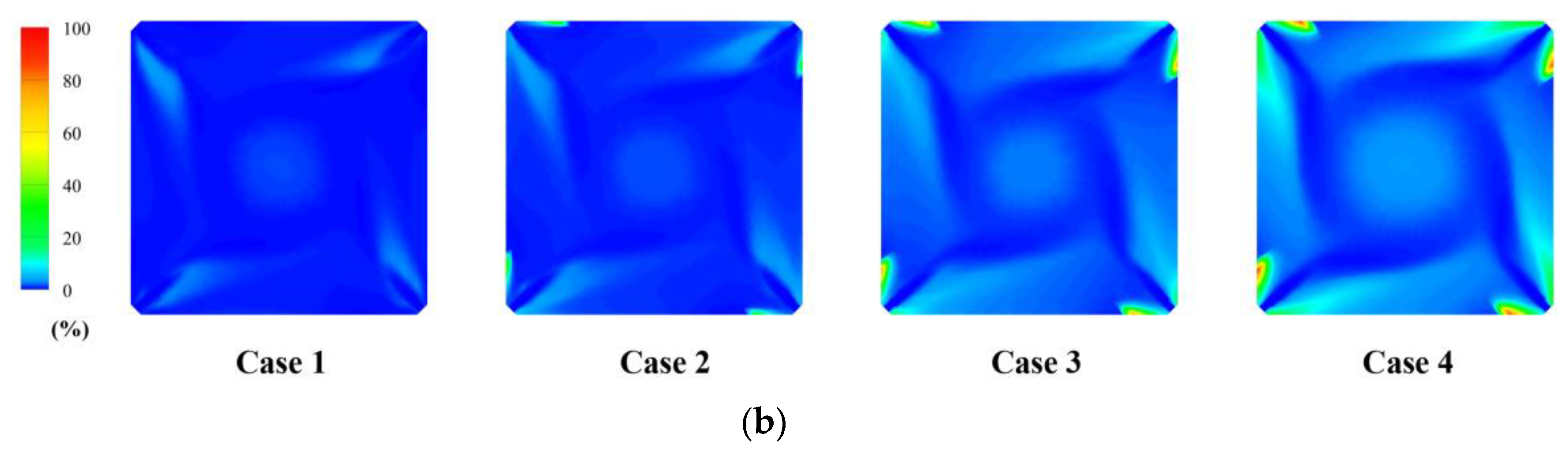
4.2. Effect of Hydrogen Blending on the Flow Field
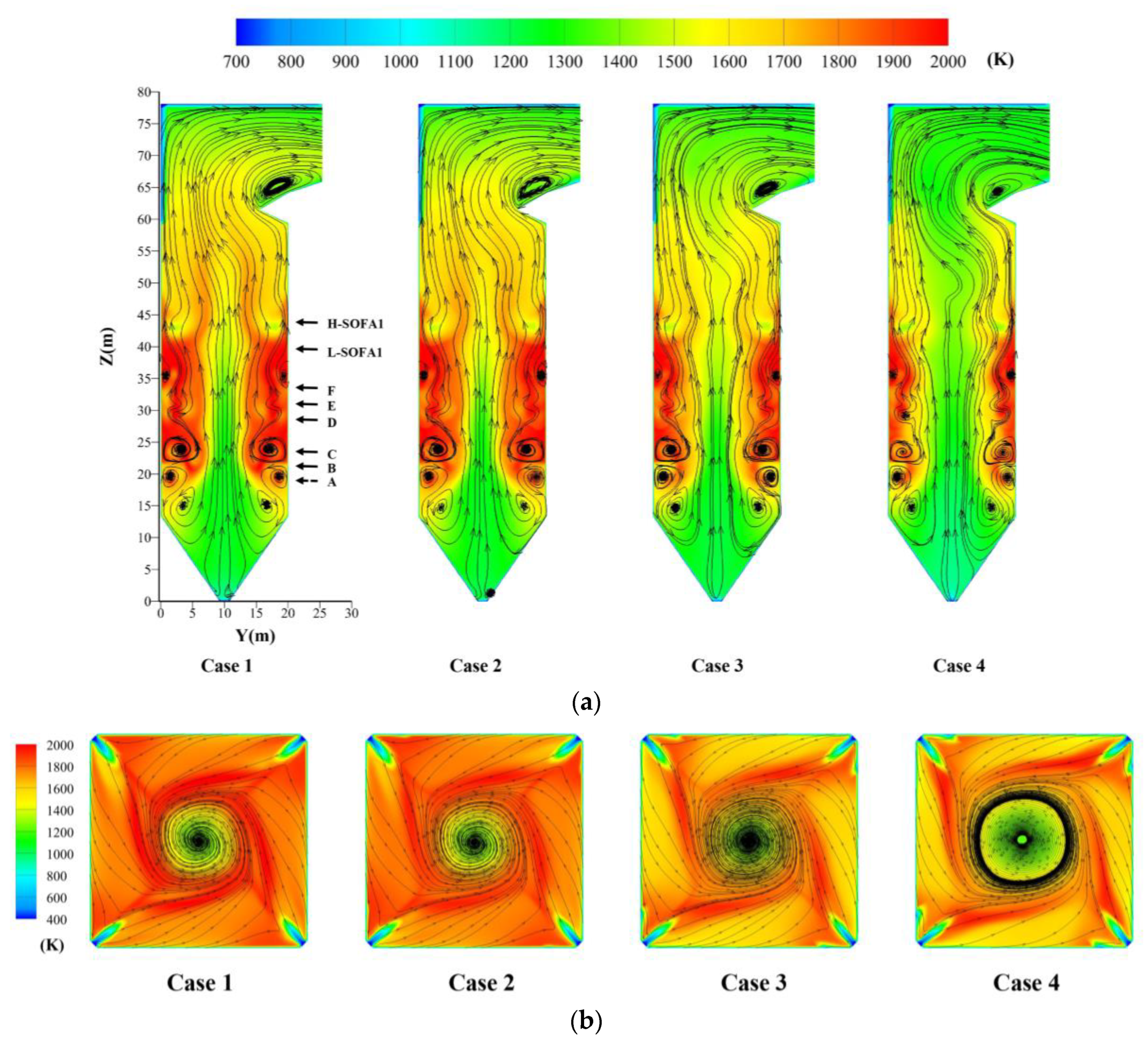
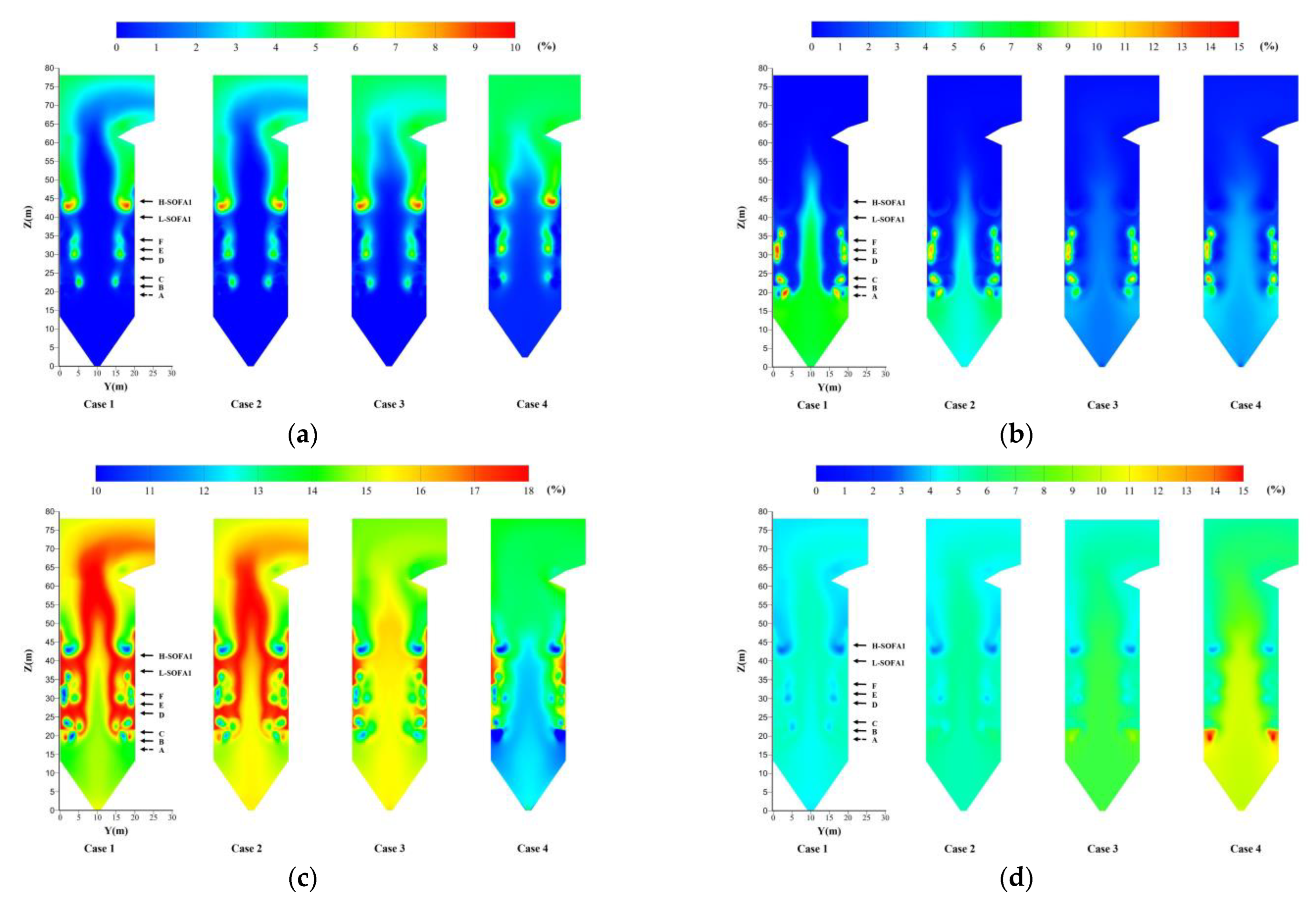
4.3. Effect of Hydrogen Blending on the Temperature
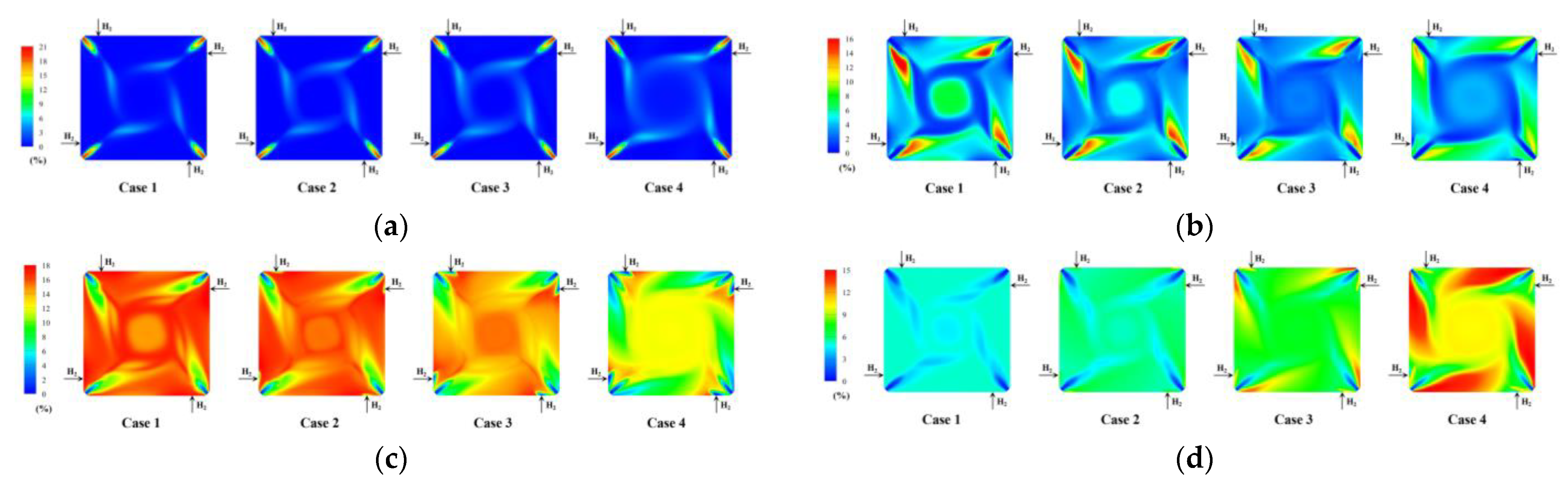
4.4. Effect of Hydrogen Blending on the Species Distribution
5. Conclusions
- (1)
- The large amount of heat released by hydrogen combustion helps the ignition of pulverized coal. The combustion of hydrogen in the B-layer burners increases the tangent diameter. It helps the mixing between air and coal.
- (2)
- More H2O will be produced after hydrogen blending. A large amount of heat is stored in H2O. The temperature has a significant reduction, which affects the heat exchange of the boiler. When the percentage of hydrogen blending reaches 10%, the maximum temperature in the furnace is reduced by about 5.3%. However, the temperature distribution in the lower part of the combustion zone is more uniform.
- (3)
- Hydrogen blending reduces CO2 production at the source. Compared with the CO2 concentration of 15.6% under the non-hydrogen blending condition, the CO2 concentration decreased to 13.6% under the 10% hydrogen blending condition.
- (4)
- As the percentage of hydrogen blending increases, the concentrations of O2 and CO at the furnace outlet increase. The combustion is not complete. The existing air distribution pattern is not suitable for the combustion of pulverized coal with hydrogen.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Zhai, R.; Hu, Y. Performance evaluation of wind-solar-hydrogen system for renewable energy generation and green hydrogen generation and storage: Energy, exergy, economic, and enviroeconomic. Energy 2023, 276, 127386. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, B.; Huang, W.; Li, Y.; Hou, L.; Xiao, J.; Mao, Z.; Li, X. A review of hydrogen generation, storage, and applications in power system. J. Energy Storage 2024, 75, 109307. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, Y.; Liu, W.; Zhao, X.; Xu, J.; Yuan, Z. Research progress of hydrogen energy and metal hydrogen storage materials. Sustain. Energy Technol. Assess. 2023, 55, 102974. [Google Scholar] [CrossRef]
- Cappelletti, A.; Martelli, F. Investigation of a pure hydrogen fueled gas turbine burner. Int. J. Hydrogen Energy 2017, 42, 10513–10523. [Google Scholar] [CrossRef]
- Xu, S.; Xi, L.; Tian, S.; Tu, Y.; Chen, S.; Zhang, S.; Liu, H. Numerical investigation of pressure and H2O dilution effects on NO formation and reduction pathways in pure hydrogen MILD combustion. Appl. Energy 2023, 350, 121736. [Google Scholar] [CrossRef]
- Jithin, E.V.; Varghese, R.J.; Velamati, R.K. Experimental and numerical investigation on the effect of hydrogen addition and N2/CO2 dilution on laminar burning velocity of methane/oxygen mixtures. Int. J. Hydrogen Energy 2020, 45, 16838–16850. [Google Scholar] [CrossRef]
- Yadav, V.K.; Ray, A.; Ravi, M. Experimental and computational investigation of the laminar burning velocity of hydrogen-enriched biogas. Fuel 2019, 235, 810–821. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheung, C.S.; Leung, C.W.; Li, X.; Li, X.; Huang, Z. Effects of fuel composition and initial pressure on laminar flame speed of H2/CO/CH4 bio-syngas. Fuel 2019, 238, 149–158. [Google Scholar] [CrossRef]
- Park, S. Hydrogen addition effect on NO formation in methane/air lean-premixed flames at elevated pressure. Int J Hydrog. Energy 2021, 46, 25712–25725. [Google Scholar] [CrossRef]
- de Persis, S.; Idir, M.; Molet, J.; Pillier, L. Effect of hydrogen addition on NOx formation in high-pressure counter-flow premixed CH4/air flames. Int. J. Hydrogen Energy 2019, 44, 23484–23502. [Google Scholar] [CrossRef]
- Donohoe, N.; Heufer, A.; Metcalfe, W.K.; Curran, H.J.; Davis, M.L.; Mathieu, O.; Plichta, D.; Morones, A.; Petersen, E.L.; Güthe, F. Ignition delay times, laminar flame speeds, and mechanism validation for natural gas/hydrogen blends at elevated pressures. Combust Flame 2014, 161, 1432–1443. [Google Scholar] [CrossRef]
- Cellek, M.S.; Pınarbaşı, A. Investigations on performance and emission characteristics of an industrial low swirl burner while burning natural gas, methane, hydrogen-enriched natural gas and hydrogen as fuels. Int. J. Hydrogen Energy 2018, 43, 1194–1207. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Pugh, D.G.; Marsh, P.; Bulat, G.; Bowen, P. Preliminary study on lean premixed combustion of ammonia-hydrogen for swirling gas turbine combustors. Int. J. Hydrogen Energy 2017, 42, 24495–24503. [Google Scholar] [CrossRef]
- Khateeb, A.A.; Guiberti, T.F.; Zhu, X.; Younes, M.; Jamal, A.; Roberts, W.L. Stability limits and NO emissions of technically-premixed ammonia-hydrogen-nitrogen-air swirl flames. Int. J. Hydrogen Energy 2020, 45, 22008–22018. [Google Scholar] [CrossRef]
- Pugh, D.; Bowen, P.; Valera-Medina, A.; Giles, A.; Runyon, J.; Marsh, R. Influence of steam addition and elevated ambient conditions on NOx reduction in a staged premixed swirling NH3/H2 flame. Proc. Combust. Inst. 2019, 37, 5401–5409. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Gutesa, M.; Xiao, H.; Pugh, D.; Giles, A.; Goktepe, B.; Marsh, R.; Bowen, P. Premixed ammonia/hydrogen swirl combustion under rich fuel conditions for gas turbines operation. Int. J. Hydrogen Energy 2019, 44, 8615–8626. [Google Scholar] [CrossRef]
- Okafor, E.C.; Tsukamoto, M.; Hayakawa, A.; Somarathne, K.D.K.A.; Kudo, T.; Tsujimura, T.; Kobayashi, H. Influence of wall heat loss on the emission characteristics of premixed ammonia-air swirling flames interacting with the combustor wall. Proc. Combust. Inst. 2021, 38, 5139–5146. [Google Scholar] [CrossRef]
- Tan, T.; Fan, W.; Zhang, R. Study on hydrogen staged combustion for gas turbine. Fuel 2023, 349, 128700. [Google Scholar] [CrossRef]
- Banihabib, R.; Lingstädt, T.; Wersland, M.; Kutne, P.; Assadi, M. Development and testing of a 100 kW fuel-flexible micro gas turbine running on 100% hydrogen. Int. J. Hydrogen Energy 2023, 49, 92–111. [Google Scholar] [CrossRef]
- Liu, X.; Bertsch, M.; Subash, A.A.; Yu, S.; Szasz, R.-Z.; Li, Z.; Petersson, P.; Bai, X.-S.; Aldén, M.; Lörstad, D. Investigation of turbulent premixed methane/air and hydrogen-enriched methane/air flames in a laboratory-scale gas turbine model combustor. Int. J. Hydrogen Energy 2021, 46, 13377–13388. [Google Scholar] [CrossRef]
- Reale, F.; Sannino, R. Water and steam injection in micro gas turbine supplied by hydrogen enriched fuels: Numerical investigation and performance analysis. Int. J. Hydrogen Energy 2021, 46, 24366–24381. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, X.; Jin, G.; Wang, X.; Yu, J.; Shan, S. New coefficients of the weighted-sum-of-gray-gases model for gas radiation characteristics of hydrogen/natural gas blends combustion. Int. Commun. Heat Mass Transf. 2023, 149, 107090. [Google Scholar] [CrossRef]
- Meziane, S.; Bentebbiche, A. Numerical study of blended fuel natural gas-hydrogen combustion in rich/quench/lean combustor of a micro gas turbine. Int. J. Hydrogen Energy 2019, 44, 15610–15621. [Google Scholar] [CrossRef]
- Bioche, K.; Bricteux, L.; Bertolino, A.; Parente, A.; Blondeau, J. Large Eddy Simulation of rich ammonia/hydrogen/air combustion in a gas turbine burner. Int. J. Hydrogen Energy 2021, 46, 39548–39562. [Google Scholar] [CrossRef]
- Bioche, K.; Blondeau, J.; Bricteux, L. Large eddy simulation investigation of pressure and wall heat loss effects on rich ammonia-hydrogen-air combustion in a gas turbine burner. Int. J. Hydrogen Energy 2022, 47, 36342–36353. [Google Scholar] [CrossRef]
- Lu, H.; Huang, S.; Li, H.; Cheng, Z.; Chang, X.; Dong, L.; Kong, D.; Jing, X. Numerical simulation of combustion characteristics in a 660 MW tangentially fired pulverized coal boiler subjected to peak-load regulation. Case Stud. Therm. Eng. 2023, 49, 103168. [Google Scholar] [CrossRef]
- Shih, T.-H.; Liou, W.W.; Shabbir, A.; Yang, Z.; Zhu, J. A new k-ϵ eddy viscosity model for high reynolds number turbulent flows. Comput. Fluids 1995, 24, 227–238. [Google Scholar] [CrossRef]
- Chang, J.; Wang, X.; Zhou, Z.; Chen, H.; Niu, Y. CFD modeling of hydrodynamics, combustion and NOx emission in a tangentially fired pulverized-coal boiler at low load operating conditions. Adv. Powder Technol. 2021, 32, 290–303. [Google Scholar] [CrossRef]
- Kobayashi, H.; Howard, J.B.; Sarofim, A.F. Coal devolatilization at high temperatures. Symp. (Int.) Combust. 1977, 16, 411–425. [Google Scholar] [CrossRef]
- Ansys Inc. Ansys Fluent Theory Guide; Ansys Inc.: Canonsburg, PA, USA, 2023. [Google Scholar]
- Baum, M.; Street, P. Predicting the combustion behaviour of coal particles. Combust. Sci. Technol. 1971, 3, 231–243. [Google Scholar] [CrossRef]
- Sazhin, S.S.; Sazhina, E.M.; Faltsi-Saravelou, O.; Wild, P. The P-1 model for thermal radiation transfer: Advantages and limitations. Fuel 1996, 75, 289–294. [Google Scholar] [CrossRef]
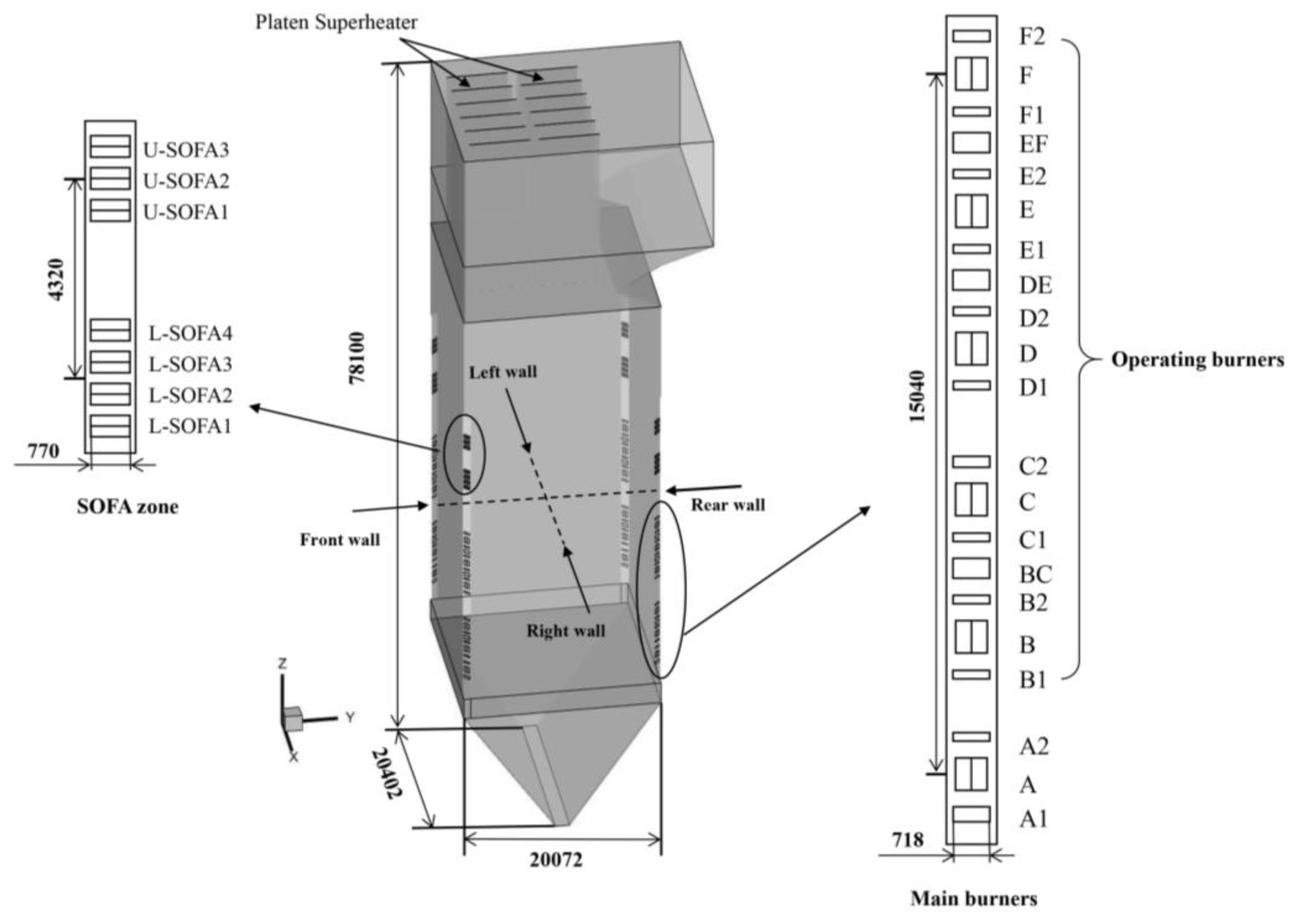
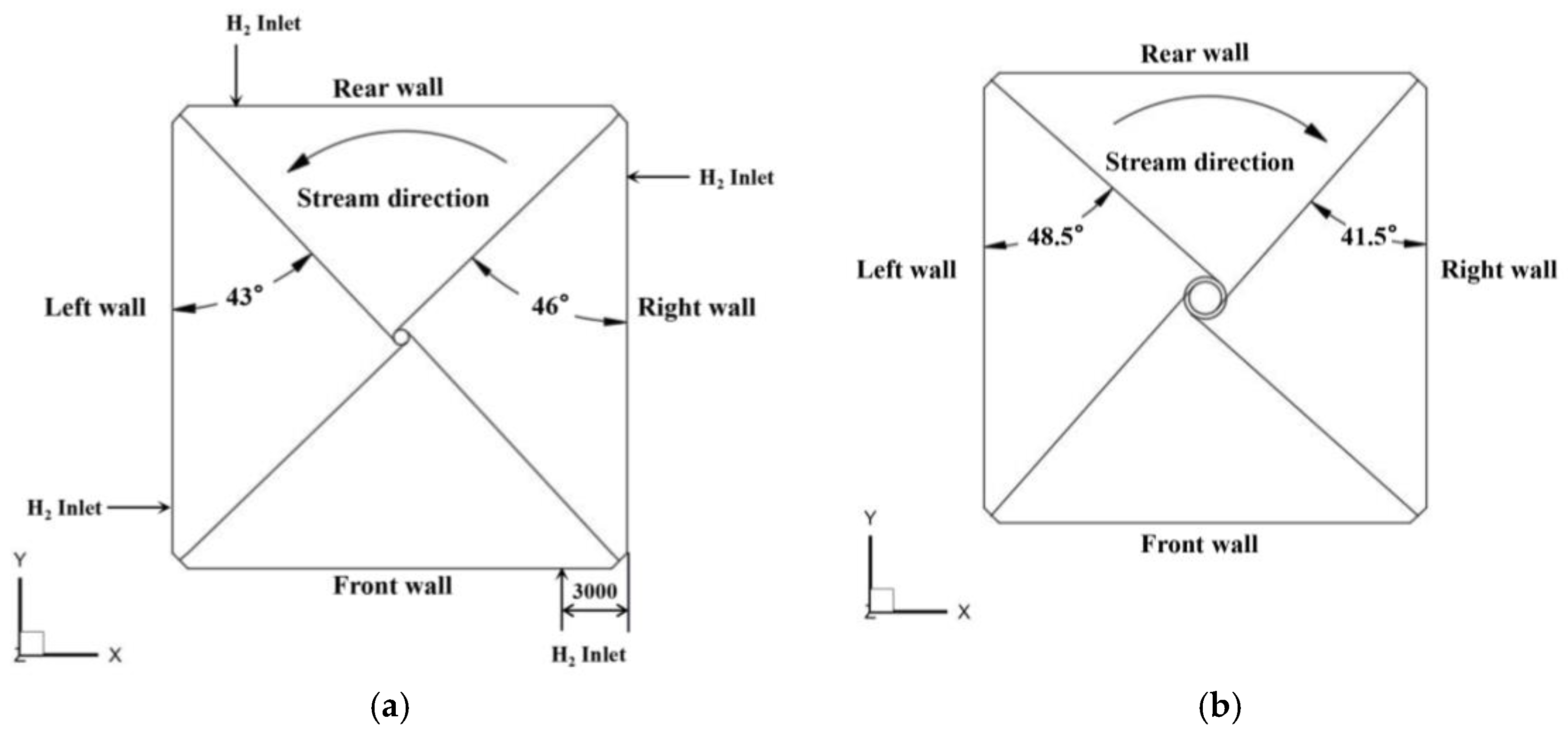






| Proximate (As received, wt%) | Ultimate (As received, wt%) | ||
|---|---|---|---|
| Moisture | 28.50–29.60 | C | 53.04–54.29 |
| Ash | 4.45–5.36 | H | 2.42–2.58 |
| Volatile | 19.69–20.30 | O | 8.00–9.17 |
| Fixed carbon | 46.45–45.65 | N | 0.55 |
| Net heating value (MJ/kg) | 18.40–19.42 | S | 0.72–0.77 |
| Case Name | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Total airflow (t/h) | 2406.05 | 2401.60 | 2383.81 | 2361.53 |
| Primary airflow (t/h) | 618.35 | 618.35 | 618.35 | 618.35 |
| Secondary airflow (t/h) | 1243.93 | 1239.48 | 1221.69 | 1199.41 |
| Over-fire air flow (t/h) | 543.77 | 543.77 | 543.77 | 543.77 |
| Total coal flow (t/h) | 305.46 | 302.41 | 290.19 | 274.91 |
| Hydrogen flow (t/h) | 0.00 | 0.40 | 1.97 | 3.95 |
| Primary air temperature (K) | 338.15 | 338.15 | 338.15 | 338.15 |
| Secondary air temperature (K) | 638.15 | 638.15 | 638.15 | 638.15 |
| Hydrogen temperature (K) | / | 300 | 300 | 300 |
| Hydrogen mixing ratio (%) | 0.00 | 1.00 | 5.00 | 10.00 |
| Excess air ratio | 1.20 | 1.20 | 1.20 | 1.20 |
| Layer of burner | B C D E F | B C D E F | B C D E F | B C D E F |
| Parameter | Simulation | Design | Measurement | Relative Error |
|---|---|---|---|---|
| O2 (vol%) | 3.46 | 3.50 | / | −1.14% |
| CO2 (vol%) | 15.65 | 16.46 | / | −4.92% |
| Gas temperature (K) | 1591.61 | 1518.22 | / | 4.83% |
| Flue temperature (K) | 1142.43 | / | 1183.55 | −3.48% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Huang, S.; Qian, B.; Wang, K.; Gao, N.; Lin, X.; Shi, Z.; Lu, H. Numerical Simulation of Hydrogen–Coal Blending Combustion in a 660 MW Tangential Boiler. Processes 2024, 12, 415. https://doi.org/10.3390/pr12020415
Dong L, Huang S, Qian B, Wang K, Gao N, Lin X, Shi Z, Lu H. Numerical Simulation of Hydrogen–Coal Blending Combustion in a 660 MW Tangential Boiler. Processes. 2024; 12(2):415. https://doi.org/10.3390/pr12020415
Chicago/Turabian StyleDong, Lijiang, Shangwen Huang, Baiyun Qian, Kaike Wang, Ning Gao, Xiang Lin, Zeqi Shi, and Hao Lu. 2024. "Numerical Simulation of Hydrogen–Coal Blending Combustion in a 660 MW Tangential Boiler" Processes 12, no. 2: 415. https://doi.org/10.3390/pr12020415




