Hydrocarbon Gas Generation from Direct and Indirect Hydrogenation of Organic Matter: Implications from Hydrothermal Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Hydrothermal Experiments
2.3. Gas Determination
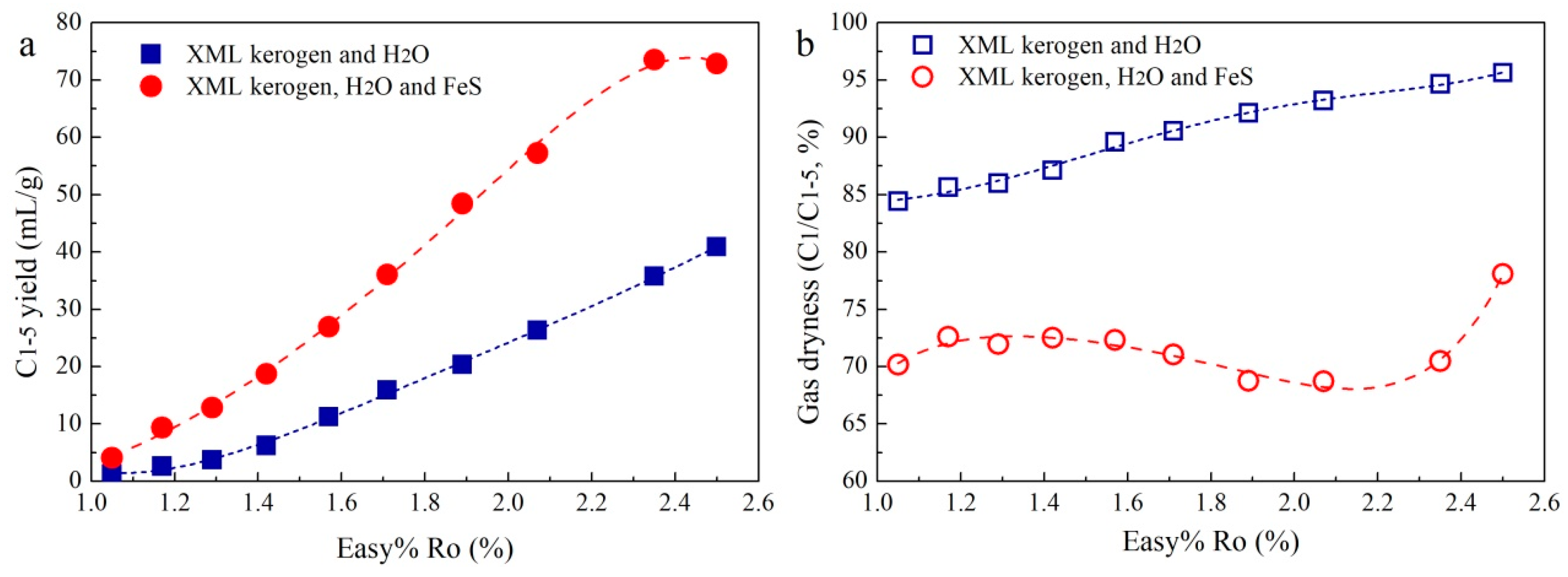
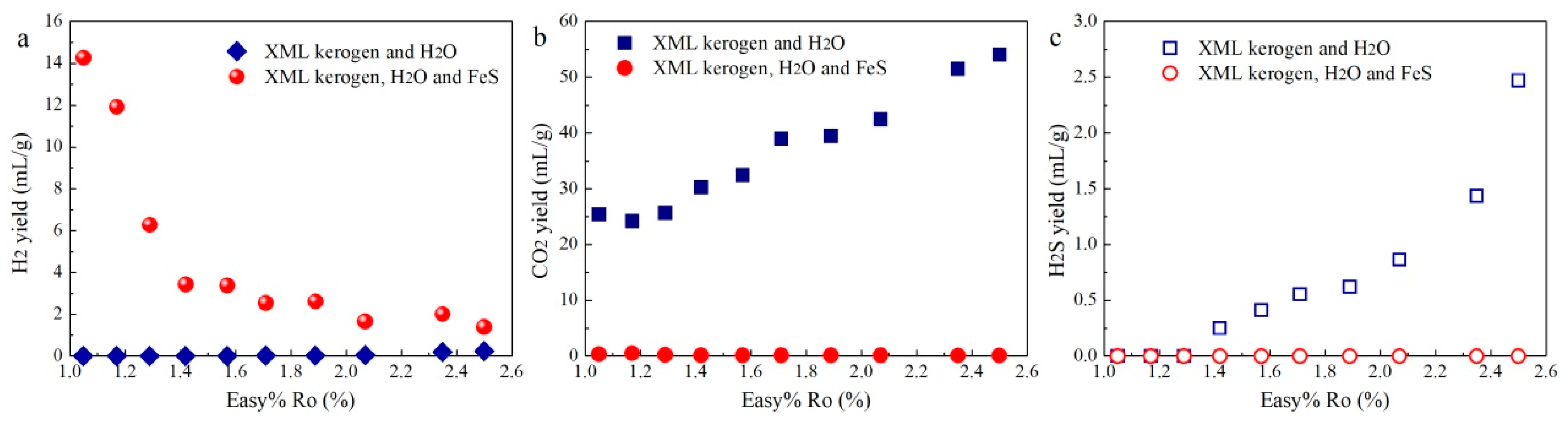
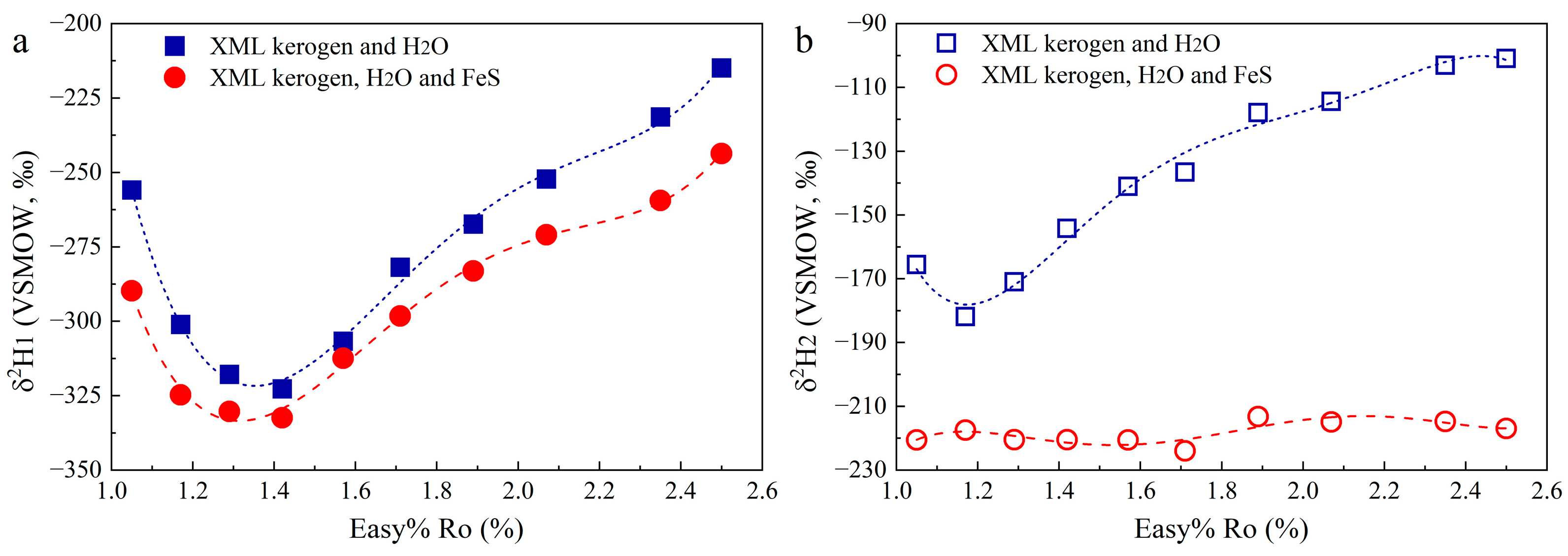
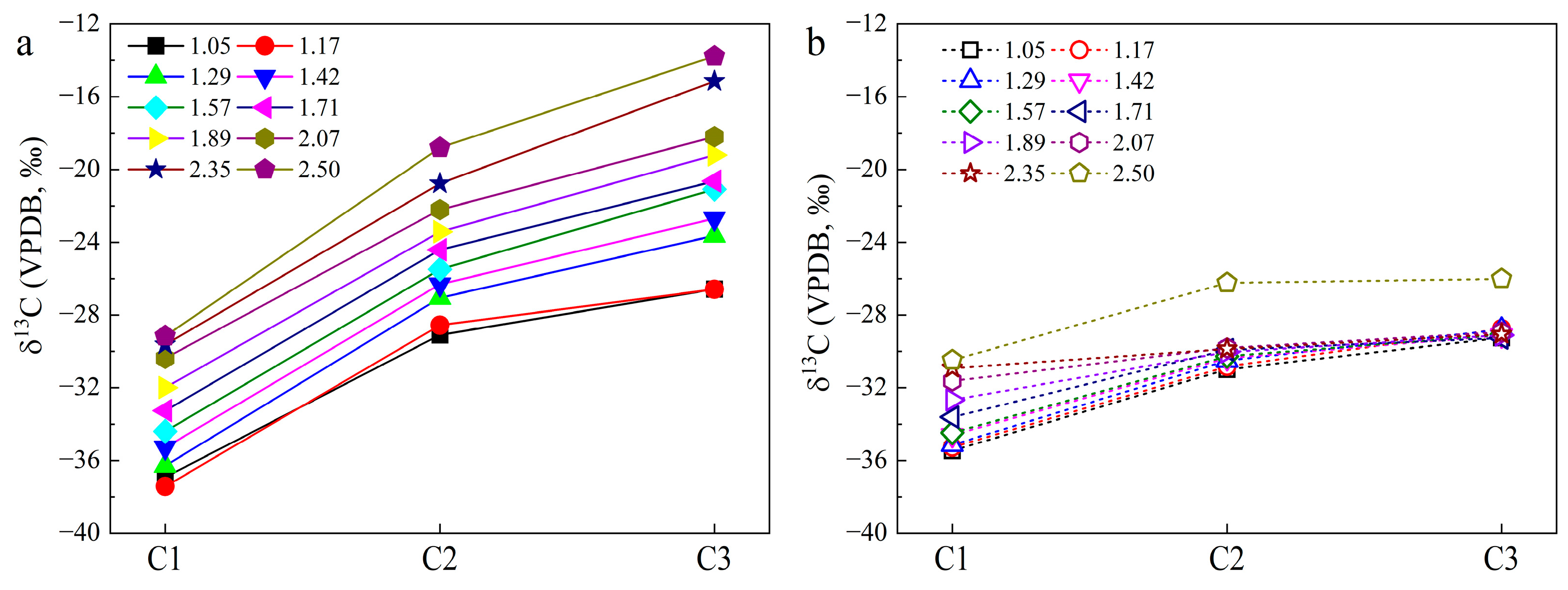
3. Results
3.1. Gas Yields and Compositions
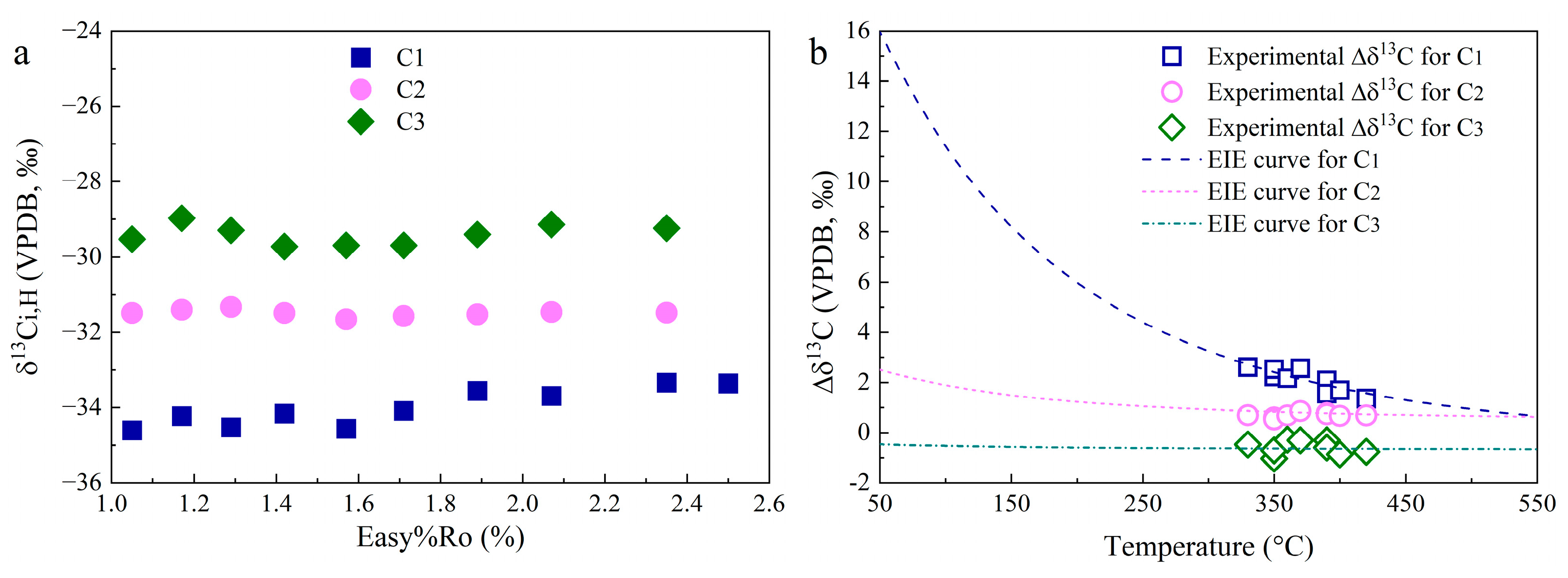
3.2. Carbon Isotopic Compositions
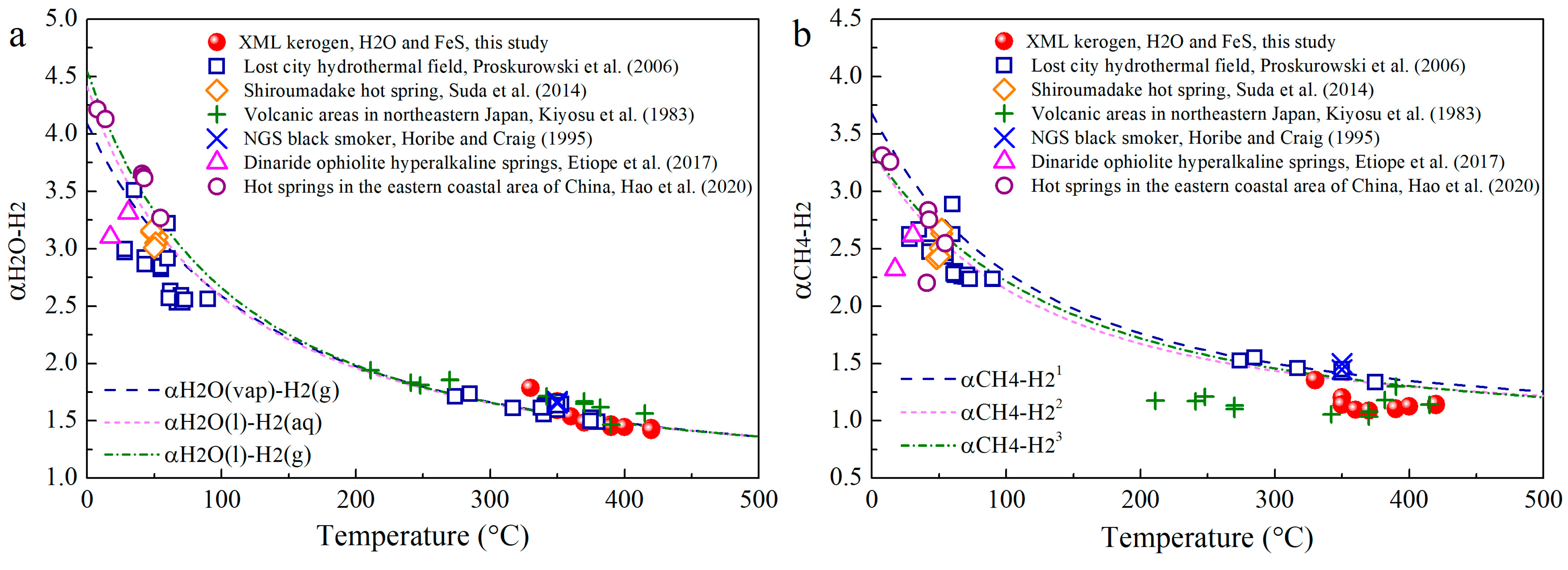
3.3. Hydrogen Isotopic Compositions
4. Discussion
4.1. Mechanisms for Gas Generation in Two Hydrogenation Reactions
4.2. Carbon Isotope Fractionation for HC Gas Generation
4.3. H Isotope Fractionation of H2O-H2-CH4 in Hydrothermal Conditions
4.4. Geological Implications for GAS Generation in Deep Formations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tissot, B.P.; Welte, D.H. Petroleum Formation and Occurrence, 2nd ed.; Spring: Berlin/Heidelberg, Germany; New York, NY, USA, 1984. [Google Scholar]
- Seewald, J.S. Organic-inorganic interactions in petroleum-producing sedimentary basins. Nature 2003, 426, 327–333. [Google Scholar] [CrossRef]
- Pan, C.C.; Geng, A.S.; Zhong, N.; Liu, J.Z.; Yu, L. Kerogen pyrolysis in the presence and absence of water and minerals: Amounts and compositions of bitumen and liquid hydrocarbons. Fuel 2009, 88, 909–919. [Google Scholar] [CrossRef]
- Hoering, T.C. Thermal reactions of kerogen with added water, heavy water and pure organic substances. Org. Geochem. 1984, 21, 267–278. [Google Scholar] [CrossRef]
- Lewan, M.D.; Roy, S. Role of water in hydrocarbon generation from Type-I kerogen in Mahogany oil shale of the Green River Formation. Org. Geochem. 2011, 42, 31–41. [Google Scholar] [CrossRef]
- Schimmelmann, A.; Boudou, J.P.; Lewan, M.D.; Wintsch, R.P. Experimental controls on D/H and 13C/12C ratios of kerogen, bitumen and oil during hydrous pyrolysis. Org. Geochem. 2001, 32, 1009–1018. [Google Scholar] [CrossRef]
- Lewan, M.D. Experiments on the role of water in petroleum formation. Geochim. Cosmochim. Acta 1997, 61, 3691–3723. [Google Scholar] [CrossRef]
- He, K.; Zhang, S.C.; Mi, J.K.; Zhang, W.L. Pyrolysis involving n-hexadecane, water and minerals: Insight into the mechanisms and isotope fractionation for water-hydrocarbon reaction. J. Anal. Appl. Pyrol. 2018, 130, 198–208. [Google Scholar] [CrossRef]
- He, K.; Zhang, S.C.; Wang, X.M.; Mi, J.K.; Zhang, W.J.; Guo, J.H.; Zhang, W.L. Pyrolysis of 1-methylnaphthalene involving water: Effects of Fe-bearing minerals on the generation, C and H isotope fractionation of methane from H2O-hydrocarbon reaction. Org. Geochem. 2021, 152, 104151. [Google Scholar] [CrossRef]
- Leif, R.N.; Simoneit, B.R.T. The role of alkenes produced during hydrous pyrolysis of a shale. Org. Geochem. 2000, 31, 1189–1208. [Google Scholar] [CrossRef]
- Gao, L.; Schimmelmann, A.; Tang, Y.C.; Mastalerz, M. Isotope rollover in shale gas observed in laboratory pyrolysis experiments: Insight to the role of water in thermogenesis of mature gas. Org. Geochem. 2014, 68, 95–106. [Google Scholar] [CrossRef]
- Reeves, E.P.; Seewald, J.S.; Sylva, S.P. Hydrogen isotope exchange between n-alkanes and water under hydrothermal conditions. Geochim. Cosmochim. Acta 2012, 77, 582–599. [Google Scholar] [CrossRef]
- Wang, D.T.; Seewald, J.S.; Reeves, E.P.; Ono, S.; Sylva, S.P. Incorporation of water-derived hydrogen into methane during artificial maturation of source rock under hydrothermal conditions. Org. Geochem. 2022, 171, 104468. [Google Scholar] [CrossRef]
- Wang, Y.; Sessions, A.L.; Nielsen, R.J.; Goddard III, W.A. Equilibrium 2H/1H fractionations in organic molecules. II: Linear alkanes, alkenes, ketones, carboxylic acids, esters, alcohols and ethers. Geochim. Cosmochim. Acta 2009, 73, 7076–7086. [Google Scholar] [CrossRef]
- Schimmelmann, A.; Lewan, M.D.; Wintsch, R.P. D/H isotope ratios of kerogen, bitumen, oil, and water in hydrous pyrolysis of source rocks containing kerogen types I, II, IIS, and III. Geochim. Cosmochim. Acta 1999, 63, 3751–3766. [Google Scholar] [CrossRef]
- Horibe, Y.; Craig, H. D/H fractionation in the system methane-hydrogen-water. Geochim. Cosmochim. Acta 1995, 59, 5209–5217. [Google Scholar] [CrossRef]
- Sessions, A.L.; Sylva, S.P.; Summons, R.E.; Hayes, J.M. Isotopic exchange of carbon-bound hydrogen over geologic timescales. Geochim. Cosmochim. Acta 2004, 68, 1545–1559. [Google Scholar] [CrossRef]
- Zhang, S.C.; He, K.; Hu, G.Y.; Mi, J.K.; Ma, Q.S.; Liu, K.Y.; Tang, Y.C. Unique chemical and isotopic characteristics and origins of natural gases in the Paleozoic marine formations in the Sichuan Basin, SW China: Isotope fractionation of deep and high mature carbonate reservoir gases. Mar. Pet. Geol. 2018, 89, 68–82. [Google Scholar] [CrossRef]
- Seewald, J.S. Aqueous geochemistry of low molecular weight hydrocarbons at elevated temperatures and pressures: Constraints from mineral buffered laboratory experiments. Geochim. Cosmochim. Acta 2001, 65, 1641–1664. [Google Scholar] [CrossRef]
- McCollom, T.M.; Seewald, J.S. A reassessment of the potential for reduction of dissolved CO2 to hydrocarbons during serpentinization of olivine. Geochim. Cosmochim. Acta 2001, 65, 3769–3778. [Google Scholar] [CrossRef]
- Milesi, V.; Prinzhofer, A.; Guyot, F.; Benedetti, M.; Rodrigues, R. Contribution of siderite-water interaction for the unconventional generation of hydrocarbon gases in the Solimões Basin, North-West Brazil. Mar. Petrol. Geol. 2016, 71, 168–182. [Google Scholar] [CrossRef]
- Kishima, N. A thermodynamic study on the pyrite-pyrrhotite-magnetite-water system at 300–500 °C with relevance to the fugacity/concentration quotient of aqueous H2S. Geochim. Cosmochim. Acta 1989, 53, 2143–2155. [Google Scholar] [CrossRef]
- Etiope, G.; Samardžić, N.; Grassa, F.; Hrvatović, H.; Miošić, N.; Skopljak, F. Methane and hydrogen in hyperalkaline groundwaters of the serpentinized Dinaride ophiolite belt, Bosnia and Herzegovina. Appl. Geochem. 2017, 84, 286–296. [Google Scholar] [CrossRef]
- Klein, F.; Grozeva, N.G.; Seewald, J.S. Abiotic methane synthesis and serpentinization in olivine-hosted fluid inclusions. Proc. Natl. Acad. Sci. USA 2019, 116, 17666–17672. [Google Scholar] [CrossRef] [PubMed]
- Milkov, A.V. Molecular hydrogen in surface and subsurface natural gases: Abundance, origins and ideas for deliberate exploration. Earth Sci. Rev. 2022, 230, 104063. [Google Scholar] [CrossRef]
- Jin, Z.J.; Zhang, L.P.; Yang, L.; Hu, W.X. Primary study of geochemistry features of deep fluids and their effectiveness on oil/gas reservoir formation in sedimentary basins. Earth Sci. J. China Univ. Geosci. 2002, 27, 659–665. [Google Scholar]
- Vacquand, C.; Deville, E.; Beaumont, V.; Guyot, F.; Sissmann, O.; Pillot, D.; Arcilla, C.; Prinzhofer, A. Reduced gas seepages in ophiolitic complexes: Evidences for multiple origins of the H2–CH4–N2 gas mixtures. Geochim. Cosmochim. Acta 2018, 223, 437–461. [Google Scholar] [CrossRef]
- Burruss, R.C.; Laughrey, C.D. Carbon and hydrogen isotopic reversals in deep basin gas: Evidence for limits to the stability of hydrocarbons. Org. Geochem. 2010, 41, 1285–1296. [Google Scholar] [CrossRef]
- Zumberge, J.; Ferworn, K.; Brown, S. Isotopic reversal (‘rollover’) in shale gases produced from the Mississippian Barnett and Fayetteville formations. Mar. Pet. Geol. 2012, 31, 43–52. [Google Scholar] [CrossRef]
- Durand, B. Kerogen: Insoluble Organic Matter from Sedimentary Rocks, 1st ed.; Bayeusaine: Paris, France, 1980; pp. 35–52. [Google Scholar]
- Zhang, S.C.; Mi, J.K.; He, K. Synthesis of hydrocarbon gases from four different carbon sources and hydrogen gas using a gold-tube system by Fischer-Tropsch method. Chem. Geol. 2013, 349–350, 27–35. [Google Scholar] [CrossRef]
- Sweeney, J.J.; Burnham, A.K. Evaluation of a simple model of vitrinite reflectance based on chemical kinetics. AAPG Bull. 1990, 74, 1559–1570. [Google Scholar]
- Kissin, Y.V. Catagenesis and composition of petroleum: Origin of n-alkanes and isoalkanes in petroleum crudes. Geochim. Cosmochim. Acta 1987, 512, 445–2457. [Google Scholar] [CrossRef]
- Wei, N.; Xu, D.; Hao, B.; Guo, S.; Guo, Y.; Wang, S. Chemical reactions of organic compounds in supercritical water gasification and oxidation. Water Res. 2021, 190, 116634. [Google Scholar] [CrossRef] [PubMed]
- Cramer, B.; Krooss, B.M.; Littke, R. Modelling isotope fractionation during primary cracking of natural gas: A reaction kinetic approach. Chem. Geol. 1998, 149, 235–250. [Google Scholar] [CrossRef]
- Tang, Y.C.; Perry, J.K.; Jenden, P.D.; Schoell, M. Mathematical modeling of stable carbon isotope ratios in natural gases. Geochim. Cosmochim. Acta 2000, 64, 2673–2687. [Google Scholar] [CrossRef]
- He, K.; Zhang, S.C.; Mi, J.K.; Zhang, W.L. The evolution of chemical groups and isotopic fractionation at different maturation stages during lignite pyrolysis. Fuel 2008, 211, 492–506. [Google Scholar] [CrossRef]
- Berner, U.; Faber, E.; Scheeder, G.; Panten, D. Primary cracking of algal and landplant kerogens: Kinetic models of isotope variations in methane, ethane and propane. Chem. Geol. 1995, 126, 233–245. [Google Scholar] [CrossRef]
- Galimov, E.M. Isotope organic geochemistry. Org. Geochem. 2006, 37, 1200–1262. [Google Scholar] [CrossRef]
- Suda, K.; Ueno, Y.; Yoshizaki, M.; Nakamura, H.; Kurokawa, K.; Nishiyama, E.; Yoshino, K.; Hongoh, Y.; Kawachi, K.; Omori, S.; et al. Origin of methane in serpentinite-hosted hydrothermal systems: The CH4–H2–H2O hydrogen isotope systematics of the Hakuba Happo hot spring. Earth Planet. Sc. Lett. 2014, 386, 112–125. [Google Scholar] [CrossRef]
- Turner, A.C.; Pester, N.J.; Bill, M.; Conrad, M.E.; Knauss, K.G.; Stolper, D.A. Experimental determination of hydrogen isotope exchange rates between methane and water under hydrothermal conditions. Geochim. Cosmochim. Acta 2022, 329, 231–255. [Google Scholar] [CrossRef]
- Pester, N.J.; Conrad, M.E.; Knauss, K.G.; De Paolo, D.J. Kinetics of D/H isotope fractionation between molecular hydrogen and water. Geochim. Cosmochim. Acta 2018, 242, 191–212. [Google Scholar] [CrossRef]
- Kiyosu, Y. Hydrogen isotopic compositions of hydrogen and methane from some volcanic areas in northeastern Japan. Earth Planet. Sci. Lett. 1983, 62, 41–52. [Google Scholar] [CrossRef]
- Proskurowski, G.; Lilley, M.D.; Kelly, D.S.; Olson, E.J. Low temperature volatile production at the Lost City Hydrothermal Field, evidence from a hydrogen stable isotope geothermometer. Chem. Geol. 2006, 229, 331–343. [Google Scholar] [CrossRef]
- Hao, Y.; Pang, Z.; Tian, J.; Wang, Y.; Li, Z.; Li, L.; Xing, L. Origin and evolution of hydrogen-rich gas discharges from a hot spring in the eastern coastal area of China. Chem. Geol. 2020, 538, 119477. [Google Scholar] [CrossRef]
- Tilley, B.; Muehlenbachs, K. Isotope reversals and universal stages and trends of gas maturation in sealed, self-contained petroleum systems. Chem. Geol. 2013, 339, 194–204. [Google Scholar] [CrossRef]
- Jenden, P.D.; Drazan, D.J.; Kaplan, I.R. Mixing of thermogenic natural gases in northern Appalachian Basin. AAPG Bull. 1993, 77, 980–998. [Google Scholar]
- Mangenot, X.; Xie, H.; Crémière, A.; Giunta, T.; Lilley, M.; Sissmann, O.; Orphan, V.; Schimmelmann, A.; Gaucher, E.C.; Girard, J.P.; et al. 2H–2H clumping in molecular hydrogen method and preliminary results. Chem. Geol. 2023, 621, 121278. [Google Scholar] [CrossRef]


| Sample | Ro | Elemental Compositions (wt%) | H/C | O/C | δ13C | δ2H | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | C | H | O | N | S | (‰, VPDB) | (‰, VSMOW) | |||
| XML | 0.60 | 49.54 | 4.05 | 6.56 | 1.69 | 12.00 | 0.98 | 0.10 | −28.5 | −111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, K.; Wang, X.; Yang, C.; Zhang, S. Hydrocarbon Gas Generation from Direct and Indirect Hydrogenation of Organic Matter: Implications from Hydrothermal Experiments. Processes 2024, 12, 458. https://doi.org/10.3390/pr12030458
He K, Wang X, Yang C, Zhang S. Hydrocarbon Gas Generation from Direct and Indirect Hydrogenation of Organic Matter: Implications from Hydrothermal Experiments. Processes. 2024; 12(3):458. https://doi.org/10.3390/pr12030458
Chicago/Turabian StyleHe, Kun, Xiaomei Wang, Chunlong Yang, and Shuichang Zhang. 2024. "Hydrocarbon Gas Generation from Direct and Indirect Hydrogenation of Organic Matter: Implications from Hydrothermal Experiments" Processes 12, no. 3: 458. https://doi.org/10.3390/pr12030458
APA StyleHe, K., Wang, X., Yang, C., & Zhang, S. (2024). Hydrocarbon Gas Generation from Direct and Indirect Hydrogenation of Organic Matter: Implications from Hydrothermal Experiments. Processes, 12(3), 458. https://doi.org/10.3390/pr12030458







