Study on Oxidation Activity of Hydrogenated Biodiesel–Ethanol–Diesel Blends
Abstract
:1. Introduction
2. Selection of Hydrogenated Biodiesel Substitution Mechanism and Validation of Ternary Fuel Mechanism Simplification
2.1. Selection of Hydrogenated Biodiesel Substitution Mechanism
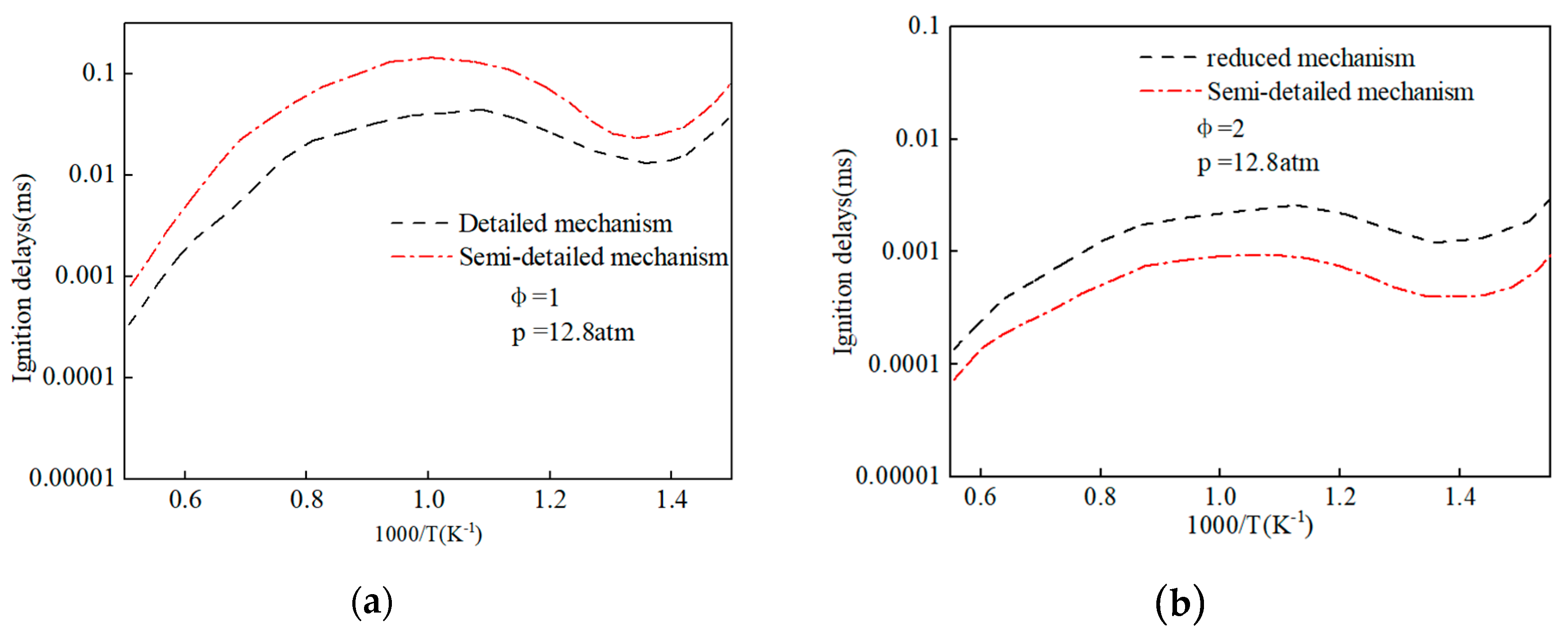
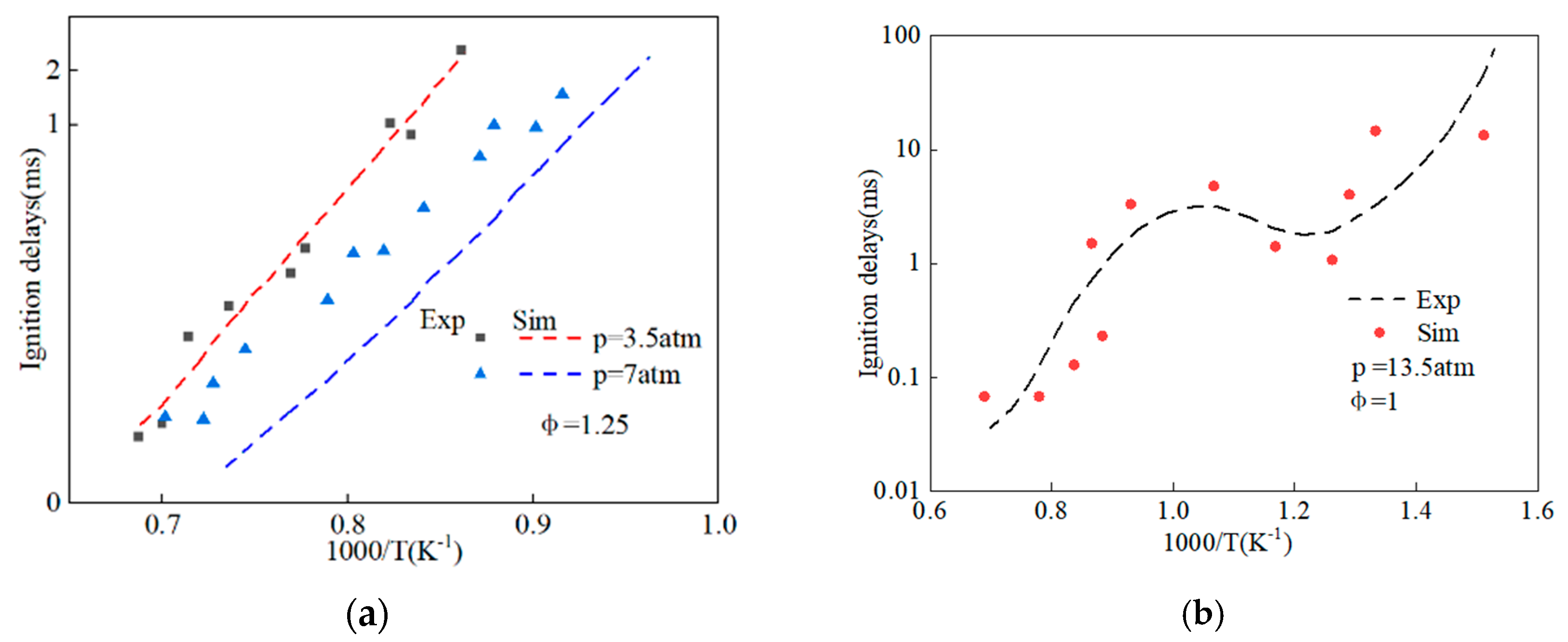
2.2. Chemical Reaction Kinetics of Methyl Oleate and Stearic Acid Methyl Ester
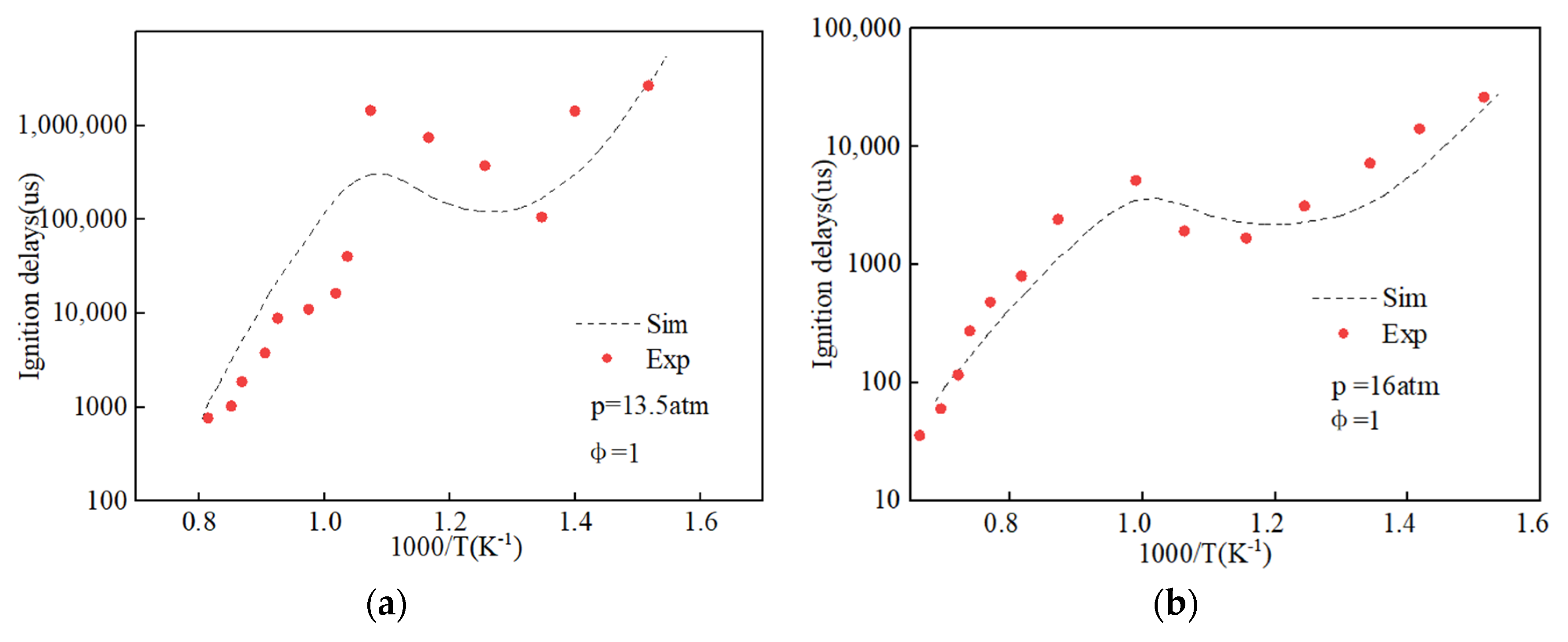
2.3. Chemical Kinetics Mechanism of Hydrogenated Biodiesel–Ethanol–Diesel
2.4. Theoretical Basis of Multi-Component Fuel Cross-Reaction
3. Results and Analysis
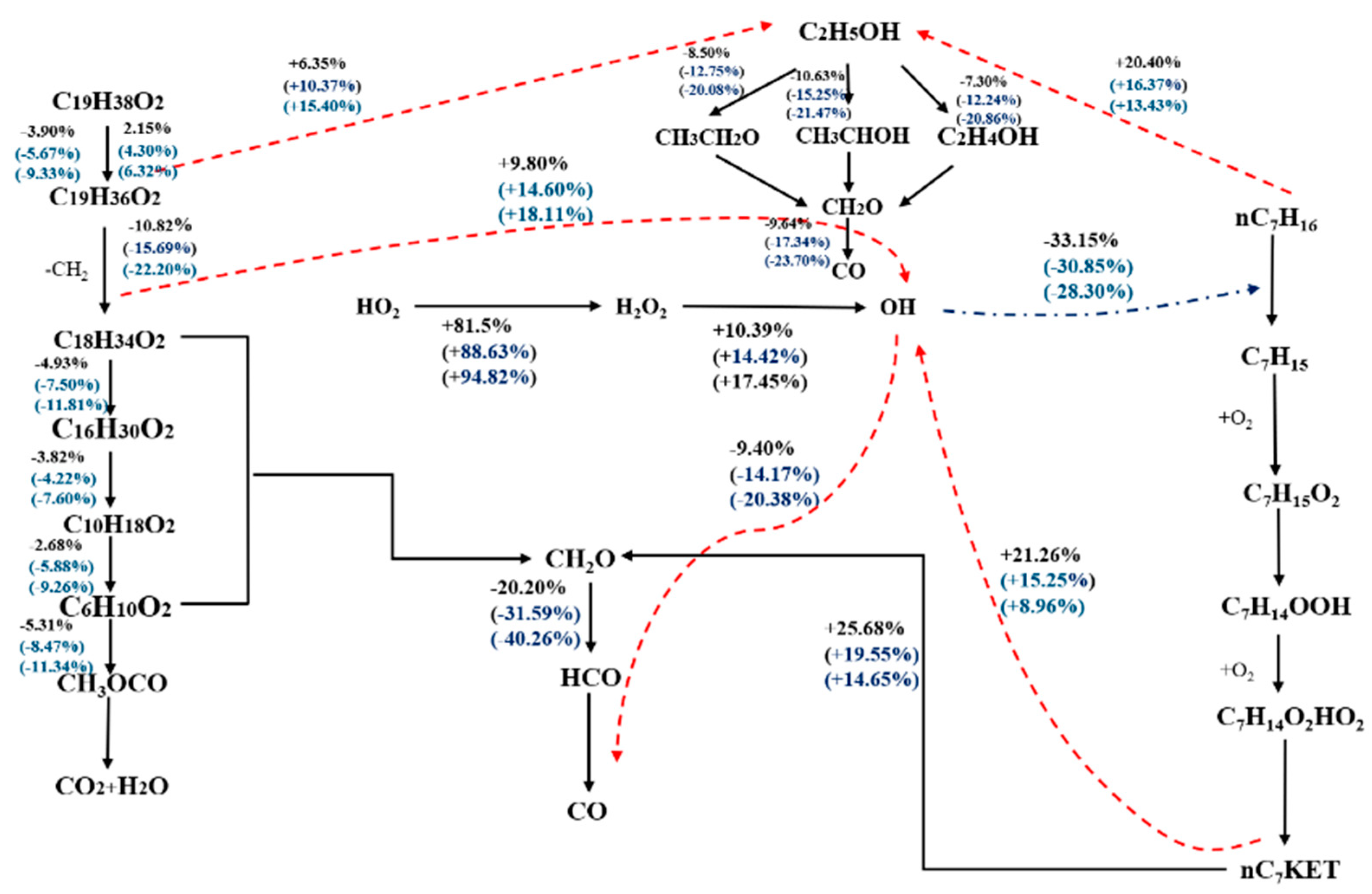
3.1. Cross-Reaction Flux Analysis of Methyl Decanoate and n-Heptane at 900 K Involving OH Radicals
3.2. Cross-Reaction Flux Analysis of Methyl Oleate and n-Heptane at 900 K under Low Temperature Conditions
3.3. Cross-Reaction Flux Analysis of n-Heptane–Methyl Oleate–Ethanol at High and Low Temperatures
3.3.1. Low-Temperature OH Cross-Reaction Flux Analysis for n-Heptane–Methyl Oleate–Ethanol
3.3.2. High-Temperature OH Cross-Reaction Flux Analysis for n-Heptane–Methyl Oleate–Ethanol
4. Conclusions
- (1)
- In the MD10 (MD20) mixed-fuel system, the total consumption rate of n-heptane is 68.93% (54.31%), with OH flowing from n-heptane to methyl decanoate. In the HB10 (HB20) mixed-fuel system, the total consumption rate of n-heptane is 77.21% (60.58%), with OH flowing from the methyl oleate and stearic acid methyl ester mixture to n-heptane.
- (2)
- At low temperatures in the HB5E5, HB10E10, and HB15E15 mixed-fuel systems, OH produced by the cracking of n-heptane is also supplied to ethanol. Due to the substantial consumption of OH by ethanol at this stage, the reactivity of the mixed-fuel system is relatively low. As the content of methyl oleate, stearic acid methyl ester, and ethanol increases, the generation of OH gradually decreases, which is unfavorable for the reaction to proceed.
- (3)
- At high temperatures, in the HB5E5, HB10E10, and HB15E15 systems, the total generation rates are respectively 24.6%, 33.29%, and 38.65%. Ethanol predominantly undergoes cracking reactions at high temperatures, producing a substantial amount of OH. This indicates that increasing the content of hydrogenated biodiesel and ethanol within a limited range can enhance the reactivity of the system, facilitating the reaction process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, W.; Li, J.; He, B.; Yan, F.; Cui, Z.; Wu, K.; Lin, L.; Qian, X.; Cheng, Y. Biodiesel production from waste chicken fat with low free fatty acids by an integrated catalytic process of composite membrane and sodium methoxide. Bioresour. Technol. 2013, 139, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Martos, F.J.; Soriano, J.A.; Braic, A.; Fernández-Yáñez, P.; Armas, O. A CFD Modelling Approach for the Operation Analysis of an Exhaust Backpressure Valve Used in a Euro 6 Diesel Engine. Energies 2023, 16, 4112. [Google Scholar] [CrossRef]
- Sathyamurthy, R.; Balaji, D.; Gorjian, S.; Muthiya, S.J.; Bharathwaaj, R.; Vasanthaseelan, S.; Essa, F.A. Performance Combus-tion and Emission Characteristics of a DI-CI Diesel Engine Fueled with Corn Oil Methyl Ester Biodiesel Blends. Sustain. Energy Technol. Assess. 2021, 43, 100981. [Google Scholar]
- Aydn, S. Comprehensive Analysis of Combustion, Performance and Emissions of Power Generator Diesel Engine Fueled with Different Source of Biodiesel Blends. Energy 2020, 205, 118074. [Google Scholar] [CrossRef]
- Szabados, G.; Berecz Ky, Á. Experimental investigation of physicochemical properties of diesel, biodiesel and TBK-biodiesel fuels and combustion and emission analysis in CI internal combustion engine. Renew. Energy 2018, 121, 568–578. [Google Scholar] [CrossRef]
- Mubarak, M.; Shaija, A.; Suchithra, T.V. Experimental Evaluation of Salvinia Molesta Oil Biodiesel/Diesel Blends Fuel on Combustion Performance and Emission Analysis of Diesel Engine. Fuel 2021, 287, 119526. [Google Scholar] [CrossRef]
- Xi, S.; Xue, J.; Wang, F.; Li, X. Reduction of Large-Size Combustion Mechanisms of n-Decane and n-Dodecane with an Improved Sensitivity Analysis Method. Combust. Flame 2020, 222, 326–335. [Google Scholar] [CrossRef]
- Nadanakumar, V.; Muthiya, J.; Prudhvi, T.; Induja, S.; Sathyamurthy, R.; Dharmaraj, V. Experimental investigation to control HC, CO & NOx emissions from diesel engines using diesel oxidation catalyst. Mater. Today Proc. 2021, 43, 434–440. [Google Scholar]
- Sundararajan, R.; Jeyaseelan, T. Effect of Biodiesel, Biodiesel Binary Blends, Hydrogenated Biodiesel and Injection Parameters on NOx and Soot Emissions in a Turbocharged Diesel Engine. Fuel 2019, 240, 101–118. [Google Scholar]
- Zuo, L. Study on Combustion and Emission Characteristics of Hydrogenated Biodiesel-Ethanol-Diesel for Diesel Engine Combustion; Jiangsu University: Zhenjiang, China, 2020. [Google Scholar]
- Yu, Q. Simulation Study on PCCI Diesel Combustion Process with Coupled DMC/Biodiesel Reaction Mechanism; Jiangsu University: Zhenjiang, China, 2021. [Google Scholar]
- Marcoberardino, G.D.; Vitali, D.; Spinelli, F.; Binotti, M.; Manzolini, G. Green Hydrogen Production from Raw Biogas: A Techno-Economic Investigation of Conventional Processes Using Pressure Swing Adsorption Unit. Processes 2018, 6, 19. [Google Scholar] [CrossRef]
- Al-Gharibeh, E.; Kumar, K. Oxidation kinetics of methyl decanoate in a motored engine. Fuel 2022, 308, 121912. [Google Scholar] [CrossRef]
- Herbinet, O.; Pitz, W.J.; Westbrook, C.K. Detailed chemical kinetic oxidation mechanism for a biodiesel surrogate. Combust. Flame 2008, 154, 507–528. [Google Scholar] [CrossRef]
- Glaude, P.E.; Herbinet, O.; Bax, S.; Biet, J.; Warth, V.; Battin-Leclerc, F. Modeling of the Oxidation of Methyl Esters—Validation for Methyl Hexanoate, Methyl Heptanoate, and Methyl Decanoate in a Jet-Stirred Reactor. Combust. Flame 2010, 157, 2035–2050. [Google Scholar] [CrossRef] [PubMed]
- Hodnett, B.K.; Verma, V. Thermodynamic vs. Kinetic Basis for Polymorph Selection. Processes 2019, 7, 272. [Google Scholar] [CrossRef]
- Zhou, Z.; Yi, Q.; Wang, R.; Wang, G.; Ma, C. Numerical Investigation on Coal Combustion in Ultralow CO2 Blast Furnace: Effect of Oxygen Temperature. Processes 2020, 8, 877. [Google Scholar] [CrossRef]
- Chang, Y. Research on the Reaction Mechanism of Fuel Skeleton for Characterization of Diesel and Biodiesel Based on Decoupling Method; Dalian University of Technology: Dalian, China, 2016. [Google Scholar]
- Zhai, Y. Experimental and Model Study on the Reaction Kinetics of Biodiesel Alternative Fuel Combustion; University of Science and Technology of China: Anhui, China, 2018. [Google Scholar]
- Mei, D.; Zhang, Q.; Ju, Z.; Gu, M.; Yuan, Y.; Chen, C.; Tao, J. Partial hydrogenated biodiesel improves the combustion and emission of diesel engine. China J. Highw. Transp. 2019, 32, 184–190. [Google Scholar]
- Leng, X.; Li, M.; He, Z.; Zhang, Y.; Zhong, W.; Xuan, T.; Duan, L.; Wang, P. Combustion characteristics of engine fueled by gasoline/hydrogenated biodiesel blend. J. Harbin Eng. Univ. 2020, 41, 1196–1202. [Google Scholar]
- Sinha, A.; Thomson, M. The chemical structures of opposed flow diffusion flames of C3 oxygenated hydrocarbons (isopropanol, dimethoxy methane, and dimethyl carbonate) and their mixtures. Combust. Flame 2004, 136, 548–556. [Google Scholar] [CrossRef]
- Adu-Mensah, D.; Mei, D.; Zuo, L.; Zhang, Q.; Wang, J. A review on partial hydrogenation of biodiesel and its influence on fuel property. Fuel 2019, 251, 660–668. [Google Scholar] [CrossRef]
- Zuo, L.; Wang, J.; Mei, D.; Dai, S.; Adu-Mensah, D. Experimental investigation on combustion and (regulated and unregulated) emissions performance of a common-rail diesel engine using partially hydrogenated biodiesel-ethanol-diesel ternary blend. Renew. Energy 2022, 185, 1272–1283. [Google Scholar] [CrossRef]
- Westbrook, C.K.; Naik, C.V.; Herbinet, O.; Pitz, W.J.; Mehl, M. Detailed chemical kinetic reaction mechanism for biodiesel components methyl stearate and methyl oleate. Proc. Combust. Inst. 2011, 33, 383–389. [Google Scholar]
- Curran, H.J.; Gaffuri, P.; Pitz, W.J.; Westbrook, C.K. A Comprehensive Modeling Study of n-Heptane Oxidation. Combust. Flame 1998, 114, 149–177. [Google Scholar] [CrossRef]
- Metcalfe, W.K.; Burke, S.M.; Ahmed, S.S.; Curran, H.J. A Hierarchical and Comparative Kinetic Modeling Study of C1 − C2 Hydrocarbon and Oxygenated Fuels. Chem. Kinet. 2013, 45, 638–675. [Google Scholar] [CrossRef]
- Fieweger, K.; Blumenthal, R.; Adomeit, G. Self-ignition of SI engine model fuels: A shock tube investigation at high pressure. Combust. Flame 1997, 109, 599–619. [Google Scholar] [CrossRef]
- Shen, H.-P.S.; Steinberg, J.; Vanderover, J.; Oehlschlaeger, M.A. A shock tube study of the ignition of n-heptane, n-decane, n-dodecane, and n-tetradecane at elevated pressures. Energy Fuels 2009, 23, 2482–2489. [Google Scholar] [CrossRef]
- Herzler, J.; Jerig, L.; Roth, P. Shock tube study of the ignition of lean n-heptane/air mixtures at intermediate temperatures and high pressures. Proc. Combust. Inst. 2005, 30, 1147–1153. [Google Scholar] [CrossRef]
- Hartmann, M.; Gushterova, I.; Fikri, M.; Schulz, C.; Schießl, R.; Maas, U. Auto-ignition of toluene-doped n-heptane and iso-octane/air mixtures: High-pressure shock-tube experiments and kinetics modeling. Combust. Flame 2011, 158, 172–178. [Google Scholar] [CrossRef]
- Jessica, L.B.; Youngchul, R.; Rolf, D.R.; Joanna, M.; Daw, C.S. Development and Validation of a Reduced Reaction Mechanism for Biodiesel-Fueled Engine Simulations. SAE Int. J. Fuels Lubr. 2009, 1, 675–702. [Google Scholar]
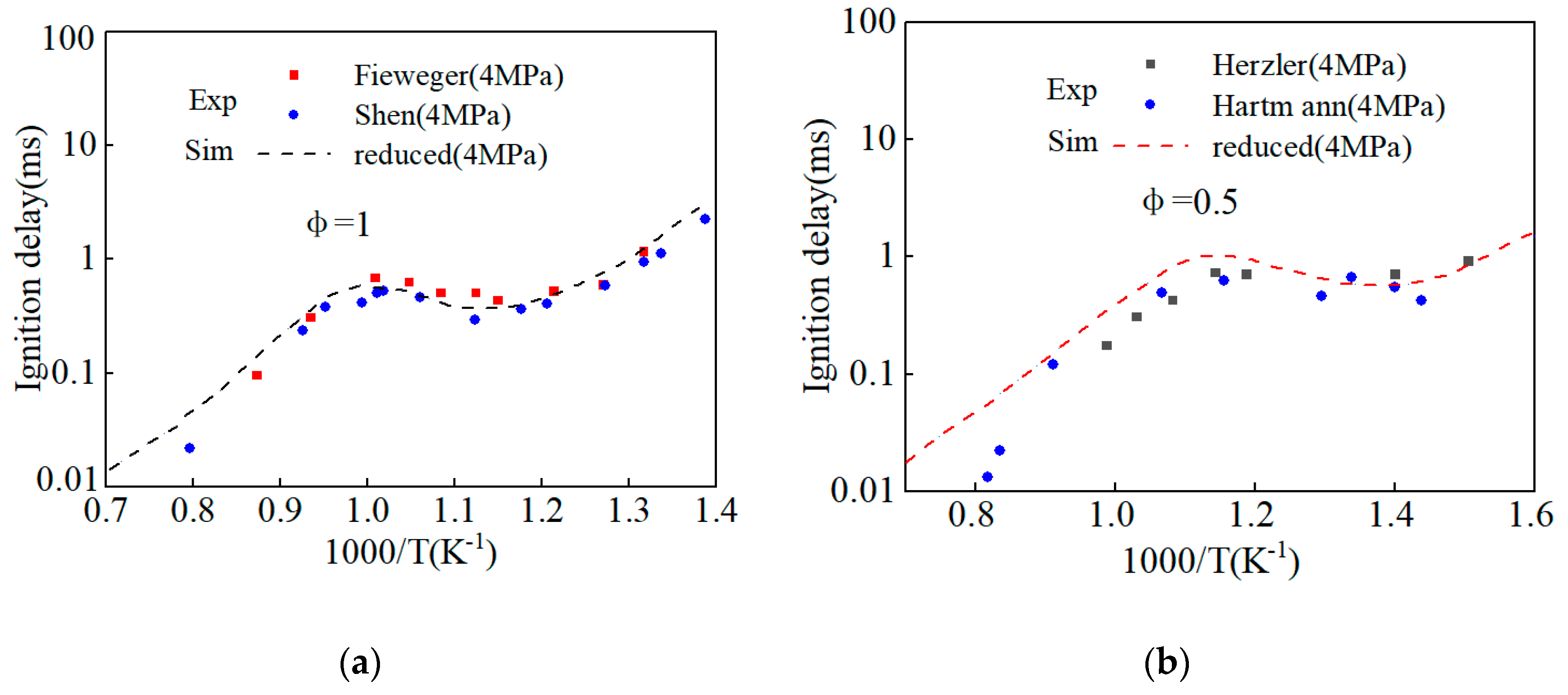



| Sample | Cetane Number |
|---|---|
| CME | 59.3 |
| UPHCME | 71.2 |
| MD | 47 |
| Methyl Oleate | 62.3 |
| Stearic Acid Methyl Ester | 104.1 |
| Composition | Molecular Formula | Peak Area/% | |
|---|---|---|---|
| CME | UPHCME | ||
| Methyl Myristic Acid Ester (C14:0) | C15H30O2 | 0.13 | 0.15 |
| Methyl Palmitate (C16:0) | C17H34O2 | 23.05 | 23.11 |
| Methyl Stearate (C18:0) | C19H38O2 | 3.17 | 10.54 |
| Trans-Methyl Oleate (trans-C18:1) | C19H36O2 | 0 | 12.18 |
| Cis-Methyl Oleate (cis-C18:1) | C19H36O2 | 21.47 | 31.62 |
| Methyl Linoleate (C18:2) | C19H34O2 | 49.94 | 20.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Chen, L.; Zhang, R.; Zhao, W. Study on Oxidation Activity of Hydrogenated Biodiesel–Ethanol–Diesel Blends. Processes 2024, 12, 462. https://doi.org/10.3390/pr12030462
Zhou J, Chen L, Zhang R, Zhao W. Study on Oxidation Activity of Hydrogenated Biodiesel–Ethanol–Diesel Blends. Processes. 2024; 12(3):462. https://doi.org/10.3390/pr12030462
Chicago/Turabian StyleZhou, Jianbo, Lyu Chen, Rui Zhang, and Weidong Zhao. 2024. "Study on Oxidation Activity of Hydrogenated Biodiesel–Ethanol–Diesel Blends" Processes 12, no. 3: 462. https://doi.org/10.3390/pr12030462





