Mechanism of Calcium Ion-Selective Channel Opening in the ChR2_L132C Mutant: A Molecular Dynamics Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Modeling Transmembrane CatCh Systems
2.2. MD Simulation Details
3. Results
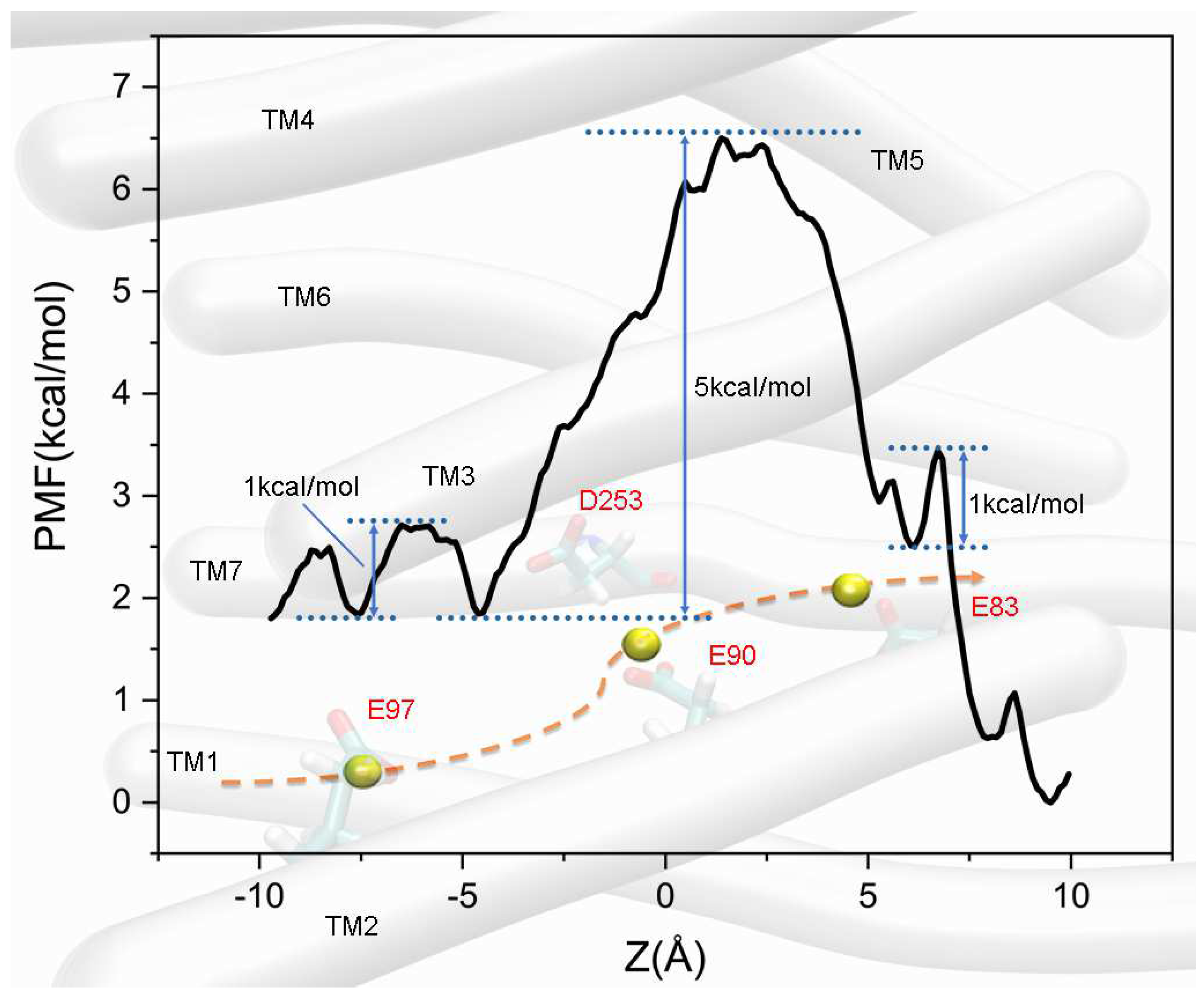
3.1. Models of CatCh-P520 and Simulation of ion Permeation
3.2. Effects of Amino Acids at CG
3.3. Comparison of Ca2+ and Na+ Permeation Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13945. [Google Scholar] [CrossRef]
- Kleinlogel, S.; Feldbauer, K.; Dempski, R.E.; Fotis, H.; Wood, P.G.; Bamann, C.; Bamberg, E. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat. Neurosci. 2011, 14, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.H.; Ganjawala, T.H.; Lu, Q.; Ivanova, E.; Zhang, Z. ChR2 mutants at L132 and T159 with improved operational light sensitivity for vision restoration. PLoS ONE 2014, 9, e98924. [Google Scholar] [CrossRef] [PubMed]
- Mager, T.; Lopez de la Morena, D.; Senn, V.; Schlotte, J.; D’Errico, A.; Feldbauer, K.; Wrobel, C.; Jung, S.; Bodensiek, K.; Rankovic, V.; et al. High frequency neural spiking and auditory signaling by ultrafast red-shifted optogenetics. Nat. Commun. 2018, 9, 1750. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Lahore, R.G.; Pampaloni, N.P.; Peter, E.; Heim, M.M.; Tillert, L.; Vierock, J.; Oppermann, J.; Walther, J.; Schmitz, D.; Owald, D.; et al. Calcium-permeable channelrhodopsins for the photocontrol of calcium signalling. Nat. Commun. 2022, 13, 7844. [Google Scholar] [CrossRef] [PubMed]
- Volkov, O.; Kovalev, K.; Polovinkin, V.; Borshchevskiy, V.; Bamann, C.; Astashkin, R.; Marin, E.; Popov, A.; Balandin, T.; Willbold, D.; et al. Structural insights into ion conduction by channelrhodopsin 2. Science 2017, 358, eaan8862. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.E.; Zhang, F.; Yizhar, O.; Ramakrishnan, C.; Nishizawa, T.; Hirata, K.; Ito, J.; Aita, Y.; Tsukazaki, T.; Hayashi, S.; et al. Crystal structure of the channelrhodopsin light-gated cation channel. Nature 2012, 482, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.E.; Kim, Y.S.; Paggi, J.M.; Evans, K.E.; Allen, W.E.; Richardson, C.; Inoue, K.; Ito, S.; Ramakrishnan, C.; Fenno, L.E.; et al. Structural mechanisms of selectivity and gating in anion channelrhodopsins. Nature 2018, 561, 349–354. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kato, H.E.; Yamashita, K.; Ito, S.; Inoue, K.; Ramakrishnan, C.; Fenno, L.E.; Evans, K.E.; Paggi, J.M.; Dror, R.O.; et al. Crystal structure of the natural anion-conducting channelrhodopsin GtACR1. Nature 2018, 561, 343–348. [Google Scholar] [CrossRef]
- Oda, K.; Vierock, J.; Oishi, S.; Rodriguez-Rozada, S.; Taniguchi, R.; Yamashita, K.; Wiegert, J.S.; Nishizawa, T.; Hegemann, P.; Nureki, O. Crystal structure of the red light-activated channelrhodopsin Chrimson. Nat. Commun. 2018, 9, 3949. [Google Scholar] [CrossRef]
- Oda, K.; Nomura, T.; Nakane, T.; Yamashita, K.; Inoue, K.; Ito, S.; Vierock, J.; Hirata, K.; Maturana, A.D.; Katayama, K.; et al. Time-resolved serial femtosecond crystallography reveals early structural changes in channelrhodopsin. eLife 2021, 10, e62389. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, W.; Zhou, S.; Ran, X.; Shang, Y.; Lo, G.V.; Dou, Y.; Yuan, S. The effect on ion channel of different protonation states of E90 in channelrhodopsin-2: A molecular dynamics simulation. RSC Adv. 2021, 11, 14542–14551. [Google Scholar] [CrossRef] [PubMed]
- Kuhne, J.; Vierock, J.; Tennigkeit, S.A.; Dreier, M.A.; Wietek, J.; Petersen, D.; Gavriljuk, K.; El-Mashtoly, S.F.; Hegemann, P.; Gerwert, K. Unifying photocycle model for light adaptation and temporal evolution of cation conductance in channelrhodopsin-2. Proc. Natl. Acad. Sci. USA 2019, 116, 9380–9389. [Google Scholar] [CrossRef] [PubMed]
- Radu, I.; Bamann, C.; Nack, M.; Nagel, G.; Bamberg, E.; Heberle, J. Conformational changes of channelrhodopsin-2. J. Am. Chem. Soc. 2009, 131, 7313–7319. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Zhang, W.; Yuan, S. The Mechanism of the Channel Opening in Channelrhodopsin-2: A Molecular Dynamics Simulation. Int. J. Mol. Sci. 2023, 24, 5667. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, D.A.; Case, D.A.; Caldwell, J.W.; Ross, W.S.; Cheatham, T.E.; Debolt, S.; Ferguson, D.; Seibel, G.; Kollman, P. Amber, a Package of Computer-Programs for Applying Molecular Mechanics, Normal-Mode Analysis, Molecular-Dynamics and Free-Energy Calculations to Simulate the Structural and Energetic Properties of Molecules. Comput. Phys. Commun. 1995, 91, 1–41. [Google Scholar] [CrossRef]
- Kalé, L.; Skeel, R.; Bhandarkar, M.; Brunner, R.; Gursoy, A.; Krawetz, N.; Phillips, J.; Shinozaki, A.; Varadarajan, K.; Schulten, K. NAMD2: Greater Scalability for Parallel Molecular Dynamics. J. Comput. Phys. 1999, 151, 283–312. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmüller, H.; MacKerell, A.D. CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Andersen, H.C. Rattle: A “velocity” version of the shake algorithm for molecular dynamics calculations. J. Comput. Phys. 1983, 52, 24–34. [Google Scholar] [CrossRef]
- Feller, S.E.; Zhang, Y.; Pastor, R.W.; Brooks, B.R. Constant pressure molecular dynamics simulation: The Langevin piston method. J. Chem. Phys. 1995, 103, 4613–4621. [Google Scholar] [CrossRef]
- Miller, B.R., 3rd; McGee, T.D., Jr.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Govorunova, E.G.; Sineshchekov, O.A.; Li, H.; Wang, Y.; Brown, L.S.; Palmateer, A.; Melkonian, M.; Cheng, S.; Carpenter, E.; Patterson, J.; et al. Cation and Anion Channelrhodopsins: Sequence Motifs and Taxonomic Distribution. mBio 2021, 12, e0165621. [Google Scholar] [CrossRef]
- Roux, B. The membrane potential and its representation by a constant electric field in computer simulations. Biophys. J. 2008, 95, 4205–4216. [Google Scholar] [CrossRef]
- Rappleye, M.; Berndt, A. Structural basis for ion selectivity and engineering in channelrhodopsins. Curr. Opin. Struct. Biol. 2019, 57, 176–184. [Google Scholar] [CrossRef]
- Jiang, D.; Zhang, J.; Xia, Z. Structural Advances in Voltage-Gated Sodium Channels. Front. Pharmacol. 2022, 13, 908867. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, Z.; Li, Z.; Qian, X.; Lu, S.; Dong, M.; Zhou, Q.; Yan, N. Structure of the voltage-gated calcium channel Ca(v)1.1 at 3.6 A resolution. Nature 2016, 537, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Noskov, S.Y.; Roux, B. Ion selectivity in potassium channels. Biophys. Chem. 2006, 124, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Yu, H.; Liu, C.; Song, C. The Ca2+ permeation mechanism of the ryanodine receptor revealed by a multi-site ion model. Nat. Commun. 2020, 11, 922. [Google Scholar] [CrossRef]
- Gao, S.; Yao, X.; Yan, N. Structure of human Ca(v)2.2 channel blocked by the painkiller ziconotide. Nature 2021, 596, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, S.; Cui, X.; Xu, H.; Qiu, Y.; Wei, Y.; Dong, Y.; Zhu, B.; Peng, C.; Liu, S.; et al. Molecular insights into the gating mechanisms of voltage-gated calcium channel CaV2.3. Nat. Commun. 2023, 14, 516. [Google Scholar] [CrossRef] [PubMed]

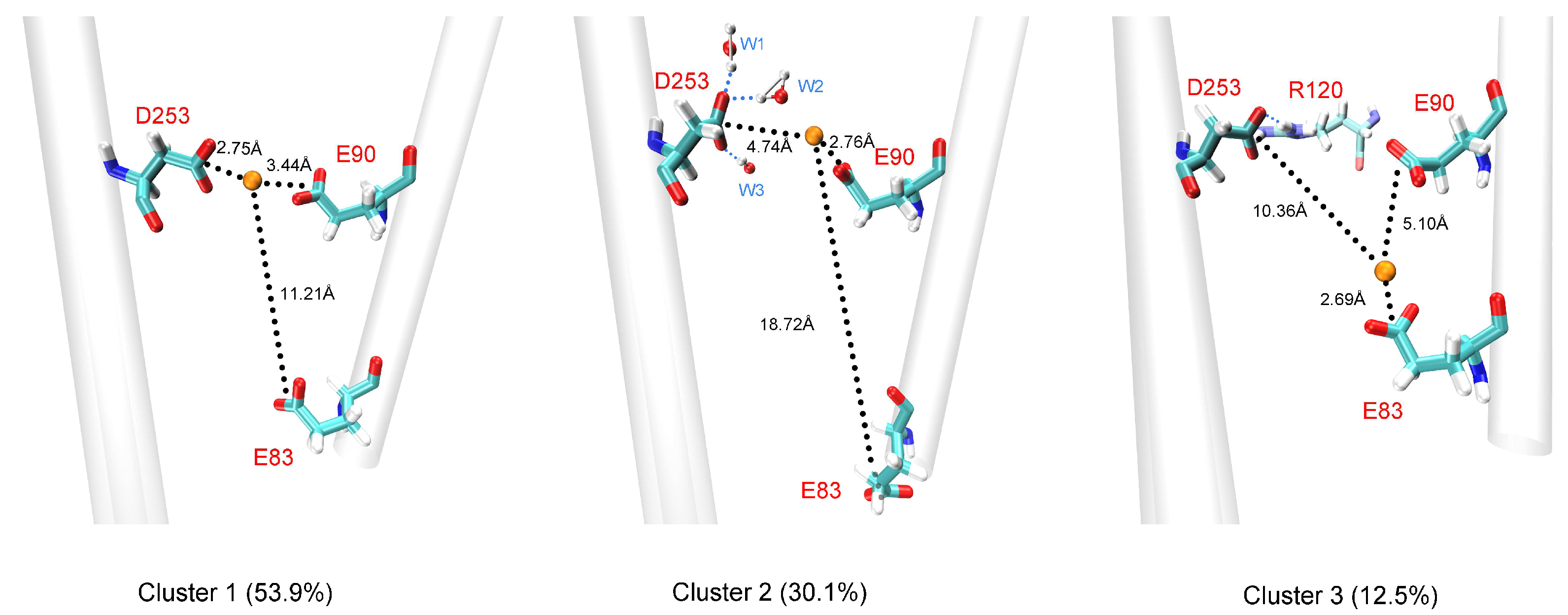

| Cluster | Percentage | Binding Free Energy (kcal/mol) | ||
|---|---|---|---|---|
| E90 | D253 | E83 | ||
| 1 | 53.9% | −12.21 | −16.65 | −0.41 |
| 2 | 30.1% | −17.05 | −1.29 | −0.23 |
| 3 | 12.5% | −1.78 | −0.40 | −18.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Zhang, W.; Yuan, S.; Dou, Y. Mechanism of Calcium Ion-Selective Channel Opening in the ChR2_L132C Mutant: A Molecular Dynamics Simulation. Processes 2024, 12, 494. https://doi.org/10.3390/pr12030494
Xu T, Zhang W, Yuan S, Dou Y. Mechanism of Calcium Ion-Selective Channel Opening in the ChR2_L132C Mutant: A Molecular Dynamics Simulation. Processes. 2024; 12(3):494. https://doi.org/10.3390/pr12030494
Chicago/Turabian StyleXu, Tao, Wenying Zhang, Shuai Yuan, and Yusheng Dou. 2024. "Mechanism of Calcium Ion-Selective Channel Opening in the ChR2_L132C Mutant: A Molecular Dynamics Simulation" Processes 12, no. 3: 494. https://doi.org/10.3390/pr12030494
APA StyleXu, T., Zhang, W., Yuan, S., & Dou, Y. (2024). Mechanism of Calcium Ion-Selective Channel Opening in the ChR2_L132C Mutant: A Molecular Dynamics Simulation. Processes, 12(3), 494. https://doi.org/10.3390/pr12030494








