The Reversible Transformation of a Vesicular Aggregate in Response to a pH Oscillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
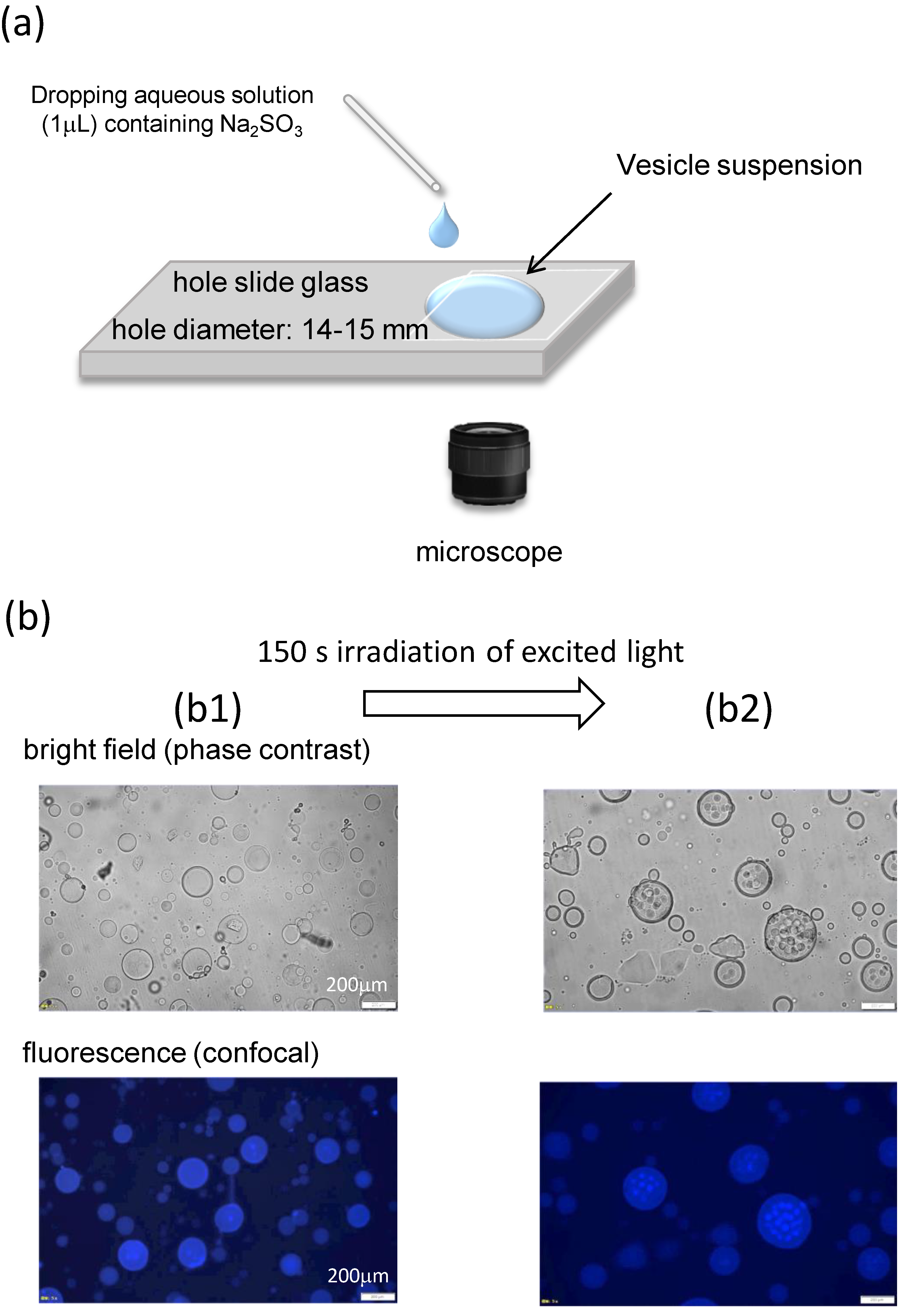
2.3. Observation of VA
3. Results and Discussions
3.1. Structure of the VA
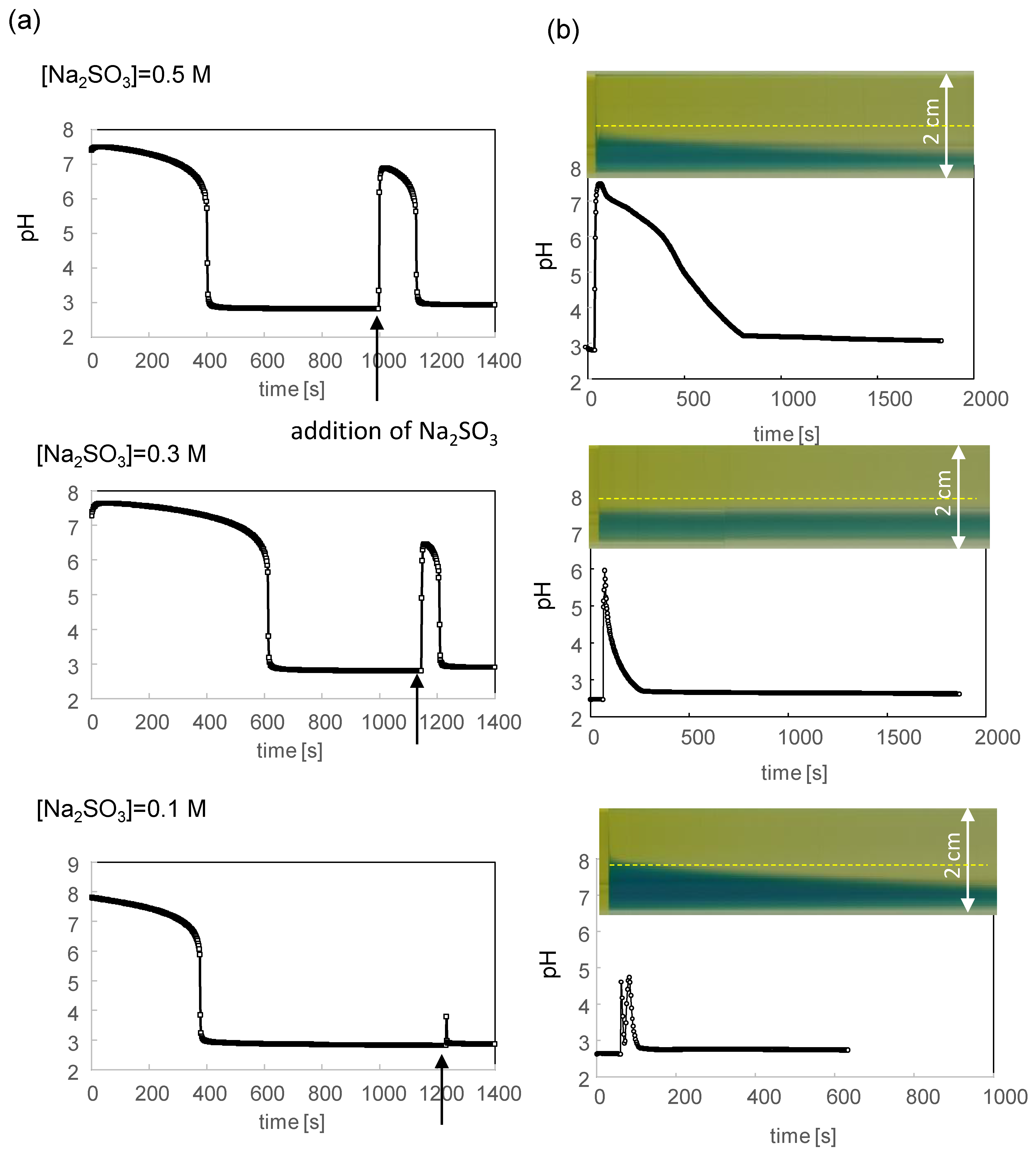
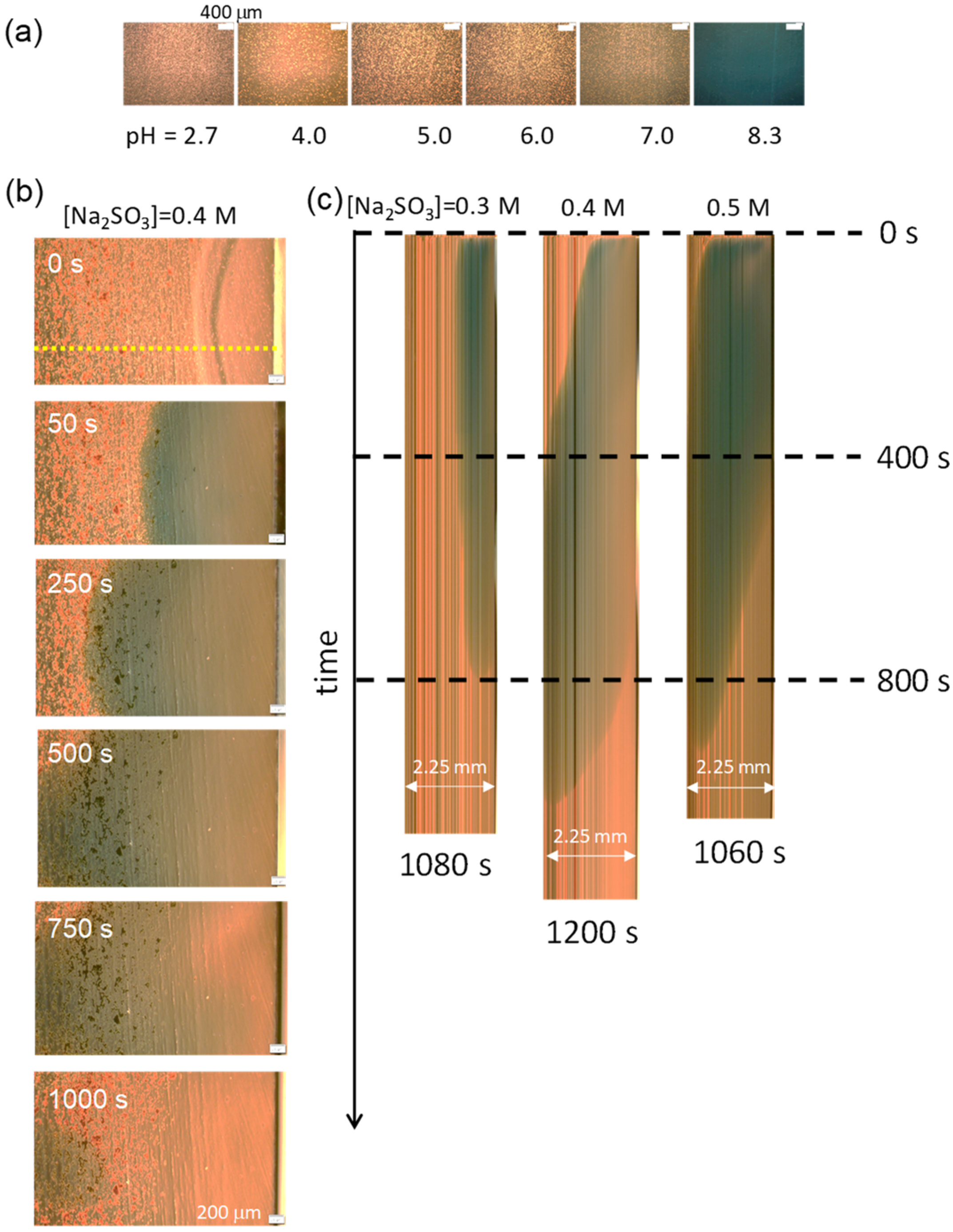
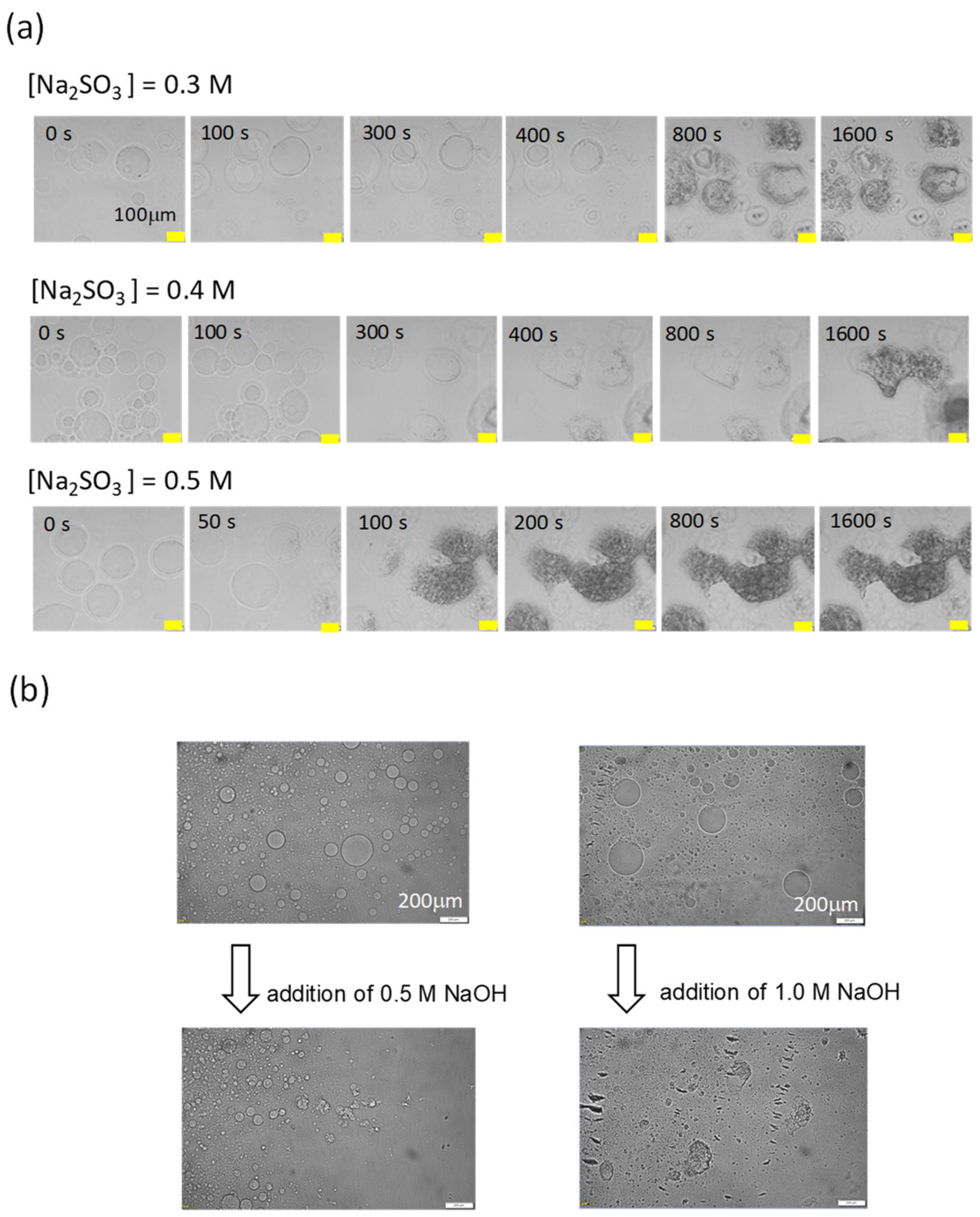
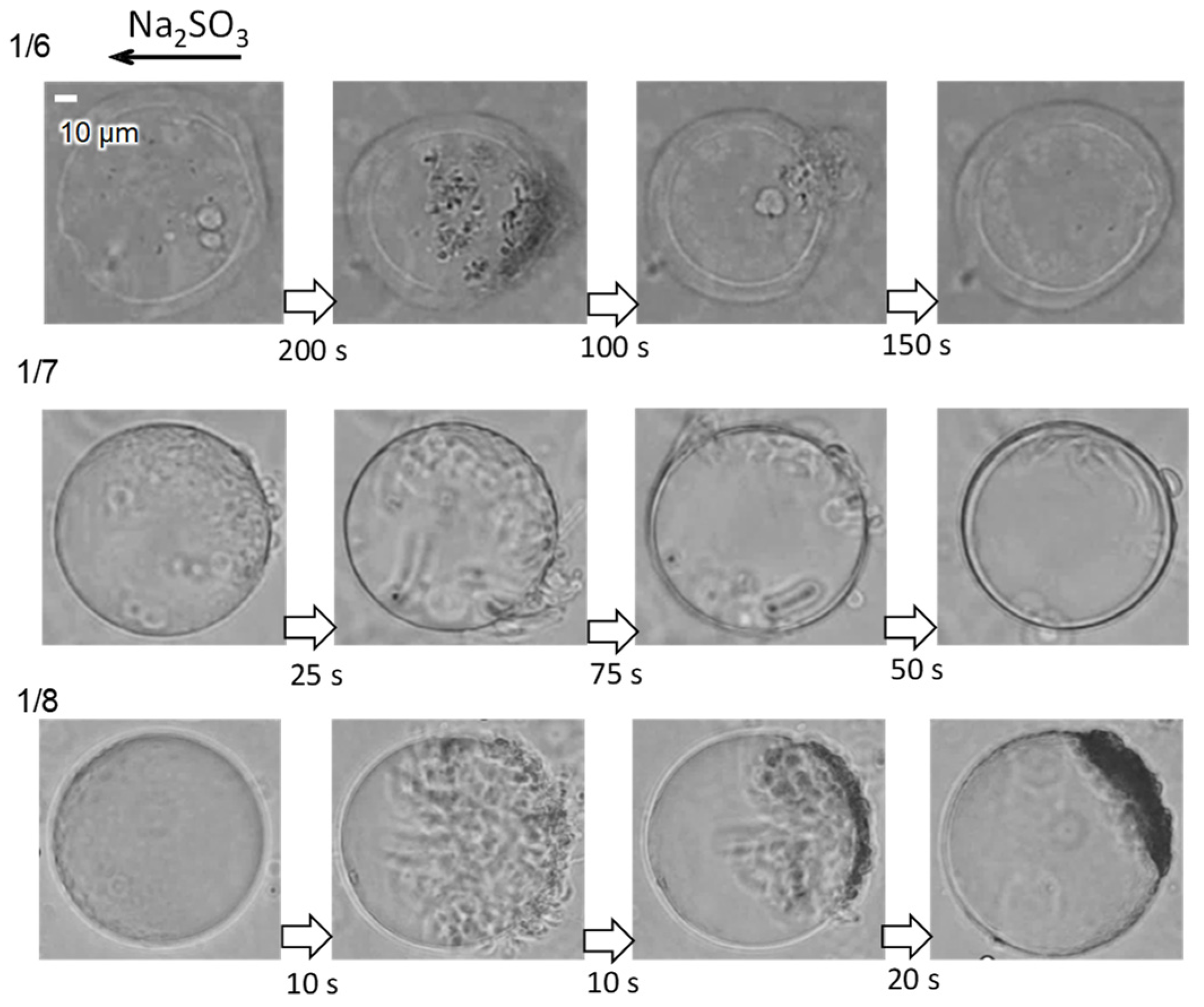
3.2. pH Oscillation and Structural Change without the Phosphate Buffer
3.3. pH Oscillation and Structural Change with the Phosphate Buffer
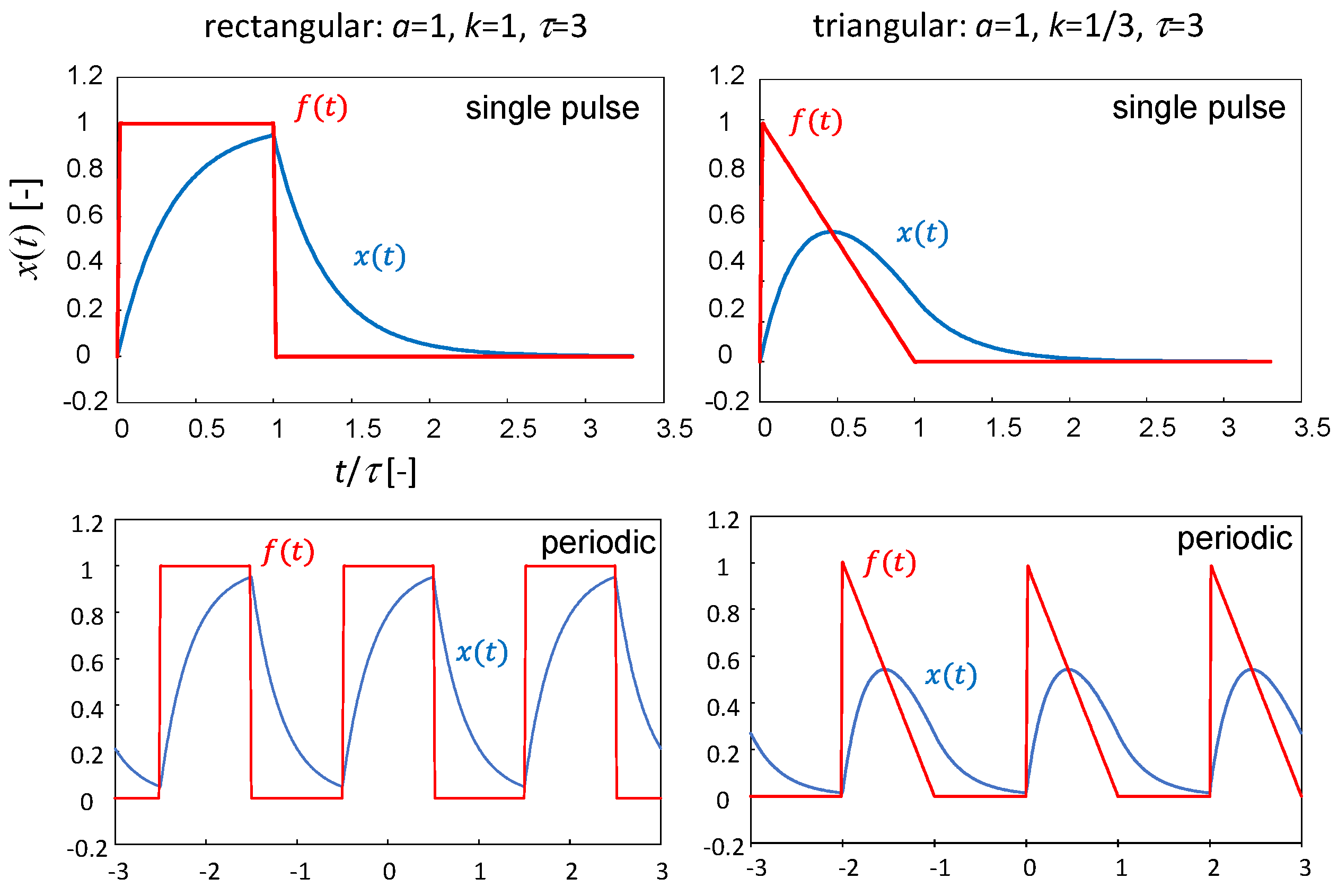
3.4. Effect of the Oscillation Pattern
- (i)
- Rectangular stimulus:
- (ii)
- Triangular stimulus:
- (i′)
- Periodic rectangles:
- (ii′)
- Periodic triangles:
4. Conclusions and the Future Perspective
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malkin, E.Z.; Bratman, S.V. Bioactive DNA from extracellular vesicles and particles. Cell Death Dis. 2020, 11, 584. [Google Scholar] [CrossRef]
- Tomasi, R.; Noël, J.-M.; Zenati, A.; Ristori, S.; Rossi, F.; Cabuil, V.; Kanoufi, F.; Abou-Hassan, A. Chemical communication between liposomes encapsulating a chemical oscillatory reaction. Chem. Sci. 2014, 5, 1854–1859. [Google Scholar] [CrossRef]
- Poros-Tarcali, E.; Perez-Mercader, J. Concurrent self-regulated autonomous synthesis and functionalization of pH-responsive giant vesicles by a chemical pH oscillator. Soft Matter 2021, 17, 4011–4018. [Google Scholar] [CrossRef]
- Noguchi, H. Disappearance, division, and route change of excitable reaction-diffusion waves in deformable membranes. Sci. Rep. 2023, 13, 6207. [Google Scholar] [CrossRef]
- Shao, Q.; Zhang, S.; Hu, Z.; Zhou, Y. Multimode Self-Oscillating Vesicle Transformers. Angew. Chem. Int. Ed. 2020, 59, 17125–17129. [Google Scholar] [CrossRef]
- Guo, J.; Poros-Tarcali, E.; Pérez-Mercader, J. Periodic Polymerization and the Generation of Polymer Giant Vesicles Autonomously Driven by pH Oscillatory Chemistry. Front. Chem. 2021, 9, 576349. [Google Scholar] [CrossRef]
- Tamate, R.; Ueki, T.; Shibayama, M.; Yoshida, R. Self-Oscillating Vesicles: Spontaneous Cyclic Structural Changes of Synthetic Diblock Copolymers. Angew. Chem. Int. Ed. 2014, 53, 11248–11252. [Google Scholar] [CrossRef] [PubMed]
- Lagzi, I.; Wang, D.; Kowalczyk, B.; Grzybowski, B.A. Vesicle-to-Micelle Oscillations and Spatial Patterns. Langmuir 2010, 26, 13770–13772. [Google Scholar] [CrossRef]
- Miele, Y.; Holló, G.; Lagzi, I.; Rossi, F. Shape Deformation, Budding and Division of Giant Vesicles and Artificial Cells: A Review. Life 2022, 12, 841. [Google Scholar] [CrossRef] [PubMed]
- Sachin Krishnan, T.V.; Sunil Kumar, P.B. Active membrane recycling induced morphology changes in vesicles. Front. Phys. 2022, 10, 1003558. [Google Scholar] [CrossRef]
- Ho, J.C.S.; Rangamani, P.; Liedberg, B.; Parikh, A.N. Mixing Water, Transducing Energy, and Shaping Membranes: Autonomously Self-Regulating Giant Vesicles. Langmuir 2016, 32, 2151–2163. [Google Scholar] [CrossRef]
- Park, H.; Sut, T.N.; Ferhan, A.R.; Yoon, B.K.; Zhdanov, V.P.; Cho, N.-J.; Jackman, J.A. pH-Modulated Nanoarchitectonics for Enhancement of Multivalency-Induced Vesicle Shape Deformation at Receptor-Presenting Lipid Membrane Interfaces. Langmuir 2023, 39, 8297–8305. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Sakuma, Y.; Imai, M.; Ziherl, P. Morphology of vesicle triplets: Shape transformation at weak and strong adhesion limits. Soft Matter 2023, 19, 4286–4296. [Google Scholar] [CrossRef] [PubMed]
- Tamemoto, N.; Noguchi, H. Pattern formation in reaction–diffusion system on membrane with mechanochemical feedback. Sci. Rep. 2020, 10, 19582. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, V.; Kakorin, S.; Neumann, E. Transient oscillation of shape and membrane conductivity changes by field pulse-induced electroporation in nano-sized phospholipid vesicles. Phys. Chem. Chem. Phys. 2013, 15, 6303–6322. [Google Scholar] [CrossRef] [PubMed]
- Nawa, E.; Yamamoto, D.; Shioi, A. Chemotactic Amoeboid-Like Shape Change of a Vesicle under a pH Gradient. Bull. Chem. Soc. Jpn. 2015, 88, 1536–1544. [Google Scholar] [CrossRef]
- Baumgart, T.; Hess, S.T.; Webb, W.W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature 2003, 425, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Tsuji, A.; Yanagisawa, M.; Saeki, D.; Juni, K.; Morimoto, Y.; Yoshikawa, K. Phase Separation on a Phospholipid Membrane Inducing a Characteristic Localization of DNA Accompanied by Its Structural Transition. J. Phys. Chem. Lett. 2010, 1, 3391–3395. [Google Scholar] [CrossRef]
- Nawa-Okita, E.; Nakao, Y.; Yamamoto, D.; Shioi, A. A Molecular Assembly Machine Working under a Quasi-Steady State pH Gradient. Bull. Chem. Soc. Jpn. 2020, 93, 604–610. [Google Scholar] [CrossRef]
- Vutukuri, H.R.; Hoore, M.; Abaurrea-Velasco, C.; van Buren, L.; Dutto, A.; Auth, T.; Fedosov, D.A.; Gompper, G.; Vermant, J. Active particles induce large shape deformations in giant lipid vesicles. Nature 2020, 586, 52–56. [Google Scholar] [CrossRef]
- Iyer, P.; Gompper, G.; Fedosov, D.A. Non-equilibrium shapes and dynamics of active vesicles. Soft Matter 2022, 18, 6868–6881. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hua, Y.; Jiang, Y.; Zhou, X.; Zhang, L. Rotational Diffusion of Soft Vesicles Filled by Chiral Active Particles. Sci. Rep. 2017, 7, 15006. [Google Scholar] [CrossRef] [PubMed]
- Paoluzzi, M.; Di Leonardo, R.; Marchetti, M.C.; Angelani, L. Shape and Displacement Fluctuations in Soft Vesicles Filled by Active Particles. Sci. Rep. 2016, 6, 34146. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, K.; Negishi, M.; Tanaka-Takiguchi, Y.; Hayashi, M.; Yoshikawa, K. Specific Transformation of Assembly with Actin Filaments and Molecular Motors in a Cell-Sized Self-Emerged Liposome. Orig. Life Evol. Biosph. 2014, 44, 325–329. [Google Scholar] [CrossRef]
- Blanchoin, L.; Boujemaa-Paterski, R.; Sykes, C.; Plastino, J. Actin Dynamics, Architecture, and Mechanics in Cell Motility. Physiol. Rev. 2014, 94, 235–263. [Google Scholar] [CrossRef]
- Cohen, O.; Safran, S.A. Physics of Spontaneous Calcium Oscillations in Cardiac Cells and Their Entrainment. Phys. Rev. Lett. 2019, 122, 198101. [Google Scholar] [CrossRef]
- Nakada, M.; Fujikami, Y.; Kawaguchi, M.; Yamamoto, D.; Shioi, A. A molecular assembly that crawls on a solid substrate with a metabolic-like process. Mol. Syst. Des. Eng. 2016, 1, 208–215. [Google Scholar] [CrossRef]
- Brochard-Wyart, F.; de Gennes, P.G.; Sandre, O. Transient pores in stretched vesicles: Role of leak-out. Phys. A Stat. Mech. Its Appl. 2000, 278, 32–51. [Google Scholar] [CrossRef]
- Nomura, F.; Nagata, M.; Inaba, T.; Hiramatsu, H.; Hotani, H.; Takiguchi, K. Capabilities of liposomes for topological transformation. Proc. Natl. Acad. Sci. USA 2001, 98, 2340–2345. [Google Scholar] [CrossRef]
- Umeda, T.; Nomura, F.; Inaba, T.; Takiguchi, K.; Hotani, H. Stepwise Shrinkage of Liposomes Driven by Thermal Fluctuations of the Membranes. ChemPhysChem 2005, 6, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Saitoh, A.; Furuta, M.; Satomi, N.; Ishino, A.; Nishida, G.; Sudo, H.; Hotani, H.; Takiguchi, K. Opening of Holes in Liposomal Membranes Is Induced by Proteins Possessing the FERM Domain. J. Mol. Biol. 2006, 362, 403–413. [Google Scholar] [CrossRef]
- Hamada, T.; Hirabayashi, Y.; Ohta, T.; Takagi, M. Rhythmic pore dynamics in a shrinking lipid vesicle. Phys. Rev. E 2009, 80, 051921. [Google Scholar] [CrossRef] [PubMed]
- Rábai, G.; Kaminaga, A.; Hanazaki, I. Mechanism of the Oscillatory Bromate Oxidation of Sulfite and Ferrocyanide in a CSTR. J. Phys. Chem. 1996, 100, 16441–16442. [Google Scholar] [CrossRef]
- Mikhailov, A.S.; Showalter, K. Control of waves, patterns and turbulence in chemical systems. Phys. Rep. 2006, 425, 79–194. [Google Scholar] [CrossRef]
- Kodama, A.; Sakuma, Y.; Imai, M.; Oya, Y.; Kawakatsu, T.; Puff, N.; Angelova, M.I. Migration of phospholipid vesicles in response to OH− stimuli. Soft Matter 2016, 12, 2877–2886. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimada, M.; Someya, R.; Okamoto, Y.; Yamamoto, D.; Shioi, A. The Reversible Transformation of a Vesicular Aggregate in Response to a pH Oscillation. Processes 2024, 12, 514. https://doi.org/10.3390/pr12030514
Shimada M, Someya R, Okamoto Y, Yamamoto D, Shioi A. The Reversible Transformation of a Vesicular Aggregate in Response to a pH Oscillation. Processes. 2024; 12(3):514. https://doi.org/10.3390/pr12030514
Chicago/Turabian StyleShimada, Moeka, Risa Someya, Yasunao Okamoto, Daigo Yamamoto, and Akihisa Shioi. 2024. "The Reversible Transformation of a Vesicular Aggregate in Response to a pH Oscillation" Processes 12, no. 3: 514. https://doi.org/10.3390/pr12030514
APA StyleShimada, M., Someya, R., Okamoto, Y., Yamamoto, D., & Shioi, A. (2024). The Reversible Transformation of a Vesicular Aggregate in Response to a pH Oscillation. Processes, 12(3), 514. https://doi.org/10.3390/pr12030514





