Synergistic Effect of Plasma-Activated Water with Micro/Nanobubbles, Ultraviolet Photolysis, and Ultrasonication on Enhanced Escherichia coli Inactivation in Chicken Meat
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Effect of PAW Combined with a Supplementary Disinfection Technique during the Soaking Process on the Survival of E. coli in Inactivation Experiment
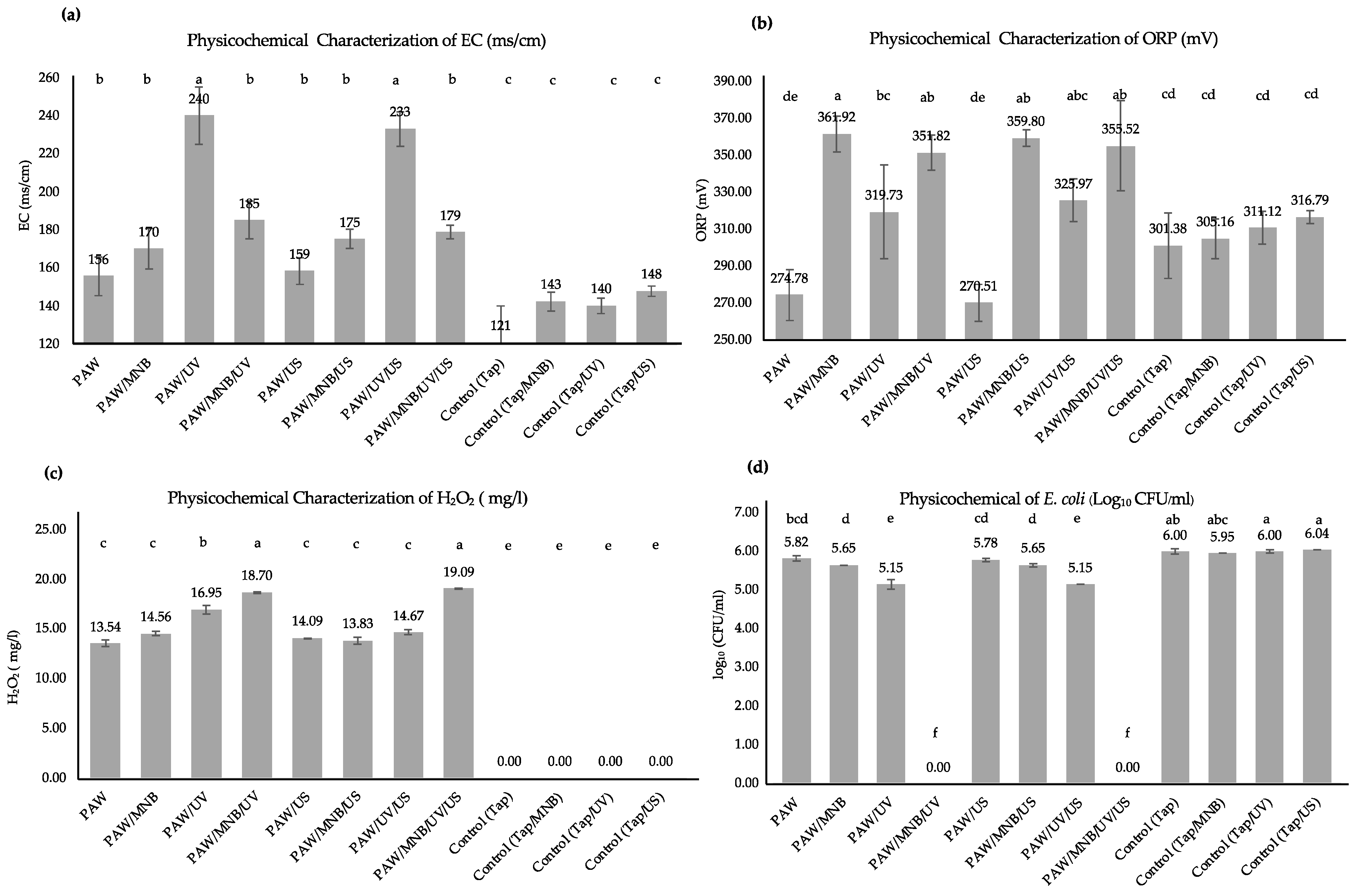
3.1.1. Physicochemical Characterization and Bactericidal Effects of PAW Combined with a Supplementary Technique
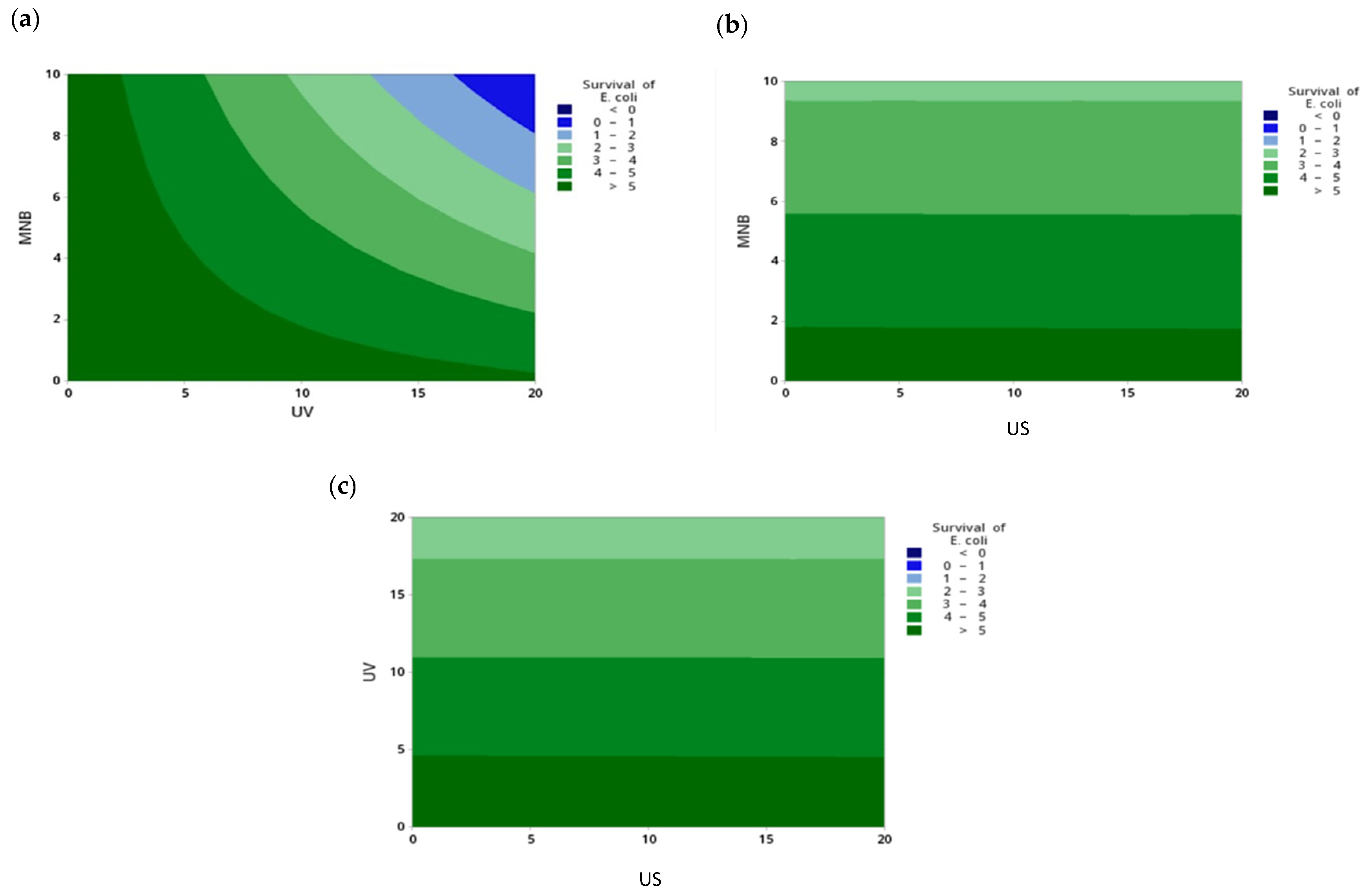
3.1.2. Statistical Evaluation of the Effect of PAW Combined with a Supplementary Technique on the Survival of E. coli
3.2. Effect of PAW Combined with a Supplementary Disinfection Technique during the Soaking Process on the Survival of E. coli in Chicken Meat in Inactivation Experiment
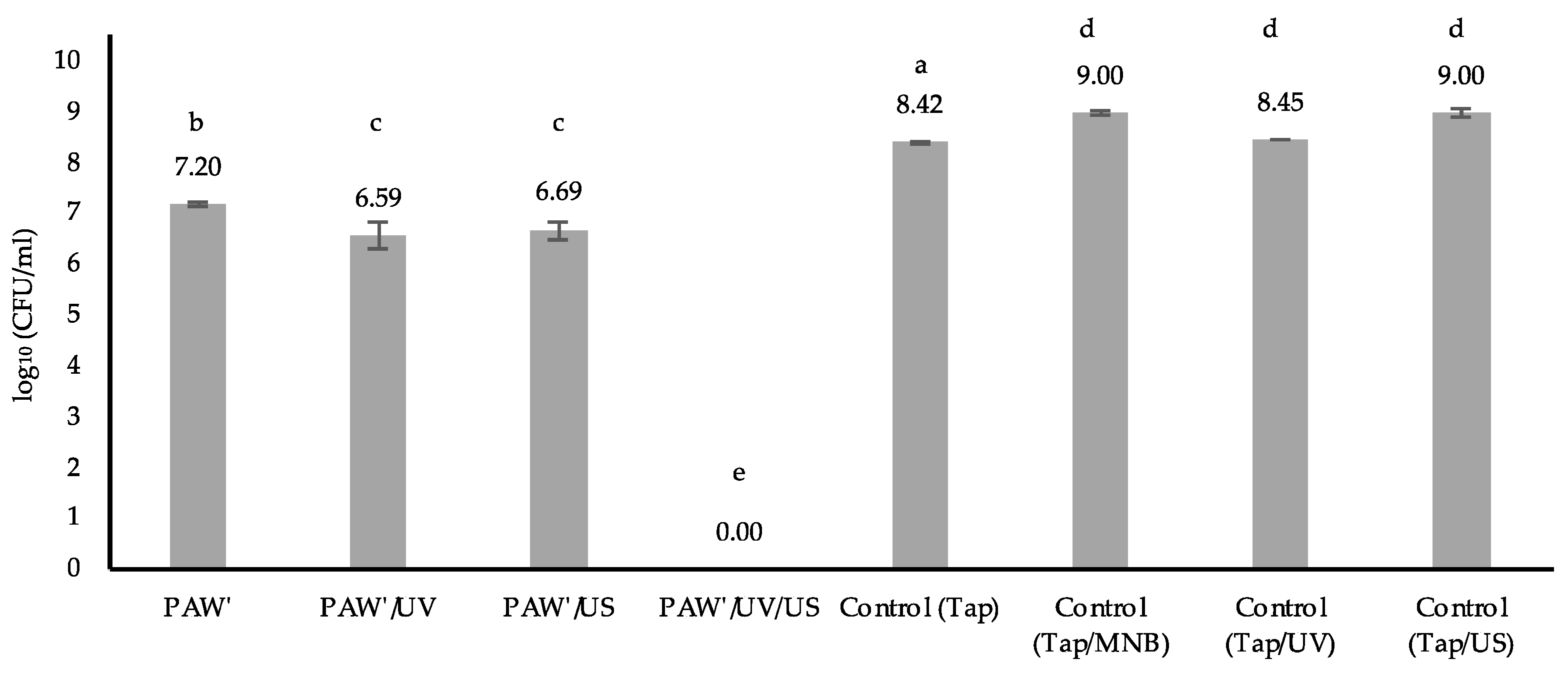
3.2.1. Test Results for the Survival of E. coli in Chicken Meat
3.2.2. Effect of Activation Time on E. coli Survival in Chicken Meat
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Artavia Oreamuno, M.A.; Siddig, K. What Does the OECD-FAO Agricultural Outlook 2017–2026 Imply for Income Distribution in the Sudan and Ethiopia; Working Paper; Humboldt-Universität zu Berlin: Berlin, Germany, 2020. [Google Scholar]
- Nakhaee, P.; Hafez, H.M. A Review on Significance of Public Health Issues Related to Poultry Campylobacteriosis. J. Poult. Sci. Avian Dis. 2023, 1, 52–66. [Google Scholar] [CrossRef]
- Rowan, N.J.; Espie, S.; Harrower, J.; Anderson, J.; Marsili, L.; MacGregor, S. Pulsed-plasma gas-discharge inactivation of microbial pathogens in chilled poultry wash water. J. Food Prot. 2007, 70, 2805–2810. [Google Scholar] [CrossRef]
- Kuraica, M.; Obradović, B.; Manojlović, D.; Ostojić, D.; Purić, J. Application of coaxial dielectric barrier discharge for potable and waste water treatment. Ind. Eng. Chem. Res 2006, 45, 882–905. [Google Scholar]
- Kondeti, V.S.K.; Phan, C.Q.; Wende, K.; Jablonowski, H.; Gangal, U.; Granick, J.L.; Hunter, R.C.; Bruggeman, P.J. Long-lived and short-lived reactive species produced by a cold atmospheric pressure plasma jet for the inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Free. Radic. Biol. Med. 2018, 124, 275–287. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, S.; Choe, W.; Yong, H.I.; Jo, C.; Kim, K. Plasma-functionalized solution: A potent antimicrobial agent for biomedical applications from antibacterial therapeutics to biomaterial surface engineering. ACS Appl. Mater. Interfaces 2017, 9, 43470–43477. [Google Scholar] [CrossRef]
- Fang, L.; Liu, J.; Ju, S.; Zheng, F.; Dong, W.; Shen, M. Experimental and theoretical evidence of enhanced ferromagnetism in sonochemical synthesized BiFeO3 nanoparticles. Appl. Phys. Lett. 2010, 97, 242501. [Google Scholar] [CrossRef]
- Perinban, S.; Orsat, V.; Raghavan, V. Nonthermal plasma–liquid interactions in food processing: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1985–2008. [Google Scholar] [CrossRef]
- Traylor, M.J.; Pavlovich, M.J.; Karim, S.; Hait, P.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Long-term antibacterial efficacy of air plasma-activated water. J. Phys. D Appl. Phys. 2011, 44, 472001. [Google Scholar] [CrossRef]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.D.; Von Woedtke, T. The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, R.; Tian, Y.; Su, B.; Wang, K.; Yu, S.; Zhang, J.; Fang, J. Sterilization efficiency of a novel electrochemical disinfectant against Staphylococcus aureus. Environ. Sci. Technol. 2016, 50, 3184–3192. [Google Scholar] [CrossRef]
- Shen, J.; Tian, Y.; Li, Y.; Ma, R.; Zhang, Q.; Zhang, J.; Fang, J. Bactericidal effects against S. aureus and physicochemical properties of plasma activated water stored at different temperatures. Sci. Rep. 2016, 6, 28505. [Google Scholar] [CrossRef]
- Ma, R.; Wang, G.; Tian, Y.; Wang, K.; Zhang, J.; Fang, J. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 2015, 300, 643–651. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, R.; Zhang, Q.; Feng, H.; Liang, Y.; Zhang, J.; Fang, J. Assessment of the physicochemical properties and biological effects of water activated by non-thermal plasma above and beneath the water surface. Plasma Process. Polym. 2015, 12, 439–449. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef]
- Kamgang-Youbi, G.; Herry, J.M.; Meylheuc, T.; Brisset, J.L.; Bellon-Fontaine, M.N.; Doubla, A.; Naïtali, M. Microbial inactivation using plasma-activated water obtained by gliding electric discharges. Lett. Appl. Microbiol. 2009, 48, 13–18. [Google Scholar] [CrossRef]
- Naïtali, M.; Kamgang-Youbi, G.; Herry, J.-M.; Bellon-Fontaine, M.-N.; Brisset, J.-L. Combined effects of long-living chemical species during microbial inactivation using atmospheric plasma-treated water. Appl. Environ. Microbiol. 2010, 76, 7662–7664. [Google Scholar] [CrossRef]
- Royintarat, T.; Meerak, J.; Seesuriyachan, P.; Boonyawan, D.; Wattanutchariya, W. Inactivation of Salmonella typhimurium in Chicken Meat by Plasma-activated water. In Proceedings of the Ph.D. Symposium on Industrial Engineering, Belgrade, Serbia, 28 September 2018. [Google Scholar]
- Royintarat, T.; Choi, E.H.; Seesuriyachan, P.; Wattanutchariya, W. Ultrasound-assisted plasma-activated water for bacterial inactivation in poultry industry. In Proceedings of the 2019 IEEE International Conference on Industrial Technology (ICIT), Melbourne, VIC, Australia, 13–15 February 2019; pp. 1028–1032. [Google Scholar]
- Royintarat, T.; Choi, E.H.; Boonyawan, D.; Seesuriyachan, P.; Wattanutchariya, W. Chemical-free and synergistic interaction of ultrasound combined with plasma-activated water (PAW) to enhance microbial inactivation in chicken meat and skin. Sci. Rep. 2020, 10, 1559. [Google Scholar] [CrossRef]
- Fan, W.; An, W.-G.; Huo, M.-X.; Yang, W.; Zhu, S.-Y.; Lin, S.-S. Solubilization and stabilization for prolonged reactivity of ozone using micro-nano bubbles and ozone-saturated solvent: A promising enhancement for ozonation. Sep. Purif. Technol. 2020, 238, 116484. [Google Scholar] [CrossRef]
- John, A.; Brookes, A.; Carra, I.; Jefferson, B.; Jarvis, P. Microbubbles and their application to ozonation in water treatment: A critical review exploring their benefit and future application. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1561–1603. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Lyu, T.; Pan, G.; Li, P. Aquatic macrophytes in morphological and physiological responses to the nanobubble technology application for water restoration. ACS ES&T Water 2020, 1, 376–387. [Google Scholar]
- Zhang, X.H.; Maeda, N.; Craig, V.S. Physical properties of nanobubbles on hydrophobic surfaces in water and aqueous solutions. Langmuir 2006, 22, 5025–5035. [Google Scholar] [CrossRef]
- Lyu, T.; Wu, S.; Mortimer, R.J.; Pan, G. Nanobubble technology in environmental engineering: Revolutionization potential and challenges. Environ. Sci. Technol. 2019, 53, 7175–7176. [Google Scholar] [CrossRef]
- Almquist, C.B.; Biswas, P. A mechanistic approach to modeling the effect of dissolved oxygen in photo-oxidation reactions on titanium dioxide in aqueous systems. Chem. Eng. Sci. 2001, 56, 3421–3430. [Google Scholar] [CrossRef]
- Churnside, J.H. Lidar signature from bubbles in the sea. Opt. Express 2010, 18, 8294–8299. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kim, H.-Y.; Lee, H.; Kim, S.-H.; Jin, H.; Bae, J.; Choi, H.-K. Discovery of potential biomarkers in human melanoma cells with different metastatic potential by metabolic and lipidomic profiling. Sci. Rep. 2017, 7, 8864. [Google Scholar] [CrossRef]
- Yamasaki, K.; Sakata, K.; Chuhjoh, K. Water Treatment Method and Water Treatment System. U.S. Patent No. 7,662,288, 16 February 2010. [Google Scholar]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef]
- Hijnen, W.; Beerendonk, E.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo) cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef]
- Elmnasser, N.; Guillou, S.; Leroi, F.; Orange, N.; Bakhrouf, A.; Federighi, M. Pulsed-light system as a novel food decontamination technology: A review. Can. J. Microbiol. 2007, 53, 813–821. [Google Scholar] [CrossRef]
- Besaratinia, A.; Yoon, J.I.; Schroeder, C.; Bradforth, S.E.; Cockburn, M.; Pfeifer, G.P. Wavelength dependence of ultraviolet radiation-induced DNA damage as determined by laser irradiation suggests that cyclobutane pyrimidine dimers are the principal DNA lesions produced by terrestrial sunlight. FASEB J. 2011, 25, 3079–3091. [Google Scholar] [CrossRef]
- Beck, S.E.; Ryu, H.; Boczek, L.A.; Cashdollar, J.L.; Jeanis, K.M.; Rosenblum, J.S.; Lawal, O.R.; Linden, K.G. Evaluating UV-C LED disinfection performance and investigating potential dual-wavelength synergy. Water Res. 2017, 109, 207–216. [Google Scholar] [CrossRef]
- Song, K.; Mohseni, M.; Taghipour, F. Mechanisms investigation on bacterial inactivation through combinations of UV wavelengths. Water Res. 2019, 163, 114875. [Google Scholar] [CrossRef]
- Sarkinas, A.; Sakalauskiene, K.; Raisutis, R.; Zeime, J.; Salaseviciene, A.; Puidaite, E.; Mockus, E.; Cernauskas, D. Inactivation of some pathogenic bacteria and phytoviruses by ultrasonic treatment. Microb. Pathog. 2018, 123, 144–148. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; Li, C.; Cui, H. Inactivation mechanism of E. coli O157:H7 under ultrasonic sterilization. Ultrason. Sonochem. 2019, 59, 104751. [Google Scholar] [CrossRef]
- Moonsub, K.; Boonyawan, D.; Tonglek, V.; Wattanutchariya, W. Enhancing Efficiency of Plasma-Activated Water Using Microbubbles/Nanobubbles Techniques. In Proceedings of the 2022 International Conference on Industrial Engineering and Operations Management, Istanbul, Turkey, 7–10 March 2022. [Google Scholar]
- Azevedo, A.; Etchepare, R.; Calgaroto, S.; Rubio, J. Aqueous dispersions of nanobubbles: Generation, properties and features. Miner. Eng. 2016, 94, 29–37. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, P.; Luan, B.; Zhang, X.; Fang, Z.; Xian, Y.; Lu, X.; Ostrikov, K.K.; Bazaka, K. Microplasma bubbles: Reactive vehicles for biofilm dispersal. ACS Appl. Mater. Interfaces 2019, 11, 20660–20669. [Google Scholar] [CrossRef]
- Perez, S.M.; Biondi, E.; Laurita, R.; Proto, M.; Sarti, F.; Gherardi, M.; Bertaccini, A.; Colombo, V. Plasma activated water as resistance inducer against bacterial leaf spot of tomato. PLoS ONE 2019, 14, e0217788. [Google Scholar] [CrossRef]
- Maher, M.F.; Nasti, R.A.; Vollbrecht, M.; Starker, C.G.; Clark, M.D.; Voytas, D.F. Plant gene editing through de novo induction of meristems. Nat. Biotechnol. 2020, 38, 84–89. [Google Scholar] [CrossRef]
- Baek, K.H.; Heo, Y.S.; Park, J.Y.; Kang, T.; Lee, Y.E.; Lim, J.; Kim, S.B.; Jo, C. Inactivation of Salmonella typhimurium by non-thermal plasma bubbles: Exploring the key reactive species and the influence of organic matter. Foods 2020, 9, 1689. [Google Scholar] [CrossRef]
- Govaert, M.; Smet, C.; Verheyen, D.; Walsh, J.L.; Van Impe, J.F. Combined effect of Cold Atmospheric Plasma and hydrogen peroxide treatment on mature Listeria monocytogenes and Salmonella typhimurium biofilms. Front. Microbiol. 2019, 10, 2674. [Google Scholar] [CrossRef]
- Hassan, M.E.; Janda, M.; Machala, Z. Transport of Gaseous Hydrogen Peroxide and Ozone into Bulk Water vs. Electrosprayed Aerosol. Water 2021, 13, 182. [Google Scholar] [CrossRef]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and sterilization using plasma technology: Fundamentals and future perspectives for biological applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Salvi, D. Evaluation of plasma-activated water (PAW) as a novel disinfectant: Effectiveness on Escherichia coli and Listeria innocua, physicochemical properties, and storage stability. LWT 2021, 149, 111847. [Google Scholar] [CrossRef]
- Xia, B.; Vyas, H.K.N.; Zhou, R.; Zhang, T.; Hong, J.; Rothwell, J.G.; Rice, S.A.; Carter, D.; Ostrikov, K.K.; Cullen, P.J. The Importance of Superoxide Anion for Escherichia coli Biofilm Removal Using Plasma-Activated Water. J. Environ. Chem. Eng. 2023, 11, 109977. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Ojha, S.; Burgess, C.M.; Sun, D.W.; Tiwari, B.K. Inactivation efficacy of plasma-activated water: Influence of plasma treatment time, exposure time and bacterial species. Int. J. Food Sci. Technol. 2021, 56, 721–732. [Google Scholar] [CrossRef]
- Feizollahi, E.; Roopesh, M. Degradation of zearalenone by atmospheric cold plasma: Effect of selected process and product factors. Food Bioprocess Technol. 2021, 14, 2107–2119. [Google Scholar] [CrossRef]
- Rathore, V.; Nema, S.K. Optimization of process parameters to generate plasma activated water and study of physicochemical properties of plasma activated solutions at optimum condition. J. Appl. Phys. 2021, 129, 084901. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Spetlikova, E.; Janda, V.; Lukes, P.; Clupek, M. Role of UV radiation, solution conductivity and pulse repetition frequency in the bactericidal effects during pulse corona discharges. In Proceedings of the WDS, Prague, Czech Republic, 1–4 June 2010; pp. 96–100. [Google Scholar]
- Lukes, P.; Clupek, M.; Babicky, V.; Sunka, P. Ultraviolet radiation from the pulsed corona discharge in water. Plasma Sources Sci. Technol. 2008, 17, 024012. [Google Scholar] [CrossRef]
- Fan, W.; Cui, J.; Li, Q.; Huo, Y.; Xiao, D.; Yang, X.; Yu, H.; Wang, C.; Jarvis, P.; Lyu, T. Bactericidal efficiency and photochemical mechanisms of micro/nano bubble-enhanced visible light photocatalytic water disinfection. Water Res. 2021, 203, 117531. [Google Scholar] [CrossRef]
- Wassmann, M.; Moeller, R.; Rabbow, E.; Panitz, C.; Horneck, G.; Reitz, G.; Douki, T.; Cadet, J.; Stan-Lotter, H.; Cockell, C.S. Survival of spores of the UV-resistant Bacillus subtilis strain MW01 after exposure to low-earth orbit and simulated martian conditions: Data from the space experiment ADAPT on EXPOSE-E. Astrobiology 2012, 12, 498–507. [Google Scholar] [CrossRef]
- Atkinson, A.J.; Apul, O.G.; Schneider, O.; Garcia-Segura, S.; Westerhoff, P. Nanobubble technologies offer opportunities to improve water treatment. Acc. Chem. Res. 2019, 52, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Oshita, S.; Kawabata, S.; Makino, Y.; Yoshimoto, T. Identification of ROS produced by nanobubbles and their positive and negative effects on vegetable seed germination. Langmuir 2016, 32, 11295–11302. [Google Scholar] [CrossRef]
- Liu, S.; Oshita, S.; Makino, Y.; Wang, Q.; Kawagoe, Y.; Uchida, T. Oxidative capacity of nanobubbles and its effect on seed germination. ACS Sustain. Chem. Eng. 2016, 4, 1347–1353. [Google Scholar] [CrossRef]
- Sampaio, A.d.G.; Chiappim, W.; Milhan, N.V.M.; Botan Neto, B.; Pessoa, R.; Koga-Ito, C.Y. Effect of the pH on the Antibacterial Potential and Cytotoxicity of Different Plasma-Activated Liquids. Int. J. Mol. Sci. 2022, 23, 13893. [Google Scholar] [CrossRef] [PubMed]
- Boudam, M.K.; Moisan, M.; Saoudi, B.; Popovici, C.; Gherardi, N.; Massines, F. Bacterial spore inactivation by atmospheric-pressure plasmas in the presence or absence of UV photons as obtained with the same gas mixture. J. Phys. D Appl. Phys. 2006, 39, 3494. [Google Scholar] [CrossRef]
- Dobrin, D.; Magureanu, M.; Mandache, N.B.; Ionita, M.-D. The effect of non-thermal plasma treatment on wheat germination and early growth. Innov. Food Sci. Emerg. Technol. 2015, 29, 255–260. [Google Scholar] [CrossRef]
- Rehman, M.U.; Jawaid, P.; Uchiyama, H.; Kondo, T. Comparison of free radicals formation induced by cold atmospheric plasma, ultrasound, and ionizing radiation. Arch. Biochem. Biophys. 2016, 605, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.-J.; Masur, K. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2019, 400, 39–62. [Google Scholar] [CrossRef]
- Reece, S.M.; Sinha, A.; Grieshop, A.P. Primary and photochemically aged aerosol emissions from biomass cookstoves: Chemical and physical characterization. Environ. Sci. Technol. 2017, 51, 9379–9390. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, Y.; Feng, H.; Ma, R.; Tian, Y.; Zhang, J.; Fang, J. A study of oxidative stress induced by non-thermal plasma-activated water for bacterial damage. Appl. Phys. Lett. 2013, 102, 203701. [Google Scholar] [CrossRef]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold plasma: A novel non-thermal technology for food processing. Food Biophys. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Matafonova, G.; Batoev, V. Review on low-and high-frequency sonolytic, sonophotolytic and sonophotochemical processes for inactivating pathogenic microorganisms in aqueous media. Water Res. 2019, 166, 115085. [Google Scholar] [CrossRef]



| Source | DF | Adj SS | Adj MS | F-Value | p Value |
|---|---|---|---|---|---|
| Model | 4 | 92.7473 | 23.1868 | 2467.43 | 0.000 |
| Blocks | 1 | 0.0003 | 0.0003 | 0.03 | 0.860 |
| Linear | 2 | 67.7220 | 33.8610 | 3603.32 | 0.000 |
| MNB | 1 | 28.0635 | 28.0635 | 2986.38 | 0.000 |
| UV | 1 | 39.6585 | 39.6585 | 4220.27 | 0.000 |
| 2-Way Interactions | 1 | 25.0250 | 25.0250 | 2663.04 | 0.000 |
| MNB*UV | 1 | 25.0250 | 25.0250 | 2663.04 | 0.000 |
| Error | 11 | 0.1034 | 0.0094 | ||
| Total | 15 | 92.8507 |
| Parameters | ||||||
|---|---|---|---|---|---|---|
| Response | Goal | Lower | Target | Upper | Weight | Importance |
| Survival of E. coli | Minimum | 0 | 6 | 1 | 1 | |
| Solution | MNB | UV | Survival of E. coli Fit | Composite Desirability | ||
| 1 | 10 | 20 | −0.0000000 | 1 | ||
| Multiple Response Prediction | ||||||
| Variable | Setting | |||||
| MNB | 10 | |||||
| UV | 20 | |||||
| Response | Fit | SE Fit | 95% CI | 95% PI | ||
| Survival of E. coli | −0.0000 | 0.0485 | (−0.1067, 0.1067) | (−0.2385, 0.2385) | ||
| Source | DF | Adj SS | Adj MS | F-Value | p Value |
|---|---|---|---|---|---|
| Model | 4 | 70.2937 | 17.5734 | 999.67 | 0.000 |
| Blocks | 1 | 0.0021 | 0.0021 | 0.12 | 0.752 |
| Linear | 2 | 51.7780 | 25.8890 | 1472.71 | 0.000 |
| UV | 1 | 26.6085 | 26.6085 | 1513.64 | 0.000 |
| US | 1 | 25.1695 | 25.1695 | 1431.78 | 0.000 |
| 2-Way Interactions | 1 | 18.5136 | 18.5136 | 1053.16 | 0.000 |
| UV*US | 1 | 18.5136 | 18.5136 | 1053.16 | 0.000 |
| Error | 3 | 0.0527 | 0.0176 | ||
| Total | 7 | 70.3465 |
| Parameters | ||||||
|---|---|---|---|---|---|---|
| Response | Goal | Lower | Target | Upper | Weight | Importance |
| Survival of E. coli | Minimum | 0 | 8.42 | 1 | 1 | |
| Solution | UV | US | Survival of E. coli Fit | Composite Desirability | ||
| 1 | 20 | 20 | 0.0000000 | 1 | ||
| Multiple Response Prediction | ||||||
| Variable | Setting | |||||
| UV | 20 | |||||
| US | 20 | |||||
| Response | Fit | SE Fit | 95% CI | 95% PI | ||
| Survival of E. coli | 0.0000 | 0.0938 | (−0.2984, 0.2984) | (−0.5168, 0.5168) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moonsub, K.; Seesuriyachan, P.; Boonyawan, D.; Wattanutchariya, W. Synergistic Effect of Plasma-Activated Water with Micro/Nanobubbles, Ultraviolet Photolysis, and Ultrasonication on Enhanced Escherichia coli Inactivation in Chicken Meat. Processes 2024, 12, 567. https://doi.org/10.3390/pr12030567
Moonsub K, Seesuriyachan P, Boonyawan D, Wattanutchariya W. Synergistic Effect of Plasma-Activated Water with Micro/Nanobubbles, Ultraviolet Photolysis, and Ultrasonication on Enhanced Escherichia coli Inactivation in Chicken Meat. Processes. 2024; 12(3):567. https://doi.org/10.3390/pr12030567
Chicago/Turabian StyleMoonsub, Kochakon, Phisit Seesuriyachan, Dheerawan Boonyawan, and Wassanai Wattanutchariya. 2024. "Synergistic Effect of Plasma-Activated Water with Micro/Nanobubbles, Ultraviolet Photolysis, and Ultrasonication on Enhanced Escherichia coli Inactivation in Chicken Meat" Processes 12, no. 3: 567. https://doi.org/10.3390/pr12030567







