Exploring the REEs Energy Footprint: Interlocking AI/ML with an Empirical Approach for Analysis of Energy Consumption in REEs Production
Abstract
1. Introduction
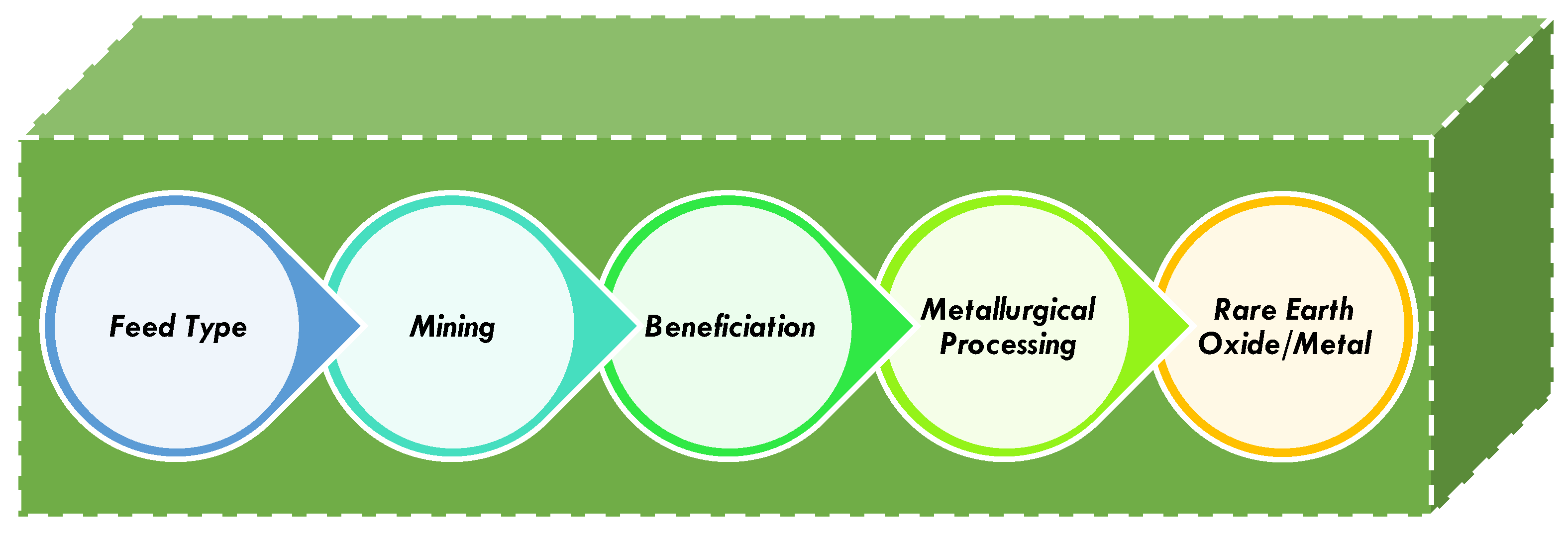
2. Methodology and Scope
3. Functional Units and Factor Determination
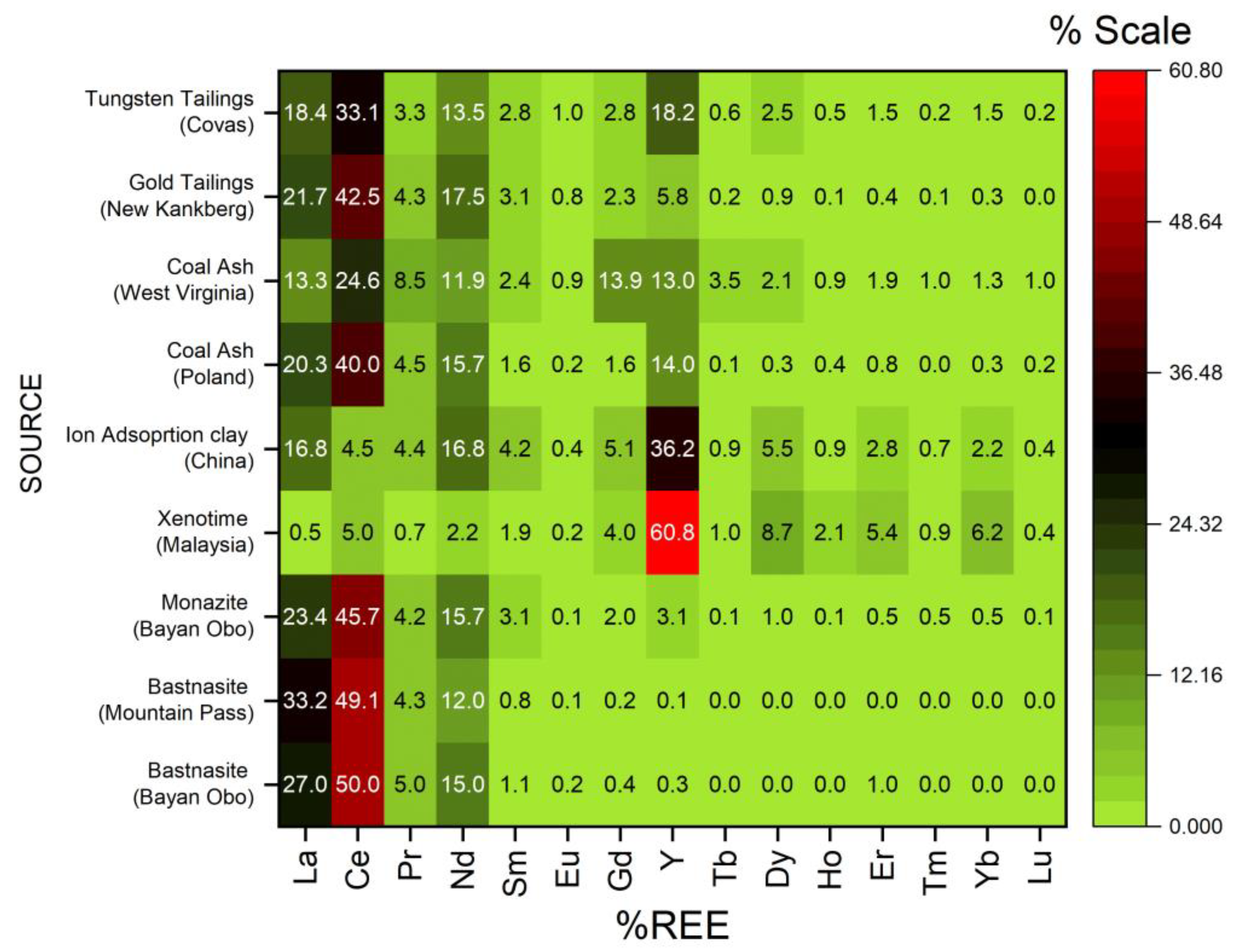
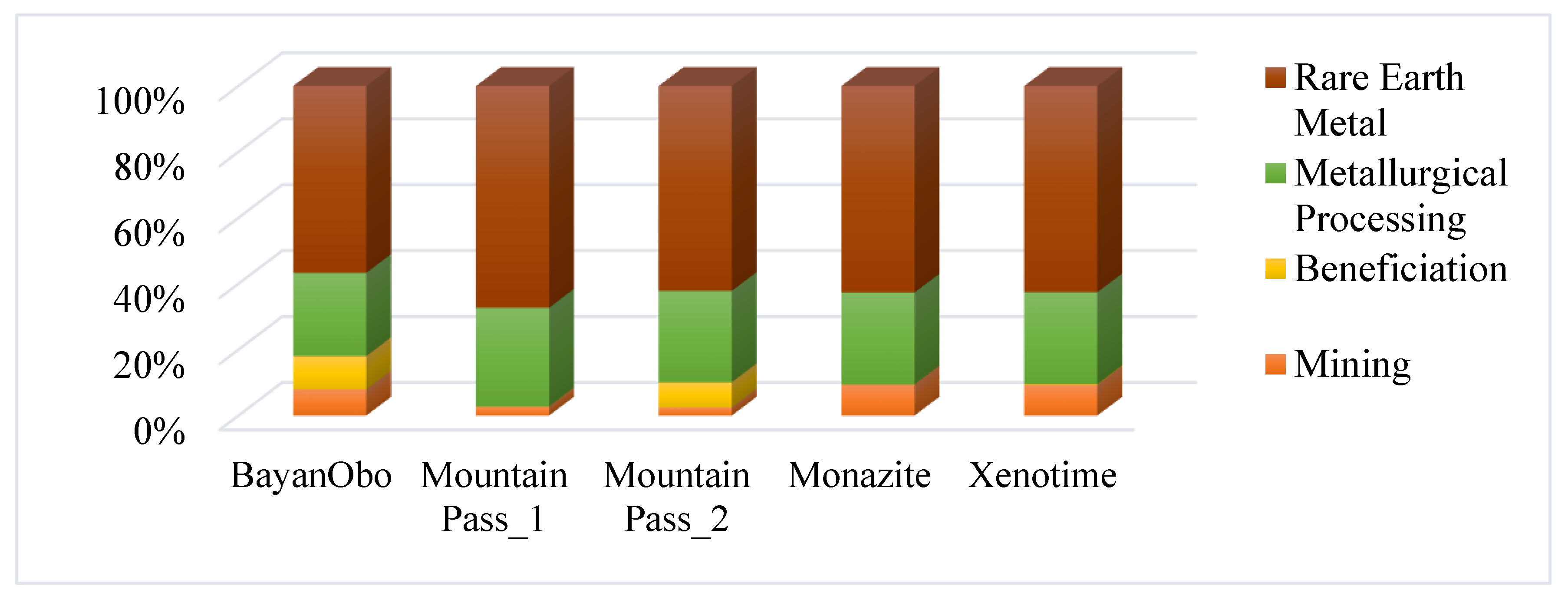
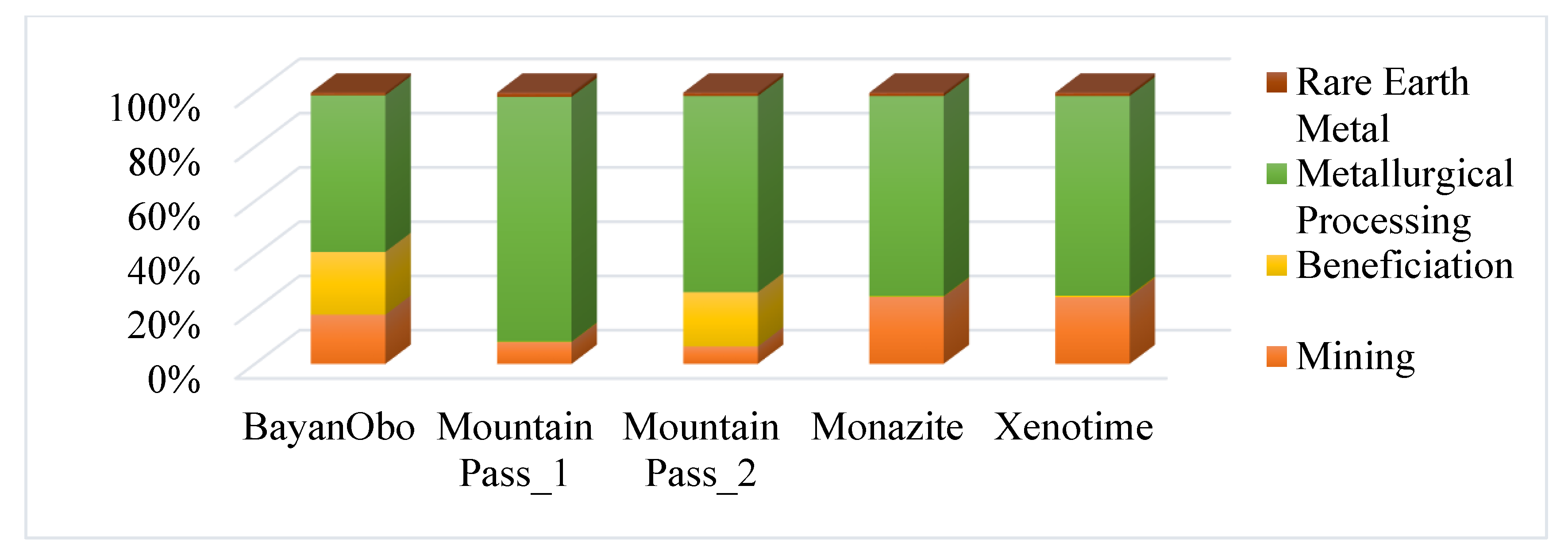
3.1. Composition of REEs in Feed Type
3.2. Mining Unit
3.3. Beneficiation Unit
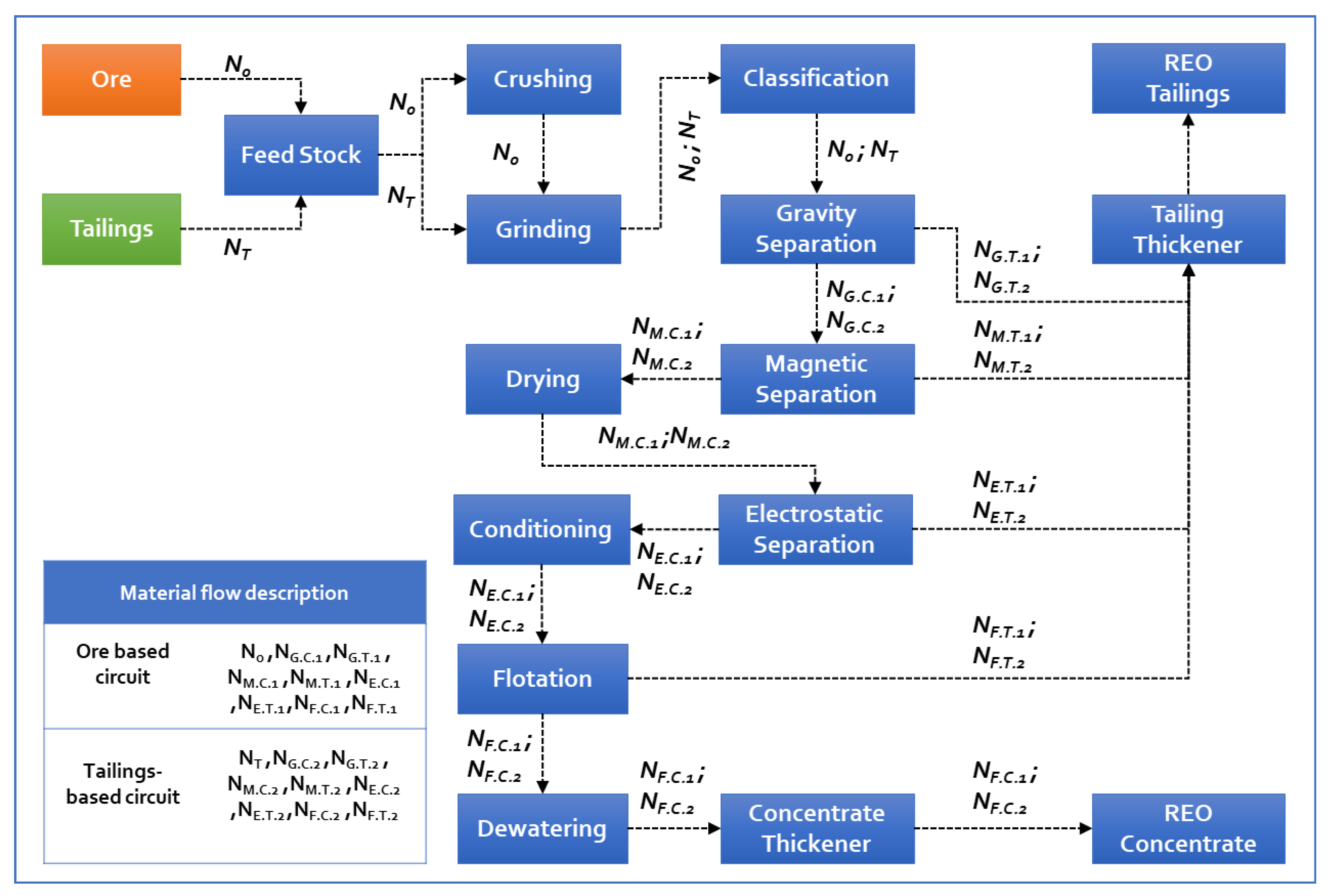
| Unit Operation | Factors | Quantity Required | Equivalent Energy | Recovery % | Material Flow (Tons) | Net Energy Consumption (KJ) |
|---|---|---|---|---|---|---|
| Crushing (c) | Electrical Energy | ae.c (KWh) | ee.c (KJ/KWh) | - | No | ae.c × ee.c × No |
| Steel (Installation) | ac.s (Kg) | ec.s (KJ/Kg) | - | No | ac.s × ec.s × No | |
| Grinding (g) | Electrical Energy | ae.g (KWh) | ee.g (KJ/KWh) | - | No, NT | (ae.g × ee.g × No) + (ae.g × ee.g × NT) |
| Steel (Installation and grinding media) | ag.s (Kg) | eg.s (KJ/Kg) | - | No, NT | (ag.s × eg.s × No) + (ag.s × eg.s × NT) | |
| Water Pumping | aw.g (KWh) | ew.g (KJ/KWh) | - | No, NT | (aw.g × ew.g × No) + (aw.g × ew.g × NT) | |
| Classification (cl) | Electrical Energy | ae.cl (KWh) | ee.cl (KJ/KWh) | - | No, NT | (ae.cl × ee.cl × No) + (ae.cl × ee.cl × NT) |
| Steel (Installation) | acl.s (Kg) | ecl.s (KJ/Kg) | - | No, NT | (acl.s × ecl.s × No) + (acl.s × ecl.s × NT) | |
| Water Pumping | aw.cl (KWh) | ew.cl (KJ/KWh) | - | No, NT | (aw.cl × ew.cl × No) + (aw.cl × ew.cl × NT) | |
| Gravity separation (gs) | Electrical Energy | ae.gs (KWh) | ee.gs (KJ/KWh) | - | No, NT | (ae.gs × ee.gs × No) + (ae.gs × ee.gs × NT) |
| Steel (Installation) | ags.s (Kg) | egs.s (KJ/Kg) | - | No, NT | (ags.s × egs.s × No) + (ags.s × egs.s × NT) | |
| Water Pumping | aw.gs (KWh) | ew.gs (KJ/KWh) | - | No, NT | (aw.gs× ew.gs × No) + (aw.gs × ew.gs × NT) | |
| Concentrate Pumping | ac.gs (KWh) | ec.gs (KJ/KWh) | Rgs | NG.C.1, NG.C.2 | (ac.gs× ec.gs × NG.C.1) + (ac.gs × ec.gs × NG.C.2) | |
| Tailings Pumping | at.gs (KWh) | et.gs (KJ/KWh) | 1-Rgs | NG.T.1, NG.T.2 | (at.gs× et.gs × NG.T.1) + (at.gs × et.gs × NG.T.2) | |
| Magnetic separation (ms) | Electrical Energy | ae.ms (KWh) | ee.ms (KJ/KWh) | - | NG.C.1, NG.C.2 | (ae.ms × ee.ms × NG.C.1) + (ae.ms × ee.ms × NG.C.2) |
| Steel (Installation) | ams.s (Kg) | ems.s (KJ/Kg) | - | NG.C.1, NG.C.2 | (ams.s × ems.s × NG.C.1) + (ams.s × ems.s × NG.C.2) | |
| Water Pumping | aw.ms (KWh) | ew.ms (KJ/KWh) | - | NG.C.1, NG.C.2 | (aw.ms× ew.ms × NG.C.1) + (aw.ms × ew.ms × NG.C.2) | |
| Concentrate Pumping | ac.ms (KWh) | ec.ms (KJ/KWh) | Rms | NM.C.1, NM.C.2 | (ac.ms× ec.ms × NM.C.1) + (ac.ms × ec.ms × NM.C.2) | |
| Tailings Pumping | at.ms (KWh) | et.ms (KJ/KWh) | 1-Rms | NM.T.1, NM.T.2 | (at.ms× et.ms × NM.T.1) + (at.ms × et.ms × NM.T.2) | |
| Drying (d) | Electrical Energy | ae.d (KWh) | ee.d (KJ/KWh) | - | NM.C.1, NM.C.2 | (ae.d × ee.d × NM.C.1) + (ae.d × ee.d × NM.C.2) |
| Fuel oil/Steam | ao.d (gal) | eo.d (KJ/gal) | - | NM.C.1, NM.C.2 | (ao.d × eo.d × NM.C.1) + (ao.d × eo.d × NM.C.2) | |
| Electrical separation (es) | Electrical Energy | ae.es (KWh) | ee.es (KJ/KWh) | - | NM.C.1, NM.C.2 | (ae.es × ee.es × NM.C.1) + (ae.es × ee.es × NM.C.2) |
| Steel (Installation) | aes.s (Kg) | ees.s (KJ/Kg) | - | NM.C.1, NM.C.2 | (aes.s × ees.s × NM.C.1) + (aes.s × ees.s × NM.C.2) | |
| Electrical Energy (Concentrate processing) | ae.c.es (KWh) | ee.c.es (KJ/KWh) | Res | NE.C.1, NE.C.2 | (ae.c.es × ee.c.es × NE.C.1) + (ae.c.es × ee.c.es × NE.C.2) | |
| Electrical Energy (Tailing processing) | ae.t.es (KWh) | ee.t.es (KJ/KWh) | 1-Res | NE.T.1, NE.T.2 | (ae.t.es × ee.t.es × NE.T.1) + (ae.t.es × ee.t.es × NE.T.2) | |
| Conditioning (con) | Electrical Energy | ae.con (KWh) | ee.con (KJ/KWh) | - | NE.C.1, NE.C.2 | (ae.con × ee.con × NE.C.1) + (ae.con × ee.con × NE.C.2) |
| Steel (Installation) | acon.s (Kg) | econ.s (KJ/Kg) | - | NE.C.1, NE.C.2 | (acon.s × econ.s × NE.C.1) + (acon.s × econ.s × NE.C.2) | |
| Water Pumping | aw.con (KWh) | ew.con (KJ/KWh) | - | NE.C.1, NE.C.2 | (aw.con × ew.con × NE.C.1) + (aw.con × ew.con × NE.C.2) | |
| Fuel oil | ao.con (gal) | eo.con (KJ/gal) | - | NE.C.1, NE.C.2 | (ao.con × eo.con × NE.C.1) + (ao.con × eo.con × NE.C.2) | |
| Chemicals | achem.con (Kg) | echem.con (KJ/Kg) | - | NE.C.1, NE.C.2 | (achem.con × echem.con × NE.C.1) + (achem.con × echem.con × NE.C.2) | |
| Electrical Energy (Slurry pumping) | ae.sl.con (KWh) | ee.sl.con (KJ/KWh) | - | NE.C.1, NE.C.2 | (ae.sl.con × ee.sl.con × NE.C.1) + (ae.sl.con × ee.sl.con × NE.C.2) | |
| Flotation (flo) | Electrical Energy | ae.flo (KWh) | ee.flo (KJ/KWh) | - | NE.C.1, NE.C.2 | (ae.flo × ee.flo × NE.C.1) + (ae.flo × ee.flo × NE.C.2) |
| Steel (Installation) | aflo.s (Kg) | eflo.s (KJ/Kg) | - | NE.C.1, NE.C.2 | (aflo.s × eflo.s × NE.C.1) + (aflo.s × eflo.s × NE.C.2) | |
| Water Pumping | aw.flo (KWh) | ew.flo (KJ/KWh) | - | NE.C.1, NE.C.2 | (aw.flo × ew.flo × NE.C.1) + (aw.flo × ew.flo × NE.C.2) | |
| Fuel oil | ao.flo (gal) | eo.flo (KJ/gal) | - | NE.C.1, NE.C.2 | (ao.flo × eo.flo × NE.C.1) + (ao.flo × eo.flo × NE.C.2) | |
| Reagents | area.flo (Kg) | erea.flo (KJ/Kg) | - | NE.C.1, NE.C.2 | (area.flo × erea.flo × NE.C.1) + (area.flo × erea.flo × NE.C.2) | |
| Concentrate Pumping | ac.flo (KWh) | ec.flo (KJ/KWh) | Rflo | NF.C.1, NF.C.2 | (ac.flo × ec.flo × NF.C.1) + (ac.flo × ec.flo × NF.C.2) | |
| Tailings Pumping | at.flo (KWh) | et.flo (KJ/KWh) | 1-Rflo | NF.T.1, NF.T.2 | (at.flo × et.flo × NF.T.1) + (at.flo × et.flo × NF.T.2) | |
| Dewatering (dew) (Thickening -Filtration) | Electrical Energy | ae.dew (KWh) | ee.dew (KJ/KWh) | - | NF.C.1, NF.C.2 | (ae.dew × ee.dew × NF.C.1) + (ae.dew × ee.dew × NF.C.2) |
| Steel (Installation) | adew.s (Kg) | edew.s (KJ/Kg) | - | NF.C.1, NF.C.2 | (adew.s × edew.s × NF.C.1) + (adew.s × edew.s × NF.C.2) | |
| Water Pumping | aw.dew (KWh) | ew.dew (KJ/KWh) | - | NF.C.1, NF.C.2 | (aw.dew × ew.dew × NF.C.1) + (aw.dew × ew.dew × NF.C.2) | |
| Concentrate Disposal | ac.dew (KWh) | ec.dew (KJ/KWh) | - | NF.C.1, NF.C.2 | (ac.dew × ec.dew × NF.C.1) + (ac.dew × ec.dew × NF.C.2) | |
| Tailing Disposal | at.dew (KWh) | et.dew (KJ/KWh) | - | NG.T.1, NG.T.2, NM.T.1, NM.T.2, NE.T.1, NE.T.2, NF.T.1, NF.T.2 | (at.dew × et.dew × (NG.T.1 + NM.T.1 + NE.T.1 + NF.T.1)) + (at.dew × et.dew × (NG.T.2 + NM.T.2 + NE.T.2 + NF.T.2)) |
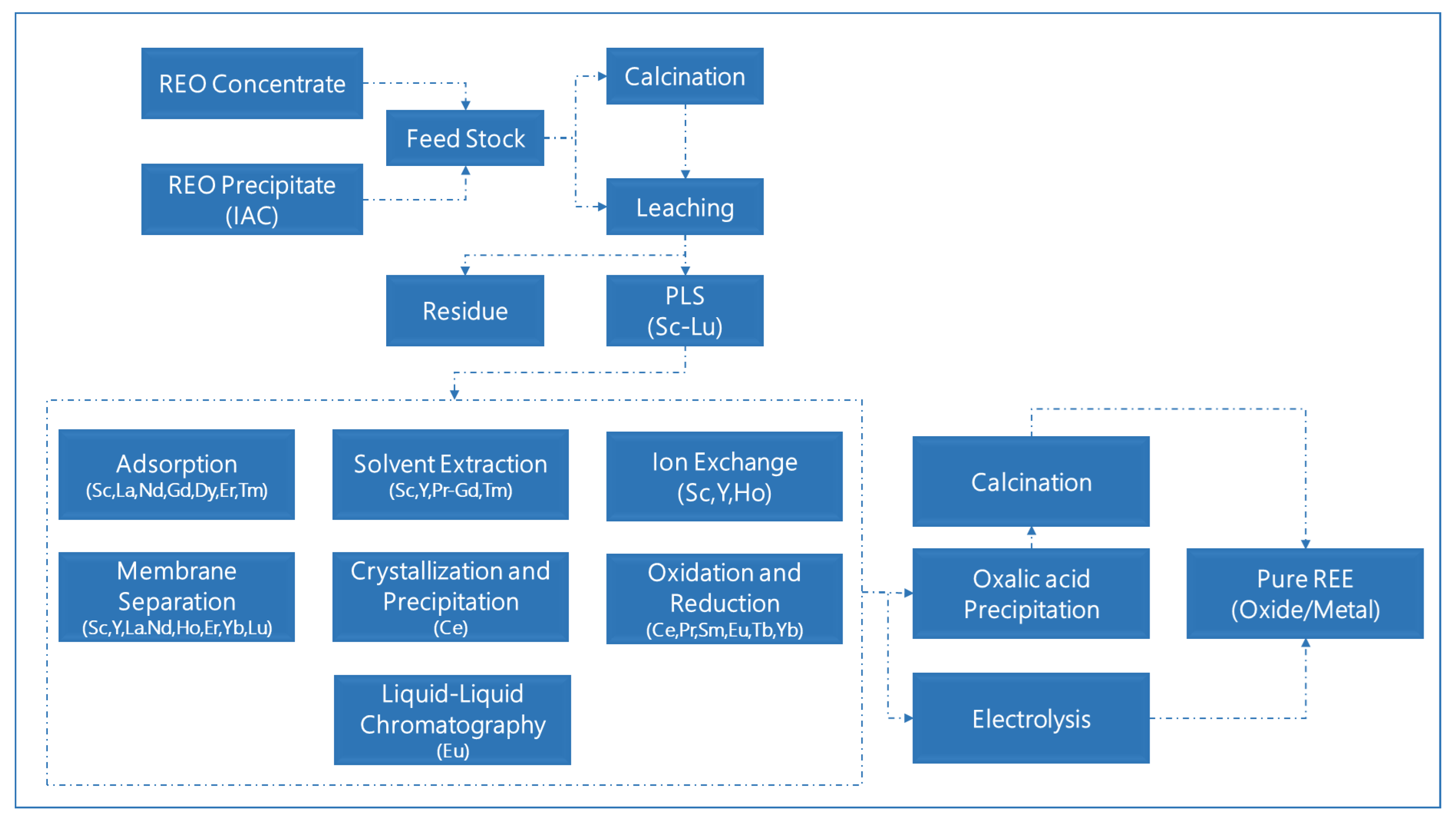
3.4. Metallurgical Processing Unit
| Separation Method | Suitable REEs |
|---|---|
| Adsorption | Sc [32], La [33], Nd [34], Gd [35,36], Dy [37,38], Er [39], Tm [40] |
| Solvent Extraction | Sc [41], Y [42], Pr [43], Nd [44], Sm [45], Eu [46], Gd [36], Tb [47], Tm [48] |
| Ion exchange | Sc [49,50], Y [51], Ho [52] |
| Membrane Separation | Sc [53], Y [54], La [55], Nd [56,57], Ho [58,59], Er [60], Yb [61,62], Lu [63,64] |
| Crystallization and Precipitation | Ce [65] |
| Oxidation and Reduction | Ce [1], Pr [1], Sm [1], Eu [1,46], Tb [1], Yb [1] |
| Liquid–Liquid Chromatography | Y [66] |
4. Analysis of Energy Consumption and Opportunities for Reduction
4.1. Data Analysis of the Literature Concerning the EC per Ton of REE Metal Produced
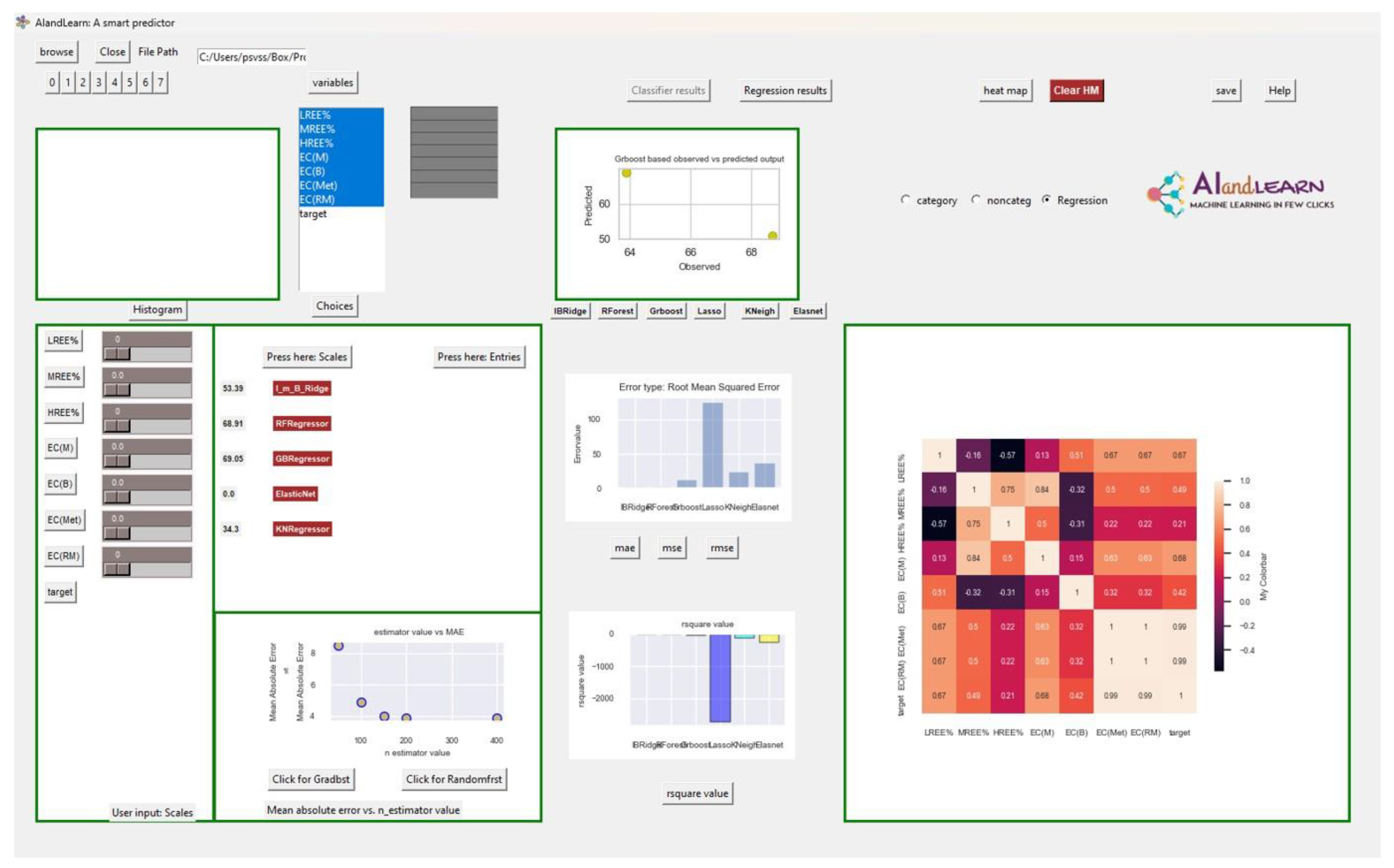
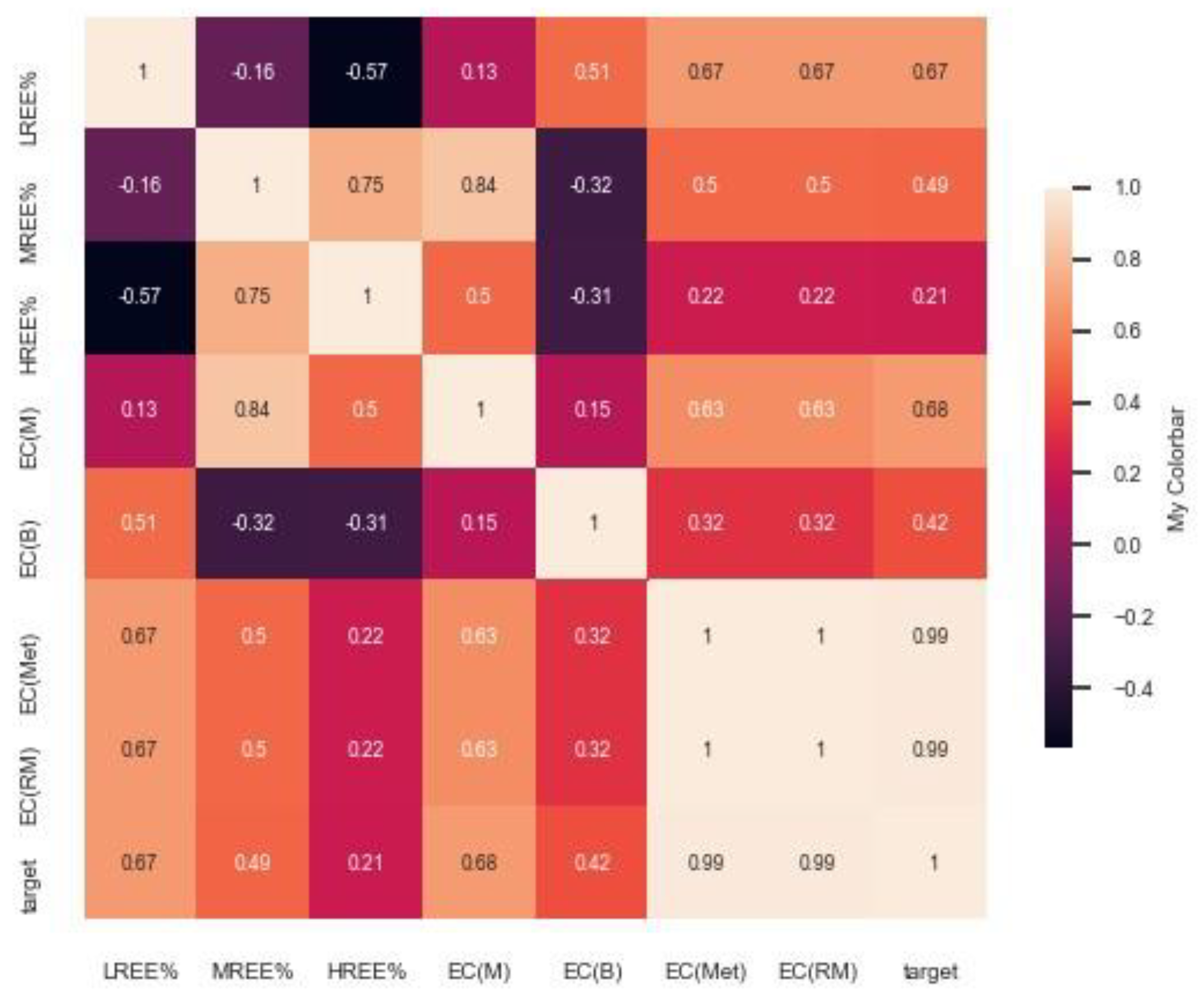
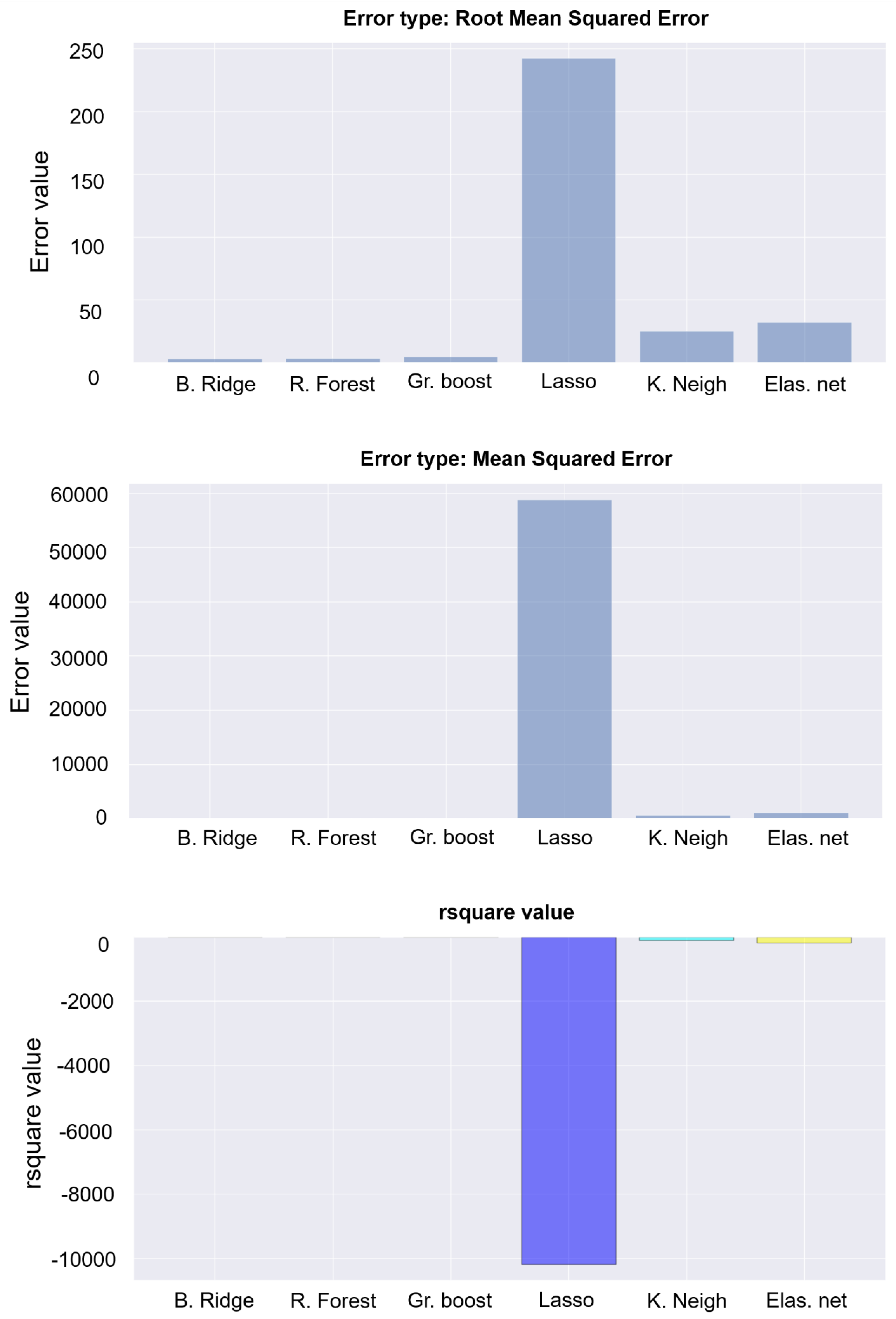
4.2. Prediction of EC for Secondary Sources Using an AI/ML-Based Approach
4.3. Perspectives on Opportunities for Energy Reduction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gupta, C.K.; Krishnamurthy, N. Extractive metallurgy of rare earths. Int. Mater. Rev. 1992, 37, 197–248. [Google Scholar] [CrossRef]
- Pathapati, S.V.S.H.; Free, M.L.; Sarswat, P.K. A Comparative Study on Recent Developments for Individual Rare Earth Elements Separation. Processes 2023, 11, 2070. [Google Scholar] [CrossRef]
- Preinfalk, C.; Morteani, G. The Industrial Applications of Rare Earth Elements. In Lanthanides, Tantalum and Niobium: Mineralogy, Geochemistry, Characteristics of Primary Ore Deposits, Prospecting, Processing and Applications Proceedings of a workshop in Berlin, November; Springer: Berlin/Heidelberg, Germany, 1989; pp. 359–370. [Google Scholar] [CrossRef]
- Vaughan, J.; Gontijo, V.L.; Valenta, R.; Alonso, E. Rare earth element sources, end-use demand trends, and hydrometallurgical separations. In Proceedings of the 26th World Mining Congress, Brisbane, Australia, 26–29 June 2023; pp. 713–723. [Google Scholar]
- Cardoso, C.E.D.; Almeida, J.C.; Lopes, C.B.; Trindade, T.; Vale, C.; Pereira, E. Recovery of rare earth elements by carbon-based nanomaterials—A review. Nanomaterials 2019, 9, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://css.umich.edu/publications/factsheets/material-resources/critical-materials-factsheet (accessed on 23 November 2023).
- Wall, F. Rare Earth Elements. In Encyclopedia of Geology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 680–693. [Google Scholar] [CrossRef]
- Julapong, P.; Numprasanthai, A.; Tangwattananukul, L.; Juntarasakul, O.; Srichonphaisarn, P.; Aikawa, K.; Park, I.; Ito, M.; Tabelin, C.B.; Phengsaart, T. Rare Earth Elements Recovery from Primary and Secondary Resources Using Flotation: A Systematic Review. Appl. Sci. 2023, 13, 8364. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Fujita, Y.; McCall, S.K.; Ginosar, D. Recycling rare earths: Perspectives and recent advances. MRS Bull. 2022, 47, 283–288. [Google Scholar] [CrossRef]
- Gaustad, G.; Williams, E.; Leader, A. Rare earth metals from secondary sources: Review of potential supply from waste and byproducts. Resour. Conserv. Recycl. 2021, 167, 105213. [Google Scholar] [CrossRef]
- Weng, Z.; Haque, N.; Mudd, G.M.; Jowitt, S.M. Assessing the energy requirements and global warming potential of the production of rare earth elements. J. Clean. Prod. 2016, 139, 1282–1297. [Google Scholar] [CrossRef]
- Broussaud, A.; Barbery, G. Applications of Energy Analysis to Mineral and Metallurgical Process Selection and Development. Int. J. Miner. Process. 1978, 5, 93–105. [Google Scholar] [CrossRef]
- Hale, R.W. Energy Use Patterns in Metallurgical and Nonmetallic Mineral Processing; Battelle Columbus Laboratories for US Bureau of Mines: Columbus, OH, USA, 1976. [Google Scholar]
- Talens, P.L.; Villalba Méndez, G. Material and energy requirement for rare earth production. JOM 2013, 65, 1327–1340. [Google Scholar] [CrossRef]
- Zaimes, G.G.; Hubler, B.J.; Wang, S.; Khanna, V. Environmental life cycle perspective on rare earth oxide production. ACS Sustain. Chem. Eng. 2015, 3, 237–244. [Google Scholar] [CrossRef]
- Vahidi, E.; Navarro, J.; Zhao, F. An initial life cycle assessment of rare earth oxides production from ion-adsorption clays. Resour. Conserv. Recycl. 2016, 113, 1–11. [Google Scholar]
- Deng, H.; Kendall, A. Life cycle assessment with primary data on heavy rare earth oxides from ion-adsorption clays. Int. J. Life Cycle Assess. 2019, 24, 1643–1652. [Google Scholar] [CrossRef]
- Grzesik, K.; Kossakowska, K.; Bieda, B.; Kozakiewicz, R. Screening Life Cycle Assessment of beneficiation processes for Rare Earth Elements recovery from secondary sources. IOP Conf. Ser. Earth Environ. Sci. 2019, 214, 012068. [Google Scholar] [CrossRef]
- Kossakowska, K.; Grzesik, K. The significance of energy consumption in environmental impact of rare earth elements recovery from tailings and mining waste. E3S Web Conf. 2019, 108, 02011. [Google Scholar] [CrossRef]
- Yang, D.; Gao, S.; Hong, J.; Ye, L.; Ma, X.; Qi, C.; Li, X. Life cycle assessment of rare earths recovery from waste fluorescent powders—A case study in China. Waste Manag. 2019, 99, 60–70. [Google Scholar] [CrossRef]
- Schreiber, A.; Marx, J.; Zapp, P. Life Cycle Assessment studies of rare earths production—Findings from a systematic review. Sci. Total Environ. 2021, 791, 148257. [Google Scholar] [CrossRef]
- Navarro, J.; Zhao, F. Life-cycle assessment of the production of rare-earth elements for energy applications: A review. Front. Energy Res. 2014, 2, 45. [Google Scholar] [CrossRef]
- Guyonnet, D.; Planchon, M.; Rollat, A.; Escalon, V.; Tuduri, J.; Charles, N.; Vaxelaire, S.; Dubois, D.; Fargier, H. Material flow analysis applied to rare earth elements in Europe. J. Clean. Prod. 2015, 107, 215–228. [Google Scholar] [CrossRef]
- Das, S.; Gaustad, G.; Sekar, A.; Williams, E. Techno-economic analysis of supercritical extraction of rare earth elements from coal ash. J. Clean. Prod. 2018, 189, 539–551. [Google Scholar] [CrossRef]
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Rare Earths, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Norgate, T.; Haque, N. Energy and greenhouse gas impacts of mining and mineral processing operations. J. Clean. Prod. 2010, 18, 266–274. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J.A. Wills’ Mineral Processing Technology; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Jeswiet, J.; Szekeres, A. Energy Consumption in Mining Comminution. Procedia CIRP 2016, 48, 140–145. [Google Scholar] [CrossRef]
- Sunil, S.R.; Rayapudi, V.; Dhawan, N. Recovery of Iron Values from Discarded Iron Ore Slimes. Min. Metall Explor. 2020, 37, 287–295. [Google Scholar] [CrossRef]
- Free, M.L. Hydrometallurgy; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Hammouda, S.B.e.n.; Sillanpää, M. Fabrication of novel metal ion imprinted xanthan gum-layered double hydroxide nanocomposite for adsorption of rare earth elements. Carbohydr. Polym. 2018, 194, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Dai, J.; Wang, Y.; Li, C.; Yan, Y. Fabrication of phosphate functionalized chiral nematic mesoporous silica films for the efficient and selective adsorption of lanthanum ions. J. Mol. Liq. 2019, 277, 786–793. [Google Scholar] [CrossRef]
- Ravi, S.; Lee, Y.-R.; Yu, K.; Ahn, J.-W.; Ahn, W.-S. Benzene triamido-tetraphosphonic acid immobilized on mesoporous silica for adsorption of Nd3+ ions in aqueous solution. Microporous Mesoporous Mater. 2018, 258, 62–71. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, E.; Zhang, F.; Dai, J.; Yan, Y. Selective adsorption and separation of gadolinium with three-dimensionally interconnected macroporous imprinted chitosan films. Cellulose 2017, 24, 977–988. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.; Torkaman, R.; Torab-Mostaedi, M.; Hemmati, A.; Ghaemi, A. High performance separation of gadolinium from samarium with the imidazolium ionic liquid through selective complexation of organophosphorus extractants. Environ. Technol. Innov. 2020, 19, 100979. [Google Scholar] [CrossRef]
- Biju, V.M.; Gladis, J.M.; Rao, T.P. Effect of γ-irradiation of ion imprinted polymer (IIP) particles for the preconcentrative separation of dysprosium from other selected lanthanides. Talanta 2003, 60, 747–754. [Google Scholar] [CrossRef]
- Liu, E.; Xu, X.; Zheng, X.; Zhang, F.; Liu, E.; Li, C. An ion imprinted macroporous chitosan membrane for efficiently selective adsorption of dysprosium. Sep. Purif. Technol. 2017, 189, 288–295. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Prasada Rao, T. Ion imprinted polymer solid phase extraction (IIP-SPE) for preconcentrative separation of erbium(III) from adjacent lanthanides and yttrium. Sep. Sci. Technol. 2006, 41, 233–246. [Google Scholar] [CrossRef]
- Kubra, K.T.; Hasan, M.; Hasan, N.; Salman, S.; Khaleque, A.; Sheikh, C.; Rehan, A.I.; Rasee, A.I.; Waliullah, R.M.; Awual, E.; et al. The heavy lanthanide of Thulium(III) separation and recovery using specific ligand-based facial composite adsorbent. Colloids Surf. A Physicochem. Eng. Asp. 2023, 667, 131415. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, Z.; Baba, Y.; Kubota, F.; Kamiya, N.; Goto, M. Synergistic extraction of rare-earth metals and separation of scandium using 2-thenoyltrifluoroacetone and tri-n-octylphosphine oxide in an ionic liquid system. J. Chem. Eng. Jpn. 2014, 47, 656–662. [Google Scholar] [CrossRef]
- Sengupta, A.; Mohapatra, P.K.; Kadam, R.M.; Manna, D.; Ghanty, T.K.; Iqbal, M.; Huskensc, J.; Verboomc, W. Diglycolamide-functionalized task specific ionic liquids for nuclear waste remediation: Extraction, luminescence, theoretical and EPR investigations. RSC Adv. 2014, 4, 46613–46623. [Google Scholar] [CrossRef]
- Matsumiya, M.; Nomizu, D.; Tsuchida, Y.; Sasaki, Y. Separation of rare earth elements by synergistic solvent extraction with phosphonium-based ionic liquids using a β-diketone extractant and a neutral ligand. Solvent Extr. Ion Exch. 2021, 39, 764–784. [Google Scholar] [CrossRef]
- Xing, L.; Ma, X.; Hu, K.; Yuan, H.; Wei, J.; Gao, H.; Nie, Y. Selective separation of Nd from La/Ce/Pr using phosphate-based ionic liquids: Solvent extraction studies and density functional theory. Miner. Eng. 2023, 191, 107967. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Pei, Y.; Ren, J.; Wang, J. PH-Controlled Selective Separation of Neodymium (III) and Samarium (III) from Transition Metals with Carboxyl-Functionalized Ionic Liquids. ACS Sustain. Chem. Eng. 2015, 3, 3167–3174. [Google Scholar] [CrossRef]
- Mishra, B.B.; Devi, N. Solvent extraction and separation of europium (III) using a phosphonium ionic liquid and an organophosphorus extractant—A comparative study. J. Mol. Liq. 2018, 271, 389–396. [Google Scholar] [CrossRef]
- Abreu, R.D.; Morais, C.A. Study on separation of heavy rare earth elements by solvent extraction with organophosphorus acids and amine reagents. Miner. Eng. 2014, 61, 82–87. [Google Scholar] [CrossRef]
- Zeng, Z.; Su, X.; Gao, Y.; Yu, G.; Ni, S.; Su, J.; Sun, X. Separation of lutetium using a novel bifunctional ionic liquid based on phosphonate functionalization. Sep. Purif. Technol. 2021, 264, 118439. [Google Scholar] [CrossRef]
- Hamaguchi, H.; Ohuchi, A.; Onuma, N.; Kuroda, R. Anion exchange behavior of rare earth elements in potassium sulfate medium. J. Chromatogr. A 1964, 16, 396–402. [Google Scholar] [CrossRef]
- Hamaguchi, H.; Ohuchi, A.; Shimizu, T.; Onuma, N.; Kuroda, R. Separation of Scandium from Yttrium, Rare Earths, Thorium, Zirconium, Uranium, and Other Elements by Anion Exchange Chromatography in Ammonium Sulfate Media. Anal. Chem. 1964, 36, 2304–2306. [Google Scholar] [CrossRef]
- Rychkov, V.N.; Kirillov, E.V.; Kirillov, S.V.; Bunkov, G.M.; Mashkovtsev, M.A.; Botalov, M.S.; Semenishchev, V.S.; Volkovich, V.A. Selective ion exchange recovery of rare earth elements from uranium mining solutions. AIP Conf. Proc. 2016, 1767, 020017. [Google Scholar]
- El-Dessouky, S.I.; El-Sofany, E.A.; Daoud, J.A. Studies on the sorption of praseodymium (III), holmium (III) and cobalt (II) from nitrate medium using TVEX-PHOR resin. J. Hazard. Mater. 2007, 143, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Cherkasov, R.A.; Garifzyanov, A.R.; Galeev, R.R.; Kurnosova, N.V.; Davletshin, R.R. Membrane transport of metal ions with lipophilic aminomethylphosphine oxides. Russ. J. Gen. Chem. 2011, 81, 1464–1469. [Google Scholar] [CrossRef]
- Kunthakudee, N.; Sunsandee, N.; Pancharoen, U.; Ramakul, P. Separation of yttrium from rare earth using hollow fiber-supported liquid membrane: Factorial design analysis. Desalination Water Treat. 2016, 57, 3985–3994. [Google Scholar] [CrossRef]
- Li, L.; Yu, B.; Davis, K.; King, A.; Dal-Cin, M.; Nicalek, A.; Du, N. Separation of Neodymium (III) and Lanthanum (III) via a Flat Sheet-Supported Liquid Membrane with Different Extractant-Acid Systems. Membranes 2022, 12, 1197. [Google Scholar] [CrossRef] [PubMed]
- Wannachod, T.; Leepipatpiboon, N.; Pancharoen, U.; Nootong, K. Separation and mass transport of Nd(III) from mixed rare earths via hollow fiber supported liquid membrane: Experiment and modeling. Chem. Eng. J. 2014, 248, 158–167. [Google Scholar] [CrossRef]
- Wannachod, T.; Leepipatpiboon, N.; Pancharoen, U.; Nootong, K. Synergistic effect of various neutral donors in D2EHPA for selective neodymium separation from lanthanide series via HFSLM. J. Ind. Eng. Chem. 2014, 20, 4152–4162. [Google Scholar] [CrossRef]
- Moreno, C.; Valiente, M. Studies on the mechanism of transport of lanthanide ions through supported liquid membranes containing di-(2-ethylhexyl) phosphoric acid (D2EHPA) as a carrier. J. Membr. Sci. 1999, 155, 155–162. [Google Scholar] [CrossRef]
- Kenichi Akiba, K.; Hashimoto, H.; Nakamura, S.; Saito, Y. Mutual separation of holmium, erbium, and yttrium by high-speed countercurrent chromatography. J. Liq. Chromatogr. 1995, 18, 2723–2741. [Google Scholar] [CrossRef]
- Kubota, F.; Kakoi, T.; Goto, M.; Furusaki, S.; Nakashio, F.; Hano, T. Permeation behavior of rare earth metals with a calix [4]arene carboxyl derivative in a hollow-fiber membrane. J. Membr. Sci. 2000, 165, 149–158. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Yan, Y.; Chen, J.; Dai, J.; Dai, X. Separation of adjacent heavy rare earth Lutetium (III) and Ytterbium (III) by task-specific ionic liquid Cyphos IL 104 embedded polymer inclusion membrane. J. Membr. Sci. 2020, 610, 118263. [Google Scholar] [CrossRef]
- Croft, C.F.; Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Separation of lanthanum (III), gadolinium (III) and ytterbium (III) from sulfuric acid solutions by using a polymer inclusion membrane. J. Membr. Sci. 2018, 545, 259–265. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J. Asymmetric Membrane Containing Ionic Liquid [A336][P507] for the Preconcentration and Separation of Heavy Rare Earth Lutetium. ACS Sustain. Chem. Eng. 2016, 4, 2644–2650. [Google Scholar] [CrossRef]
- Gaikwad, A.G. Behavior of the transport and separation of lanthanum, yttrium and lutetium metal ions through a celluose fiber supported solid membrane. Maced. J. Chem. Chem. Eng. 2012, 31, 255–269. [Google Scholar] [CrossRef]
- Yang, P.; Zhuang, Q.; Li, Y.; Gu, J. Green separation of rare earth elements by valence-selective crystallization of MOFs. Chem. Commun. 2019, 55, 14902–14905. [Google Scholar] [CrossRef]
- Dybczyński, R.S.; Kulisa, K.; Pyszynska, M.; Bojanowska-Czajka, A. New reversed phase-high performance liquid chromatographic method for selective separation of yttrium from all rare earth elements employing nitrilotriacetate complexes in anion exchange mode. J. Chromatogr. A 2015, 1386, 74–80. [Google Scholar] [CrossRef]
- Zhang, Z.; Allen, L.; Podder, P.; Free, M.L.; Sarswat, P.K. Recovery and enhanced upgrading of rare earth elements from coal-based resources: Bioleaching and precipitation. Minerals 2021, 11, 484. [Google Scholar] [CrossRef]
- Noble, A.; Tech, V. Development of a Cost-Effective Extraction Process for the Recovery of Heavy and Critical Rare Earth Elements from the Clays and Shales Associated with Coal; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2020. [Google Scholar]
- Sarswat, P.K.; Leake, M.; Allen, L.; Free, M.L.; Hu, X.; Kim, D.; Noble, A.; Luttrell, G.H. Efficient recovery of rare earth elements from coal based resources: A bioleaching approach. Mater. Today Chem. 2020, 16, 100246. [Google Scholar] [CrossRef]
- Azimi, G.; Forsberg, K.; Ouchi, T.; Kim, H.; Alam, S.; Baba, A.A. Rare Metal Technology 2020; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Sarswat, P.K.; Podder, P.; Zhang, Z.; Free, M.L. Autogenous acid production using a regulated bio-oxidation method for economical recovery of REEs and critical metals from coal-based resources. Appl. Surf. Sci. Adv. 2022, 11, 100283. [Google Scholar] [CrossRef]


| Factors | Quantity Needed | Equivalent Energy | Net Energy Consumption |
|---|---|---|---|
| Blasting Explosive | a1 (Kg) | e1 (KJ/Kg) | a1 × e1 |
| Diesel | a2 (gal) | e2 (KJ/gal) | a2 × e2 |
| Electricity | a3 (KWh) | e3 (KJ/KWh) | a3 × e3 |
| Bayesian Ridge Model Scales | Ideal Situation | Bastnasite (Bayan Obo) | Bastnasite (Mountain Pass_1) | Coal Ash (Poland) | Coal Ash (West Virginia) | Gold Tailings (New Kankberg) | Tungsten Tailings (Covas) |
|---|---|---|---|---|---|---|---|
| Predicted Scales | 53.39 | 142.06 | 130.41 | 141.68 | 136.75 | 144.14 | 138.05 |
| Normalized scales | 1.00 | 2.66 | 2.44 | 2.65 | 2.56 | 2.70 | 2.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathapati, S.V.S.H.; Singh, R.S.; Free, M.L.; Sarswat, P.K. Exploring the REEs Energy Footprint: Interlocking AI/ML with an Empirical Approach for Analysis of Energy Consumption in REEs Production. Processes 2024, 12, 570. https://doi.org/10.3390/pr12030570
Pathapati SVSH, Singh RS, Free ML, Sarswat PK. Exploring the REEs Energy Footprint: Interlocking AI/ML with an Empirical Approach for Analysis of Energy Consumption in REEs Production. Processes. 2024; 12(3):570. https://doi.org/10.3390/pr12030570
Chicago/Turabian StylePathapati, Subbu Venkata Satyasri Harsha, Rahulkumar Sunil Singh, Michael L. Free, and Prashant K. Sarswat. 2024. "Exploring the REEs Energy Footprint: Interlocking AI/ML with an Empirical Approach for Analysis of Energy Consumption in REEs Production" Processes 12, no. 3: 570. https://doi.org/10.3390/pr12030570
APA StylePathapati, S. V. S. H., Singh, R. S., Free, M. L., & Sarswat, P. K. (2024). Exploring the REEs Energy Footprint: Interlocking AI/ML with an Empirical Approach for Analysis of Energy Consumption in REEs Production. Processes, 12(3), 570. https://doi.org/10.3390/pr12030570










