Abstract
The use of edible oils and fats in dairy products is becoming increasingly important in the food industry because of their complementary functional properties. Most of these products are produced using food-grade enzymes as processing aids because processes involving enzymes are considered mild and environmentally friendly for regulatory purposes. The poor stability and recovery of enzymes in their native state limit their performance, and to enhance their activity, stability, and reusability, enzymes are often immobilised—a process that involves attaching them to a solid support. Additionally, immobilisation enables enzymes to selectively target specific substrates or products, making them highly efficient. These features have led to the increased use of immobilised enzymes in dairy and lipid processing and enzymes have been used to produce a broad range of products such as whey protein concentrates and isolates, peptide–lipid conjugates, lipid concentrates, structured lipids, and human milk fat substitutes. Therefore, this article reviews the current progress on different enzyme preparations and their use in lipid and dairy processing. It also summarises opportunities in enzyme-catalysed valorisation of dairy and lipid waste streams with the ultimate goals of sustainable food production and reductions in waste.
1. Introduction
Enzymes are proteins that act as biological catalysts in living organisms, including plants, animals, and microorganisms [1]. They play a crucial role in accelerating reactions, enabling the various biochemical processes necessary for life. Enzymes are highly specific in their actions, catalysing a particular reaction or a group of related reactions [2]. They function by lowering the activation energy required for a reaction to occur, making it easier and faster for the reaction to take place [3]. Enzymes are used extensively in industrial processes, such as food and beverage production, pharmaceuticals, and bioremediation [4]. However, to ensure their efficient and cost-effective use, enzymes often need to be stabilised for repeated reuse. This necessity underscores the significance of enzyme immobilisation, a vital process for enhancing their stability and repeated reuse in industrial applications.
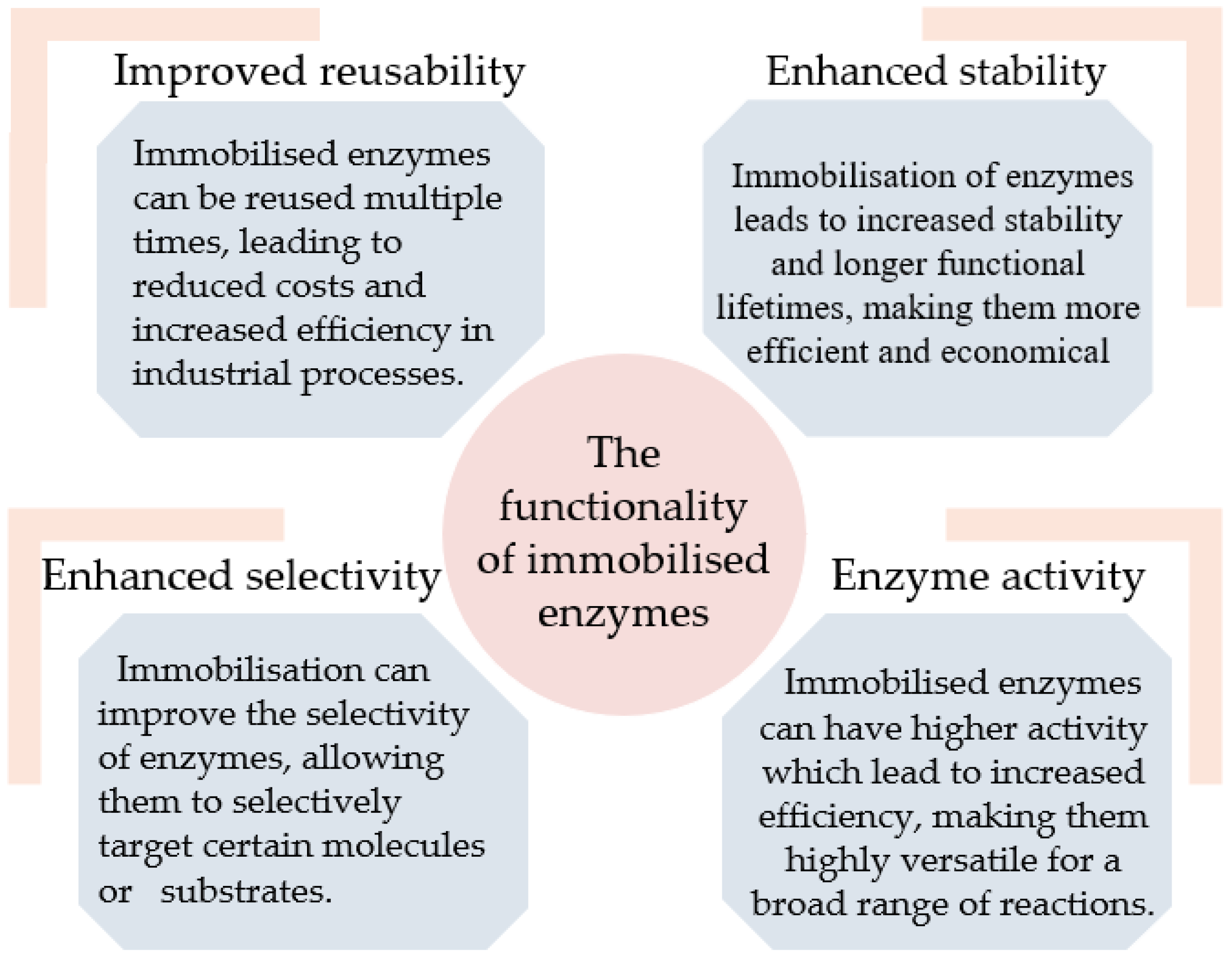
Immobilisation is a technique used in biotechnology to attach enzymes onto a support material or carrier, allowing them to function in a variety of different applications [5]. Immobilised enzymes have become an important tool in many industries due to their unique advantages over free enzymes, including increased stability, reusability, and selectivity (Figure 1). Immobilisation protects enzymes from degradation, extreme temperatures, and other harsh conditions, thereby extending their functional life and efficiency [5,6,7,8].
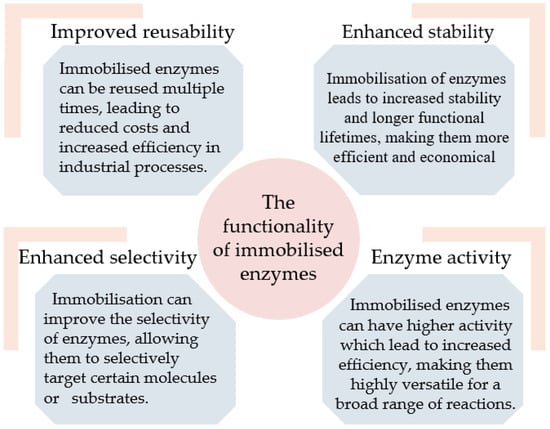
Figure 1.
The functionality of immobilised enzymes.
Enzymes play a major role in lipid and dairy processing and offer various benefits over chemical processes. Enzymes have been used for milk modifications to produce several dairy-based products such as whey protein isolate (WPI), whey protein concentrate (WPC), hydrolysed infant formula, and milk fat [9,10,11,12]. For instance, whey was obtained via enzymatic coagulation of cow milk by rennet [13] and whey protein hydrolysates were produced by the hydrolysis of cow and goat milk using different proteases including alcalase, neutrase, trypsin, bromelain, pepsin, papain, and proteinase [14,15,16]. Enzymes have also been used to produce bioactive peptides from milk proteins and dairy by-products [17,18], as well as structured and specialty lipids with functional properties via processes such as hydrolysis, esterification, and interesterification [19,20,21]. This suggests that food-grade enzymes and appropriate immobilisation supports are needed for these applications. An ideal support material or matrix for immobilisation must be robust, inert, stable, and affordable [22,23].
Several studies have been carried out on the fortification of dairy products with functional lipids, resulting in improved health and sensory qualities. These functional lipids are mostly added in pure forms, and they include omega-3, -6, and -9 polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic (EPA), docosahexaenoic (DHA), alpha-linolenic (ALA), linoleic (LA), and oleic (OA) acids. A recent study found that yoghurt fortified with PUFAs showed neuroprotective effects against aluminium-chloride-mediated neurotoxicity and enhanced brain dopamine and serotonin [24]. Also, yoghurts fortified with EPA and DHA showed high bioaccessibility values as a result of efficient intestinal absorption [25]. Other dairy products fortified with omega-3 fatty acids [26,27,28] and other functional lipids have been reported (Table 1). Since pure forms of these PUFAs are often required, they must be extracted or concentrated from oils. Industrial production of PUFA concentrates is achieved by environmentally unfriendly methods such as fractional distillation and urea complexation techniques, which lead to partial oxidation and polymerisation of these lipids [29]. The use of enzymes for concentrating PUFAs has been reported as the preferred method as they are milder and environmentally friendly [21,30]. In addition, enzymes are used as food processing aids and are regarded as natural for regulatory purposes [31,32].

Table 1.
Dairy product fortification with functional lipids.
Therefore, this review focuses on different enzyme immobilisation techniques using various supports, their use in lipid and dairy processing, and the valorisation of their waste products.
2. Enzyme Immobilisation Methods
Enzyme immobilisation is a process of attaching enzymes to a solid support, resulting in an enhanced activity, stability, reusability, and efficiency [41,42]. Immobilisation can also significantly reduce the cost and loss of enzymes during reactions as immobilised enzymes are easier to recover than non-immobilised ones. There are several methods of enzyme immobilisation, including adsorption, entrapment, covalent bonding, and crosslinking. The choice of an immobilisation method depends on many factors, such as the specific characteristics of the enzyme and its applications [43].
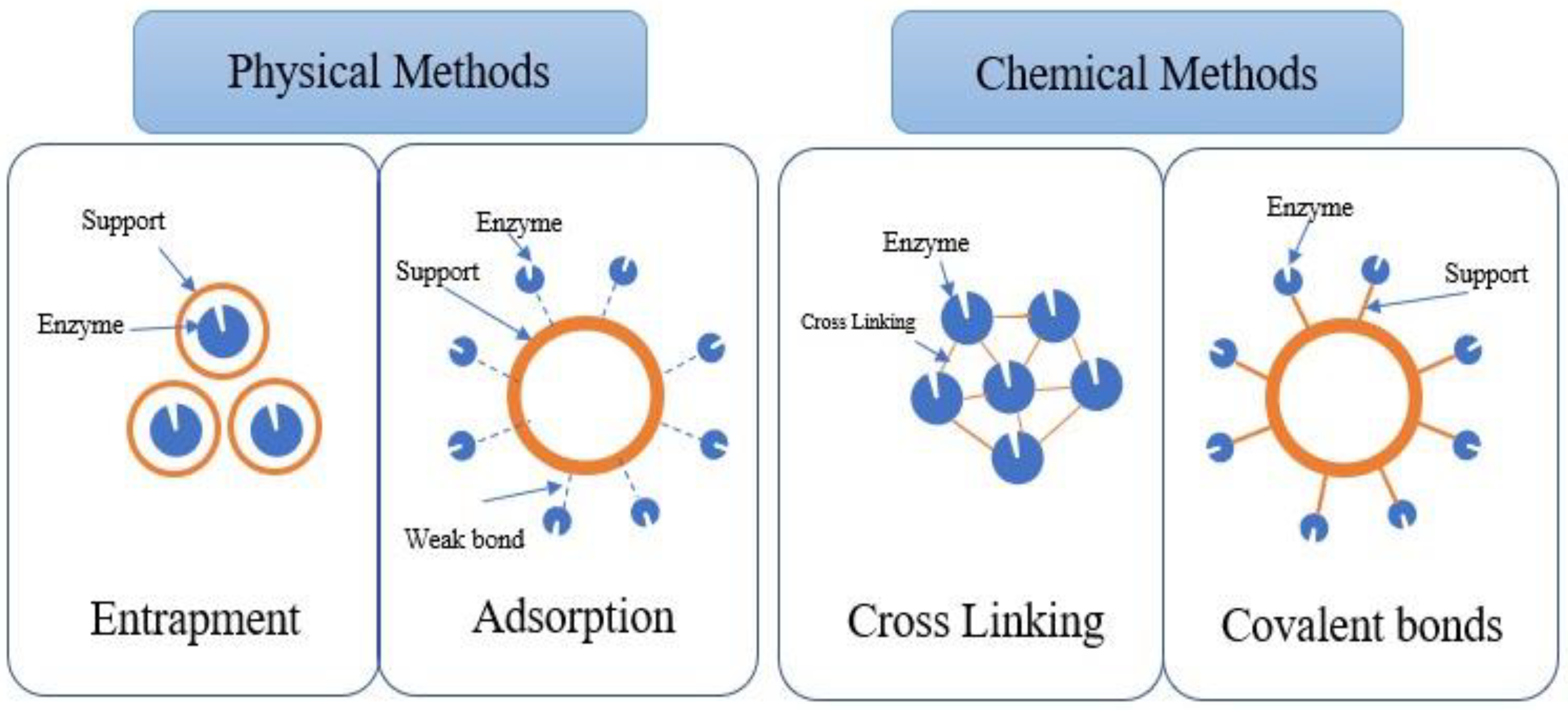
Enzyme immobilisation can be achieved through physical and chemical methods, and the strength of enzyme attachment to the support differs (Figure 2). For instance, in the physical method, enzymes can be adsorbed on the surface of the support (adsorption) or entrapped within the support matrix (entrapment) without forming any chemical bonds with the support [44]. Meanwhile, the chemical method involves the formation of covalent (chemical) bonds between the enzyme and the support material. They can also be chemically bonded to the functional groups on the support material via crosslinkers [45,46].
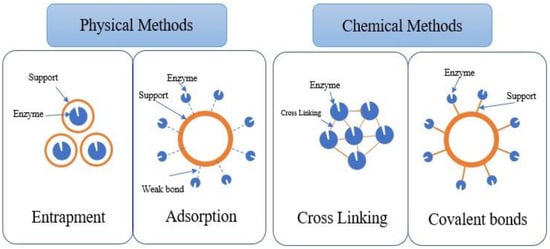
Figure 2.
Enzyme immobilisation methods.
2.1. Physical Methods of Enzyme Immobilisation
2.1.1. Adsorption
The adsorption method involves attaching enzymes to a solid support through weak interactions such as van der Waals forces, hydrogen bonding, and hydrophobic interactions [47]. With this method, enzymes attach to the surface of the support material without forming strong covalent bonds. The adsorption method is simple and no chemical modification of the enzyme or the support material is required. It has been reported that enzymes immobilised by this interfacial adsorption method often have a high activity because they are attached to the support in an open conformation [48].
A previous study found that Candida antacrtica lipase A immobilised on octadecyl resin via physical adsorption showed a higher immobilisation yield and enzyme activity than when immobilised covalently [41]. However, the immobilised enzyme could be reused for five cycles before a sharp decline in activity [41]. This is the major drawback of immobilisation by adsorption, as enzymes can easily leach from the support due to the weak bonding, thus reducing the stability and reusability of the immobilised enzymes. Despite this drawback, the method is still being used in some applications where a simple immobilisation method like adsorption is desired.
2.1.2. Entrapment
Immobilisation by entrapment involves trapping the enzyme within a porous support material that allows free diffusion of the substrates and product but not the enzyme [44]. The support material must have the appropriate pore sizes for the enzyme to be entrapped. A highly porous material or a material with too large pores will cause enzyme leaching and a low immobilisation efficiency and activity [49,50]. To prevent leaching, the enzyme is mixed with a polymer, which forms a complex structure within the material, thus trapping the enzyme [49]. It is important to note that the entrapped enzyme is not attached to the support, but the support acts as a physical barrier that prevents the enzyme from leaching.
A β-fructofuranosidase enzyme from Aspergillus japonicus was immobilised by entrapment on Amberlite IRA 900 resin (a food-grade resin) for the synthesis of fructooligosaccharide (FOS) [51]. The authors found that the immobilised enzyme had a higher FOS yield, activity, and reusability in up to 12 batch cycles [51]. Several studies have shown that enzymes immobilised by entrapment often have a high degree of reusability, which is desired in industrial applications [44,49,50,51]. However, others have reported that the process of entrapment can cause enzyme rigidity and inactivation [52,53].
2.2. Chemical Methods of Enzyme Immobilisation
2.2.1. Covalent Bond
Covalent bond formation is widely used for enzyme immobilisation as it ensures a strong and permanent attachment of the enzyme to the support material, thus preventing enzyme leaching [44]. This method greatly relies on the amino or carboxyl functional group of the enzymes and the reactive group of the support through various types of linkages and interactions [54]. The most common active groups on the support include glutaraldehyde, epoxide, glyoxyl, and vinyl sulfone [55,56]. The covalent binding method is irreversible, and enzymes immobilised this way are always stable as they do not detach from the support during use. Therefore, this is the ideal immobilisation method when the complete removal of the enzyme from the final product is desired [57]. Covalent bonding also improves the selectivity, reusability, thermostability, and organic solvent tolerance of enzymes, making it highly versatile in many applications [54,55,58].
A commercial alkaline protease from Bacillus licheniformis protease immobilised covalently on chitosan modified by glutaraldehyde and ethylenediamine showed higher enzyme activity at 70 °C compared to the value exhibited at 60 °C by the free enzyme. The immobilised enzyme was also highly effective in hydrolysing casein, haemoglobin, soy proteins, and gelatin compared to the free enzyme [59]. Protease (Novo-Pro D) immobilised on agarose activated with a glyoxyl group was used to hydrolyse casein ten times without any decrease in activity [60]. Others have also reported multiple reuses of enzymes immobilised covalently [16,61,62].
2.2.2. Crosslinking
The crosslinking method involves forming a covalent bond between enzymes and further attaching them to the support material [44]. Generally, this immobilisation technique is straightforward, and it involves two steps. First, enzymes are precipitated using precipitants such as ammonium sulfate, ethanol, tert-butanol, acetone, acetonitrile, glycol, and iso-propanol [2,63]. Afterwards, the precipitated enzymes are crosslinked to form a crosslinked enzyme aggregate or crystal (CLEA or CLEC, respectively). Examples of crosslinkers include glutaraldehyde, benzoquinone, dextran-polyaldehyde, polyethyleneimine, and carbodiimide [44]. However, glutaraldehyde is the most reported crosslinker. The crosslinking process using glutaraldehyde ensures the stable bond of the enzyme to the support, allowing for efficient immobilisation [6]. Crosslinking is a well-established immobilisation technique, and the immobilised enzymes are always stable and highly reusable. The only drawback with this method is that enzymes can be easily denatured during precipitation as some are not solvent-tolerant [64]. Consequently, choosing a precipitant that will not denature the enzyme is essential.
A recent study found that immobilisation of lipase from Pseudomonas stutzeri via crosslinking resulted in a more robust and highly stable enzyme that is more active in deep eutectic solvents (DESs) than the free enzyme that was not active in these solvents [65]. In another study, invertase was immobilised on chitosan using glutaraldehyde as the crosslinker [66]. The results showed that the enzyme had higher pH stability, thermal stability, and reusability, retaining 70% of its activity after nine batches of reuse. It was highly effective in producing bioethanol [66].
3. Immobilised Enzymes in Dairy Processing
Enzymes have been widely used as processing aids during the production of dairy products including WPC, WPI, infant formula, and milk fat (Table 2). The use of immobilised enzymes in the production of these products is discussed in this section.

Table 2.
Enzymes in dairy product manufacturing.
3.1. Whey Protein Concentrate
WPC is a popular supplement and ingredient in various food products due to its high protein content (66.5–95.8%) and nutritional value [96]. Traditional methods of producing WPC involve the use of various filtration and separation processes to concentrate the protein content from whey, a by-product of cheese making [97]. Enzyme immobilisation techniques have provided opportunities for more efficient and environmentally friendly methods of producing WPC. Immobilised enzymes in the production of whey protein concentrate can potentially lower production costs in the long run due to enzyme reusability. Additionally, the process can be tailored to achieve specific protein concentrations and meet the desired product specifications [68].
The most used enzymes to produce WPC are lactase and β-galactosidase, which are mixed with the whey to hydrolyse the lactose [98]. The enzymes break down the lactose into glucose and galactose while leaving the proteins intact. Studies describing WPC production via hydrolysis using immobilised lactase and β-galactosidase have been reported [68,69,99]. After the hydrolysis, the solid supports with the immobilised enzymes are separated from the liquid phase via centrifugation. The liquid phase containing the whey proteins is then concentrated using various methods such as evaporation, ultrafiltration, or spray drying to produce whey protein concentrate. During concentration, the liquid is reduced in volume, resulting in a higher protein content [42,98].
Immobilised enzymes have emerged as a promising solution to produce WPC, offering a multitude of advantages over free enzymes. β-galactosidase from Kluyveromyces lactis was immobilised on calcium alginate spheres by entrapment and used for the production of WPC from bovine, goat, and buffalo whey [68]. The authors found that the storage and thermal stability of the enzyme increased after immobilisation and it showed a high operational stability, which allowed multiple reuses for more than 10 cycles [68]. In another study, Kluyveromyces lactis β-galactosidase immobilised on chitosan activated with glutaraldehyde, hydrolysed 43% of milk lactose compared to the 38% hydrolysed by the free (non-immobilised) enzyme [100]. These enzymes experience enhanced stability when immobilised, enabling multiple reuses and specificity, leading to targeted hydrolysis of lactose while preserving whey proteins and minimising by-product formation [68,98,100].
3.2. Whey Protein Isolate
Whey protein isolate (WPI) is a highly purified form of whey protein, recognised for its exceptional nutritional profile and effectiveness in supporting muscle recovery and growth [101]. Derived from milk during the cheese-making process, WPI undergoes further processing to remove most of the fats and carbohydrates, resulting in a product that contains an impressive concentration of protein, typically over 90% [102]. This purity not only makes it an ideal choice for athletes but also makes it a suitable option for individuals who may be lactose intolerant, as the lactose content is significantly reduced, as well as its rapid absorption and rich essential amino acid content [103].
WPI production using enzymes involves a specialised process known as enzymatic hydrolysis or proteolysis. This process is used to break down the whey protein into its constituent peptides and amino acids, followed by further purification to isolate the whey protein in its purest form [104]. However, using immobilised enzymes is a highly efficient and advanced process, with these methods carefully chosen to ensure the highest quality of the final product, with minimal impact on the nutritional value and bioactivity of the whey proteins [88].
One of the key benefits of using immobilised enzymes for WPI production is the control over the enzymatic reactions. The immobilised enzymes function under controlled conditions, such as temperature and pH, leading to consistent and predictable outcomes. This control results in a purer form of WPI with a higher protein content, fewer impurities, and a more refined amino acid profile. Moreover, immobilised enzymes have an extended operational life compared to free enzymes, reducing the need for frequent enzyme replacements and making the production process more cost-effective and sustainable [105]. Additionally, the immobilised enzymes can be easily recovered and reused, reducing the overall demand for enzymes. As consumer demand for high-quality protein supplements continues to grow, WPI production using immobilised enzymes offers a promising solution to meet these needs efficiently and sustainably [59].
3.3. Bioactive Peptide Synthesis
Proteases have been used in dairy processing to hydrolyse milk proteins into smaller peptides and free amino acids [81] to improve the digestibility of proteins and absorption in humans [82] and to introduce the desired sensory characteristics [83]. For example, in cheese making, proteases were used to break down casein into peptides, which enhances the flavour and texture of the cheese during the production process [83]. Apart from improved sensory characteristics, several studies have shown that milk-derived peptides can possess a broad range of functional properties, such as antioxidant and antimicrobial properties. For instance, bioactive peptides with antioxidant and antimicrobial properties have been produced from goat milk via hydrolysis using proteases derived from Aspergillus oryzae and Aspergillus flavipes [106].
Peptide synthesis plays a significant role in dairy processing, particularly in the production of dairy products with improved functional and nutritional properties. Bioactive peptides are important in the process of cheese making as they selectively impede the activity of proteolytic enzymes found in lactic acid bacteria. As a result, these peptides have a direct impact on the quality of the cheese produced [107]. Immobilising enzymes on a support material enhances their stability and reusability, allowing for efficient and controlled enzymatic reactions. In dairy processing, immobilised enzymes are utilised to selectively cleave or modify milk proteins, leading to the generation of bioactive peptides with desirable functionalities, such as antioxidant and antimicrobial activity. This enzymatic approach offers advantages in terms of specificity, efficiency, and the ability to generate peptides with tailored properties, contributing to the development of innovative dairy products with enhanced health benefits and sensory attributes [108].
3.4. Hydrolysed Infant Formula
Hydrolysed milk is commonly used for children genetically predisposed to a cow’s milk allergy [109]. A recent study found that partially hydrolysed milk can reduce the skin barrier function in infants and reduce the risk of atopic dermatitis [110]. Lactose intolerance has been widely reported to be common among infants and children [111]. While this condition may be transient in some infants, it could be severe in others [112]. So, the intake of lactose-free milk has been suggested as a possible solution as it does not adversely affect normal growth in term infants [113,114]. Immobilised enzymes can be used to produce some of these specialty milk-based foods [82].
Lactase can catalyse the hydrolysis of lactose and is used to produce lactose-reduced and lactose-free dairy foods. For instance, lactase immobilised on chitosan was used to produce low-dose-lactose milk [115]. The immobilised enzyme was used for ten successive hydrolysis cycles, making the process highly economical with higher performance than using the free enzyme [115]. Several other studies have shown that immobilised enzymes can be used to hydrolyse lactose to produce a broad range of lactose-free dairy-based foods for infants [116,117].
A recent study used immobilised lipase to hydrolyse lipids in infant formula to understand if this can improve intestinal structures in preterm infants [118]. Another study found an enhanced absorption of long-chain PUFAs following consumption of functional milk formula partially hydrolysed with immobilised lipase [119]. The authors found that this process helped improve lipid absorption, tissue accretion, and the intestinal structure. These findings indicate that food-grade enzymes immobilised on different support materials can be used to produce hydrolysed infant formula.
3.5. Butter, Cheese, and Related Milk Fat Products
Milk fat can be used to make a broad range of products including butter, cheese, milk powder, and cream. It has been reported that by 2025, about 35 million tonnes of milk fat will be produced globally and enzymes will play a major role in this production [120].
Cheese making involves the coagulation of milk via proteolysis or acidification reactions. The enzymatic coagulation process involves rennet that is often isolated and purified from calf stomachs [121]. However, plant and microbial (fungi) sources have also been reported and have gained wide acceptance because of the growing demand for cheese and the reduction in animal rennet production [122].
Rennet obtained from Rhizomucor miehei was immobilised on alginate-chitosan nanoparticles for cheese making [122]. The immobilised rennet can be recovered after use, unlike the free form that remains in the curd, which may accelerate the degradation of caseins, thereby influencing the flavour and texture of the finished cheese [122]. An immobilised chymosin, a major protease in rennet, was used for the production of rennet-free Gouda cheese [123]. A recent study also found that chymosin immobilised on eggshell membranes was more effective than the free enzyme in cheese making [124].
4. Immobilised Enzymes for Lipid Processing and Modification
Lipids are a diverse group of biomolecules that include fats, oils, waxes, and other related compounds. One of the key enzymes involved in lipid processing is lipase. Lipases are a class of enzymes that catalyse the hydrolysis, esterification, and interesterification of lipids [8,19,125,126]. Immobilised lipases play crucial roles in lipid modification for a broad range of applications in the food industry (Table 3).

Table 3.
Immobilised lipases for lipid hydrolysis, esterification, and interesterification.
4.1. Production of Omega-3 Fatty Acid Concentrates for Dairy Product Fortification
Hydrolysis is the primary function of lipases and involves the breakdown of triacylglycerols into free fatty acids. Immobilised lipases have been widely reported for the hydrolysis of oil to produce concentrates of specific fatty acids. Lipases have positional or fatty acid specificities, which allow them to hydrolyse some fatty acids selectively from the glycerol backbone of oils [41,144]. For instance, in fish and thraustochytrid oils, studies have shown that DHA is predominantly located at the central position (sn-2) on the glycerol backbone [145,146]. So, to produce concentrates of DHA from these oils, positional selective lipases that discriminate against fatty acids in the sn-2 position would be ideal.
Immobilised lipases with positional selectivity have been used to produce concentrates of omega-3 EPA and DHA via hydrolysis. Recently, lipase from Alcaligenes species immobilised on Accurel MP1000 was used to concentrate 70% EPA from microalga oil. Highly pure forms of EPA like this are being used for cheese and yoghurt fortification [33,39] and in nutrition drinks [147]. Also, Candida antarctica lipase A (CAL-A) immobilised on octadecyl-activated resin was used for concentrating 89% DHA by hydrolysing saturated fatty acids from thraustochytrid oil. The high level of saturated fatty acids in this oil makes it an excellent oil for DHA concentration [41]. This shows that lipases can be used to produce high-quality DHA concentrate for food fortification, such as infant formula and other dairy-based products such as butter, cheese, and ice cream [24,26,27,28,148].
4.2. Production of Flavour Esters, Structured Lipids, and Human Milk Fat Substitutes
Lipases also possess esterification activities, enabling them to catalyse the synthesis of esters from fatty acids. Lipases are commonly used to produce flavour esters and a broad range of food additives [32]. In a recent study, the rancid and tart off-flavour of goat milk was removed via the esterification of goat milk fat using immobilised Thermomyces lanuginosus lipase. Esters with sweet, floral, and fruity flavours were produced and the unpleasant odour in the original goat milk was decreased by 75% [149].
Interesterification is a process in which fatty acids are rearranged within triglyceride molecules, resulting in the modification of the physical and chemical properties of lipids [150]. Lipases are widely used for interesterification reactions in the production of structured lipids, which are tailored fats with specific fatty acid compositions [138,151]. These modified lipids offer improved nutritional profiles, enhanced functional properties, and better stability compared to natural fats and oils [150]. Interesterification is commonly applied in the food industry to produce margarine, shortenings, and other specialty fats [152].
Human breast milk fat consists of saturated, monounsaturated, and long-chain polyunsaturated fatty acids that are beneficial for the growth and development of infants. The distribution of these fatty acids on the glycerol backbone of human breast milk fat determines their bioavailability when consumed by infants [153,154]. Several studies have shown that enzymes such as lipases can be used to produce human milk fat (HMF) substitutes (HMFSs) [155,156,157]. HMFSs are synthesised to mimic HMF which has palmitic and oleic acids with a unique positional distribution. HMFs are generally triacylglycerols (TAGs) that contain mainly palmitic acid esterified at the sn-2 position on the glycerol backbone, while oleic acids and other unsaturated fats are located at sn-1 and sn-3 positions [158]. Since breast milk fat is rich in long-chain PUFAs such as EPA, DHA, and ARA, which are precursors of prostaglandins and eicosanoids that play regulatory roles [159], lipases can be used to produce HMFSs that are rich in these fatty acids because of their positional selectivity [41,138].
Immobilised lipases play a crucial role in the esterification (conjugation) of fatty acids with other non-lipid compounds. The conjugation of oils, lipids, or fatty acids with other compounds such as polyphenols has been reported, resulting in the formation of phenolipids [5,6,7,8]. These phenolipids possess unique properties that combine the beneficial attributes of both polyphenols and lipids [160]. They can exhibit an enhanced antioxidant activity, improved solubility in lipophilic environments, and increased bioavailability. Phenolipids have found applications in various industries, including pharmaceuticals, cosmetics, and food [160,161].
5. Immobilised Enzymes for Bioconversion of Dairy and Lipid Waste
5.1. Enzymes for Dairy Waste Processing
The utilisation of immobilised enzymes for the treatment of dairy waste has gained considerable attention in recent years due to their potential to enhance waste management practices and contribute to sustainability in the dairy industry. They can also be used under harsher reaction conditions, such as non-neutral pH and higher temperatures, which are typical of dairy processing waste streams [162,163]. The immobilisation material or matrix for enzymes used in dairy waste processing must be robust, inert, and stable (Table 4). This can reduce the cost and increase the sustainability of dairy waste processing [164]. Because enzymes are more specific, efficient, and environmentally friendly than traditional chemical processes, they can selectively break down complex organic molecules in dairy waste into simpler compounds that can be further processed into valuable products [165].
Lactose hydrolysis, achieved through immobilised lactase enzymes, stands out as a prominent application. By converting lactose into its constituent sugars, immobilised lactase facilitates the production of lactose-free dairy products and reduces the environmental impact of dairy waste streams [166]. Furthermore, immobilised enzymes like lipases hold promise in modifying the lipid profile of dairy waste, enabling the development of novel value-added products or facilitating lipid breakdown for more efficient waste treatment [167]. The interaction between immobilised enzymes and dairy waste treatment not only addresses economic and environmental concerns but also fosters improvement in dairy by-product utilisation.
The efficacy of immobilised enzymes in dairy waste treatment is underscored by the diverse array of solid supports that can be employed to immobilise these biocatalysts. Calcium alginate, chitosan beads, and agarose gels are examples of widely used matrices that offer favourable environments for enzyme activity while allowing efficient separation and recovery [168]. These immobilisation matrices facilitate targeted enzymatic hydrolysis processes, such as lactose breakdown and lipid modification, which are crucial for value creation and waste reduction [169].
The use of immobilised enzymes in dairy waste processing has been widely reported. For instance, dairy by-products are used as the main ingredient for the production of kashk, an Iranian dairy product produced enzymatically [170]. A highly potent protease isolated from Bacillus cereus was immobilised on magnetic nanoparticles and used to treat wastewater from yoghurt production with a protein recovery yield of 44% [171]. Another study proposed a β-galactosidase-enzyme-mediated process for valorisation of lactose-rich whey [172]. The authors described how β-galactosidase immobilised on polystyrene nanofibers was used to convert lactose into galactooligosaccharides (GOSs) in batch and repeated batch systems [172].
Therefore, the use of immobilised enzymes in dairy waste treatment aligns with the broader movement toward sustainable and circular practices in the food industry.

Table 4.
Enzyme immobilisation support for dairy waste processing.
Table 4.
Enzyme immobilisation support for dairy waste processing.
| Type of Support | Enzyme | Application in Dairy Waste Processing | Reference |
|---|---|---|---|
| Calcium alginate | Lactase | Hydrolysis of lactose in dairy waste for lactose-intolerant products. | [173] |
| Agarose gel | Peptidase | Production of bioactive peptides from dairy by-products. | [170] |
| Modified glass beads | β-galasctosidase | Extraction of bioactive compounds from dairy waste for use as functional food ingredients. | [174] |
| Zeolite nanoparticles | Laccase | Removal of harmful pollutants from dairy waste to protect the environment. | [175] |
| Magnetic nanoparticles | Protease | Removal of pathogens from dairy waste streams. | [176] |
| Methacrylatic monoliths | β-galactosidase | Prebiotic production from a waste product of the dairy industry. | [177] |
| Magnetic nanoparticles | Lipase | Applied to produce biodiesel from dairy waste. | [167] |
| Magnetite nanoparticles | protease | Hydrolysis of protein from dairy wastewater to produce whey protein isolate. | [171] |
By converting waste materials into useful products or reducing the environmental burden of waste disposal, immobilised enzyme technologies contribute to the optimisation of resource utilisation and promote a greener dairy sector. However, challenges and considerations persist in the application of immobilised enzymes for dairy waste treatment. The choice of immobilisation method, enzyme selection, and support material must be carefully tailored to the specific characteristics of the waste stream and the desired treatment outcomes. Factors such as enzyme loading, operational stability, and economic feasibility require a thorough assessment to ensure practical implementation [178].
5.2. Enzymes for Lipid Waste Processing
The application of immobilised enzymes in treating lipid-rich waste presents a promising avenue for enhancing the management of industrial wastes and by-products. Immobilised enzymes offer distinct advantages over free enzymes, making them well suited to address the challenges associated with lipid-rich waste streams. Lipase, a widely studied enzyme, stands out as a key biocatalyst in this context (Table 5). Immobilised lipases can hydrolyse the triglycerides present in the lipid-rich waste, breaking them down into valuable fatty acids and glycerol [179]. This process not only facilitates the conversion of lipid waste into potentially valuable materials for various applications but also reduces the environmental burden of untreated lipid wastes [180].
By converting lipid waste into valuable products, such as fatty acids that can be used as biofuels or biochemicals, immobilised enzyme approaches contribute to the optimisation of resource utilisation and the reduction in environmental impacts. Nevertheless, challenges remain in the widespread adoption of immobilised enzyme-based strategies for lipid-rich waste treatment [181]. Ongoing research focuses on refining immobilisation techniques, exploring alternative enzyme sources, and assessing the environmental implications of immobilised-enzyme-based processes [182]. As industries seek to address the environmental and economic concerns associated with lipid waste, the integration of immobilised enzymes holds potential for advancing sustainable practices and resource-efficient waste management [183].
Several studies have shown that lipases can be used for lipid waste processing. Immobilised lipases have been used for the remediation of hazardous pollutants from lipid-rich waste via hydrolysis of fats and oils [176,182]. A new bioremediation method was developed for the removal of oils in wastewater using Acinetobacter haemolyticus lipase. The lipase, which was immobilised on an eggshell membrane, was highly effective in hydrolysing olive, canola, cottonseed, palm, and peanut oils from wastewater while retaining its activity after 19 reuses [184]. Aspergillus niger lipase immobilised on the microporous acrylic resin was used for the deacidification of high-acid soy sauce residue oil [185]. This enzyme exhibited a high deacidification activity, thermostability, and reusability. It was also able to convert the free fatty acid in soy source residue oil to diacylglycerol-enriched oil that could be used for other food applications [185].
Lipid waste from different food processes has been widely used to produce biodiesel. Biodiesel is usually prepared using edible vegetable oils via transesterification, which can be catalysed by lipases [186]. Interestingly, old or used cooking oil can be a good source of oil for biodiesel production, and several studies have shown that spent frying or cooking oils could be used to make biodiesel [187,188,189,190,191].
Therefore, lipases are versatile in the bioprocessing of lipid waste for value addition, which is consistent with a circular economy, in which food processing wastes are kept in use while minimising negative environmental impacts and footprints.

Table 5.
Enzyme immobilisation support for lipid waste processing.
Table 5.
Enzyme immobilisation support for lipid waste processing.
| Type of Support | Enzyme | Application in Lipid-Rich Waste Processing | Reference |
|---|---|---|---|
| Eggshell membrane | Lipase | Hydrolysis of lipids in waste with a high oleic acid composition | [184] |
| Chitosan beads | Lipase | Modification of lipid profiles in waste for value-added products | [192] |
| Silica cellulose | Lipase | Remediation of hazardous pollutants from lipid-rich waste | [176] |
| Calcium alginate beads | Lipase | Breakdown of fats and oils in lipid waste | [180] |
| Magnetic nanoparticles | Lipase | Conversion of lipid waste into biodiesel or bioproducts | [193] |
| Microporous acrylic resins | Lipase | Biotransformation of lipid waste components into valuable compounds | [185] |
| Kieselguhr (diatomaceous earth) | Lipase | Production of lipolytic enzymes for lipid waste treatment | [194] |
| Rice straw | Lipase | Breakdown of waste oil | [195] |
6. Conclusions and Future Research Direction
Enzyme use in dairy and lipid processing is becoming increasingly attractive due to numerous advantages. Enzymes offer greater advantages over the use of chemicals because reactions involving enzymes can be performed under milder conditions, such as low temperatures and near-neutral pH, and products are considered natural for regulatory purposes. Also, because the reaction conditions are mild, the product quality is enhanced and fewer side products are formed, making this an environmentally friendly technique. In addition, since enzymes are highly specific in their actions, they can be used to produce a broad range of dairy and lipid-based products with a high purity and functionality. In this review, the numerous applications of immobilised enzymes for lipid and dairy processing have been discussed. The selection of an appropriate immobilisation method and support material was also addressed, as this is crucial to maintaining the enzyme’s stability and activity. Despite the numerous advantages of enzyme immobilisation, there is still a low uptake of this technology in several dairy and lipid processing applications.
In any event, enzyme immobilisation remains a promising technology with significant potential for industrial applications, as it offers a range of potential benefits in various applications in lipid and dairy processing. The prospects of immobilised enzymes for industrial-scale applications hold significant promise as they can be applied to a broad range of catalytic applications in dairy and lipid processing through an enhanced operational efficiency, environmental sustainability, and economic viability. To utilise them at an industrial scale, several challenges must be addressed. Further research is needed to optimise immobilisation techniques, ensuring that enzyme activity, stability, and selectivity are not compromised during the immobilisation process. Also, more food-grade immobilisation supports must be investigated to avoid contamination when used for dairy and lipid processing. Further studies should focus on the development of scalable and cost-effective immobilisation methods for viable large-scale industrial use.
Funding
This research was funded by a PhD scholarship received from the University of Ha’il, Kingdom of Saudi Arabia.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Fawzih Alzahrani gratefully acknowledges the support from the University of Ha’il, Kingdom of Saudi Arabia, and the University of Newcastle, Australia.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| PUFA | Polyunsaturated fatty acid |
| EPA | Eicosapentaenoic acid |
| WPI | Whey protein isolate |
| WPC | Whey protein concentrate |
| DHA | Docosahexaenoic acid |
| ALA | Alpha-linolenic acid |
| LA | Linoleic acid |
| OA | Oleic acid |
| FOS | Fructooligosaccharide |
| CLEA | Crosslinked enzyme aggregate |
| CLEC | Crosslinked enzyme crystal |
| CAL-A | Candida antarctica lipase A |
| HMF | Human milk fat |
| TAGs | Triacylglycerols |
| ARA | Arachidonic acid |
References
- Akanbi, T.O.; Ji, D.; Agyei, D. Revisiting the scope and applications of food enzymes from extremophiles. J. Food Biochem. 2020, 44, e13475. [Google Scholar] [CrossRef]
- Chen, N.; Chang, B.; Shi, N.; Yan, W.; Lu, F.; Liu, F. Cross-linked enzyme aggregates immobilization: Preparation, characterization, and applications. Crit. Rev. Biotechnol. 2023, 43, 369–383. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, X.; Fan, K. Nanozymes inspired by natural enzymes. Acc. Mater. Res. 2021, 2, 534–547. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A.; Sachan, S. Enzymes used in the food industry: Friends or foes? In Enzymes in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 827–843. [Google Scholar]
- Silva, J.M.F.; Dos Santos, K.P.; Dos Santos, E.S.; Rios, N.S.; Gonçalves, L.R.B. Immobilization of Thermomyces lanuginosus lipase on a new hydrophobic support (Streamline phenyl™): Strategies to improve stability and reusability. Enzym. Microb. Technol. 2023, 163, 110166. [Google Scholar] [CrossRef]
- Abellanas-Perez, P.; Carballares, D.; Fernandez-Lafuente, R.; Rocha-Martin, J. Glutaraldehyde modification of lipases immobilized on octyl agarose beads: Roles of the support enzyme loading and chemical amination of the enzyme on the final enzyme features. Int. J. Biol. Macromol. 2023, 248, 125853. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Akanbi, T.O.; Marshall, S.N.; Barrow, C.J. Polydatin-fatty acid conjugates are effective antioxidants for stabilizing omega 3-containing bulk fish oil and fish oil emulsions. Food Chem. 2019, 301, 125297. [Google Scholar] [CrossRef]
- Eberhardt, A.; López, E.C.; Marino, F.; Mammarella, E.J.; Manzo, R.M.; Sihufe, G.A. Whey protein hydrolysis with microbial proteases: Determination of kinetic parameters and bioactive properties for different reaction conditions. Int. J. Dairy Technol. 2021, 74, 489–504. [Google Scholar] [CrossRef]
- Amigo-Benavent, M.; FitzGerald, R.J. Impact of thermal inactivation conditions on the residual proteolytic activity and the viscosity properties of whey protein concentrate enzymatic hydrolysates. Food Hydrocoll. 2022, 124, 107333. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Mora-Cortes, W.G.; Leandro-Roldan, M.M.; González-Velázquez, D.A.; Torres-Llanez, M.J.; Ramírez-Suarez, J.C.; González-Córdova, A.F.; Vallejo-Córdoba, B. Production of whey protein hydrolysates with angiotensin-converting enzyme-inhibitory activity using three new sources of plant proteases. Biocatal. Agric. Biotechnol. 2020, 28, 101724. [Google Scholar] [CrossRef]
- Lermen, A.M.; Clerici, N.J.; Borchartt Maciel, D.; Daroit, D.J. Characterization and application of a crude bacterial protease to produce antioxidant hydrolysates from whey protein. Prep. Biochem. Biotechnol. 2023, 53, 12–21. [Google Scholar] [CrossRef]
- León-López, A.; Pérez-Marroquín, X.A.; Estrada-Fernández, A.G.; Campos-Lozada, G.; Morales-Peñaloza, A.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Milk whey hydrolysates as high value-added natural polymers: Functional properties and applications. Polymers 2022, 14, 1258. [Google Scholar] [CrossRef]
- Du, X.; Jing, H.; Wang, L.; Huang, X.; Wang, X.; Wang, H. Characterization of structure, physicochemical properties, and hypoglycemic activity of goat milk whey protein hydrolysate processed with different proteases. LWT 2022, 159, 113257. [Google Scholar] [CrossRef]
- Wróblewska, B.; Karamać, M.; Amarowicz, R.; Szymkiewicz, A.; Troszyńska, A.; Kubicka, E. Immunoreactive properties of peptide fractions of cow whey milk proteins after enzymatic hydrolysis. Int. J. Food Sci. Technol. 2004, 39, 839–850. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Gonçalves, L.R.; Amorim, K.P.; Pinheiro, B.B.; Ramos, M.V.; Souza, P.F.; Oliveira, J.S.; Freitas, D.C.; Freitas, C.D. Immobilization and characterization of latex cysteine peptidases on different supports and application for cow’s milk protein hydrolysis. Process Biochem. 2022, 117, 180–190. [Google Scholar] [CrossRef]
- Monari, S.; Ferri, M.; Russo, C.; Prandi, B.; Tedeschi, T.; Bellucci, P.; Zambrini, A.V.; Donati, E.; Tassoni, A. Enzymatic production of bioactive peptides from scotta, an exhausted by-product of ricotta cheese processing. PLoS ONE 2019, 14, e0226834. [Google Scholar] [CrossRef]
- Kleekayai, T.; Cermeño, M.; FitzGerald, R.J. The production of bioactive peptides from milk proteins. In Agents of Change: Enzymes in Milk and Dairy Products; Springer: Berlin/Heidelberg, Germany, 2021; pp. 447–497. [Google Scholar]
- Gunathilake, T.; Akanbi, T.O.; Barrow, C.J. Lipase-produced omega-3 acylglycerols for the fortification and stabilization of extra virgin olive oil using hydroxytyrosyl palmitate. Future Foods 2021, 4, 100045. [Google Scholar] [CrossRef]
- Aryee, A.N.; Akanbi, T.O.; Nwachukwu, I.D.; Gunathilake, T. Perspectives on preserving lipid quality and strategies for value enhancement. Curr. Opin. Food Sci. 2022, 44, 100802. [Google Scholar] [CrossRef]
- Xia, Q.; Akanbi, T.O.; Wang, B.; Li, R.; Liu, S.; Barrow, C.J. Investigation of enhanced oxidation stability of microencapsulated enzymatically produced tuna oil concentrates using complex coacervation. Food Funct. 2020, 11, 10748–10757. [Google Scholar] [CrossRef]
- Gan, J.; Bagheri, A.R.; Aramesh, N.; Gul, I.; Franco, M.; Almulaiky, Y.Q.; Bilal, M. Covalent organic frameworks as emerging host platforms for enzyme immobilization and robust biocatalysis–A review. Int. J. Biol. Macromol. 2021, 167, 502–515. [Google Scholar] [CrossRef]
- Taheri-Kafrani, A.; Kharazmi, S.; Nasrollahzadeh, M.; Soozanipour, A.; Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.-A.; Razmjou, A.; Yek, S.M.-G.; Varma, R.S. Recent developments in enzyme immobilization technology for high-throughput processing in food industries. Crit. Rev. Food Sci. Nutr. 2021, 61, 3160–3196. [Google Scholar] [CrossRef]
- Ramadan, M.M.; El-Said, M.M.; El-Messery, T.M.; Mohamed, R.S. Development of flavored yoghurt fortified with microcapsules of triple omega 3-6-9 for preventing neurotoxicity induced by aluminum chloride in rats. J. Food Process. Preserv. 2021, 45, e15759. [Google Scholar] [CrossRef]
- Guadarrama-Flores, B.; Matencio, A.; Navarro-Orcajada, S.; Martínez-Lede, I.; Conesa, I.; Vidal-Sánchez, F.J.; García-Carmona, F.; López-Nicolás, J.M. Development if healthy milk and yogurt products for reducing metabolic diseases using cyclodextrin and omega-3 fatty acids from fish oil. Food Funct. 2022, 13, 5528–5535. [Google Scholar] [CrossRef] [PubMed]
- Pandule, V.S.; Sharma, M.; HC, D. Omega-3 fatty acid-fortified butter: Preparation and characterisation of textural, sensory, thermal and physico-chemical properties. Int. J. Dairy Technol. 2021, 74, 181–191. [Google Scholar] [CrossRef]
- Muñoz-Tébar, N.; De la Vara, J.; de Elguea-Culebras, G.O.; Cano, E.; Molina, A.; Carmona, M.; Berruga, M.I. Enrichment of sheep cheese with chia (Salvia hispanica L.) oil as a source of omega-3. LWT 2019, 108, 407–415. [Google Scholar] [CrossRef]
- Gowda, A.; Sharma, V.; Goyal, A.; Singh, A.; Arora, S. Process optimization and oxidative stability of omega-3 ice cream fortified with flaxseed oil microcapsules. J. Food Sci. Technol. 2018, 55, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Akanbi, T.O.; Adcock, J.L.; Barrow, C.J. Selective concentration of EPA and DHA using Thermomyces lanuginosus lipase is due to fatty acid selectivity and not regioselectivity. Food Chem. 2013, 138, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Secundo, F.; Sun, J.; Mao, X. Advances in enzyme biocatalysis for the preparation of functional lipids. Biotechnol. Adv. 2022, 61, 108036. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, T.; Akanbi, T.O.; Bucher, T.; Barrow, C.J. Enzymes in nutrition, baby foods, and food safety. In Value-Addition in Food Products and Processing through Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 153–161. [Google Scholar]
- Gunathilake, T.; Akanbi, T.O.; Van Vuong, Q.; Scarlett, C.J.; Barrow, C.J. Enzyme technology in the production of flavors and food additives. In Value-Addition in Food Products and Processing through Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 45–55. [Google Scholar]
- Gumus, C.E.; Gharibzahedi, S.M.T. Yogurts supplemented with lipid emulsions rich in omega-3 fatty acids: New insights into the fortification, microencapsulation, quality properties, and health-promoting effects. Trends Food Sci. Technol. 2021, 110, 267–279. [Google Scholar] [CrossRef]
- Jia, H.-X.; Qu, Y.-Z.; Chen, W.-L.; Su, M.-Y. Effect of high DHA and ARA fortification on lipid oxidation of infant formula powder. CyTA-J. Food 2024, 22, 2300812. [Google Scholar] [CrossRef]
- Madrigal, C.; Soto-Méndez, M.J.; Leis, R.; Hernández-Ruiz, Á.; Valero, T.; Lara Villoslada, F.; Martínez de Victoria, E.; Moreno, J.M.; Ortega, R.M.; Ruiz-López, M.D. Dietary intake, nutritional adequacy and food sources of total fat and fatty acids, and relationships with personal and family factors in Spanish children aged one to< 10 Years: Results of the EsNuPI study. Nutrients 2020, 12, 2467. [Google Scholar]
- Moghadam, F.V.; Pourahmad, R.; Mortazavi, A.; Davoodi, D.; Azizinezhad, R. Use of fish oil nanoencapsulated with gum arabic carrier in low fat probiotic fermented milk. Food Sci. Anim. Resour. 2019, 39, 309. [Google Scholar] [CrossRef]
- Zahran, H.; Mabrouk, A.M.; Salama, H.H. Evaluation of yoghurt fortified with encapsulated echium oil rich in stearidonic acid as a low-fat dairy food. Egypt. J. Chem. 2022, 65, 29–41. [Google Scholar] [CrossRef]
- Zahed, O.; Khosravi-Darani, K.; Mortazavian, A.M.; Mohammadi, A. Bacterial conjugated linoleic acid bio-fortification of synbiotic yogurts using Propionibacterium freudenreichii as adjunct culture. Ital. J. Food Sci. 2021, 33, 1–11. [Google Scholar] [CrossRef]
- Villamil, R.-A.; Guzmán, M.-P.; Ojeda-Arredondo, M.; Cortés, L.Y.; Archila, E.G.; Giraldo, A.; Mondragón, A.-I. Cheese fortification through the incorporation of UFA-rich sources: A review of recent (2010–2020) evidence. Heliyon 2021, 7, e05785. [Google Scholar] [CrossRef] [PubMed]
- Lehaçani, S.M.; Al-Abdullah, B. Characterisation of soft white cheese fortified with flaxseed oil to enhance its quality, lipid profile and health benefits. Riv. Ital. Delle Sostanze Grasse 2023, 100, 165–175. [Google Scholar]
- Akanbi, T.O.; Barrow, C.J. Candida antarctica lipase A effectively concentrates DHA from fish and thraustochytrid oils. Food Chem. 2017, 229, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme immobilization technologies and industrial applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Mohammadi, Z.B.; Zhang, F.; Kharazmi, M.S.; Jafari, S.M. Nano-biocatalysts for food applications; immobilized enzymes within different nanostructures. Crit. Rev. Food Sci. Nutr. 2022, 63, 11351–11369. [Google Scholar] [CrossRef] [PubMed]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme entrapment, biocatalyst immobilization without covalent attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Sicard, C. In Situ Enzyme Immobilization by Covalent Organic Frameworks. Angew. Chem. 2023, 135, e202213405. [Google Scholar] [CrossRef]
- Guisan, J.M.; Fernandez-Lorente, G.; Rocha-Martin, J.; Moreno-Gamero, D. Enzyme immobilization strategies for the design of robust and efficient biocatalysts. Curr. Opin. Green Sustain. Chem. 2022, 35, 100593. [Google Scholar] [CrossRef]
- Du, X.; Wang, L.; Li, Y.; Wu, J.; Chen, G.; Liang, H.; Gao, D. Cooperative adsorption-degradation of benzo[a]pyrene by extracellular enzymes of white rot fungi immobilized on macroporous adsorption resin. Int. Biodeterior. Biodegrad. 2023, 178, 105564. [Google Scholar] [CrossRef]
- Binhayeeding, N.; Yunu, T.; Pichid, N.; Klomklao, S.; Sangkharak, K. Immobilisation of Candida rugosa lipase on polyhydroxybutyrate via a combination of adsorption and cross-linking agents to enhance acylglycerol production. Process Biochem. 2020, 95, 174–185. [Google Scholar] [CrossRef]
- Cao, Y.-P.; Zhi, G.-Y.; Han, L.; Chen, Q.; Zhang, D.-H. Biosynthesis of benzyl cinnamate using an efficient immobilized lipase entrapped in nano-molecular cages. Food Chem. 2021, 364, 130428. [Google Scholar] [CrossRef]
- Vetrano, A.; Gabriele, F.; Germani, R.; Spreti, N. Characterization of lipase from Candida rugosa entrapped in alginate beads to enhance its thermal stability and recyclability. New J. Chem. 2022, 46, 10037–10047. [Google Scholar] [CrossRef]
- Bedzo, O.K.; Trollope, K.; Gottumukkala, L.D.; Coetzee, G.; Görgens, J.F. Amberlite IRA 900 versus calcium alginate in immobilization of a novel, engineered β-fructofuranosidase for short-chain fructooligosaccharide synthesis from sucrose. Biotechnol. Prog. 2019, 35, e2797. [Google Scholar] [CrossRef]
- Fopase, R.; Paramasivam, S.; Kale, P.; Paramasivan, B. Strategies, challenges and opportunities of enzyme immobilization on porous silicon for biosensing applications. J. Environ. Chem. Eng. 2020, 8, 104266. [Google Scholar] [CrossRef]
- Nawawi, N.N.; Hashim, Z.; Rahman, R.A.; Murad, A.M.A.; Bakar, F.D.A.; Illias, R.M. Entrapment of porous cross-linked enzyme aggregates of maltogenic amylase from Bacillus lehensis G1 into calcium alginate for maltooligosaccharides synthesis. Int. J. Biol. Macromol. 2020, 150, 80–89. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R. Immobilization of lipases–A review. Part I: Enzyme immobilization. Chem. Biochem. Eng. Rev. 2019, 6, 157–166. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of enzymes via immobilization: Multipoint covalent attachment and other stabilization strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef] [PubMed]
- Wahab, R.A.; Elias, N.; Abdullah, F.; Ghoshal, S.K. On the taught new tricks of enzymes immobilization: An all-inclusive overview. React. Funct. Polym. 2020, 152, 104613. [Google Scholar] [CrossRef]
- Hassan, M.E.; Yang, Q.; Xiao, Z.; Liu, L.; Wang, N.; Cui, X.; Yang, L. Impact of immobilization technology in industrial and pharmaceutical applications. 3 Biotech 2019, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Mei, P.; Mu, Z.; Li, B.; Feng, X.; Zhang, Y.; Wang, B. Enhancing enzyme activity by the modulation of covalent interactions in the confined channels of covalent organic frameworks. Angew. Chem. 2022, 134, e202201378. [Google Scholar] [CrossRef]
- Ramalho, E.X.; de Castro, R.J.S. Covalent bonding immobilization of a Bacillus licheniformis protease on chitosan and its application in protein hydrolysis. Biocatal. Agric. Biotechnol. 2023, 50, 102713. [Google Scholar] [CrossRef]
- Lopes, L.A.; Novelli, P.K.; Fernandez-Lafuente, R.; Tardioli, P.W.; Giordano, R.L.C. Glyoxyl-activated agarose as support for covalently link Novo-Pro D: Biocatalysts performance in the hydrolysis of casein. Catalysts 2020, 10, 466. [Google Scholar] [CrossRef]
- Kızıldağ, S.; Işık, C.; Teke, M. Milk lactose removal by β-galactosidase immobilized on eggshell membrane. Eur. Food Res. Technol. 2023, 249, 2125–2136. [Google Scholar] [CrossRef]
- Rios, N.S.; Mendez-Sanchez, C.; Arana-Peña, S.; Rueda, N.; Ortiz, C.; Gonçalves, L.R.; Fernandez-Lafuente, R. Immobilization of lipase from Pseudomonas fluorescens on glyoxyl-octyl-agarose beads: Improved stability and reusability. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2019, 1867, 741–747. [Google Scholar] [CrossRef]
- Rojas, M.J.; Amaral-Fonseca, M.; Zanin, G.M.; Fernandez-Lafuente, R.; Giordano, R.d.L.C.; Tardioli, P.W. Preparation of crosslinked enzyme aggregates of a thermostable cyclodextrin glucosyltransferase from Thermoanaerobacter sp. critical effect of the crosslinking agent. Catalysts 2019, 9, 120. [Google Scholar] [CrossRef]
- Reis, C.L.B.; Sousa, E.Y.A.d.; Serpa, J.d.F.; Oliveira, R.C.; Santos, J.C.S.d. Design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Química Nova 2019, 42, 768–783. [Google Scholar] [CrossRef]
- Guajardo, N.; Ahumada, K.; de María, P.D. Immobilization of pseudomonas stutzeri lipase through cross-linking aggregates (CLEA) for reactions in deep eutectic solvents. J. Biotechnol. 2021, 337, 18–23. [Google Scholar] [CrossRef]
- Malhotra, I.; Basir, S.F. Application of invertase immobilized on chitosan using glutaraldehyde or tris (Hydroxymethyl) phosphine as cross-linking agent to produce bioethanol. Appl. Biochem. Biotechnol. 2020, 191, 838–851. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.-H.; Tavano, O.; Berenguer-Murcia, A.; Fernandez-Lafuente, R. Use of Alcalase in the production of bioactive peptides: A review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef]
- Argenta, A.B.; Nogueira, A.; Scheer, A.d.P. Hydrolysis of whey lactose: Kluyveromyces lactis β-galactosidase immobilisation and integrated process hydrolysis-ultrafiltration. Int. Dairy J. 2021, 117, 105007. [Google Scholar] [CrossRef]
- Hackenhaar, C.R.; Spolidoro, L.S.; Flores, E.E.E.; Klein, M.P.; Hertz, P.F. Batch synthesis of galactooligosaccharides from co-products of milk processing using immobilized β-galactosidase from Bacillus circulans. Biocatal. Agric. Biotechnol. 2021, 36, 102136. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, X.; Xiong, B.; Zhang, T.; Zeng, X.; Wu, Z.; Sun, Y.; Pan, D. Production and transepithelial transportation of angiotensin-I-converting enzyme (ACE)-inhibitory peptides from whey protein hydrolyzed by immobilized Lactobacillus helveticus proteinase. J. Dairy Sci. 2019, 102, 961–975. [Google Scholar] [CrossRef]
- Bustamante, S.Z.; Gonzalez, J.G.; Sforza, S.; Tedeschi, T. Bioactivity and peptide profile of whey protein hydrolysates obtained from Colombian double-cream cheese production and their products after gastrointestinal digestion. LWT 2021, 145, 111334. [Google Scholar] [CrossRef]
- Rocha, G.F.; Cotabarren, J.; Obregón, W.D.; Fernández, G.; Rosso, A.M.; Parisi, M.G. Milk-clotting and hydrolytic activities of an aspartic protease from Salpichroa origanifolia fruits on individual caseins. Int. J. Biol. Macromol. 2021, 192, 931–938. [Google Scholar] [CrossRef]
- Balan, A.; Murthy, V.V.; Kadeppagari, R.K. Immobilized enzymes for bioconversion of waste to wealth. In Biotechnology for Zero Waste: Emerging Waste Management Techniques; Wiley: Hoboken, NJ, USA, 2022; pp. 33–46. [Google Scholar]
- Wafaa, N.; Elbarbary, H.; Ibrahim, E.; Mohamed, H.; Jenssen, H. Effect of enzyme type and hydrolysis time on antibacterial and antioxidant activity of whey protein hydrolysates. Iraqi J. Agric. Sci. 2022, 53, 1340–1357. [Google Scholar]
- Zhu, H.; Zhang, Y.; Yang, T.; Zheng, D.; Liu, X.; Zhang, J.; Zheng, M. Preparation of immobilized Alcalase based on metal affinity for efficient production of bioactive peptides. LWT 2022, 162, 113505. [Google Scholar] [CrossRef]
- da Cruz, C.Z.P.; de Mendonça, R.J.; Guimaraes, L.H.S.; dos Santos Ramos, M.A.; Garrido, S.S.; de Paula, A.V.; Monti, R.; Massolini, G. Assessment of the bioactive potential of cheese whey protein hydrolysates using immobilized alcalase. Food Bioprocess Technol. 2020, 13, 2120–2130. [Google Scholar] [CrossRef]
- Mao, Y.; Krischke, M.; Hengst, C.; Kulozik, U. Influence of salts on hydrolysis of β-lactoglobulin by free and immobilised trypsin. Int. Dairy J. 2019, 93, 106–115. [Google Scholar] [CrossRef]
- Xu, L.; Gong, Y.; Gern, J.E.; Lucey, J.A. Influence of whey protein hydrolysis in combination with dextran glycation on immunoglobulin E binding capacity with blood sera obtained from patients with a cow milk protein allergy. J. Dairy Sci. 2020, 103, 1141–1150. [Google Scholar] [CrossRef]
- Salvado, A.F.; Leitão, J.H.; Fonseca, L.P. Enzymatic production of bioactive peptides from whey proteins: Their active role and potential health benefits. In Enzymes for Solving Humankind’s Problems: Natural and Artificial Systems in Health, Agriculture, Environment and Energy; Springer: Berlin/Heidelberg, Germany, 2021; pp. 473–506. [Google Scholar]
- Kazemi-Taskooh, Z.; Varidi, M. Food-based iron delivery systems: A review. Trends Food Sci. Technol. 2021, 116, 75–89. [Google Scholar] [CrossRef]
- Siar, E.-H.; Morellon-Sterling, R.; Zidoune, M.N.; Fernandez-Lafuente, R. Use of glyoxyl-agarose immobilized ficin extract in milk coagulation: Unexpected importance of the ficin loading on the biocatalysts. Int. J. Biol. Macromol. 2020, 144, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Morellon-Sterling, R.; El-Siar, H.; Tavano, O.L.; Berenguer-Murcia, Á.; Fernández-Lafuente, R. Ficin: A protease extract with relevance in biotechnology and biocatalysis. Int. J. Biol. Macromol. 2020, 162, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.S.P.d.; de Andrade, C.J.; Koblitz, M.G.B.; Fai, A.E.C. Microbial peptidase in food processing: Current state of the art and future trends. Catal. Lett. 2023, 153, 114–137. [Google Scholar] [CrossRef]
- Zeng, J.; Zou, J.; Zhao, J.; Lin, K.; Zhang, L.; Yi, H.; Gong, P. Chymosin pretreatment accelerated papain catalysed hydrolysis for decreasing casein antigenicity by exposing the cleavage site at tyrosine residues. Food Chem. 2023, 404, 134777. [Google Scholar] [CrossRef]
- Sun, S.; Gao, Y.; Chen, J.; Liu, R. Identification and release kinetics of peptides from tilapia skin collagen during alcalase hydrolysis. Food Chem. 2022, 378, 132089. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Chen, Y.; Yu, J.; Yang, Z.; Wang, X.; Wang, X. Progress in enrichment of n-3 polyunsaturated fatty acid: A review. Crit. Rev. Food Sci. Nutr. 2022, 63, 11310–11326. [Google Scholar] [CrossRef]
- Ghide, M.K.; Li, K.; Wang, J.; Abdulmalek, S.A.; Yan, Y. Immobilization of Rhizomucor miehei lipase on magnetic multiwalled carbon nanotubes towards the synthesis of structured lipids rich in sn-2 palmitic acid and sn-1, 3 oleic acid (OPO) for infant formula use. Food Chem. 2022, 390, 133171. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Qin, A.; Yang, F.; Liu, D.; Li, H.; Yu, J. Milk protein hydrolysates obtained with immobilized alcalase and neutrase on magnetite nanoparticles: Characterization and antigenicity study. J. Food Sci. 2022, 87, 3107–3116. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H.; El-Shibiny, S. Reduction of milk protein antigenicity by enzymatic hydrolysis and fermentation. A review. Food Rev. Int. 2021, 37, 276–295. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ikram, S. Zinc oxide nanoparticles-impregnated chitosan surfaces for covalent immobilization of trypsin: Stability & kinetic studies. Int. J. Biol. Macromol. 2022, 207, 205–221. [Google Scholar]
- Rivera del Rio, A.; Boom, R.M.; Janssen, A.E. Effect of fractionation and processing conditions on the digestibility of plant proteins as food ingredients. Foods 2022, 11, 870. [Google Scholar] [CrossRef]
- Katić, K.; Banjanac, K.; Simović, M.; Ćorović, M.; Milivojević, A.; Marinković, A.; Bezbradica, D. Development of protease nanobiocatalysts and their application in hydrolysis of sunflower meal protein isolate. Int. J. Food Sci. Technol. 2021, 56, 4287–4297. [Google Scholar] [CrossRef]
- Li, J.; Hartinger, C.; Zhu, F. Physicochemical properties of infant formula model emulsions stabilised by different whey protein hydrolysates and characteristics of interfacial peptides. Food Hydrocoll. 2023, 145, 109035. [Google Scholar] [CrossRef]
- Budžaki, S.; Velić, N.; Ostojčić, M.; Stjepanović, M.; Rajs, B.B.; Šereš, Z.; Maravić, N.; Stanojev, J.; Hessel, V.; Strelec, I. Waste management in the agri-food industry: The conversion of eggshells, spent coffee grounds, and brown onion skins into carriers for Lipase immobilization. Foods 2022, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Jasińska, K.; Zieniuk, B.; Jankiewicz, U.; Fabiszewska, A. Bio-Based Materials versus Synthetic Polymers as a Support in Lipase Immobilization: Impact on Versatile Enzyme Activity. Catalysts 2023, 13, 395. [Google Scholar] [CrossRef]
- Pellegrino, L.; Hogenboom, J.A.; Rosi, V.; Sindaco, M.; Gerna, S.; D’Incecco, P. Focus on the Protein Fraction of Sports Nutrition Supplements. Molecules 2022, 27, 3487. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy by-products: A review on the valorization of whey and second cheese whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Bella, K.; Pilli, S.; Venkateswara Rao, P.; Tyagi, R. Bio-conversion of whey lactose using enzymatic hydrolysis with β-galactosidase: An experimental and kinetic study. Environ. Technol. 2022, 45, 1234–1247. [Google Scholar] [CrossRef]
- Shafi, A.; Ahmed, F.; Husain, Q. β-Galactosidase mediated synthesized nanosupport for the immobilization of same enzyme: Its stability and application in the hydrolysis of lactose. Int. J. Biol. Macromol. 2021, 184, 57–67. [Google Scholar] [CrossRef]
- de Freitas, M.d.F.M.; Hortêncio, L.C.; de Albuquerque, T.L.; Rocha, M.V.P.; Gonçalves, L.R.B. Simultaneous hydrolysis of cheese whey and lactulose production catalyzed by β-galactosidase from Kluyveromyces lactis NRRL Y1564. Bioprocess Biosyst. Eng. 2020, 43, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, N. Clinical applications of whey protein. In Nutrition Science, Marketing Nutrition, Health Claims, and Public Policy; Elsevier: Amsterdam, The Netherlands, 2023; pp. 13–22. [Google Scholar]
- Srikanth, D.; Gopi, D.; Sunil, C.; Michael, K.; Rawson, A. Proteins as Fat Replacers in the Food Industry. In Fat Mimetics Food Applications; Wiley: Hoboken, NJ, USA, 2023; pp. 133–154. [Google Scholar]
- Sharma, K.; Saini, R.; Patil, S. A Systematic Review on the Emerging Role of Protein Powder. Int. J. Res. Eng. Sci. 2022, 10, 88–90. [Google Scholar]
- Mora, L.; Toldrá, F. Advanced enzymatic hydrolysis of food proteins for the production of bioactive peptides. Curr. Opin. Food Sci. 2022, 49, 100973. [Google Scholar] [CrossRef]
- Yu, A.E. Continuous hydrolysis of milk proteins in membrane reactors of various configurations. Foods Raw Mater. 2021, 9, 271–281. [Google Scholar]
- Zanutto-Elgui, M.R.; Vieira, J.C.S.; do Prado, D.Z.; Buzalaf, M.A.R.; de Magalhães Padilha, P.; de Oliveira, D.E.; Fleuri, L.F. Production of milk peptides with antimicrobial and antioxidant properties through fungal proteases. Food Chem. 2019, 278, 823–831. [Google Scholar] [CrossRef]
- Smacchi, E.; Gobbetti, M. Bioactive peptides in dairy products: Synthesis and interaction with proteolytic enzymes. Food Microbiol. 2000, 17, 129–141. [Google Scholar] [CrossRef]
- Punia, H.; Tokas, J.; Malik, A.; Sangwan, S.; Baloda, S.; Singh, N.; Singh, S.; Bhuker, A.; Singh, P.; Yashveer, S. Identification and detection of bioactive peptides in milk and dairy products: Remarks about agro-foods. Molecules 2020, 25, 3328. [Google Scholar] [CrossRef] [PubMed]
- van Esch, B.C.; Knipping, K.; Jeurink, P.; van der Heide, S.; Dubois, A.E.; Willemsen, L.E.; Garssen, J.; Knippels, L.M. In vivo and in vitro evaluation of the residual allergenicity of partially hydrolysed infant formulas. Toxicol. Lett. 2011, 201, 264–269. [Google Scholar] [CrossRef]
- Holvoet, S.; Nutten, S.; Dupuis, L.; Donnicola, D.; Bourdeau, T.; Hughes-Formella, B.; Simon, D.; Simon, H.-U.; Carvalho, R.S.; Spergel, J.M. Partially hydrolysed whey-based infant formula improves skin barrier function. Nutrients 2021, 13, 3113. [Google Scholar] [CrossRef]
- Heine, R.G.; AlRefaee, F.; Bachina, P.; De Leon, J.C.; Geng, L.; Gong, S.; Madrazo, J.A.; Ngamphaiboon, J.; Ong, C.; Rogacion, J.M. Lactose intolerance and gastrointestinal cow’s milk allergy in infants and children–common misconceptions revisited. World Allergy Organ. J. 2017, 10, 1–8. [Google Scholar] [CrossRef]
- del Carmen Tocaa, M.; Fernándezb, A.; Orsic, M.; Tabaccod, O.; Vinderolae, G. Lactose intolerance: Myths and facts. An update. Arch. Argent. Pediatr. 2022, 120, 59–66. [Google Scholar]
- Lasekan, J.B.; Jacobs, J.; Reisinger, K.S.; Montalto, M.B.; Frantz, M.P.; Blatter, M.M. Lactose-free milk protein-based infant formula: Impact on growth and gastrointestinal tolerance in infants. Clin. Pediatr. 2011, 50, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Dipasquale, V.; Serra, G.; Corsello, G.; Romano, C. Standard and specialized infant formulas in Europe: Making, marketing, and health outcomes. Nutr. Clin. Pract. 2020, 35, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Belfiore, L.A.; Tambourgi, E.B.; Paulino, A.T. Production of low-dosage lactose milk using lactase immobilised in hydrogel. Int. Dairy J. 2019, 92, 77–83. [Google Scholar] [CrossRef]
- Schulz, P.; Rizvi, S.S. Hydrolysis of lactose in milk: Current status and future products. Food Rev. Int. 2021, 39, 2875–2894. [Google Scholar] [CrossRef]
- Selvarajan, E.; Nivetha, A.; Subathra Devi, C.; Mohanasrinivasan, V. Nanoimmobilization of β-galactosidase for lactose-free product development. In Nanoscience and Biotechnology for Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 199–223. [Google Scholar]
- Zaworski, K.; Woliński, J.; Słupecka-Ziemilska, M.; Pierzynowski, S.; Pierzynowska, K. Pre-digestion of the lipids in infant formula affects gut maturation of the preterm pig. PLoS ONE 2022, 17, e0265144. [Google Scholar] [CrossRef] [PubMed]
- Goncharova, K.; Kirko, S.; Grujic, D.; Kardas, M.; Grochowska-Niedworok, E.; Prykhodko, O.; Woliński, J.; Ushakova, G.; Lozinska, L.; Pierzynowski, S.G. Enhanced absorption of long-chain polyunsaturated fatty acids following consumption of functional milk formula, pre-digested with immobilized lipase ex vivo, in an exocrine pancreatic insufficient (EPI) pig model. J. Funct. Foods 2017, 34, 422–430. [Google Scholar] [CrossRef]
- Mohan, M.S.; O’Callaghan, T.F.; Kelly, P.; Hogan, S.A. Milk fat: Opportunities, challenges and innovation. Crit. Rev. Food Sci. Nutr. 2021, 61, 2411–2443. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.; Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L. Enzymatic coagulation of milk. In Fundamentals of Cheese Science; Springer: Berlin/Heidelberg, Germany, 2017; pp. 185–229. [Google Scholar]
- Hosseini, S.; Varidi, M. Optimization of microbial rennet encapsulation in alginate–chitosan nanoparticles. Food Chem. 2021, 352, 129325. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-H.; Yun, S.-E.; Mun, S.-P. The role of immobilized rennet on carbon cloth in flavor development during ripening of Gouda cheese. Food Sci. Biotechnol. 2016, 25, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Kessi, E.; Arias, J. Recombinant Camel Chymosin Effectively Acts on Milk Coagulation after Immobilization on Eggshell Membranes. ACS Food Sci. Technol. 2022, 2, 808–814. [Google Scholar] [CrossRef]
- He, W.S.; Sun, Y.; Li, Z.; Yang, H.; Li, J.; Wang, Q.; Tan, C.; Zou, B. Enhanced antioxidant capacity of lipoic acid in different food systems through lipase-mediated esterification with phytosterols. J. Sci. Food Agric. 2022, 102, 7115–7125. [Google Scholar] [CrossRef]
- Mustafa, A.; Ramadan, R.; Niikura, F.; Inayat, A.; Hafez, H. Highly selective synthesis of glyceryl monostearate via lipase catalyzed esterification of triple pressed stearic acid and glycerin. Sustain. Energy Technol. Assess. 2023, 57, 103200. [Google Scholar] [CrossRef]
- Abdel-Mageed, H.M.; Nada, D.; Radwan, R.A.; Mohamed, S.A.; Gohary, N.A.E. Optimization of catalytic properties of Mucor racemosus lipase through immobilization in a biocompatible alginate gelatin hydrogel matrix for free fatty acid production: A sustainable robust biocatalyst for ultrasound-assisted olive oil hydrolysis. 3 Biotech 2022, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- Savickaite, A.; Sadauskas, M.; Gudiukaite, R. Immobilized GDEst-95, GDEst-lip and GD-95RM lipolytic enzymes for continuous flow hydrolysis and transesterification reactions. Int. J. Biol. Macromol. 2021, 173, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Moreira, K.d.S.; de Oliveira, A.L.; Júnior, L.S.d.M.; Monteiro, R.R.; da Rocha, T.N.; Menezes, F.L.; Fechine, L.M.; Denardin, J.C.; Michea, S.; Freire, R.M. Lipase from Rhizomucor miehei immobilized on magnetic nanoparticles: Performance in fatty acid ethyl ester (FAEE) optimized production by the Taguchi method. Front. Bioeng. Biotechnol. 2020, 8, 693. [Google Scholar] [CrossRef]
- Dong, T.; Zhou, X.; Dai, Y.; Yang, X.; Zhang, W.; Yu, D.; Liu, T. Application of magnetic immobilized enzyme of nano dialdehyde starch in deacidification of rice bran oil. Enzym. Microb. Technol. 2022, 161, 110116. [Google Scholar] [CrossRef]
- Bilal, M.; Fernandes, C.D.; Mehmood, T.; Nadeem, F.; Tabassam, Q.; Ferreira, L.F.R. Immobilized lipases-based nano-biocatalytic systems—A versatile platform with incredible biotechnological potential. Int. J. Biol. Macromol. 2021, 175, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Sabi, G.J.; Gama, R.S.; Fernandez-Lafuente, R.; Cancino-Bernardi, J.; Mendes, A.A. Decyl esters production from soybean-based oils catalyzed by lipase immobilized on differently functionalized rice husk silica and their characterization as potential biolubricants. Enzym. Microb. Technol. 2022, 157, 110019. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, M.; Ponticorvo, E.; Cirillo, C.; Castaldo, R.; De Pasquale, S.; Gentile, G.; Sarno, M. Wax esters from waste fish oil catalysed by immobilized Candida rugosa lipase. Process Biochem. 2023, 130, 386–400. [Google Scholar] [CrossRef]
- Ungcharoenwiwat, P.; Aran, H. Enzymatic synthesis of coconut oil based wax esters by immobilized lipase EQ3 and commercial lipozyme RMIM. Electron. J. Biotechnol. 2020, 47, 10–16. [Google Scholar] [CrossRef]
- Simões, T.; Ferreira, J.; Lemos, M.F.; Augusto, A.; Félix, R.; Silva, S.F.; Ferreira-Dias, S.; Tecelão, C. Argan oil as a rich source of linoleic fatty acid for dietetic structured lipids production. Life 2021, 11, 1114. [Google Scholar] [CrossRef]
- Mota, D.A.; Rajan, D.; Heinzl, G.C.; Osorio, N.M.; Gominho, J.; Krause, L.C.; Soares, C.M.; Nampoothiri, K.M.; Sukumaran, R.K.; Ferreira-Dias, S. Production of low-calorie structured lipids from spent coffee grounds or olive pomace crude oils catalyzed by immobilized lipase in magnetic nanoparticles. Bioresour. Technol. 2020, 307, 123223. [Google Scholar] [CrossRef]
- Brisola, J.; Andrade, G.J.S.; Oliveira, S.A.d.; Viana, R.M.R.; Tischer, P.C.d.S.F.; Tischer, C.A. Covalent Immobilization of Lipase on Bacterial Cellulose Membrane and Nanocellulose. Mater. Res. 2022, 25, e20210350. [Google Scholar] [CrossRef]
- Xia, Q.; Akanbi, T.O.; Li, R.; Wang, B.; Yang, W.; Barrow, C.J. Lipase-catalysed synthesis of palm oil-omega-3 structured lipids. Food Funct. 2019, 10, 3142–3149. [Google Scholar] [CrossRef]
- Fotiadou, R.; Lefas, D.; Vougiouklaki, D.; Tsakni, A.; Houhoula, D.; Stamatis, H. Enzymatic Modification of Pomace Olive Oil with Natural Antioxidants: Effect on Oxidative Stability. Biomolecules 2023, 13, 1034. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, M.; Yan, X.; Zhang, G.; Xia, J.; Zeng, Z.; Yu, P.; Deng, Q.; Gong, D. Green synthesis of polydopamine functionalized magnetic mesoporous biochar for lipase immobilization and its application in interesterification for novel structured lipids production. Food Chem. 2022, 379, 132148. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Lee, W.J.; Lai, O.M.; Tan, C.P.; Wang, Y. Production of Structured triacylglycerol via enzymatic interesterification of medium-chain triacylglycerol and soybean oil using a pilot-scale solvent-free packed bed reactor. J. Am. Oil Chem. Soc. 2020, 97, 271–280. [Google Scholar] [CrossRef]
- Hwang, J.; Aum, J.; Lee, S.J.; Mun, J.M.; Kim, S.W.; Chung, M.-Y.; Kim, I.-H.; Kim, B.H. Immobilized Candida antarctica lipase B as an sn-1, 3 regiospecific biocatalyst for the interesterification of triacylglycerols with fatty acid ethyl esters. Food Sci. Biotechnol. 2023, 33, 159–170. [Google Scholar] [CrossRef]
- Miotti Jr, R.H.; Cortez, D.V.; De Castro, H.F. Transesterification of palm kernel oil with ethanol catalyzed by a combination of immobilized lipases with different specificities in continuous two-stage packed-bed reactor. Fuel 2022, 310, 122343. [Google Scholar] [CrossRef]
- Xia, Q.; Akanbi, T.O.; Wang, B.; Li, R.; Yang, W.; Barrow, C.J. Investigating the mechanism for the enhanced oxidation stability of microencapsulated omega-3 concentrates. Mar. Drugs 2019, 17, 143. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Zhang, W.; Zhang, Y.; Zhang, S.; Pang, X.; Lu, J.; Lv, J. Dietary Schizochytrium microalgae affect the fatty acid profile of goat milk: Quantification of docosahexaenoic Acid (DHA) and its distribution at Sn-2 position. Foods 2022, 11, 2087. [Google Scholar] [CrossRef] [PubMed]
- Dovale-Rosabal, G.; Rodríguez, A.; Espinosa, A.; Barriga, A.; Aubourg, S.P. Synthesis of EPA-and DHA-enriched structured acylglycerols at the sn-2 position starting from commercial salmon oil by enzymatic lipase catalysis under supercritical conditions. Molecules 2021, 26, 3094. [Google Scholar] [CrossRef] [PubMed]
- Khoonin, W.; Shantavasinkul, P.C.; Santivarangkna, C.; Trachootham, D. Loss of Eicosapentaenoic Acid (EPA) after Retort Sterilization of the EPA-BCAA Fortified Complete Nutrition Drink. Foods 2022, 11, 2023. [Google Scholar] [CrossRef] [PubMed]
- Akanbi, T.O.; Sinclair, A.J.; Barrow, C.J. Pancreatic lipase selectively hydrolyses DPA over EPA and DHA due to location of double bonds in the fatty acid rather than regioselectivity. Food Chem. 2014, 160, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; Stevenson, R.J.; Ang, X.; Peng, Y.; Quek, S.Y. Lipase-catalyzed modification of milk fat: A promising way to alter flavor notes of goat milk products. LWT 2021, 145, 111286. [Google Scholar] [CrossRef]
- Singh, P.K.; Chopra, R.; Garg, M.; Dhiman, A.; Dhyani, A. Enzymatic Interesterification of Vegetable Oil: A Review on Physicochemical and Functional Properties, and Its Health Effects. J. Oleo Sci. 2022, 71, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Akanbi, T.O.; Barrow, C.J. Lipase-catalysed incorporation of EPA into emu oil: Formation and characterisation of new structured lipids. J. Funct. Foods 2015, 19, 801–809. [Google Scholar] [CrossRef]
- Nagpal, T.; Sahu, J.K.; Khare, S.K.; Bashir, K.; Jan, K. Trans fatty acids in food: A review on dietary intake, health impact, regulations and alternatives. J. Food Sci. 2021, 86, 5159–5174. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, Q.; Jiang, C.; Evivie, S.E.; Cao, T.; Wang, Z.; Zhao, L.; Liang, S.; Li, B.; Huo, G. Infant formula supplemented with 1, 3-olein-2-palmitin regulated the immunity, gut microbiota, and metabolites of mice colonized by feces from healthy infants. J. Dairy Sci. 2022, 105, 6405–6421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Y.; Li, Y.; Lin, X.; Hong, Z.; Huang, J.; Zhang, X.; Yang, Y.; Su, Y. Effects of Sn-2-palmitate-enriched formula feeding on infants’ growth, stool characteristics, stool fatty acid soap contents and bone mineral content: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2023, 63, 10256–10266. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Pan, Y.; Qiao, M.; Yuan, Y. Synthesis of human milk fat substitutes based on enzymatic preparation of low erucic acid acyl-donors from rapeseed oil. Food Chem. 2022, 387, 132907. [Google Scholar] [PubMed]
- Li, Y.; Zhang, Y.; Zhou, Y.; Zhang, Y.; Zheng, M. A novel and controllable method for simultaneous preparation of human milk fat substitutes (OPL, OPO and LPL): Two-step enzymatic ethanolysis-esterification strategy. Food Res. Int. 2023, 163, 112168. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, Z.; Hua, L.; Zou, F.; Cheng, X.; Wang, X. Preparation of human milk fat substitutes similar to human milk fat by enzymatic acidolysis and physical blending. LWT 2021, 140, 110818. [Google Scholar] [CrossRef]
- Soares, C.M.; Barbosa, M.S.; Santos, S.B.; Mattedi, S.; Lima, Á.S.; Pereira, M.M.; Tecelão, C.; Ferreira-Dias, S. Production of Human Milk Fat Substitutes by Lipase-Catalyzed Acidolysis: Immobilization, Synthesis, Molecular Docking and Optimization Studies. Catalysts 2023, 13, 825. [Google Scholar] [CrossRef]
- Bryś, J.; Górska, A.; Ostrowska-Ligęza, E.; Wirkowska-Wojdyła, M.; Bryś, A.; Brzezińska, R.; Dolatowska-Żebrowska, K.; Małajowicz, J.; Ziarno, M.; Obranović, M. Human milk fat substitutes from lard and hemp seed oil mixtures. Appl. Sci. 2021, 11, 7014. [Google Scholar] [CrossRef]
- Arzola-Rodríguez, S.I.; Muñoz-Castellanos, L.-N.; López-Camarillo, C.; Salas, E. Phenolipids, amphipilic phenolic antioxidants with modified properties and their spectrum of applications in development: A review. Biomolecules 2022, 12, 1897. [Google Scholar] [CrossRef]
- Akanbi, T.O.; Barrow, C.J. Lipase-produced hydroxytyrosyl eicosapentaenoate is an excellent antioxidant for the stabilization of omega-3 bulk oils, emulsions and microcapsules. Molecules 2018, 23, 275. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; Rahman, U.U.; Soares, B.C.V.; Souza, S.L.Q.; Pimentel, T.C.; Scudino, H.; Guimaraes, J.T.; Esmerino, E.A.; et al. Treatment and utilization of dairy industrial waste: A review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Paul, A.; Kumar, V.; Sar, T.; Kumar, D.; Sarsaiya, S.; Liu, H.; Zhang, Z.; Binod, P.; Sindhu, R. Recent trends and developments on integrated biochemical conversion process for valorization of dairy waste to value added bioproducts: A review. Bioresour. Technol. 2022, 344, 126193. [Google Scholar]
- Zanuso, E.; Gomes, D.G.; Ruiz, H.A.; Teixeira, J.A.; Domingues, L. Enzyme immobilization as a strategy towards efficient and sustainable lignocellulosic biomass conversion into chemicals and biofuels: Current status and perspectives. Sustain. Energy Fuels 2021, 5, 4233–4247. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Sustainable bioconversion of food waste into high-value products by immobilized enzymes to meet bio-economy challenges and opportunities–A review. Food Res. Int. 2019, 123, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Charles, M. Applications of Lactase and Immobilized Lactase; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Sarnoab, M.; Iuliano, M. Biodiesel production from dairy waste scum by using an efficient nano-biocatalyst. Chem. Eng. 2020, 79, 181–186. [Google Scholar]
- Mehrotra, T.; Dev, S.; Banerjee, A.; Chatterjee, A.; Singh, R.; Aggarwal, S. Use of immobilized bacteria for environmental bioremediation: A review. J. Environ. Chem. Eng. 2021, 9, 105920. [Google Scholar] [CrossRef]
- Cieh, N.L.; Mokhtar, M.N.; Baharuddin, A.S.; Mohammed, M.A.P.; Wakisaka, M. Progress on lipase immobilization technology in edible oil and fat modifications. Food Rev. Int. 2023, 40, 457–503. [Google Scholar] [CrossRef]
- Pourjoula, M.; Picariello, G.; Garro, G.; D’Auria, G.; Nitride, C.; Ghaisari, A.R.; Ferranti, P. The protein and peptide fractions of kashk, a traditional Middle East fermented dairy product. Food Res. Int. 2020, 132, 109107. [Google Scholar] [CrossRef]
- Hashemabadi, M.; Badoei-Dalfard, A. Fabrication of magnetic CLEA-protease nanocomposite: High progression in biotechnology and protein waste management. Catal. Lett. 2019, 149, 1753–1764. [Google Scholar] [CrossRef]
- Misson, M.; Saallah, S.; Zhang, H. Nanofiber-immobilized β-galactosidase for dairy waste conversion into galacto-oligosaccharides. In Advances in Waste Processing Technology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 37–48. [Google Scholar]
- Wang, Z.; Qi, J.; Goddard, J.M. Concentrated sugar solutions protect lactase from thermal inactivation. Int. Dairy J. 2021, 123, 105168. [Google Scholar] [CrossRef]
- Ng, H.S.; Kee, P.E.; Yim, H.S.; Chen, P.-T.; Wei, Y.-H.; Lan, J.C.-W. Recent advances on the sustainable approaches for conversion and reutilization of food wastes to valuable bioproducts. Bioresour. Technol. 2020, 302, 122889. [Google Scholar] [CrossRef]
- Ekeoma, B.C.; Ekeoma, L.N.; Yusuf, M.; Haruna, A.; Ikeogu, C.K.; Merican, Z.M.A.; Kamyab, H.; Pham, C.Q.; Vo, D.-V.N.; Chelliapan, S. Recent advances in the biocatalytic mitigation of emerging pollutants: A comprehensive review. J. Biotechnol. 2023, 369, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.; Devi, M.K.; Kumar, P.S. Advances in the application of immobilized enzyme for the remediation of hazardous pollutant: A review. Chemosphere 2022, 299, 134390. [Google Scholar] [CrossRef] [PubMed]
- Pottratz, I.; Schmidt, C.; Müller, I.; Hamel, C. Immobilization of β-Galactosidase on Monolithic Discs for the Production of Prebiotics Galacto-oligosaccharides. Chem. Ing. Tech. 2021, 93, 838–843. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Multi-faceted strategy based on enzyme immobilization with reactant adsorption and membrane technology for biocatalytic removal of pollutants: A critical review. Biotechnol. Adv. 2019, 37, 107401. [Google Scholar] [CrossRef]
- Zieniuk, B.; Białecka-Florjańczyk, E.; Wierzchowska, K.; Fabiszewska, A. Recent advances in the enzymatic synthesis of lipophilic antioxidant and antimicrobial compounds. World J. Microbiol. Biotechnol. 2022, 38, 11. [Google Scholar] [CrossRef] [PubMed]
- Nimkande, V.D.; Bafana, A. A review on the utility of microbial lipases in wastewater treatment. J. Water Process Eng. 2022, 46, 102591. [Google Scholar] [CrossRef]
- Rather, A.H.; Khan, R.S.; Wani, T.U.; Beigh, M.A.; Sheikh, F.A. Overview on immobilization of enzymes on synthetic polymeric nanofibers fabricated by electrospinning. Biotechnol. Bioeng. 2022, 119, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Alcaide, M.F.; Godínez-Alemán, J.A.; González-González, R.B.; Iqbal, H.M.; Parra-Saldívar, R. Environmental impact and mitigation of micro (nano) plastics pollution using green catalytic tools and green analytical methods. Green Anal. Chem. 2022, 3, 100031. [Google Scholar] [CrossRef]
- Chakraborty, D.; Chatterjee, S.; Althuri, A.; Mohan, S.V. Sustainable enzymatic treatment of organic waste in a framework of circular economy. Bioresour. Technol. 2022, 370, 128487. [Google Scholar] [CrossRef] [PubMed]
- Işık, C.; Saraç, N.; Teke, M.; Uğur, A. A new bioremediation method for removal of wastewater containing oils with high oleic acid composition: Acinetobacter haemolyticus lipase immobilized on eggshell membrane with improved stabilities. New J. Chem. 2021, 45, 1984–1992. [Google Scholar] [CrossRef]
- Feng, K.; Huang, Z.; Peng, B.; Dai, W.; Li, Y.; Zhu, X.; Chen, Y.; Tong, X.; Lan, Y.; Cao, Y. Immobilization of Aspergillus niger lipase onto a novel macroporous acrylic resin: Stable and recyclable biocatalysis for deacidification of high-acid soy sauce residue oil. Bioresour. Technol. 2020, 298, 122553. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Sun, S.; Liu, J. Conversion of waste frying palm oil into biodiesel using free lipase A from Candida antarctica as a novel catalyst. Fuel 2020, 267, 117323. [Google Scholar] [CrossRef]
- Altinok, F.; Albayrak, S.; Arslan, N.P.; Taskin, M.; Aygun, E.; Sisecioglu, M.; Adiguzel, A. Application of Anoxybacillus gonensins UF7 lipase as a catalyst for biodiesel production from waste frying oils. Fuel 2023, 334, 126672. [Google Scholar] [CrossRef]
- Nezhad, M.K.; Aghaei, H. Tosylated cloisite as a new heterofunctional carrier for covalent immobilization of lipase and its utilization for production of biodiesel from waste frying oil. Renew. Energy 2021, 164, 876–888. [Google Scholar] [CrossRef]
- Patchimpet, J.; Simpson, B.K.; Sangkharak, K.; Klomklao, S. Optimization of process variables for the production of biodiesel by transesterification of used cooking oil using lipase from Nile tilapia viscera. Renew. Energy 2020, 153, 861–869. [Google Scholar] [CrossRef]
- Parandi, E.; Safaripour, M.; Abdellattif, M.H.; Saidi, M.; Bozorgian, A.; Nodeh, H.R.; Rezania, S. Biodiesel production from waste cooking oil using a novel biocatalyst of lipase enzyme immobilized magnetic nanocomposite. Fuel 2022, 313, 123057. [Google Scholar] [CrossRef]
- Parandi, E.; Safaripour, M.; Mosleh, N.; Saidi, M.; Nodeh, H.R.; Oryani, B.; Rezania, S. Lipase enzyme immobilized over magnetic titanium graphene oxide as catalyst for biodiesel synthesis from waste cooking oil. Biomass Bioenergy 2023, 173, 106794. [Google Scholar] [CrossRef]
- Sharma, T.; Xia, C.; Sharma, A.; Raizada, P.; Singh, P.; Sharma, S.; Sharma, P.; Kumar, S.; Lam, S.; Nadda, A.K. Mechano-chemical and biological energetics of immobilized enzymes onto functionalized polymers and their applications. Bioengineered 2022, 13, 10518–10539. [Google Scholar]
- Almeida, F.L.; Travalia, B.M.; Goncalves, I.S.; Forte, M.B.S. Biodiesel production by lipase-catalyzed reactions: Bibliometric analysis and study of trends. Biofuels Bioprod. Biorefining 2021, 15, 1141–1159. [Google Scholar] [CrossRef]
- Skliar, V.; Krusir, G.; Zakharchuk, V. Investigation of enzymatic hydrolysis of fatty waste processing of sunflower seeds by immobilized lipase. Grain Prod. Mix. Fodder’s 2020, 20, 5–11. [Google Scholar] [CrossRef]
- Kumari, M.; Chattopadhyay, S. The evaluation of the performance of rice husk and rice straw as potential matrix to obtain the best lipase immobilized system: Creating wealth from wastes. Prep. Biochem. Biotechnol. 2023, 53, 763–772. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).