Synthesis, Characterization and Power Factor Estimation of SnSe Thin Film for Energy Harvesting Applications
Abstract
:1. Introduction
2. Experimental Details
2.1. Chemicals
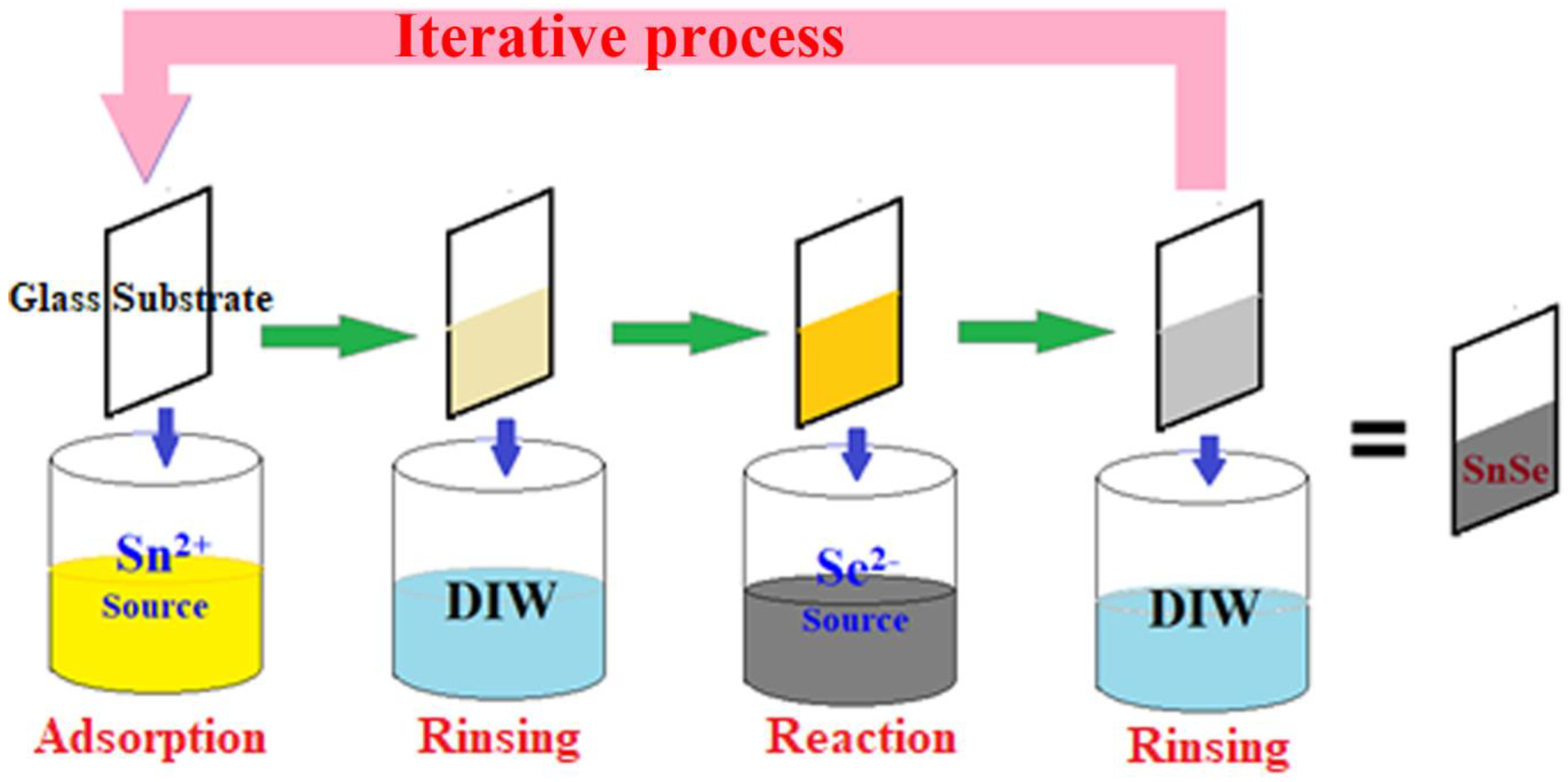
2.2. Preparation of SnSe Thin Film
2.3. Thin Film Characterization
2.4. Thermoelectric Characterization
3. Results and Discussion
3.1. Structural Analysis
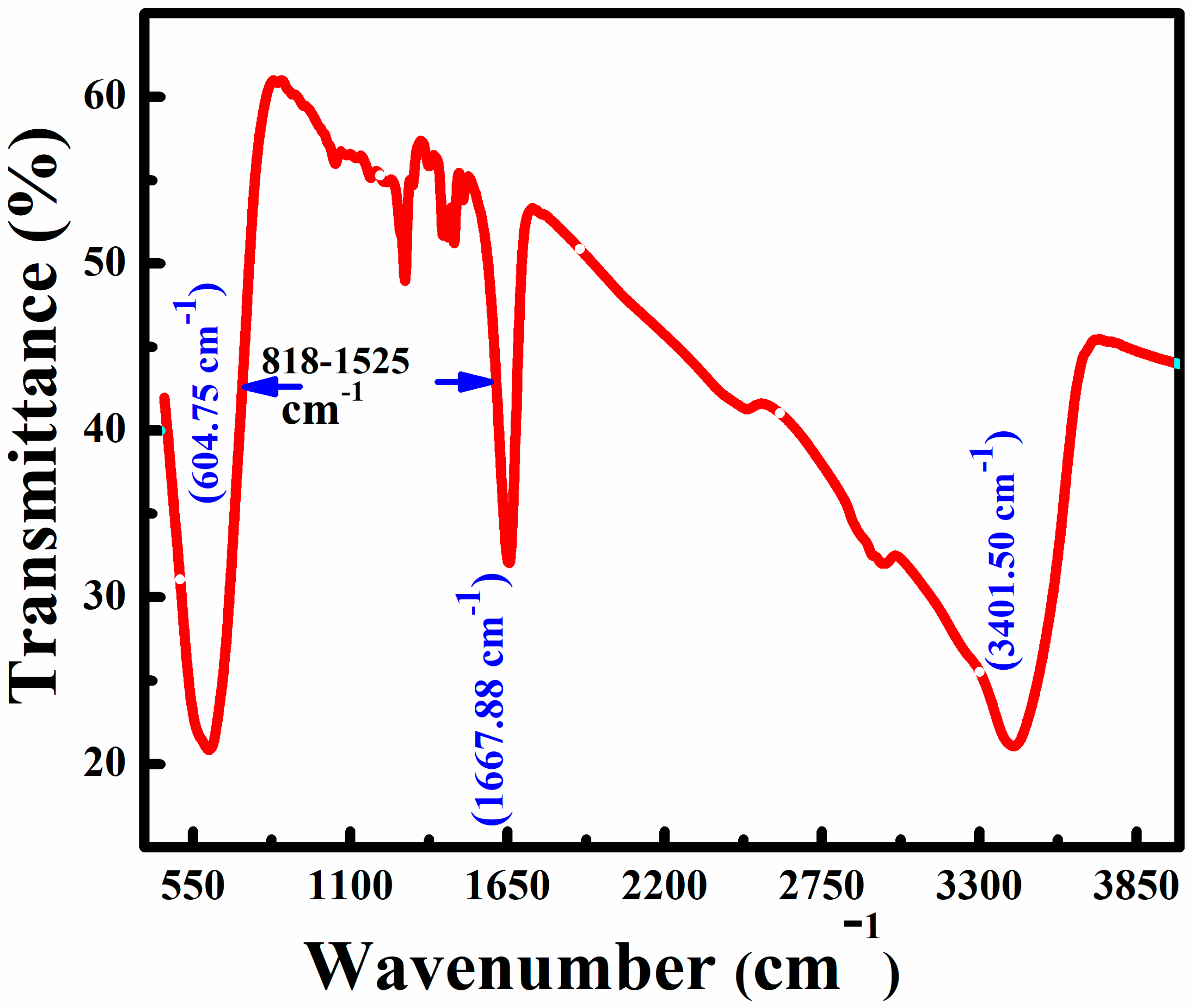
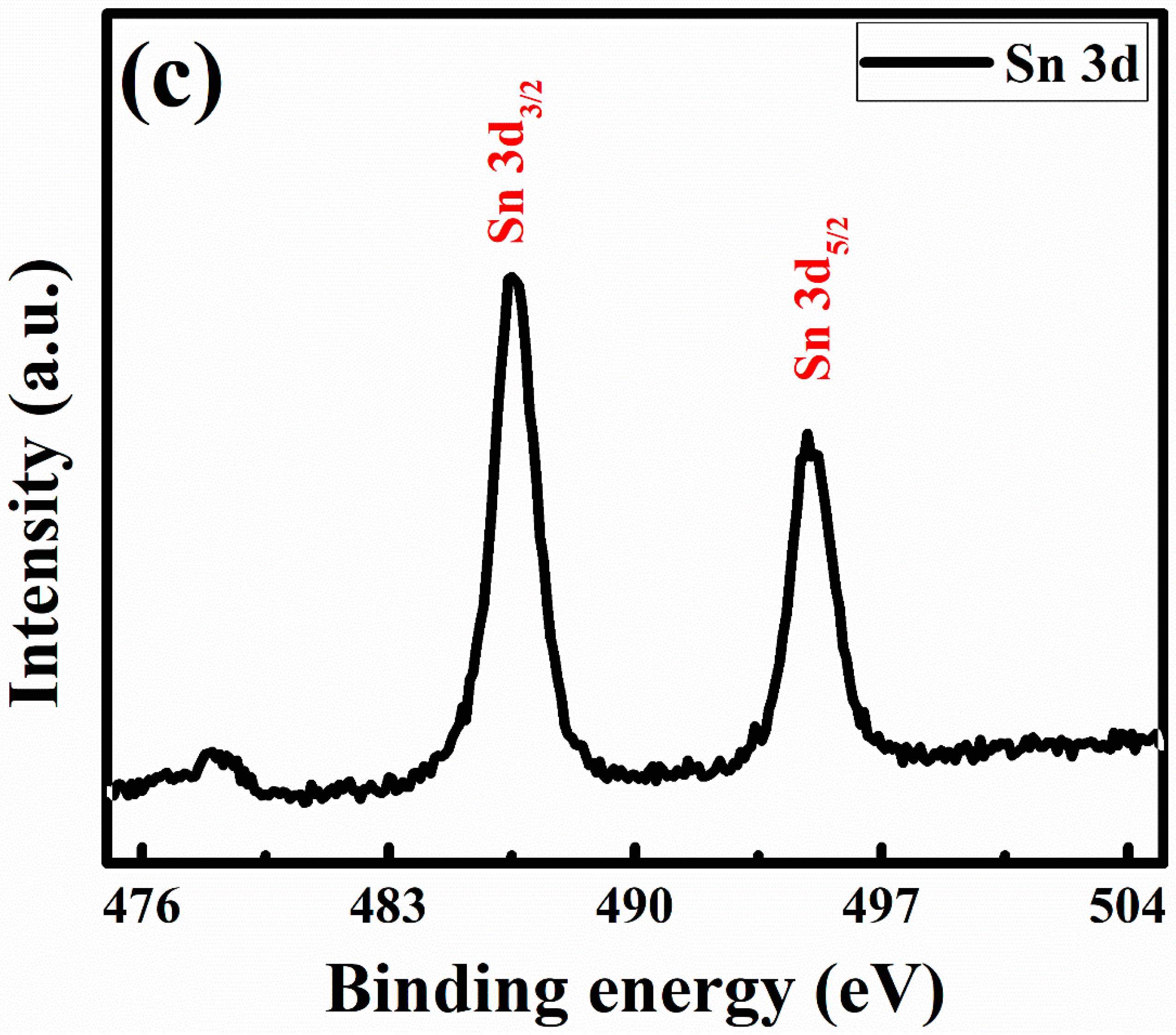
3.2. Surface and Composition Investigations
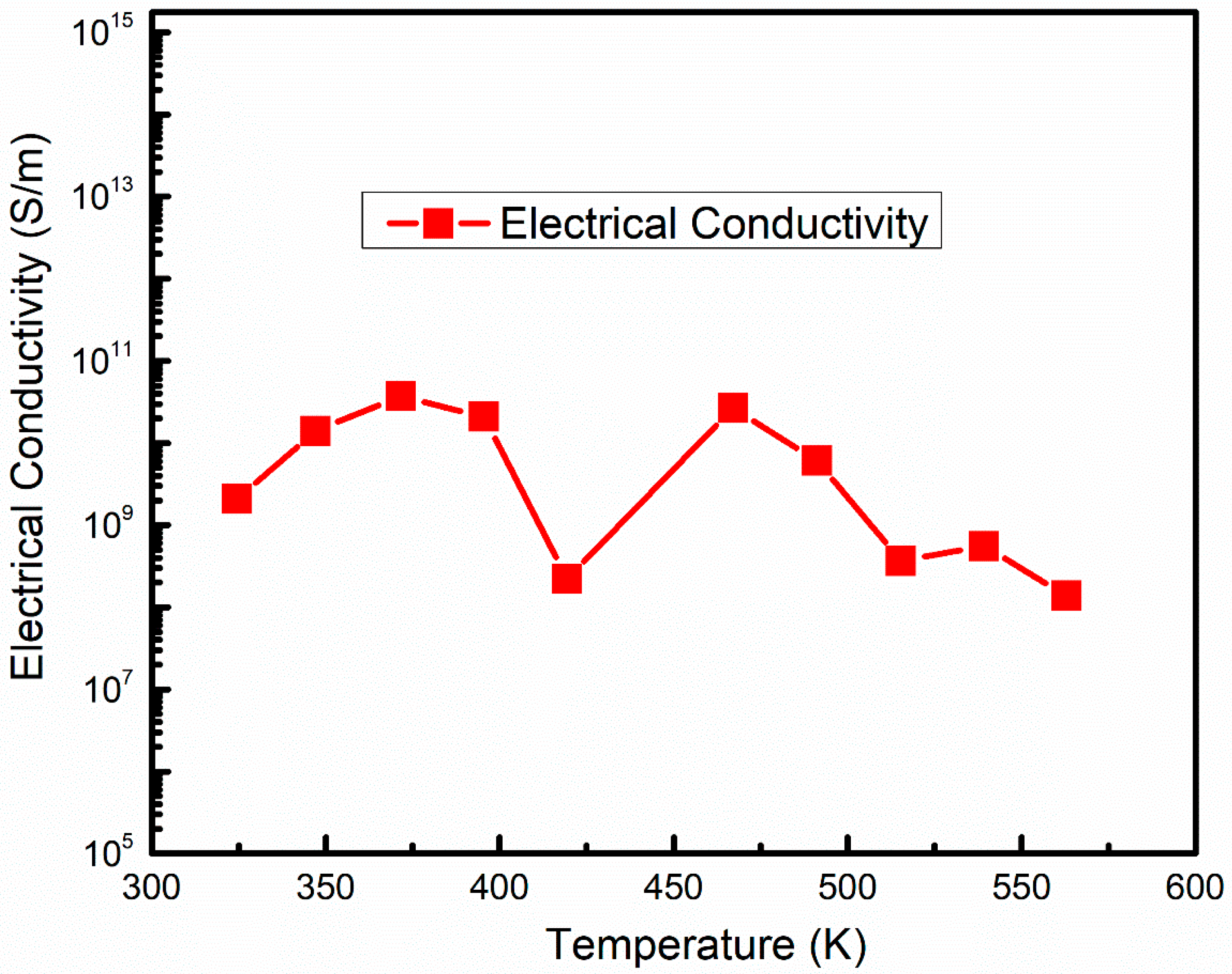
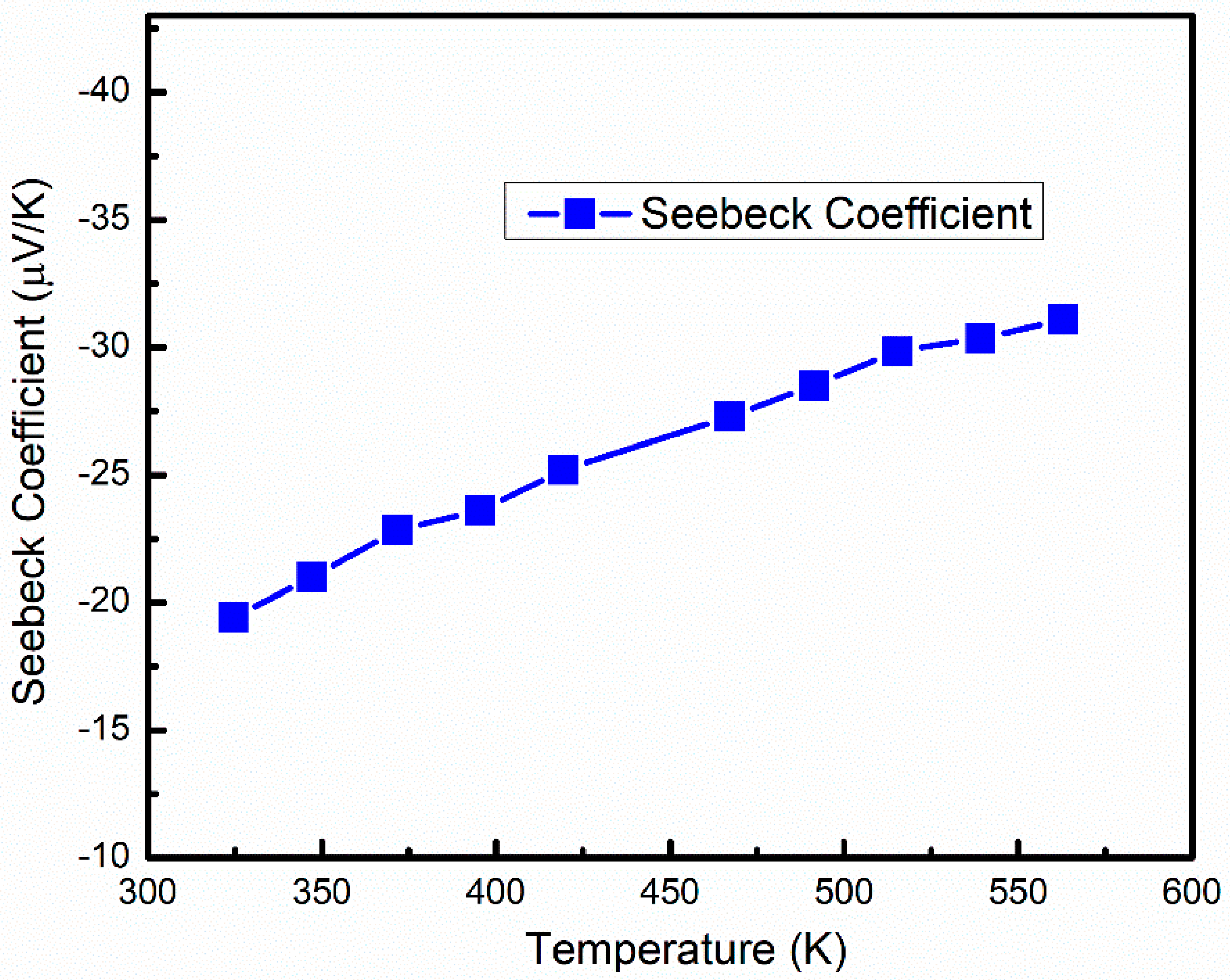
3.3. Thermoelectric Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-G.; Shi, X.; Zhao, L.-D.; Zou, J. High-performance SnSe thermoelectric materials: Progress and future challenge. Prog. Mater. Sci. 2018, 97, 283–346. [Google Scholar] [CrossRef]
- Hao, F.; Qiu, P.; Tang, Y.; Bai, S.; Xing, T.; Chu, H.-S.; Zhang, Q.; Lu, P.; Zhang, T.; Ren, D.; et al. High efficiency Bi2Te3-based materials and devices for thermoelectric power generation between 100 and 300 °C. Energy Environ. Sci. 2016, 9, 3120–3127. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Lo, S.-H.; Zhang, Y.; Sun, H.; Tan, G.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-D.; Chang, C.; Tan, G.; Kanatzidis, M.G. SnSe: A remarkable new thermoelectric material. Energy Environ. Sci. 2016, 9, 3044–3060. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Tan, G.; Hao, S.; He, J.; Pei, Y.; Chi, H.; Wang, H.; Gong, S.; Xu, H.; Dravid, V.P.; et al. Ultrahigh power factor and thermoelectric performance in hole-doped single-crystal SnSe. Science 2016, 351, 141–144. [Google Scholar] [CrossRef]
- Asfandiyar; Li, Z.L.; Sun, F.H.; Tang, H.C.; Dong, J.F.; Li, J.F. Enhanced thermoelectric properties of p-type SnS0.2Se0.8 solid solution doped with Ag. J. Alloys Compd. 2018, 745, 172–178. [Google Scholar] [CrossRef]
- Hamad, B. Electronic and Thermoelectric Properties of SnSe1-x S (x) (x=0, 0.25, 0.5, 0.75, and 1) Alloys: First-Principles Calculations. J. Electron. Mater. 2018, 47, 4047–4055. [Google Scholar] [CrossRef]
- Li, Q.Q.; Zhang, L.L.; Yin, J.Z.; Sheng, Z.H.; Chu, X.Z.; Wang, F.; Zhu, F.X. Study on the thermoelectric performance of polycrystal SnSe with Se vacancies. J. Alloys Compd. 2018, 745, 513–518. [Google Scholar] [CrossRef]
- Melendez, J.J.; Gonzalez-Romero, R.L. zT factors in Ag- and Na-doped SnSe: Chemical potentials, relaxation times and predictions for other dopant species. J. Alloys Compd. 2018, 757, 70–78. [Google Scholar] [CrossRef]
- Peng, Z.; He, D.Q.; Mu, X.; Zhou, H.Y.; Li, C.C.; Ma, S.F.; Ji, P.X.; Hou, W.K.; Wei, P.; Zhu, W.T.; et al. Preparation and Enhanced Thermoelectric Performance of Cu2Se-SnSe Composite Materials. J. Electron. Mater. 2018, 47, 3350–3357. [Google Scholar] [CrossRef]
- Shu, Y.J.; Su, X.L.; Xie, H.Y.; Zheng, G.; Liu, W.; Yan, Y.G.; Luo, T.T.; Yang, X.; Yang, D.W.; Uher, C.; et al. Modification of Bulk Heterojunction and CI Doping for High-Performance Thermoelectric SnSe2/SnSe Nanocomposites. ACS Appl. Mater. Interfaces 2018, 10, 15793–15802. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Ren, Z.; Zhang, Q. Recent progress towards high performance of tin chalcogenide thermoelectric materials. J. Mater. Chem. A 2018, 6, 2432–2448. [Google Scholar] [CrossRef]
- Wei, T.-R.; Tan, G.; Wu, C.-F.; Chang, C.; Zhao, L.-D.; Li, J.-F.; Snyder, G.J.; Kanatzidis, M.G. Thermoelectric transport properties of polycrystalline SnSe alloyed with PbSe. Appl. Phys. Lett. 2017, 110, 053901. [Google Scholar] [CrossRef]
- Tang, G.; Wei, W.; Zhang, J.; Li, Y.; Wang, X.; Xu, G.; Chang, C.; Wang, Z.; Du, Y.; Zhao, L.-D. Realizing High Figure of Merit in Phase-Separated Polycrystalline Sn1–xPbxSe. J. Am. Chem. Soc. 2016, 138, 13647–13654. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xu, J.; Liu, G.-Q.; Yang, J.; Tan, X.; Liu, Z.; Qin, H.; Shao, H.; Jiang, H.; Liang, B.; et al. Enhanced thermoelectric performance in p-type polycrystalline SnSe benefiting from texture modulation. J. Mater. Chem. C 2016, 4, 1201–1207. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, L.; Linseis, V.; Qin, B.; Chen, W.; Zhao, L.-D.; Zhu, H. High-quality textured SnSe thin films for self-powered, rapid-response photothermoelectric application. Nano Energy 2020, 72, 104742. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, X.; Lei, L.; Nie, Y.; Xiang, G. Rapid synthesis of thermoelectric SnSe thin films by MPCVD. RSC Adv. 2020, 10, 11990–11993. [Google Scholar] [CrossRef] [PubMed]
- van de Putte, M.W.; Huijben, M. Epitaxial tin selenide thin film thermoelectrics. Appl. Surf. Sci. 2023, 623, 157034. [Google Scholar] [CrossRef]
- Price, L.S.; Parkin, I.P.; Hardy, A.M.E.; Clark, R.J.H.; Hibbert, T.G.; Molloy, K.C. Atmospheric Pressure Chemical Vapor Deposition of Tin Sulfides (SnS, Sn2S3, and SnS2) on Glass. Chem. Mater. 1999, 11, 1792–1799. [Google Scholar] [CrossRef]
- Hartman, K.; Johnson, J.L.; Bertoni, M.I.; Recht, D.; Aziz, M.J.; Scarpulla, M.A.; Buonassisi, T. SnS thin-films by RF sputtering at room temperature. Thin Solid Films 2011, 519, 7421–7424. [Google Scholar] [CrossRef]
- Ogah, O.E.; Reddy, K.R.; Zoppi, G.; Forbes, I.; Miles, R.W. Annealing studies and electrical properties of SnS-based solar cells. Thin Solid Films 2011, 519, 7425–7428. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, D.; Ji, Y.; Zhang, H.; Shen, X. Two-dimensional SnS nanosheets fabricated by a novel hydrothermal method. J. Mater. Sci. 2005, 40, 591–595. [Google Scholar] [CrossRef]
- Teghil, R.; Santagata, A.; Marotta, V.; Orlando, S.; Pizzella, G.; Giardini-Guidoni, A.; Mele, A. Characterization of the plasma plume and of thin film epitaxially produced during laser ablation of SnSe. Appl. Surf. Sci. 1995, 90, 505–514. [Google Scholar] [CrossRef]
- Gregory, B.W.; Stickney, J.L. Electrochemical atomic layer epitaxy (ECALE). J. Electroanal. Chem. Interfacial Electrochem. 1991, 300, 543–561. [Google Scholar] [CrossRef]
- Burton, M.R.; Boyle, C.A.; Liu, T.; McGettrick, J.; Nandhakumar, I.; Fenwick, O.; Carnie, M.J. Full Thermoelectric Characterization of Stoichiometric Electrodeposited Thin Film Tin Selenide (SnSe). ACS Appl. Mater. Interfaces 2020, 12, 28232–28238. [Google Scholar] [CrossRef] [PubMed]
- Klochko, N.P.; Klepikova, K.S.; Zhadan, D.O.; Petrushenko, S.I.; Kopach, V.R.; Khrypunov, G.S.; Lyubov, V.M.; Dukarov, S.V.; Nikitin, V.O.; Maslak, M.O.; et al. Structure, optical, electrical and thermoelectric properties of solution-processed Li-doped NiO films grown by SILAR. Mater. Sci. Semicond. Process. 2018, 83, 42–49. [Google Scholar] [CrossRef]
- Kishore Kumar, D.; Loskot, J.; Kříž, J.; Bennett, N.; Upadhyaya, H.M.; Sadhu, V.; Venkata Reddy, C.; Reddy, K.R. Synthesis of SnSe quantum dots by successive ionic layer adsorption and reaction (SILAR) method for efficient solar cells applications. Sol. Energy 2020, 199, 570–574. [Google Scholar] [CrossRef]
- Farhad, S.F.U.; Hossain, M.A.; Tanvir, N.I.; Akter, R.; Patwary, M.A.M.; Shahjahan, M.; Rahman, M.A. Structural, optical, electrical, and photoelectrochemical properties of cuprous oxide thin films grown by modified SILAR method. Mater. Sci. Semicond. Process. 2019, 95, 68–75. [Google Scholar] [CrossRef]
- Deshpande, N.G.; Sagade, A.A.; Gudage, Y.G.; Lokhande, C.D.; Sharma, R. Growth and characterization of tin disulfide (SnS2) thin film deposited by successive ionic layer adsorption and reaction (SILAR) technique. J. Alloys Compd. 2007, 436, 421–426. [Google Scholar] [CrossRef]
- Aldawood, S.; AlGamdi, S.; Salman, S.; Garawi, M.; Alkhuraiji, T.; Ali, S. Influence of γ-ray exposure and dose dependent characteristics of (n)PbS–(p)Si hetero-structure. J. Mater. Sci. Mater. Electron. 2021, 32, 11616–11627. [Google Scholar] [CrossRef]
- Zainal, Z.; Saravanan, N.; Anuar, K.; Hussein, M.Z.; Yunus, W.M.M. Chemical bath deposition of tin selenide thin films. Mater. Sci. Eng. B 2004, 107, 181–185. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, H.; Han, M.; Dai, Z.; Pang, H.; Zheng, Y.; Lan, Y.Q.; Bao, J.; Zhu, J. Two-dimensional tin selenide nanostructures for flexible all-solid-state supercapacitors. ACS Nano 2014, 8, 3761–3770. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jin, J.; Jung, I.G.; Kim, J.M.; Kim, H.J.; Son, S.U. SnSe2 nanoplate–graphene composites as anode materials for lithium ion batteries. Chem. Commun. 2011, 47, 5241–5243. [Google Scholar] [CrossRef] [PubMed]
- Pejjai, B.; Minnam Reddy, V.R.; Seku, K.; Pallavolu, M.R.; Park, C. Eco-friendly synthesis of SnSe nanoparticles: Effect of reducing agents on the reactivity of a Se-precursor and phase formation of SnSe NPs. New J. Chem. 2018, 42, 4843–4853. [Google Scholar] [CrossRef]
- Rashad, M.M.; Shalan, A.E.; Lira-Cantú, M.; Abdel-Mottaleb, M.S.A. Enhancement of TiO2 nanoparticle properties and efficiency of dye-sensitized solar cells using modifiers. Appl. Nanosci. 2013, 3, 167–174. [Google Scholar] [CrossRef]
- Arokiya Mary, T.; Fernandez, A.C.; Sakthivel, P.; Jesudurai, J.G.M. A study on the role of surfactant on the layered growth of SnSe2 for electrical applications. J. Mater. Sci. Mater. Electron. 2016, 27, 11041–11048. [Google Scholar] [CrossRef]
- Tanhaei, M.; Mahjoub, A.R.; Safarifard, V. Energy-efficient sonochemical approach for the preparation of nanohybrid composites from graphene oxide and metal-organic framework. Inorg. Chem. Commun. 2019, 102, 185–191. [Google Scholar] [CrossRef]
- Gainza, J.; Serrano-Sánchez, F.; Rodrigues, J.E.F.S.; Huttel, Y.; Dura, O.J.; Koza, M.M.; Fernández-Díaz, M.T.; Meléndez, J.J.; Márkus, B.G.; Simon, F.; et al. High-Performance n-type SnSe Thermoelectric Polycrystal Prepared by Arc-Melting. Cell Rep. Phys. Sci. 2020, 1, 100263. [Google Scholar] [CrossRef]
- Shi, W.; Gao, M.; Wei, J.; Gao, J.; Fan, C.; Ashalley, E.; Li, H.; Wang, Z. Tin Selenide (SnSe): Growth, Properties, and Applications. Adv. Sci. 2018, 5, 1700602. [Google Scholar] [CrossRef]
- Kumar, M.; Rani, S.; Parmar, R.; Amati, M.; Gregoratti, L.; Ghosh, A.; Pathak, S.; Kumar, A.; Wang, X.; Singh, V.N. The ultra-high thermoelectric power factor in facile and scalable single-step thermal evaporation fabricated composite SnSe/Bi thin films. J. Mater. Chem. C 2022, 10, 18017–18024. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, B.; Wang, D.-Y.; Hong, T.; Gao, X.; Zhao, L. Realizing high thermoelectric properties in p-type polycrystalline SnSe by inducing DOS distortion. Rare Met. 2021, 40, 2819–2828. [Google Scholar] [CrossRef]
- Duong, A.T.; Nguyen, D.L.; Nguyen, M.N.; Nguyen, T.M.H.; Nguyen, A.D.; Pham, A.T.; Ullah, F.; Tahir, Z.; Kim, Y.S.; Trung, D.Q.; et al. High thermoelectric power factor in SnSe2 thin film grown on Al2O3 substrate. Mater. Res. Express 2019, 6, 066420. [Google Scholar] [CrossRef]
- Gu, W.-H.; Zhang, Y.-X.; Guo, J.; Cai, J.-F.; Zhu, Y.-K.; Zheng, F.; Jin, L.; Xu, J.; Feng, J.; Ge, Z.-H. Realizing high thermoelectric performance in n-type SnSe polycrystals via (Pb, Br) co-doping and multi-nanoprecipitates synergy. J. Alloys Compd. 2021, 864, 158401. [Google Scholar] [CrossRef]
- Li, F.; Wang, W.; Qiu, X.; Zheng, Z.; Fan, P.; Luo, J.; Li, B. Optimization of thermoelectric properties of n-type Ti, Pb co-doped SnSe. Inorg. Chem. Front. 2017, 4, 1721–1729. [Google Scholar] [CrossRef]
- Song, L.; Zhang, J.; Iversen, B.B. Enhanced thermoelectric properties of SnSe thin films grown by single-target magnetron sputtering. J. Mater. Chem. A 2019, 7, 17981–17986. [Google Scholar] [CrossRef]
- Marfoua, B.; Hong, J. Graphene Induced High Thermoelectric Performance in ZnO/Graphene Heterostructure. Adv. Mater. Interfaces 2023, 10, 2202387. [Google Scholar] [CrossRef]
- Urmila, K.S.; Namitha, T.A.; Rajani, J.; Philip, R.R.; Pradeep, B. Optoelectronic properties and Seebeck coefficient in SnSe thin films. J. Semicond. 2016, 37, 093002. [Google Scholar] [CrossRef]
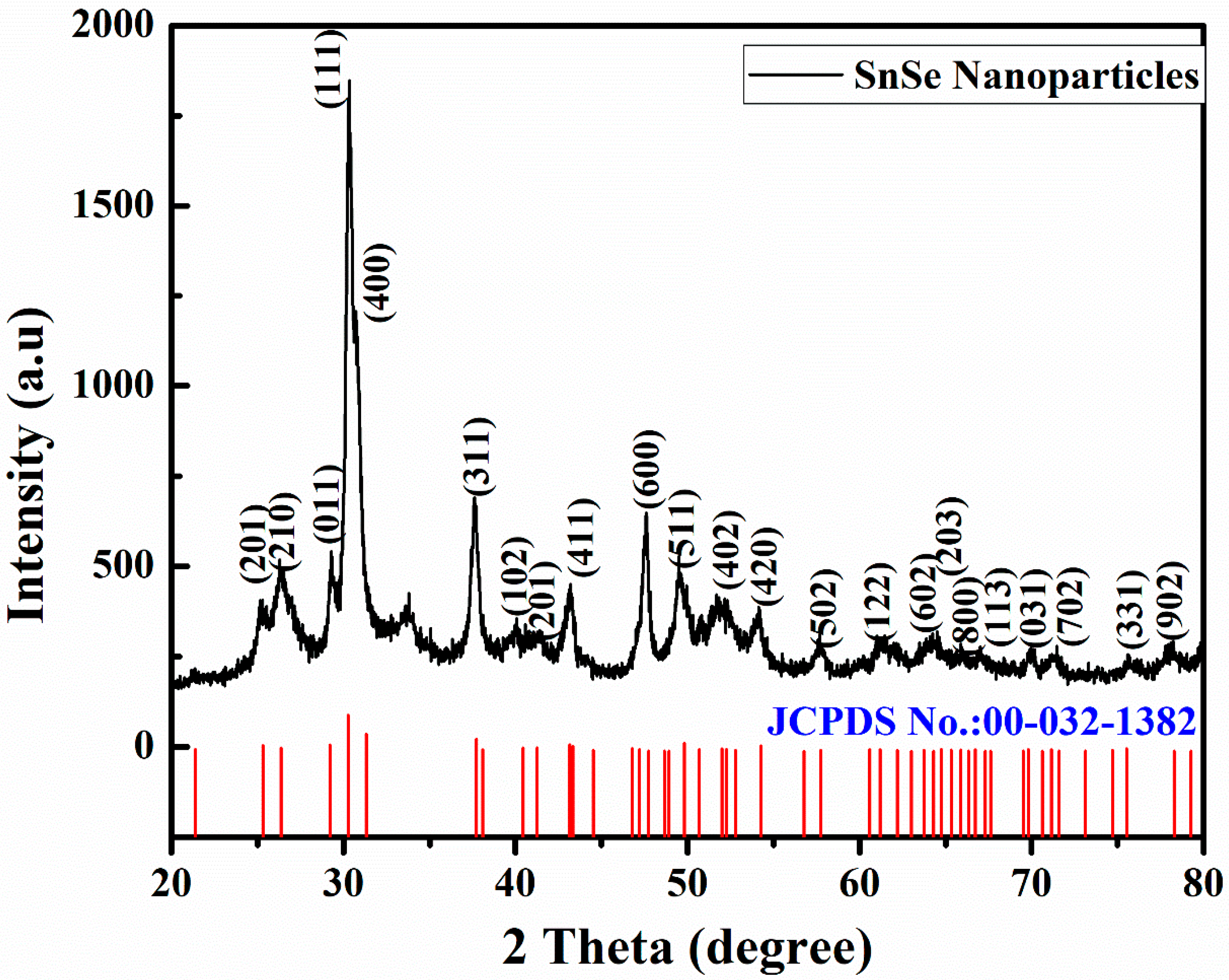




| Sample | Crystallite Dimension (nm) | Microstrain (ε × 10−2) | Deformation Density (δ) (m−2 × 1015) | Lattice Constants | ||
|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | ||||
| SnSe | 108.54 | 11.83 | 8.48 | 4.173 | 4.786 | 11.239 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, K.; Almutairi, Z.; Ali, S.M.; Almuzaiqer, R.; Wan, C.; Sayeed, A. Synthesis, Characterization and Power Factor Estimation of SnSe Thin Film for Energy Harvesting Applications. Processes 2024, 12, 665. https://doi.org/10.3390/pr12040665
Ahmad K, Almutairi Z, Ali SM, Almuzaiqer R, Wan C, Sayeed A. Synthesis, Characterization and Power Factor Estimation of SnSe Thin Film for Energy Harvesting Applications. Processes. 2024; 12(4):665. https://doi.org/10.3390/pr12040665
Chicago/Turabian StyleAhmad, Kaleem, Zeyad Almutairi, Syed Mansoor Ali, Redhwan Almuzaiqer, Chunlei Wan, and Abdul Sayeed. 2024. "Synthesis, Characterization and Power Factor Estimation of SnSe Thin Film for Energy Harvesting Applications" Processes 12, no. 4: 665. https://doi.org/10.3390/pr12040665






