Research on the Control and Performance of Integrated Self-Assembled Micro-Scale Structure of NC-Coated CL-20
Abstract
:1. Introduction
2. Materials and Experiment
2.1. Material and Instruments
2.2. The Preparation Methodology
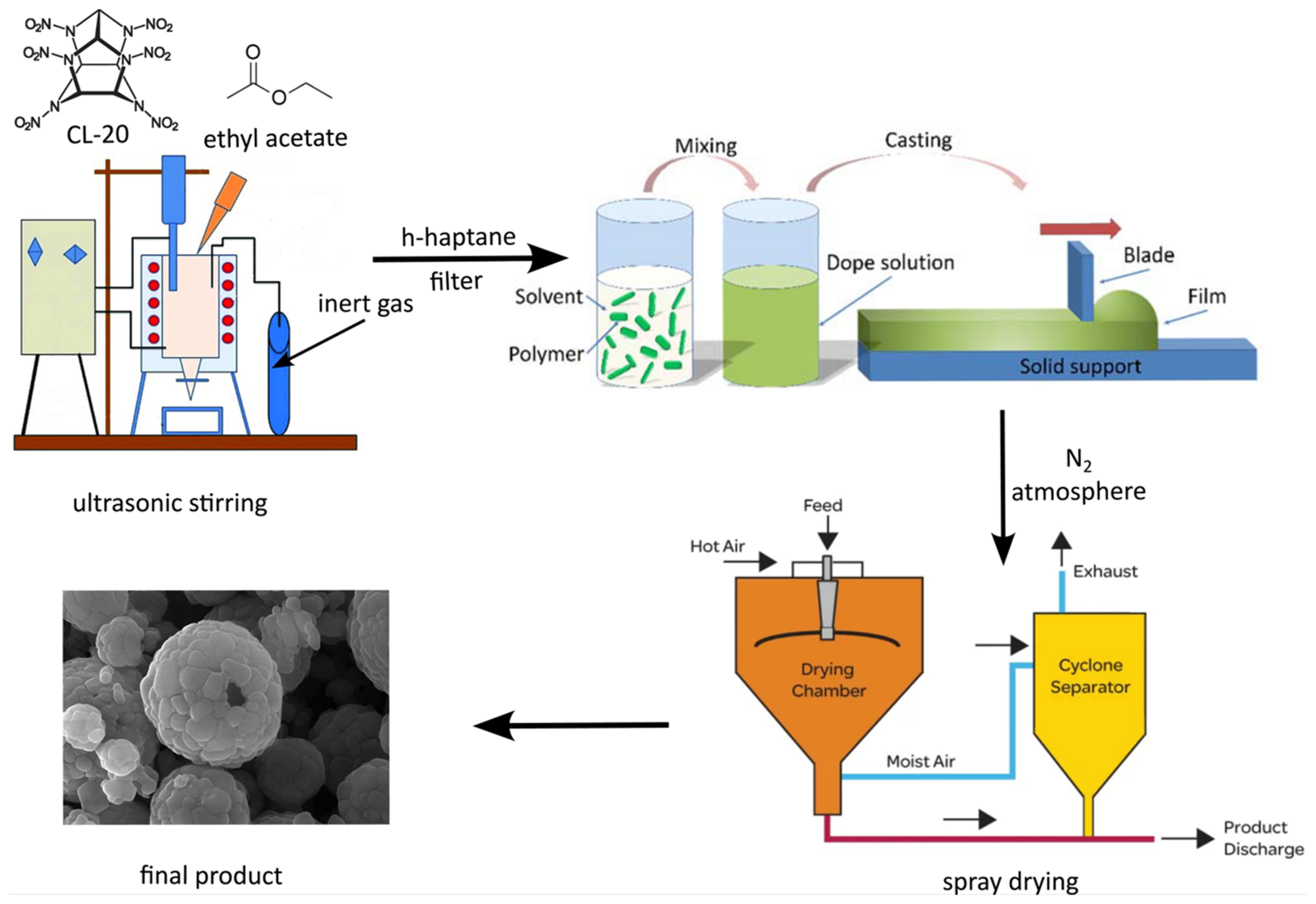
2.2.1. Preparation of Refined CL-20 via Jet Thinning Method (“Solvent/Non-Solvent” Treatment)
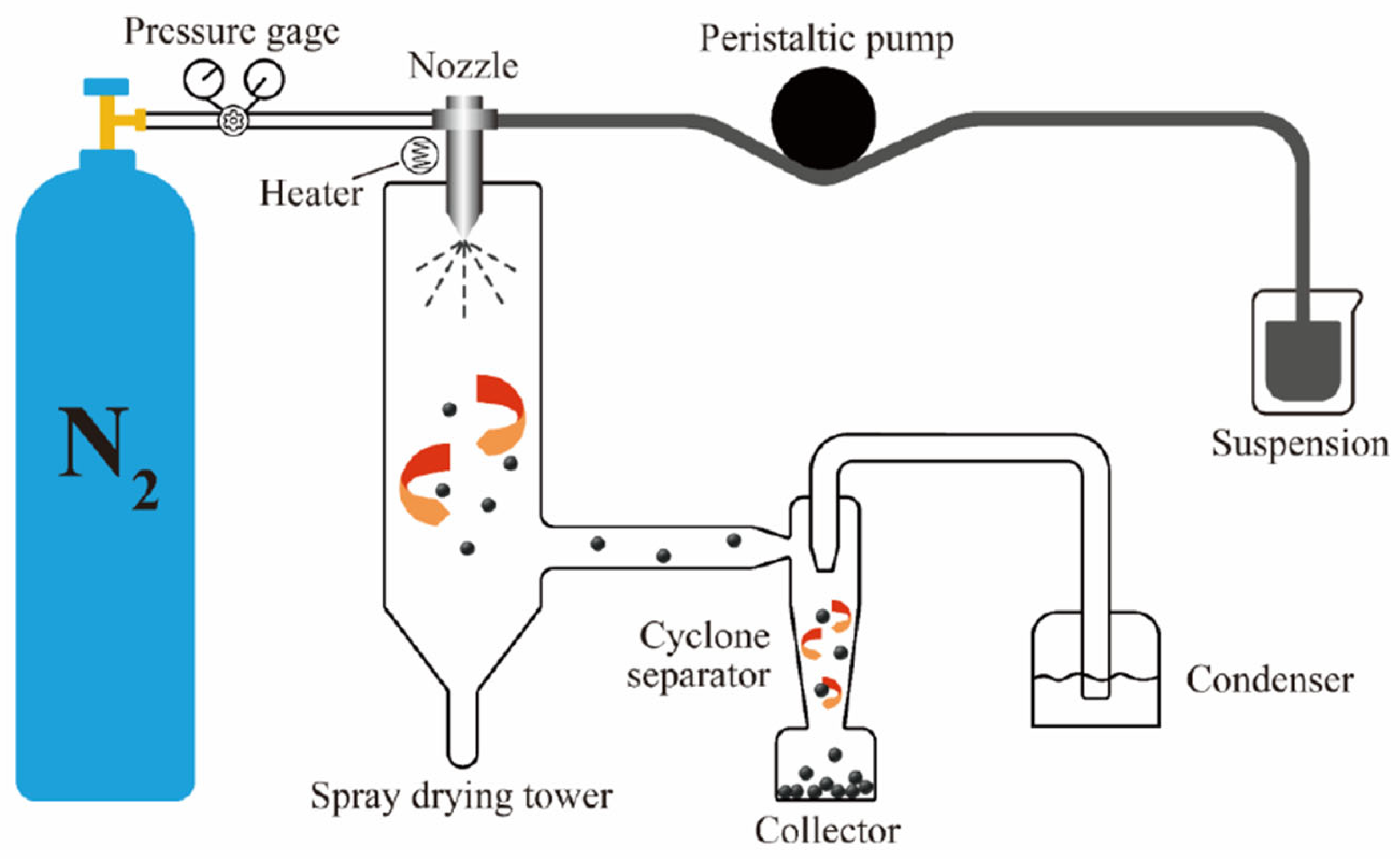
2.2.2. Preparation of Ultra-Fine CL-20/NC Microsphere via Spray-Drying Method
3. Result and Discussion
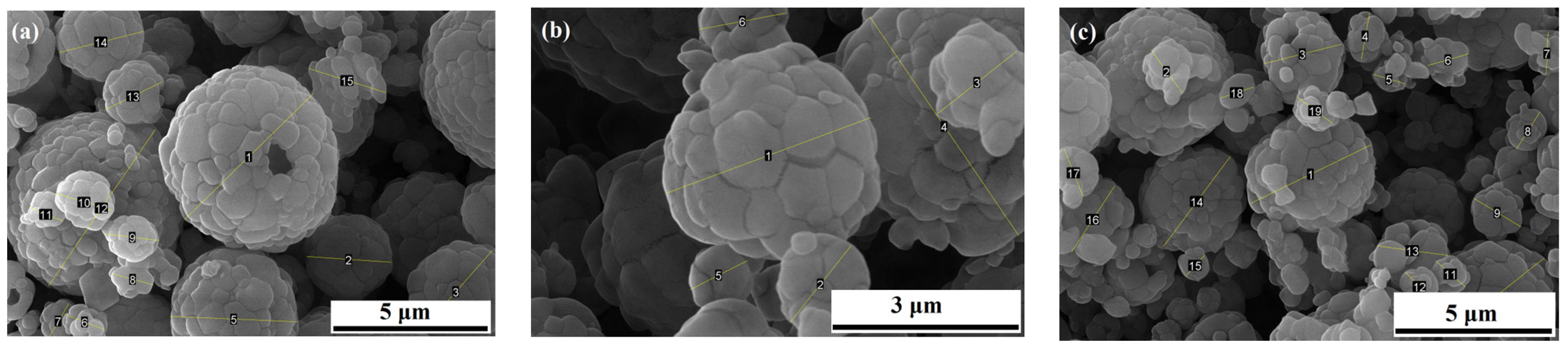
3.1. Morphology Analysis
3.1.1. SEM Analysis
3.1.2. Statistical Analysis
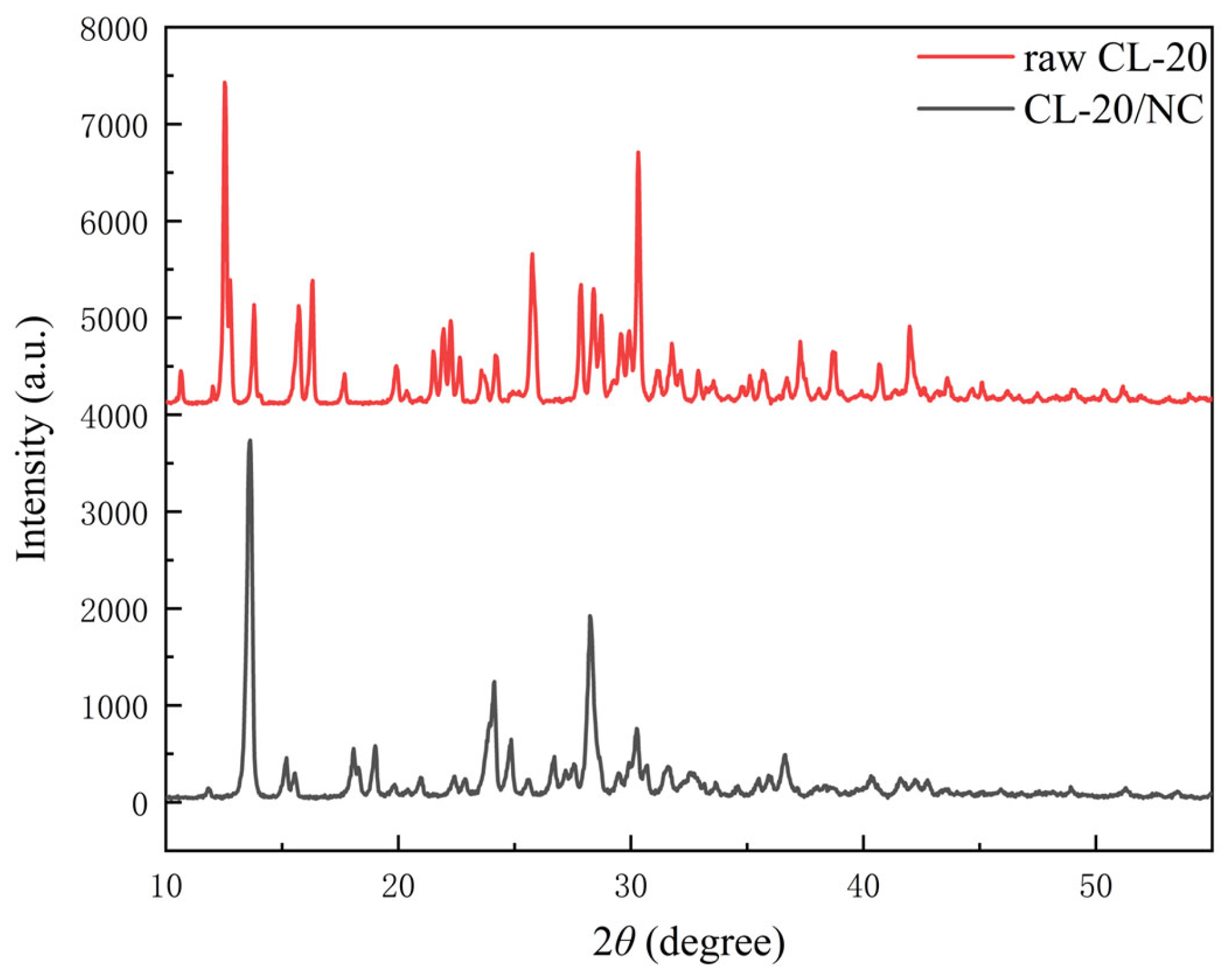
3.2. XRD Analysis
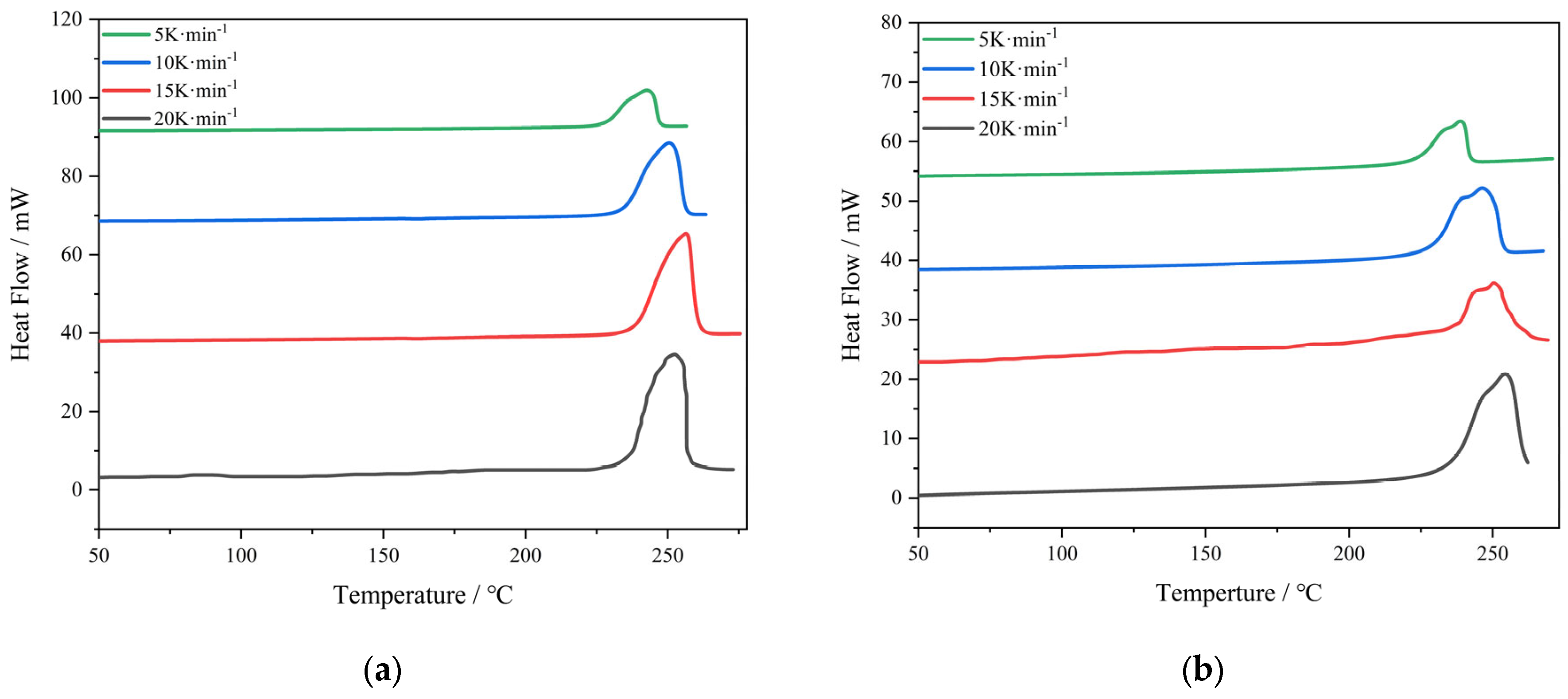
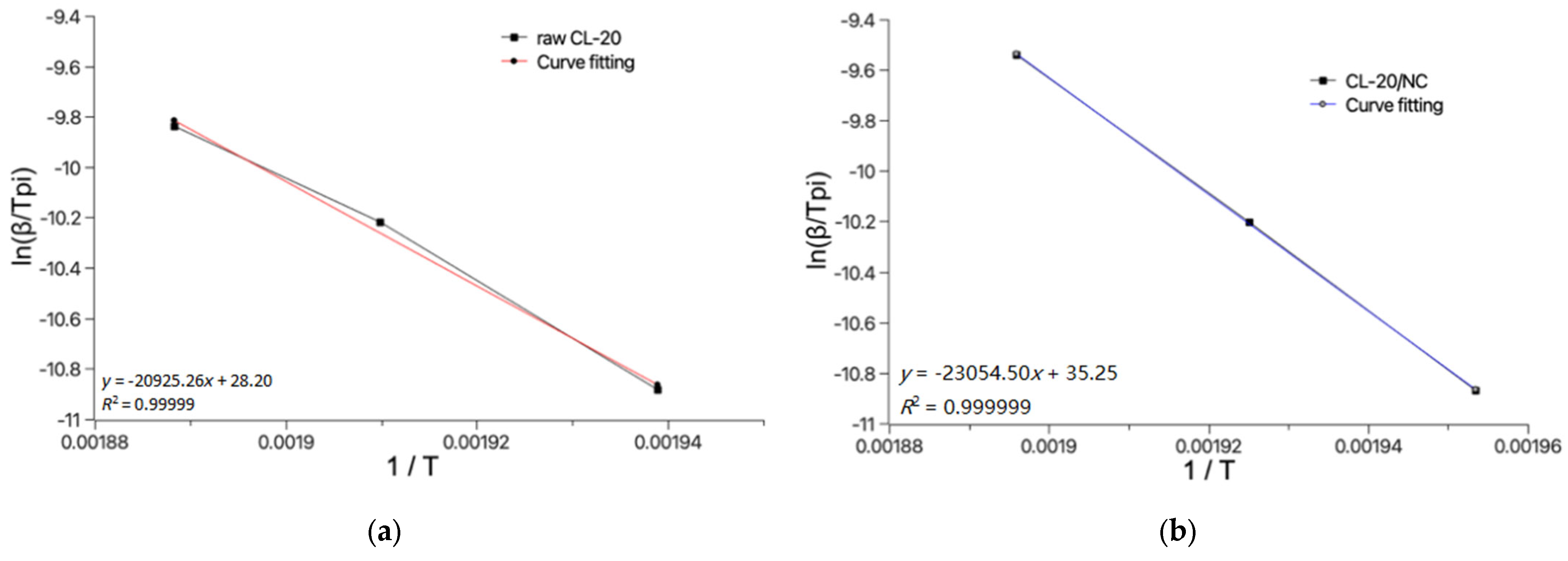
3.3. DSC Analysis
3.4. Impact Sensitivity Study and Detonation Performance
| Sample | Drop Height (H50)/cm | VoD/m·s−1 |
|---|---|---|
| raw CL-20 | 15 | 9200 |
| CL-20/NC | 47.0 | 9180 |
| HMX | 11 | 9100 |
4. Mechanism of the Desensitization of the Obtained Composites
5. Conclusions and Expectations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mahnaz, F.; Mostafa-Al-Momin, M.; Rubel, M.; Ferdous, M.; Azam, M.S. Mussel-inspired immobilization of Au on bare and graphene-wrapped Ni nanoparticles toward highly efficient and easily recyclable catalysts. RSC Adv. 2019, 9, 30358–30369. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; An, Q.; Goddard, W.A., III; Zybin, S.V.; Huang, F. Compressive Shear Reactive Molecular Dynamics Studies Indicating That Cocrystals of TNT/CL-20 Decrease Sensitivity. J. Phys. Chem. C 2014, 118, 30202–30208. [Google Scholar] [CrossRef]
- Greenberg, B.L.; Kalyon, D.M.; Erol, M.; Mezger, M.; Lee, K.; Lusk, S. Analysis of Slurry-Coating Effectiveness of CL-20 Using Grazing Incidence X-ray Diffraction. J. Energ. Mater. 2003, 21, 185–199. [Google Scholar] [CrossRef]
- Lee, K.E.; Hatch, R.L.; Braithwaite, P. Method of Making High Performance Explosive Formulations Containing CL-20. U.S. Patent No. 6,217,799, 17 April 2001. [Google Scholar]
- Zhigach, A.; Leipunskii, I.O.; Berezkina, N.G.; Pshechenkov, P.A.; Zotova, E.S.; Kudrov, B.V.; Gogulya, M.F.; Brazhnikov, M.A.; Kuskov, M.L. Aluminized nitramine-based nanocomposites: Manufacturing technique and structure study. Combust. Explos. Shock Waves 2009, 45, 666–677. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, Y.; Jiu, Y.; Du, M.; Ge, Z.; Chai, C. Study on the Influence of Hard Segment Content of Thermoplastic Polyurethane on the Insensitivity Effect of CL-20. Chin. J. Energ. Mater. 2007, 15, 395–399. [Google Scholar]
- Yu, W.; Wang, J.; Hou, C. Preparation and Characterization of Hexanitrohexaoxaisowurtzitane/Estane Microspheres. Sci. Technol. Eng. 2016, 16, 192–194. [Google Scholar] [CrossRef]
- Ye, B.Y.; Wang, J.Y.; An, C.W.; Yu, B.S.; Ji, W.; Li, H.Q. Preparation and Properties of CL-20 Based Composite Energetic Materials. J. Solid Rocket Tech. 2017, 2, 69–73. [Google Scholar]
- Wang, S.-W.; Song, X.-D.; Wu, Z.-K.; Xiao, L.; Zhang, G.-P.; Hu, Y.-B.; Hao, G.-Z.; Jiang, W.; Zhao, F.-Q. Simulation of the plasticizing behavior of composite modified double-base (CMDB) propellant in grooved calendar based on adaptive grid technology. Def. Technol. 2021, 17, 1954–1966. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Li, X. Preparation and Properties of RDX-Nitrocellulose Microspheres. Cent. Eur. J. Energ. Mater. 2016, 13, 871–881. [Google Scholar]
- Ma, X.; Li, Y.; Hussain, I.; Shen, R.; Yang, G.; Zhang, K. Core–Shell Structured Nanoenergetic Materials: Preparation and Fundamental Properties. Adv. Mater. 2020, 32, 2001291. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Stepanov, V.; Di Stasio, A.R.; Surapaneni, A.; Lee, W.Y. Investigation of the crystallization of RDX during spray drying. Powder Technol. 2015, 274, 333–337. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Z.; Qiao, S.; Piao, J.; Li, H. Morphology and properties of CL-20/MTNP cocrystal prepared via facile spray drying. FirePhysChem 2022, 3, 158–163. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, Y.; Wang, K.-X.; Wang, T.-P.; Li, Y.; Zhu, S.-G.; Zhang, L.; Yi, Z.-X.; Guan, H.; Zhu, C.-G. Tuning the Energetic Performance of CL-20 by Surface Modification Using Tannic Acid and Energetic Coordination Polymers. ACS Omega 2022, 7, 10469–10475. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Xue, L.; Zhao, F.Q.; Hu, R.Z.; Gao, H.X. A Simple Method to Estimate the Critical Temperature of Thermal Explosion for Energetic Materials Using Nonisothermal DSC. J. Energ. Mater. 2010, 28, 17–34. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, R.; Yi, X.; Li, F. The estimation of critical temperatures of thermal explosion for energetic materials using non-isothermal DSC. Thermochim. Acta 1994, 244, 171–176. [Google Scholar] [CrossRef]
- GJB/772 A-97; Experimental Methods of Sensitivity and Safety. National Military Standard of China: Beijing, China, 1997. (In Chinese)
- Li, X.; Han, Z.; Sun, S. Existence of positive solutions of nonlinear fractional q-difference equation with parameter. Adv. Differ. Equ. 2013, 2013, 260. [Google Scholar] [CrossRef]
- Bowden, F.P.; Yoffe, A.D. Initiation and Growth of Explosion in Liquids and Solids; Cambridge University Press: Cambridge, UK, 1985. [Google Scholar]
- Field, J.E.; Bourne, N.K.; Palmer, S.J.P.; Walley, M. Hot-spot ignition mechanisms for explosives and propellants. R. Soc. 1992, 339, 1654. [Google Scholar] [CrossRef]

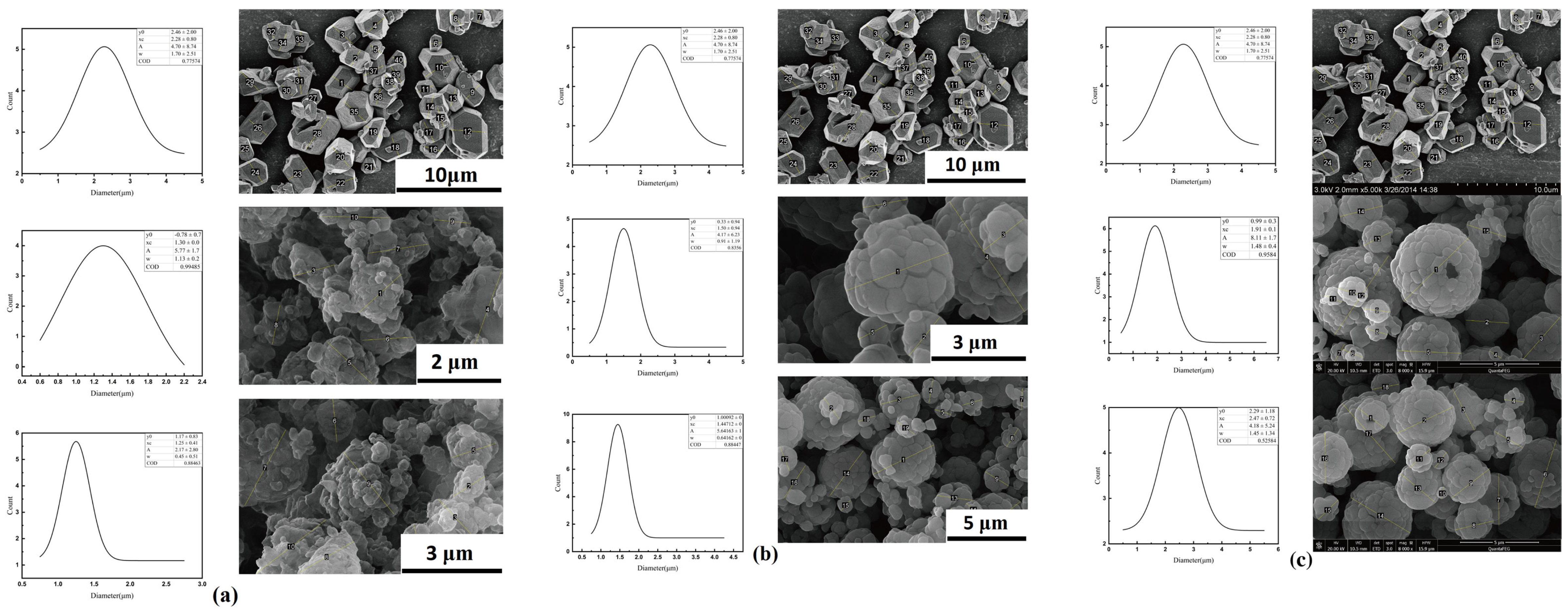
| Sample | Nozzle Diameter/mm | Injection Rate/L·h−1 | y0/μm | xc/μm | A/μm | FWHM/μm |
|---|---|---|---|---|---|---|
| ingredient | 2.46 ± 2.00 | 2.28 ± 0.80 | 4.70 ± 8.74 | 1.70 ± 2.51 | ||
| 1 | Ultrasonic nozzle | 300 | 1.17 ± 0.83 | 1.25 ± 0.41 | 2.17 ± 1.80 | 0.45 ± 0.31 |
| 2 | 0.7 | 300 | −0.78 ± 0.07 | 1.30 ± 0.02 | 5.77 ± 1.79 | 1.13 ± 0.20 |
| 3 | 1.4 | 340 | 0.33 ± 0.04 | 1.50 ± 0.94 | 4.17 ± 6.23 | 0.91 ± 0.19 |
| 4 | 1.4 | 378 | 1.00 ± 0.63 | 1.45 ± 0.08 | 5.64 ± 1.55 | 0.64 ± 0.24 |
| 5 | 2.0 | 420 | 0.99 ± 0.33 | 1.91 ± 0.10 | 8.10 ± 1.72 | 1.48 ± 0.46 |
| 6 | 2.0 | 450 | 2.29 ± 1.18 | 2.47 ± 0.72 | 4.18 ± 2.24 | 1.45 ± 0.34 |
| Sample | Peak Value/a.u. | 2θ/° | FWHM/° | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak | #1 | #2 | #3 | #1 | #2 | #3 | #1 | #2 | #3 |
| raw CL-20 | 3346 | 1053 | 1575 | 12.53 | 13.79 | 25.75 | 0.1376 | 0.1318 | 0.2201 |
| CL-20/NC | 3596 | 302 | 1246 | 13.63 | 15.55 | 24.13 | 0.2262 | 0.5298 | 0.3404 |
| Sample | 1 E/kJ·mol−1 | 2 A | 3 Te/K | 4 Tb/K |
|---|---|---|---|---|
| raw CL-20 | 173.25 | 1.37 × 1017 | 523.60 | 526.72 |
| CL-20/NC | 192.01 | 1.60 × 1019 | 528.44 | 534.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Hao, Y.; Su, L.; Wang, J.; Li, X.; Shi, X. Research on the Control and Performance of Integrated Self-Assembled Micro-Scale Structure of NC-Coated CL-20. Processes 2024, 12, 675. https://doi.org/10.3390/pr12040675
Wang H, Hao Y, Su L, Wang J, Li X, Shi X. Research on the Control and Performance of Integrated Self-Assembled Micro-Scale Structure of NC-Coated CL-20. Processes. 2024; 12(4):675. https://doi.org/10.3390/pr12040675
Chicago/Turabian StyleWang, Haoran, Yibo Hao, Lei Su, Jingyu Wang, Xiaodong Li, and Xiaofeng Shi. 2024. "Research on the Control and Performance of Integrated Self-Assembled Micro-Scale Structure of NC-Coated CL-20" Processes 12, no. 4: 675. https://doi.org/10.3390/pr12040675





