Generation Mechanism of Hydroxyl Free Radicals in Micro–Nanobubbles Water and Its Prospect in Drinking Water
Abstract
:1. Introduction
2. Generation Process and Characteristics of MNBs in Water
2.1. The Generation Process of MNBs in Water
| Generation Methods | Generation Process | Influence Factor | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Hydrodynamic cavitation | When a large pressure difference is generated in the moving fluid, hydrodynamic cavitation will be observed, resulting in MNBs. | Pressure difference | High efficiency and low energy consumption | Bubble size is not easy to control | [43,67,68,69] |
| Ultrasonic cavitation | A sound field is applied to make the liquid generate tensile stress and negative pressure. If the pressure is too saturated, MBs will be generated. | Ultrasonic time, frequency | The bubble size is small and uniform | Complex operation for large-scale treatment | |
| Optic cavitation | A certain wavelength of light is irradiated on the photocatalysis material, which makes the electrons transit, and MNBs precipitate. | Wavelength of light | No secondary pollution | High cost and not conducive to mass production | |
| Jet dispersion method | The air–liquid mixture is formed after the air compressor is injected or inhaled by itself and then injected at high speed, relying on the turbulence between the air and liquid to generate MNBs. | Air intake | Rapid generation of MNBs with uniform size | The air intake is difficult to control | |
| Compressed air passing through diffusion plate method | The pressurized air enters the liquid phase through the micropores with a certain size on the special diffusion plate, and the gas forms MNBs under the shear of the micropores. | Size of micropore | Relatively simple operation and easy to form MNBs | Expensive device, and pores are easy to block | |
| Mechanical force high-speed shearing air method | The larger bubbles in the liquid are divided into MNBs by using the shear effect generated by the high-speed rotating impeller. | Impeller rotation | Rapid generation of a large number of MNBs | Unstable bubble size and high energy consumption | |
| Dissolved gas release method | First, the gas is pressurized to supersaturate and dissolve it, and then is decompressed to be released, thus producing MNBs. | Pressure and nozzle cavitation mode | Simple operation and low energy consumption | Discontinuous gas dissolution and release and low efficiency | |
| Aeration method | Various micro–nanobubble generators are directly used to aerate in water, producing MNBs. | MNB generator type | Easy to operate, non-toxic, and residue free | The instrument is expensive | |
| Chemical reaction method | Chemical reagents are added to the solution to make it react violently, producing MNBs. | Type of reactant | High bubble generation efficiency | Cause secondary pollution | |
| Electro-chemical method | Water is electrolyzed through electrodes to form MNBs on the positive and negative plates. | Voltage size and electrolytic time | The size of bubbles can be controlled | High energy consumption and low efficiency |
2.2. Characteristics of MNBs in Water
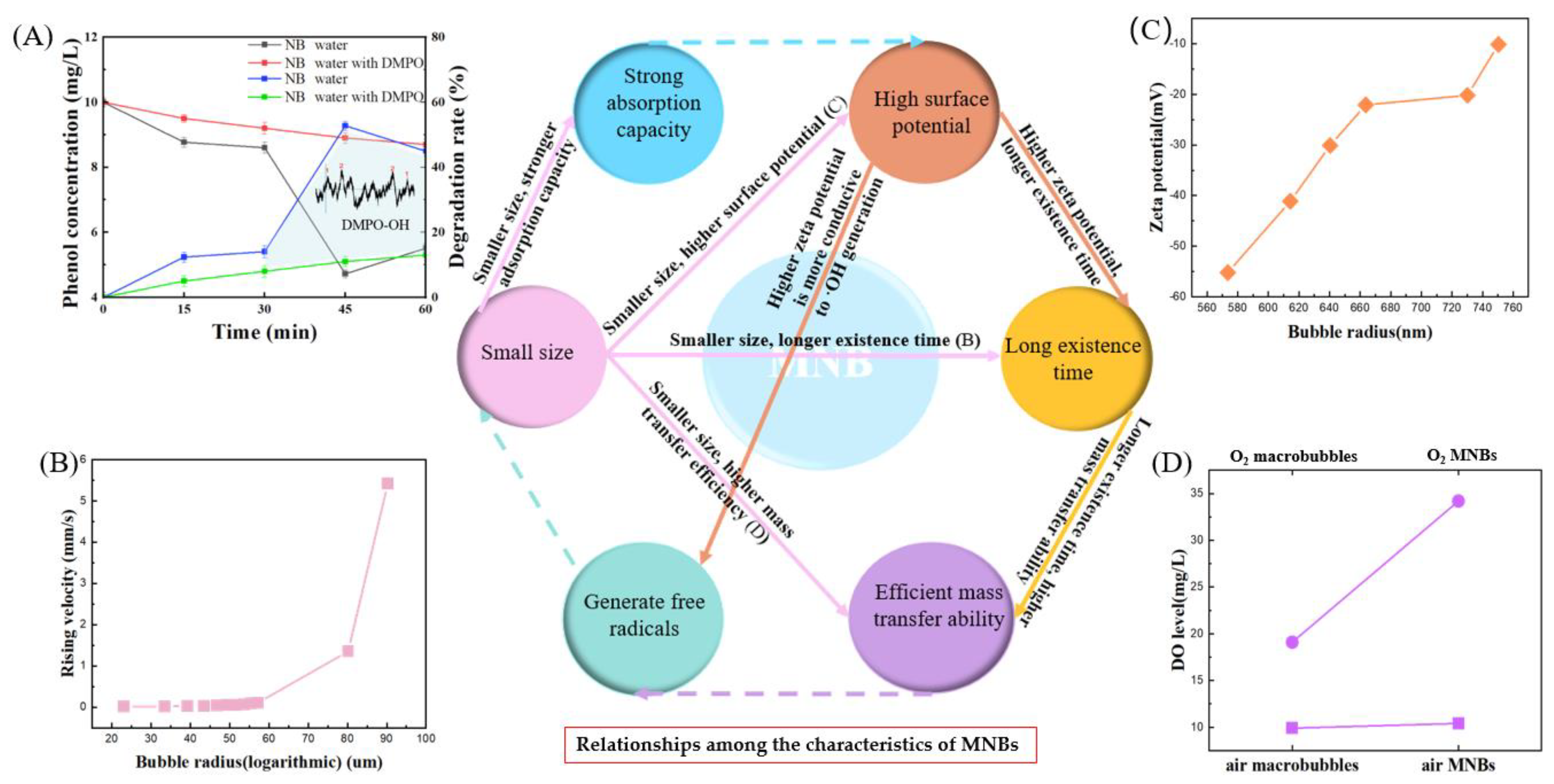
3. Characteristics of MNB Collapse and Influencing Factors of Hydroxyl Radical Generation in MNBW
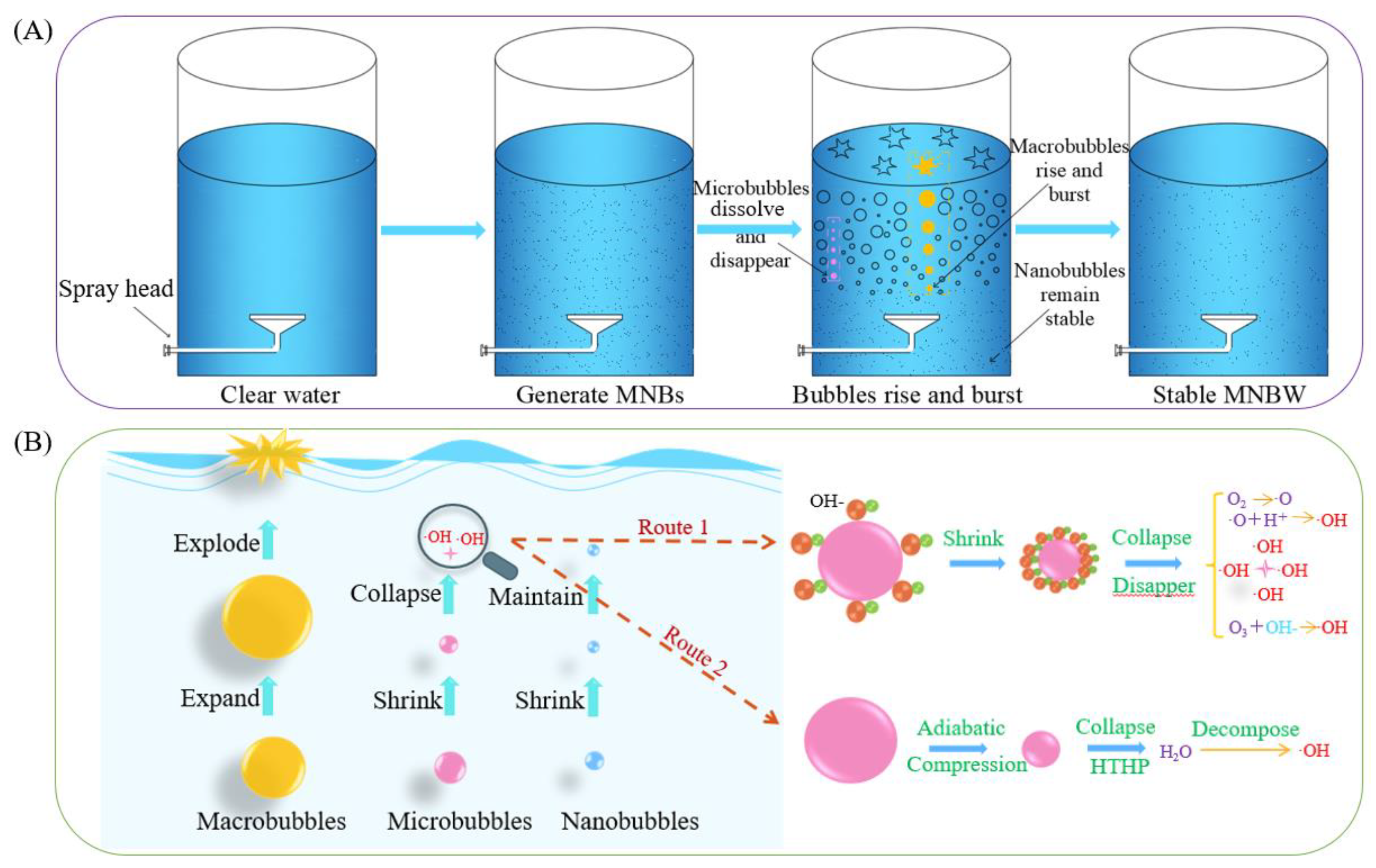
3.1. Characteristics of MNB Collapse
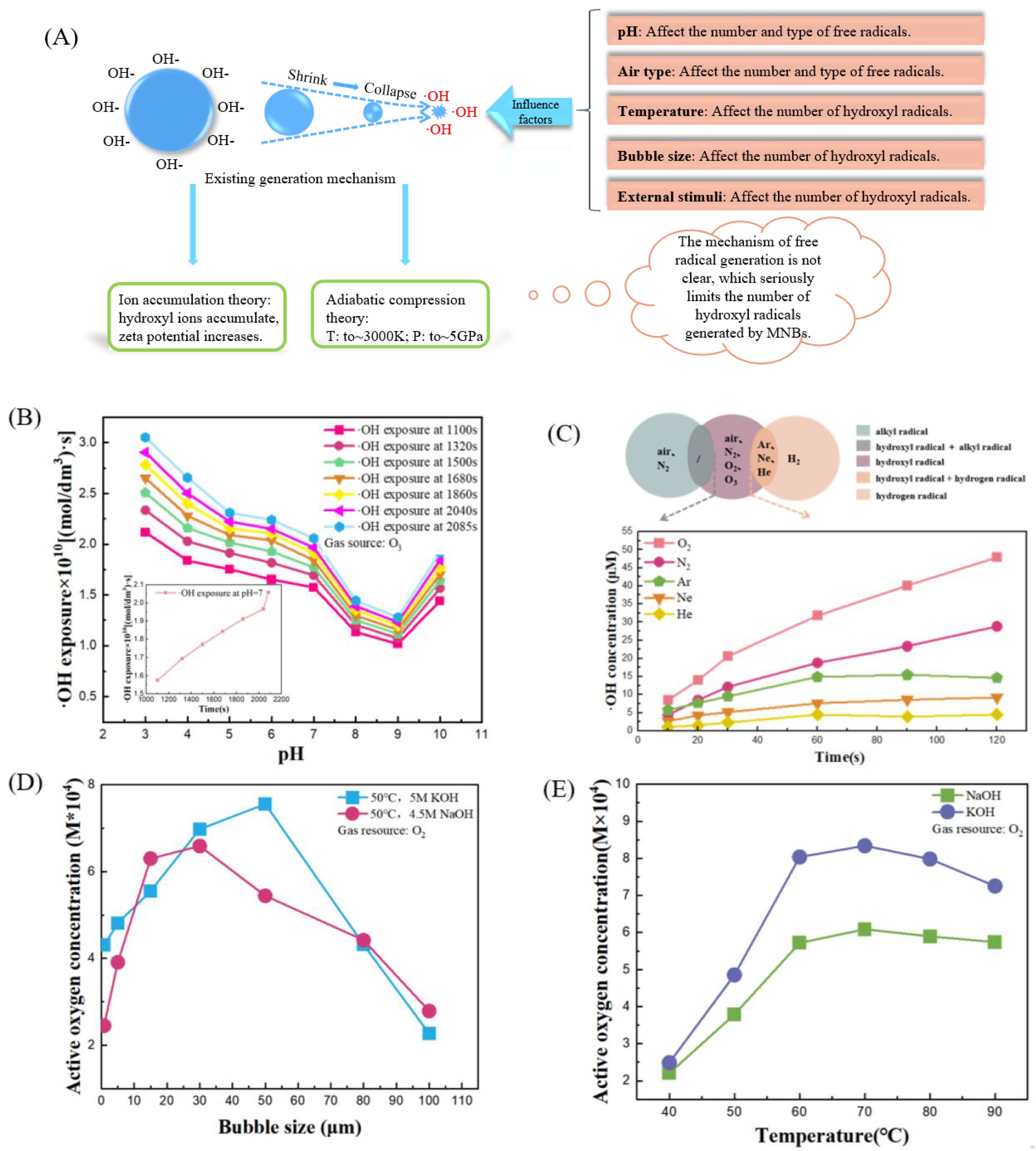
3.2. Influencing Factors of ·OH Generation in MNBW
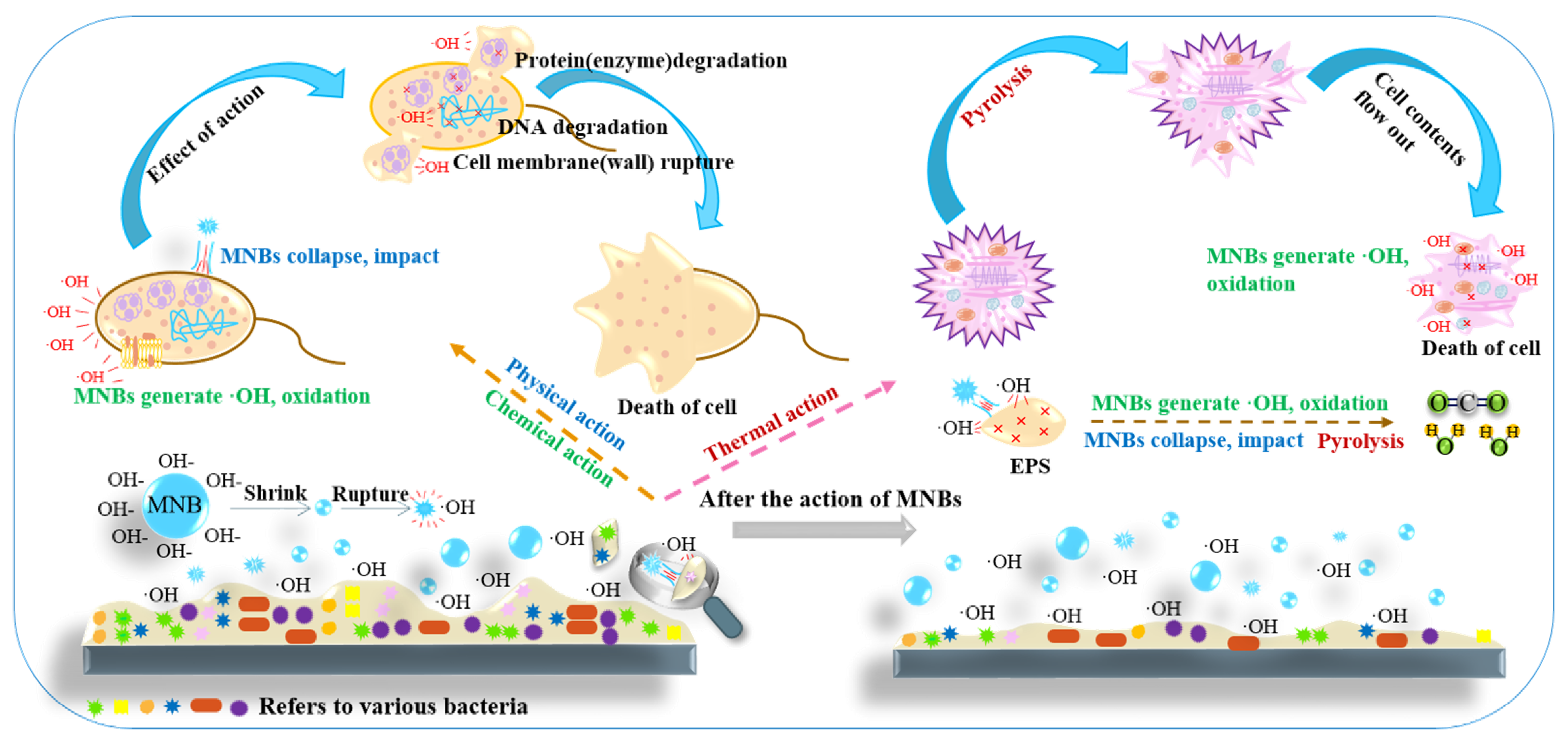
4. Effect of MNB Mechanism on Pollutants and Biofilms in Water
4.1. MNBs Remove Pollutants from Water
4.2. Control Mechanism of MNBs on Pipe Biofilm Growth
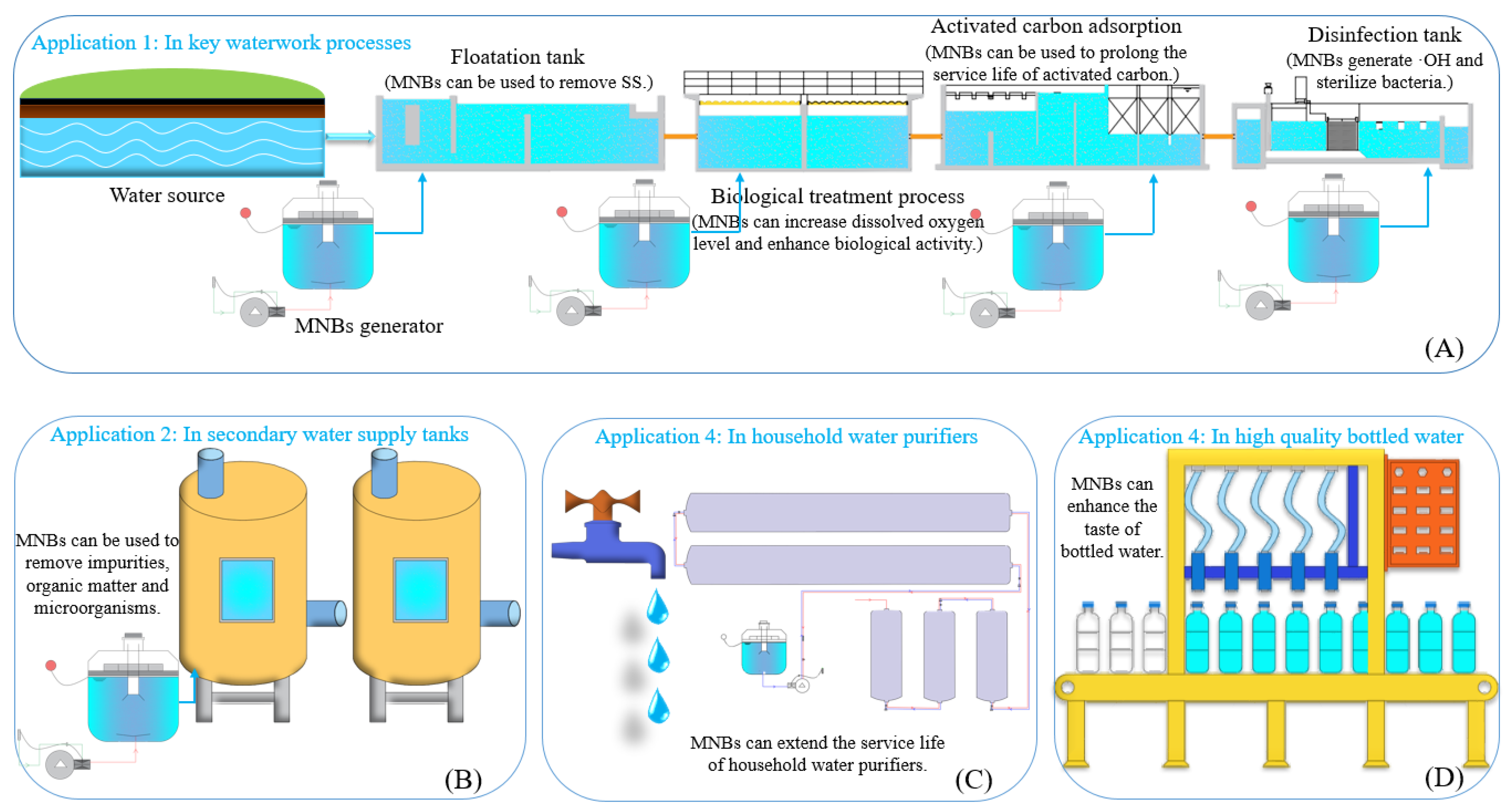
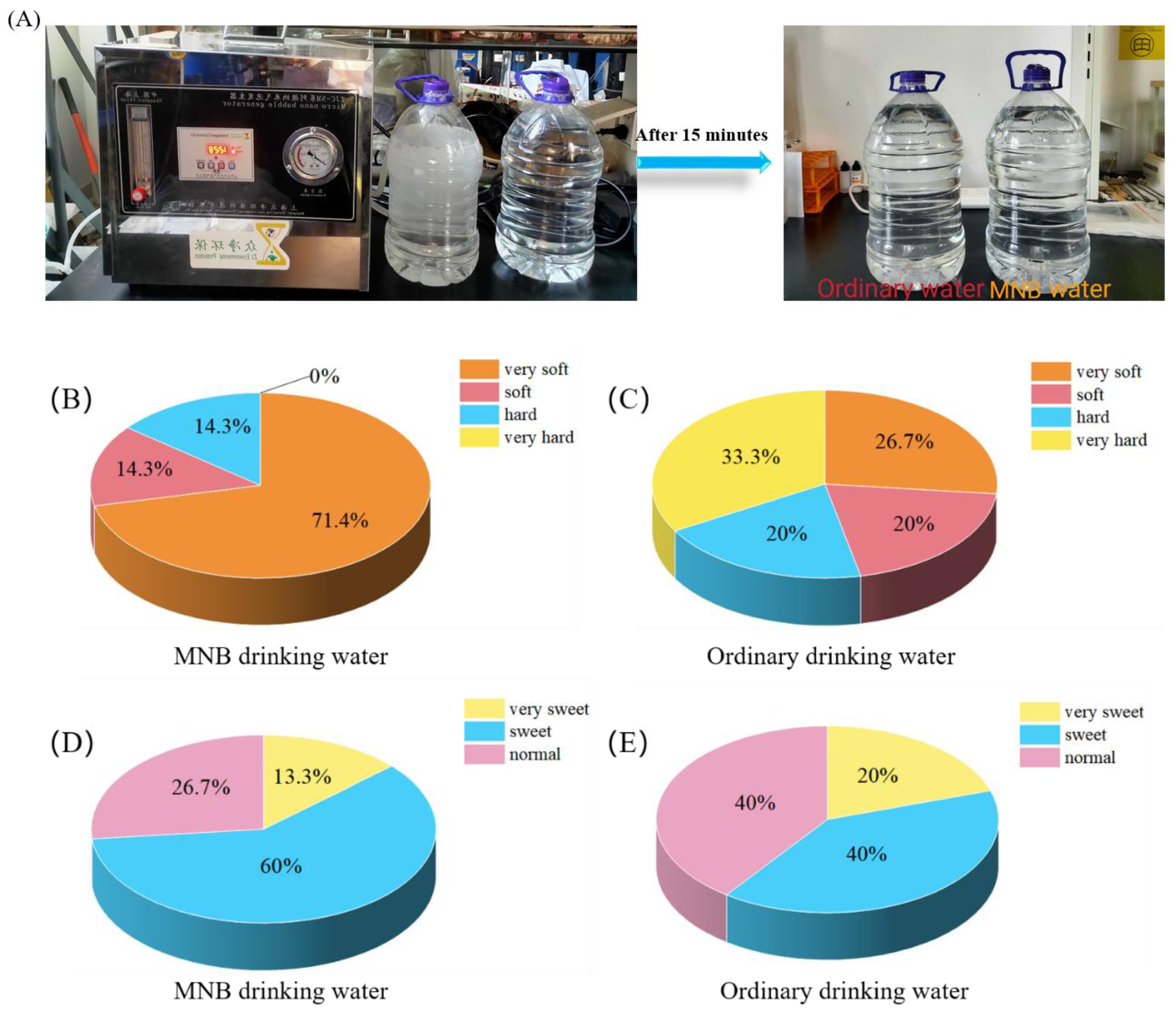
5. Application Prospect of MNBs in Drinking Water
6. Limitations and Prospects of MNBs
- The long-term stable existence of MNBs in water and the ·OH generation mechanism are highly controversial. Existing studies on the above two aspects remain at the surface and speculation level; hence, further discussion is needed.
- The relationship between the synergistic and antagonistic effects of MNBs on microorganisms remains unclear, because the MNBs can generate substantial oxidizing ·OH to destroy microorganisms and provide great potential for water disinfection. Moreover, due to a high mass transfer efficiency, MNBs have a good biological activity and can promote the biological purification function of water. These two statements are contradictory. Therefore, to effectively apply MNB technology, it is essential to investigate the circumstances under which either the synergistic or antagonistic effects of MNBs on microorganisms prevail.
- It is difficult to quantitatively determine the ·OH generated by MNBs. Recently, the detection methods of ·OH are all indirect methods, which are complicated in operation, and are inevitably interfered by many factors in the detection process, resulting in considerable errors. Future research should focus on the direct detection of ·OH to reduce unnecessary interference items.
- MNBs generate a limited number of ·OH. The ability of MNBs to generate free radicals is only one of its many outstanding properties, and the ·OH generated is only one of the many free radical products. At present, studies on the influence of various factors on the generation of ·OH by MNBs are relatively simple. They should continue to explore how to promote the generation of ·OH by MNBs and simultaneously control the factors that affect ·OH generation under optimal conditions.
- NB generation devices are expensive. NBs are superior to MBs in all aspects, but due to the high energy consumption and high price of NB generation devices, the application of NBs in various fields is limited to a certain extent. Hence, developing practical NB generation devices with a low energy consumption, low cost, excellent performance, and easy promotion is also a new potential direction of current research.
- The study of MNB characteristics is not comprehensive enough. At present, the research on the characteristics of MNBs mainly focuses on the well-known aspects of free radical generation and high mass transfer efficiency. Other characteristics of MNBs, such as heat transfer and viscosity, are unknown and require more analysis.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mekal, A.D.; El-Shazly, M.M.; Ragab, M.; Marzouk, E.R. Comparison of modern and 40-year-old drinking water pipeline in northern Sinai region, Egypt: Characteristics and health risk assessment. J. Trace Elem. Miner. 2023, 5, 10078. [Google Scholar] [CrossRef]
- Chang, L.; Lee, J.H.W.; Fung, Y.S. Prediction of lead leaching from galvanic corrosion of lead-containing components in copper pipe drinking water supply systems. J. Hazard. Mater. 2022, 436, 129169. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, H.; Yao, L.; Wei, Z.; Lou, L.; Shan, Y.; Endalkachew, S.-D.; Mallikarjuna, N.; Hu, B.; Zhou, X. The spatial distribution of pollutants in pipe-scale of large-diameter pipelines in a drinking water distribution system. J. Hazard. Mater. 2016, 317, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Barton, N.A.; Farewell, T.S.; Hallett, S.H.; Acland, T.F. Improving pipe failure predictions: Factors affecting pipe failure in drinking water networks. Water Res. 2019, 164, 114926. [Google Scholar] [CrossRef]
- Kim, J.R.; Huling, S.G.; Kan, E. Effects of temperature on adsorption and oxidative degradation of bisphenol A in an acid-treated iron-amended granular activated carbon. Chem. Eng. J. 2015, 262, 1260–1267. [Google Scholar] [CrossRef]
- Yüksel, S.; Kabay, N.; Yüksel, M. Removal of bisphenol A (BPA) from water by various nanofiltration (NF) and reverse osmosis (RO) membranes. J. Hazard. Mater. 2013, 263, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, H.; Li, Q.; Cheng, C.; Shen, H.; Zhang, Z.; Zhang, Z.; Wang, H. Removal of refractory organics in wastewater by coagulation/flocculation with green chlorine-free coagulants. Sci. Total Environ. 2021, 787, 147654. [Google Scholar] [CrossRef]
- Baig, S.; Liechti, P.A. Ozone treatment for biorefractory COD removal. Water Sci. Technol. 2001, 43, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Moreira, F.C.; Garcia-Segura, S.; Vilar, V.J.P.; Boaventura, R.A.; Brillas, E. Decolorization and mineralization of Sunset Yellow FCF azo dye by anodic oxidation, electro-Fenton, UVA photoelectro-Fenton and solar photoelectro-Fenton processes. Appl. Catal. B Environ. 2013, 142, 877–890. [Google Scholar] [CrossRef]
- Subramanian, G.; Prakash, H. Photo augmented copper-based Fenton disinfection under visible LED light and natural sunlight irradiation. Water Res. 2021, 190, 116719. [Google Scholar] [CrossRef]
- Li, T.; Shang, C.; Xiang, Y.; Yin, R.; Pan, Y.; Fan, M.; Yang, X. ClO2 pre-oxidation changes dissolved organic matter at the molecular level and reduces chloro-organic byproducts and toxicity of water treated by the UV/chlorine process. Water Res. 2022, 216, 118341. [Google Scholar] [CrossRef]
- Xu, M.Y.; Lin, Y.L.; Zhang, T.Y.; Hu, C.Y.; Tang, Y.L.; Deng, J.; Xu, B. Chlorine dioxide-based oxidation processes for water purification: A review. J. Hazard. Mater. 2022, 436, 129195. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Lo, W.; Chan, G.; Sillanpää, M.E. Biological processes for treatment of landfill leachate. J. Environ. Monit. 2010, 12, 2032–2047. [Google Scholar] [CrossRef] [PubMed]
- Ilmasari, D.; Kamyab, H.; Yuzir, A.; Riyadi, F.A.; Khademi, T.; Al-Qaim, F.F.; Kirpichnikova, I.; Krishnan, S. A Review of the Biological Treatment of Leachate: Available Technologies and Future Requirements for the Circular Economy Implementation. Biochem. Eng. J. 2022, 187, 108605. [Google Scholar] [CrossRef]
- Zhang, T. The development of drinking water treatment technology. China High-Tech Enterp. 2013, 23, 6–8. [Google Scholar]
- Zhai, H.; He, X.; Zhang, Y.; Du, T.; Adeleye, A.S.; Li, Y. Disinfection byproduct formation in drinking water sources: A case study of Yuqiao reservoir. Chemosphere 2017, 181, 224–231. [Google Scholar] [CrossRef]
- Pokhrel, D.; Viraraghavan, T. Biological filtration for removal of arsenic from drinking water. J. Environ. Manag. 2009, 90, 1956–1961. [Google Scholar] [CrossRef]
- Schijven, J.F.; van den Berg, H.H.J.L.; Colin, M.; Dullemont, Y.; Hijnen, W.A.M.; Magic-Knezev, A.; Oorthuizen, W.A.; Wubbels, G. A mathematical model for removal of human pathogenic viruses and bacteria by slow sand filtration under variable operational conditions. Water Res. 2013, 47, 2592–2602. [Google Scholar] [CrossRef]
- Hedegaard, M.J.; Albrechtsen, H.J. Microbial pesticide removal in rapid sand filters for drinking water treatment–potential and kinetics. Water Res. 2014, 48, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, D.; Liang, Y.; Zeng, H.; Zhang, J. Autotrophic nitrogen removal process in a potable water treatment biofilter that simultaneously removes Mn and NH4+-N. Bioresour. Technol. 2014, 172, 226–231. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Choo, K.-H.; Pramanik, S.K.; Suja, F.; Jegatheesan, V. Comparisons between biological filtration and coagulation processes for the removal of dissolved organic nitrogen and disinfection by-products precursors. Int. Biodeterior. Biodegrad. 2015, 104, 164–169. [Google Scholar] [CrossRef]
- Li, Z.; Dvorak, B.; Li, X. Removing 17β-estradiol from drinking water in a biologically active carbon (BAC) reactor modified from a granular activated carbon (GAC) reactor. Water Res. 2012, 46, 2828–2836. [Google Scholar] [CrossRef]
- Yapsakli, K.; Mertoglu, B.; Çeçen, F. Identification of nitrifiers and nitrification performance in drinking water biological activated carbon (BAC) filtration. Process Biochem. 2010, 45, 1543–1549. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zhang, Z.Y.; Ma, L.P.; Liu, N.; Wu, B.; Zhang, Y.; Li, A.M.; Cheng, S.P. Influences of hydraulic loading rate on SVOC removal and microbial community structure in drinking water treatment biofilters. J. Hazard. Mater. 2010, 178, 652–657. [Google Scholar] [CrossRef] [PubMed]
- McKie, M.J.; Andrews, S.A.; Andrews, R.C. Conventional drinking water treatment and direct biofiltration for the removal of pharmaceuticals and artificial sweeteners: A pilot-scale approach. Sci. Total Environ. 2016, 544, 10–17. [Google Scholar] [CrossRef]
- Akcay, M.U.; Avdan, Z.Y.; Inan, H. Effect of biofiltration process on the control of THMs and HAAs in drinking water. Desalination Water Treat. 2016, 57, 2546–2554. [Google Scholar] [CrossRef]
- Tekerlekopoulou, A.G.; Vayenas, D.V. Ammonia, iron and manganese removal from potable water using trickling filters. Desalination 2007, 210, 225–235. [Google Scholar] [CrossRef]
- Hasan, H.A.; Abdullah, S.R.S.; Kamarudin, S.K.; Kofli, N.T. Response surface methodology for optimization of simultaneous COD, NH4+–N and Mn2+ removal from drinking water by biological aerated filter. Desalination 2011, 275, 50–61. [Google Scholar] [CrossRef]
- Han, M.; Zhao, Z.-W.; Gao, W.; Cui, F.-Y. Study on the factors affecting simultaneous removal of ammonia and manganese by pilot-scale biological aerated filter (BAF) for drinking water pre-treatment. Bioresour. Technol. 2013, 145, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhao, Z.; Gao, W.; Tian, Y.; Cui, F. Effective combination of permanganate composite chemicals (PPC) and biological aerated filter (BAF) to pre-treat polluted drinking water source. Desalination Water Treat. 2016, 57, 28240–28249. [Google Scholar] [CrossRef]
- He, S.; Wang, J.; Ye, L.; Zhang, Y.; Yu, J. Removal of diclofenac from surface water by electron beam irradiation combined with a biological aerated filter. Radiat. Phys. Chem. 2014, 105, 104–108. [Google Scholar] [CrossRef]
- Marsidi, N.; Abu Hasan, H.; Abdullah, S.R.S. A review of biological aerated filters for iron and manganese ions removal in water treatment. J. Water Process Eng. 2018, 23, 1–12. [Google Scholar] [CrossRef]
- Buttiglieri, G.; Malpei, F.; Daverio, E.; Melchiori, M.; Nieman, H.; Ligthart, J. Denitrification of drinking water sources by advanced biological treatment using a membrane bioreactor. Desalination 2005, 178, 211–218. [Google Scholar] [CrossRef]
- Li, X.; Chu, H.P. Membrane bioreactor for the drinking water treatment of polluted surface water supplies. Water Res. 2003, 37, 4781–4791. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, A.R.; Carvalho, G.; Velizarov, S.; Crespo, J.G.; Reis, M.A. Kinetics of nitrate and perchlorate removal and biofilm stratification in an ion exchange membrane bioreactor. Water Res. 2012, 46, 4556–4568. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.T.; Velizarov, S.; Reis, M.A.M.; Crespo, J.G. Removal of bromate from drinking water using the ion exchange membrane bioreactor concept. Environ. Sci. Technol. 2008, 42, 7702–7708. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.G.; Wen, D.H.; Shi, D.W.; Tang, X.Y. Reduction of precursors of chlorination by-products in drinking water using fluidized-bed biofilm reactor at low temperature. Biomed. Environ. Sci. 2006, 19, 360. [Google Scholar]
- Tian, J.-Y.; Liang, H.; Li, X.; You, S.-J.; Tian, S.; Li, G.-B. Membrane coagulation bioreactor (MCBR) for drinking water treatment. Water Res. 2008, 42, 3910–3920. [Google Scholar] [CrossRef]
- Tian, J.-Y.; Chen, Z.-L.; Yang, Y.-L.; Liang, H.; Nan, J.; Wang, Z.-Z.; Li, G.-B. Hybrid process of BAC and sMBR for treating polluted raw water. Bioresour. Technol. 2009, 100, 6243–6249. [Google Scholar] [CrossRef] [PubMed]
- Haris, S.; Qiu, X.; Klammler, H.; Mohamed, M.M. The use of micro-nano bubbles in groundwater remediation: A comprehensive review. Groundw. Sustain. Dev. 2020, 11, 100463. [Google Scholar] [CrossRef]
- Sakr, M.; Mohamed, M.M.; Maraqa, M.A.; Hamouda, M.A.; Hassan, A.A.; Ali, J.; Jung, J. A critical review of the recent developments in micro–nano bubbles applications for domestic and industrial wastewater treatment. Alex. Eng. J. 2022, 61, 6591–6612. [Google Scholar] [CrossRef]
- Tan, K.A.; Mohan, Y.; Liew, K.J.; Chong, S.H.; Poh, P.E. Development of an effective cleaning method for metallic parts using microbubbles. J. Clean. Prod. 2020, 261, 121076. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, G.; Li, G. Effect of nanobubble application on performance and structural characteristics of microbial aggregates. Sci. Total Environ. 2021, 765, 142725. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, X.; Wang, H.; Hu, B.; Lei, Z.; Kobayashi, M.; Adachi, Y.; Shimizu, K.; Zhang, Z. Effects of nanobubble water on the growth of Lactobacillus acidophilus 1028 and its lactic acid production. RSC Adv. 2019, 9, 30760–30767. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fan, W.; Huo, M.; Zhao, H.; Lu, Y. Hydroxyl radical generation and contaminant removal from water by the collapse of microbubbles under different hydrochemical conditions. Water Air Soil Pollut. 2018, 229, 86. [Google Scholar] [CrossRef]
- Mezule, L.; Tsyfansky, S.; Yakushevich, V.; Juhna, T. A simple technique for water disinfection with hydrodynamic cavitation: Effect on survival of Escherichia coli. Desalination 2009, 248, 152–159. [Google Scholar] [CrossRef]
- Zhu, J.; Wakisaka, M. Effect of air nanobubble water on the growth and metabolism of Haematococcus lacustris and Botryococcus braunii. J. Nutr. Sci. Vitaminol. 2019, 65, S212–S216. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, Y.H.; Jung, I.H.; Lee, J.H. Effect of Nano Bubble Oxygen and Hydrogen Water on Microalgae. Appl. Chem. Eng. 2014, 25, 324–329. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, G. Mass transfer of nanobubble aeration and its effect on biofilm growth: Microbial activity and structural properties. Sci. Total Environ. 2020, 703, 134976. [Google Scholar] [CrossRef] [PubMed]
- Hanotu, J.; Kong, D.; Zimmerman, W.B. Intensification of yeast production with microbubbles. Food Bioprod. Process. 2016, 100, 424–431. [Google Scholar] [CrossRef]
- Hu, L.; Xia, Z. Application of ozone micro-nano-bubbles to groundwater remediation. J. Hazard. Mater. 2018, 342, 446–453. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; Zhang, X.; Li, G.; Sun, J.; Zhang, Y.; Li, M.; Hu, J. Nanobubbles influence on BSA adsorption on mica surface. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Film. 2006, 38, 990–995. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; Zhang, X.; Sun, J.; Dong, Y.; Hu, J. In situ AFM observation of BSA adsorption on HOPG with nanobubble. Chin. Sci. Bull. 2007, 52, 1913–1919. [Google Scholar] [CrossRef]
- Liu, C.; Tang, Y. Application research of micro and nano bubbles in water pollution control. E3S Web Conf. EDP Sci. 2019, 136, 06028. [Google Scholar] [CrossRef]
- Nakashima, T.; Kobayashi, Y.; Hirata, Y. Method to exterminate blue-green algae in a large pond and to improve plant growth by micro-nano bubbles in activated water. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 938, Lisbon, Portugal, 22 August 2010; pp. 391–400. [Google Scholar]
- Minamikawa, K.; Takahashi, M.; Makino, T.; Tago, K.; Hayatsu, M. Irrigation with oxygen-nanobubble water can reduce methane emission and arsenic dissolution in a flooded rice paddy. Environ. Res. Lett. 2015, 10, 084012. [Google Scholar] [CrossRef]
- Baram, S.; Weinstein, M.; Evans, J.F.; Berezkin, A.; Sade, Y.; Ben-Hur, M.; Bernstein, N.; Mamane, H. Drip irrigation with nanobubble oxygenated treated wastewater improves soil aeration. Sci. Hortic. 2022, 291, 110550. [Google Scholar] [CrossRef]
- Ebina, K.; Shi, K.; Hirao, M.; Hashimoto, J.; Kawato, Y.; Kaneshiro, S.; Morimoto, T.; Koizumi, K.; Yoshikawa, H. Oxygen and air nanobubble water solution promote the growth of plants, fishes, and mice. PLoS ONE 2013, 8, e65339. [Google Scholar] [CrossRef]
- Li, H.; Hu, L.; Song, D.; Lin, F. Characteristics of micro-nano bubbles and potential application in groundwater bioremediation. Water Environ. Res. 2014, 86, 844–851. [Google Scholar] [CrossRef]
- Sumikura, M.; Hidaka, M.; Murakami, H.; Nobutomo, Y.; Murakami, T. Ozone micro-bubble disinfection method for wastewater reuse system. Water Sci. Technol. 2007, 56, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Q.; Ma, H.; Huang, P.; Li, J.; Kikuchi, T. Effect of micro-bubbles on coagulation flotation process of dyeing wastewater. Sep. Purif. Technol. 2010, 71, 337–346. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced oxidation processes: Performance, advantages, and scale-up of emerging technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef] [PubMed]
- Temesgen, T.; Bui, T.T.; Han, M.; Kim, T.-I.; Park, H. Micro and nanobubble technologies as a new horizon for water-treatment techniques: A review. Adv. Colloid Interface Sci. 2017, 246, 40–51. [Google Scholar] [CrossRef]
- Huang, C.H. Preparation of Nanobubbles by Ultrasonic Method and Its Effect on Electric Double Layer on Electrode Surface. Master’s Thesis, Shanghai Normal University, Shanghai, China, 2016. [Google Scholar]
- Maeda, Y.; Hosokawa, S.; Baba, Y.; Tomiyama, A.; Ito, Y. Generation mechanism of micro-bubbles in a pressurized dissolution method. Exp. Therm. Fluid Sci. 2015, 60, 201–207. [Google Scholar] [CrossRef]
- Etchepare, R.; Azevedo, A.; Calgaroto, S.; Rubio, J. Removal of ferric hydroxide by flotation with micro and nanobubbles. Sep. Purif. Technol. 2017, 184, 347–353. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, A.R.; Zhang, L.J. Characteristics of micro-nanobubbles and their applications in soil environment improvement. J. Environ. Eng. Technol. 2022, 12, 1324–1332. [Google Scholar]
- Wu, M.; Song, H.; Liang, X.; Huang, N.; Li, X. Generation of micro-nano bubbles by self-developed swirl-type micro-nano bubble generator. Chem. Eng. Process.-Process Intensif. 2022, 181, 109136. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Yin, J.; Wang, D. Investigation on the effect of geometrical parameters on the performance of a venturi type bubble generator. Nucl. Eng. Des. 2017, 325, 90–96. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, L.; Mo, Z.; Du, M.; Huang, J.; Bao, J.; Tang, J.; Xie, G. Effects of the divergent angle on bubble transportation in a rectangular Venturi channel and its performance in producing fine bubbles. Int. J. Multiph. Flow 2019, 114, 192–206. [Google Scholar] [CrossRef]
- Takahashi, M.; Kawamura, T.; Yamamoto, Y.; Ohnari, H.; Himuro, S.; Shakutsui, H. Effect of shrinking microbubble on gas hydrate formation. J. Phys. Chem. B 2003, 107, 2171–2173. [Google Scholar] [CrossRef]
- Azevedo, A.; Etchepare, R.; Calgaroto, S.; Rubio, J. Aqueous dispersions of nanobubbles: Generation, properties and features. Miner. Eng. 2016, 94, 29–37. [Google Scholar] [CrossRef]
- Seddon, J.R.T.; Lohse, D.; Ducker, W.A.; Craig, V.S.J. A deliberation on nanobubbles at surfaces and in bulk. ChemPhysChem 2012, 13, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zou, Z.; Wang, S.; Wang, X.; Wang, L.; Dong, Y.; Zhao, H.; Zhang, L.; Hu, J. Formation and stability of bulk nanobubbles generated by ethanol–water exchange. ChemPhysChem 2017, 18, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Chen, H.; Li, Z.X.; Fang, H.P.; Hu, J. The longevity of nanobubbles stems from their high internal density. Sci. Sin. G Ser. 2007, 37, 556–560. [Google Scholar]
- Zhang, X.H.; Maeda, N.; Craig, V.S.J. Physical properties of nanobubbles on hydrophobic surfaces in water and aqueous solutions. Langmuir 2006, 22, 5025–5035. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, G.; Tsuruta, T.; Cheng, P. Molecular dynamics simulation on bubble formation in a nanochannel. Int. J. Heat Mass Transf. 2006, 49, 4437–4443. [Google Scholar] [CrossRef]
- Ushikubo, F.Y.; Enari, M.; Furukawa, T.; Nakagawa, R.; Makino, Y.; Kawagoe, Y.; Oshita, S. Zeta-potential of micro-and/or nano-bubbles in water produced by some kinds of gases. IFAC Proc. Vol. 2010, 43, 283–288. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W.; Kato, K. Dynamic equilibrium model for a bulk nanobubble and a microbubble partly covered with hydrophobic material. Langmuir 2016, 32, 11101–11110. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. High temperature and pressure inside a dissolving oxygen nanobubble. Ultrason. Sonochemistry 2019, 55, 308–312. [Google Scholar] [CrossRef]
- Li, H.; Hu, L.; Song, D.; Al-Tabbaa, A. Subsurface transport behavior of micro-nano bubbles and potential applications for groundwater remediation. Int. J. Environ. Res. Public Health 2014, 11, 473–486. [Google Scholar] [CrossRef]
- Hong, T.; Ye, C.; Li, C.H.; Zhang, B.J.; Zhou, L. Treatment effect of microbubble aeration technology on black-odor river water. J. Environ. Eng. Technol. 2011, 1, 20–25. [Google Scholar]
- Temesgen, T. Enhancing Gas-Liquid Mass Transfer and (Bio) Chemical Reactivity Using Ultrafine/Nanobubble in Water and Waste Water Treatments. Ph.D. Thesis, Department of Civil and Environmental Engineering, Seoul National University, Seoul, Republic of Korea, 2017. [Google Scholar]
- Zhang, M.; Qiu, L.; Liu, G. Basic characteristics and application of micro-nano bubbles in water treatment. IOP Conf. Ser. Earth Environ. Sci. 2020, 510, 042050. [Google Scholar] [CrossRef]
- Ljunggren, S.; Eriksson, J.C. The lifetime of a colloid-sized gas bubble in water and the cause of the hydrophobic attraction. Colloids Surf. A Physicochem. Eng. Asp. 1997, 129, 151–155. [Google Scholar] [CrossRef]
- Henry, W. Experiments on the quantity of gases absorbed by water, at different temperatures, and under different pressures. In Abstracts of the Papers Printed in the Philosophical Transactions of the Royal Society of London; The Royal Society: London, UK, 1832; pp. 103–104. [Google Scholar]
- Shen, D.; Xie, Z.; Shentu, J.; Long, Y.; Lu, L.; Li, L.; Qi, S. Enhanced oxidation of aromatic hydrocarbons by ozone micro-nano bubble water: Mechanism and influencing factors. J. Environ. Chem. Eng. 2023, 11, 110281. [Google Scholar] [CrossRef]
- Jin, N.; Zhang, F.; Cui, Y.; Sun, L.; Gao, H.; Pu, Z.; Yang, W. Environment-friendly surface cleaning using micro-nano bubbles. Particuology 2022, 66, 1–9. [Google Scholar] [CrossRef]
- Takahashi, M.; Chiba, K.; Li, P. Free-radical generation from collapsing microbubbles in the absence of a dynamic stimulus. J. Phys. Chem. B 2007, 111, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Ishikawa, H.; Asano, T.; Horibe, H. Effect of microbubbles on ozonized water for photoresist removal. J. Phys. Chem. C 2012, 116, 12578–12583. [Google Scholar] [CrossRef]
- Kohno, M.; Mokudai, T.; Ozawa, T.; Niwano, Y. Free radical formation from sonolysis of water in the presence of different gases. J. Clin. Biochem. Nutr. 2011, 49, 96–101. [Google Scholar] [CrossRef]
- Jia, W.; Ren, S.; Hu, B. Effect of water chemistry on zeta potential of air bubbles. Int. J. Electrochem. Sci. 2013, 8, 5828–5837. [Google Scholar] [CrossRef]
- Takahashi, M. The ζ potential of microbubbles in aqueous solutions—Electrical properties of the gas-water interface. J. Phys. Chem. 2005, 109, 21858–21864. [Google Scholar] [CrossRef]
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Colloids Surf. A Physicochem. Eng. Asp. 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Khaled Abdella Ahmed, A.; Sun, C.; Hua, L.; Zhang, Z.; Zhang, Y.; Marhaba, T.; Zhang, W. Colloidal properties of air, oxygen, and nitrogen nanobubbles in water: Effects of ionic strength, natural organic matters, and surfactants. Environ. Eng. Sci. 2018, 35, 720–727. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Aluthgun Hewage, S.; Batagoda, J.H. Stability of nanobubbles. Environ. Eng. Sci. 2018, 35, 1216–1227. [Google Scholar] [CrossRef]
- Hamamoto, S.; Takemura, T.; Suzuki, K.; Nishimura, T. Effects of pH on nano-bubble stability and transport in saturated porous media. J. Contam. Hydrol. 2018, 208, 61–67. [Google Scholar] [CrossRef]
- Li, H. Study on the Water, Fertilization and Aeration Distribution Characteristics of Aerated Drip Irrigation, and the Clogging of Emitters. Ph.D. Thesis, Jiangsu University, Zhenjiang, China, 2020. [Google Scholar]
- Yan, C.C.; Cun, H.H.; Zhang, H.Y.; Chen, L.; Liu, Y.L. Numerical simulation of effects of microbubble growth and collapse on adjacent microspheres. Chin. J. Appl. Mech. 2022, 36, 580–587. [Google Scholar]
- Kröninger, D.; Köhler, K.; Kurz, T.; Lauterborn, W. Particle tracking velocimetry of the flow field around a collapsing cavitation bubble. Exp. Fluids 2010, 48, 395–408. [Google Scholar] [CrossRef]
- Zwaan, E.; Le Gac, S.; Tsuji, K.; Ohl, C.-D. Controlled cavitation in microfluidic systems. Phys. Rev. Lett. 2007, 98, 254501. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Extreme conditions in a dissolving air nanobubble. Phys. Rev. E 2016, 94, 013106. [Google Scholar] [CrossRef]
- Yasui, K. Alternative model of single-bubble sonoluminescence. Phys. Rev. E 1997, 56, 6750. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Sivakumar, M.; Iida, Y. Theoretical study of single-bubble sonochemistry. J. Chem. Phys. 2005, 122, 224706. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Su, H.; Zhang, B. Hydrodynamic cavitation as a promising route for wastewater treatment—A review. Chem. Eng. J. 2021, 412, 128685. [Google Scholar] [CrossRef]
- Sun, X.; Park, J.J.; Kim, H.S.; Lee, S.H.; Seong, S.J.; Om, A.S.; Yoon, J.Y. Experimental investigation of the thermal and disinfection performances of a novel hydrodynamic cavitation reactor. Ultrason. Sonochemistry 2018, 49, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Khuntia, S.; Majumder, S.K.; Ghosh, P. Quantitative prediction of generation of hydroxyl radicals from ozone microbubbles. Chem. Eng. Res. Des. 2015, 98, 231–239. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Lv, Y.; Wang, S.; Zheng, S.; Du, H.; Zhang, Y. Effect of microbubble diameter, alkaline concentration and temperature on reactive oxygen species concentration. J. Chem. Technol. Biotechnol. 2017, 92, 1738–1745. [Google Scholar] [CrossRef]
- Li, P.; Takahashi, M.; Chiba, K. Enhanced free-radical generation by shrinking microbubbles using a copper catalyst. Chemosphere 2009, 77, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Chiba, K.; Li, P. Formation of hydroxyl radicals by collapsing ozone microbubbles under strongly acidic conditions. J. Phys. Chem. B 2007, 111, 11443–11446. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, C.; Libra, J.A.; Saupe, A. Ozonation of Water and Waste Water: A Practical Guide to Understanding Ozone and Its Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Li, P.; Takahashi, M.; Chiba, K. Degradation of phenol by the collapse of microbubbles. Chemosphere 2009, 75, 1371–1375. [Google Scholar] [CrossRef]
- Kondo, T.; Gamson, J.; Mitchell, J.B.; Riesz, P. Free radical formation and cell lysis induced by ultrasound in the presence of different rare gases. Int. J. Radiat. Biol. 1988, 54, 955–962. [Google Scholar] [CrossRef]
- Fan, W.; Li, Y.; Lyu, T.; Yu, J.; Chen, Z.; Jarvis, P.; Huo, Y.; Xiao, D.; Huo, M. A modelling approach to explore the optimum bubble size for micro-nanobubble aeration. Water Res. 2023, 228, 119360. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Guo, P.; Guo, W.; Li, G. Chemical effect of swirling jet-induced cavitation: Degradation of rhodamine B in aqueous solution. Ultrason. Sonochemistry 2008, 15, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y. Degradation of alachlor in aqueous solution by using hydrodynamic cavitation. J. Hazard. Mater. 2009, 161, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.H.; Doraiswamy, L.K. Sonochemistry: Science and engineering. Ind. Eng. Chem. Res. 1999, 38, 1215–1249. [Google Scholar] [CrossRef]
- Frontistis, Z.; Mantzavinos, D. Sonodegradation of 17α-ethynylestradiol in environmentally relevant matrices: Laboratory-scale kinetic studies. Ultrason. Sonochemistry 2012, 19, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Maruyama, A.; Eguchi, T.; Hirakawa, T.; Murakami, Y. Influence of microbubbles on free radical generation by ultrasound in aqueous solution: Dependence of ultrasound frequency. J. Phys. Chem. B 2015, 119, 12887–12893. [Google Scholar] [CrossRef] [PubMed]
- Makuta, T.; Aizawa, Y.; Suzuki, R. Sonochemical reaction with microbubbles generated by hollow ultrasonic horn. Ultrason. Sonochemistry 2013, 20, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J. Formation and Characterization of Bulk Micro-/Nanobubbles. Master’s Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2020. [Google Scholar]
- Tasaki, T.; Wada, T.; Fujimoto, K.; Kai, S.; Ohe, K.; Oshima, T.; Bada, Y.; Kukizaki, M. Degradation of methyl orange using short-wavelength UV irradiation with oxygen microbubbles. J. Hazard. Mater. 2009, 162, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Duan, Y.; Fan, W.; Guo, T.; Huo, M.; Yang, W.; Zhu, S.; An, W. Intensifying ozonation treatment of municipal secondary effluent using a combination of microbubbles and ultraviolet irradiation. Environ. Sci. Pollut. Res. 2019, 26, 21915–21924. [Google Scholar] [CrossRef]
- Lu, J.; Huang, X.; Zhang, Z.; Pang, H.; Chen, K.; Xia, H.; Sui, Y.; Chen, R.; Zhao, Z. Co-coagulation of micro-nano bubbles (MNBs) for enhanced drinking water treatment: A study on the efficiency and mechanism of a novel cleaning process. Water Res. 2022, 226, 119245. [Google Scholar] [CrossRef]
- Xia, Z.; Hu, L. Treatment of organics contaminated wastewater by ozone micro-nano-bubbles. Water 2018, 11, 55. [Google Scholar] [CrossRef]
- Xia, Z.; Hu, L. Remediation of organics contaminated groundwater by ozone micro-nano bubble. Jpn. Geotech. Soc. Spec. Publ. 2016, 2, 1978–1981. [Google Scholar] [CrossRef]
- Achar, J.C.; Nam, G.; Jung, J.; Klammler, H.; Mohamed, M.M. Microbubble ozonation of the antioxidant butylated hydroxytoluene: Degradation kinetics and toxicity reduction. Environ. Res. 2020, 186, 109496. [Google Scholar] [CrossRef] [PubMed]
- Jabesa, A.; Ghosh, P. Removal of diethyl phthalate from water by ozone microbubbles in a pilot plant. J. Environ. Manag. 2016, 180, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Quan, X.; Li, R.; Wu, J.; Zhao, Q. Ozonation of phenol-containing wastewater using O3/Ca(OH)2 system in a micro bubble gas-liquid reactor. Ozone Sci. Eng. 2018, 40, 173–182. [Google Scholar] [CrossRef]
- Li, P.; Tsuge, H.; Itoh, K. Oxidation of dimethyl sulfoxide in aqueous solution using microbubbles. Ind. Eng. Chem. Res. 2009, 48, 8048–8053. [Google Scholar] [CrossRef]
- Teo, K.C.; Xu, Y.; Yang, C. Sonochemical degradation for toxic halogenated organic compounds. Ultrason. Sonochemistry 2001, 8, 241–246. [Google Scholar] [CrossRef]
- Kalumuck, K.M.; Chahine, G.L. The use of cavitating jets to oxidize organic compounds in water. J. Fluids Eng. 2000, 122, 465–470. [Google Scholar] [CrossRef]
- Xia, Z.; Hu, L.; Kusaba, S.; Song, D. Remediation of TCE contaminated site by ozone micro-nano-bubbles. In The International Congress on Environmental Geotechnics; Springer: Singapore, 2018; pp. 796–803. [Google Scholar]
- Kim, I.K.; Huang, C.P. Sonochemical degradation of polycyclic aromatic sulfur hydrocarbons (PASHs) in aqueous solutions exemplified by benzothiophene. J. Chin. Inst. Eng. 2005, 28, 1107–1118. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Zhu, K.Q.; Xi, B.S. Numercial study of cavitation erosion on a rigid wall. Chin. J. Appl. Mech. 2004, 21, 22–25. [Google Scholar]
- Lakretz, A.; Ron, E.Z.; Mamane, H. Biofilm control in water by a UV-based advanced oxidation process. Biofouling 2011, 27, 295–307. [Google Scholar] [CrossRef]
- Gogate, P.R.; Kabadi, A.M. A review of applications of cavitation in biochemical engineering/biotechnology. Biochem. Eng. J. 2009, 44, 60–72. [Google Scholar] [CrossRef]
- Shirgaonkar, I.Z.; Lothe, R.R.; Pandit, A.B. Comments on the mechanism of microbial cell disruption in high-pressure and high-speed devices. Biotechnol. Prog. 1998, 14, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.; Joyce, E.; Phull, S.; Lorimer, J. Potential uses of ultrasound in the biological decontamination of water. Ultrason. Sonochemistry 2003, 10, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Shen, Y.; Liu, Y.Z.; Wang, X.; Xiao, Y.; Li, Y. Effects and mechanism of using Nanobubble to inhibit biofouling and scaling in biogas slurry drip irrigation emitters. Trans. Chin. Soc. Agric. Eng. 2022, 38, 79–87. [Google Scholar]
- Wang, X.Y. Mechanism and Application Research of the Emitters Clogging Control Method by Micro-Nano Bubbles of Drip Irrigation Systems with Biogas Slurry. Master’s Thesis, Shihezi University, Shihezi, China, 2020. [Google Scholar]
- Agarwal, A.; Ng, W.J.; Liu, Y. Cleaning of biologically fouled membranes with self-collapsing microbubbles. Biofouling 2013, 29, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Khadre, M.A.; Yousef, A.E.; Kim, J.G. Microbiological aspects of ozone applications in food: A review. J. Food Sci. 2001, 66, 1242–1252. [Google Scholar] [CrossRef]
- Zhu, Z.; Shan, L.; Li, X.; Hu, F.; Yuan, Y.; Zhong, D.; Zhang, J. Effects of interspecific interactions on biofilm formation potential and chlorine resistance: Evaluation of dual-species biofilm observed in drinking water distribution systems. J. Water Process. Eng. 2020, 38, 101564. [Google Scholar] [CrossRef]
- Bimakr, F.; Ginige, M.P.; Kaksonen, A.H.; Sutton, D.C.; Puzon, G.J.; Cheng, K.Y. Assessing graphite and stainless-steel for electrochemical sensing of biofilm growth in chlorinated drinking water systems. Sens. Actuators B Chem. 2018, 277, 526–534. [Google Scholar] [CrossRef]
- McEwan, C.; Kamila, S.; Owen, J.; Nesbitt, H.; Callan, B.; Borden, M.; Nomikou, N.; Hamoudi, R.A.; Taylor, M.A.; Stride, E.; et al. Combined sonodynamic and antimetabolite therapy for the improved treatment of pancreatic cancer using oxygen loaded microbubbles as a delivery vehicle. Biomaterials 2016, 80, 20–32. [Google Scholar] [CrossRef]
- McEwan, C.; Owen, J.; Stride, E.; Fowley, C.; Nesbitt, H.; Cochrane, D.; Coussios, C.; Borden, M.; Nomikou, N.; McHale, A.P.; et al. Oxygen carrying microbubbles for enhanced sonodynamic therapy of hypoxic tumours. J. Control. Release 2015, 203, 51–56. [Google Scholar] [CrossRef]

| Treatment Methods | Pollutants Removed from Drinking Water | Advantages | Disadvantages | References | |
|---|---|---|---|---|---|
| Physico- chemical methods | Adsorption | Organic pollutants (bisphenol A) | Simple and effective | Adsorbent regeneration and high cost; the adsorption capacity of the regenerated adsorbent decreases, and the service life is short | [5] |
| Membrane separation technology | Particles, sediment, algae, bacteria, protozoa, small colloid, virus, dissolved organic matter, divalent ions monovalent ions, and COD | No secondary pollution | High energy consumption, complex equipment, and high intake water quality requirements; membrane fouling | [6] | |
| Coagulation/flocculation | Refractory organics | Economical and practical | Produce secondary pollution | [7] | |
| Ultrasonic decomposition | Particles and organic pollutants | Short reaction time and simple process facilities | Relatively low efficiency | [17] | |
| Photocatalytic technology | Dissolved organic carbon (DOC) and bacteria | Semiconductors are cheap, can mineralize refractory compounds, and are clean and safe | Still in the development stage and immature | [9] | |
| Chemical methods | Electrochemical advanced oxidation processes (EAOPs) | Organic micropollutants | Has environmental compatibility, versatility, high efficiency and safety | Relatively low efficiency; formation of stable by-products | [10,11] |
| O3-based oxidation process | Organic pollutants (chlorophenols) and bacteria | Economical and efficient, harmless to most organisms, and no harmful by-product generation | Harmful to human health; high energy demand | [8,9] | |
| H2O2-based oxidation process | Organic pollutants (chlorophenols) and bacteria | Safe, efficient, and easy to use; widely used to prevent pollution and improve biodegradability | The reaction process is affected by many factors, and the reaction time is long | [9] | |
| Chlorine-based oxidation process | Organic matter, bacteria micropollutants, and viruses | Chlorine remains in the water as residual chlorine, and the activity is persistent High yield of active species, broad-spectrum, safe, and effective | Taste and smell are not ideal; forms more than 40 DBPs; disinfection effect is not ideal, and is used for secondary disinfection | [12,13,14,18] | |
| Biological methods | Biological sand filtration (BSF) | Viruses, bacteria, heavy metals, nitrogenous compounds, pesticides, organic chemicals, dissolved organic carbon (DOC), NOM, etc. | Easy operation, efficient and reliable operation, and low cost | Microorganisms have a high selectivity to pollutants, and the biodegradation time is long, and the equipment is complex; The uncontrolled growth of microorganisms may lead to health problems; The application of biological sand filtration has high requirements on terrain and limited application scenarios. | [19,20,21,22,23] |
| Biological activated carbon (BAC) | Nitrogenous compounds, organic carbon, and micropollutants. | The dual functions of adsorption and biodegradation improve the effectiveness of drinking water | [24,25,26,27,28] | ||
| Trickling filter (TF) | NH3-N, Fe, and Mn | No external air supply required | [29] | ||
| Biological aerated filter (BAF) | COD, NH4+-N, Fe, Mn, and diclofenac | Economical and effective | [30,31,32,33,34] | ||
| Membrane bioreactor (MBR) | Nitrate, total organic carbon (TOC), deamination, macropollutants, and anionic micropollutants (perchlorate, bromate, and nitrate) | Overcomes the problem of microbial contamination and supports the growth of selected microorganisms | [35,36,37,38] | ||
| Fluidized bed biofilm reactor (FBBR) | TOC, THM, and ammonia | No backwash required and easy to manage | [39] | ||
| Integrated/ combining technologies | Microorganisms, particles, nitrate, phosphate, organic matter, and ammonium | Higher treatment efficiency; improve the quality of the treated water and reduce membrane pollution | [40,41] | ||
| Application Fields | Main Function | Gas Type | Bubble Size (nm) | Bubble Concentration (One/mL) | Characteristics of Applied MNBs | References |
|---|---|---|---|---|---|---|
| Biochemical process | Promote the growth of microalgae and increase the output of many high-value products. | Air | <200 | / | ④ | [49,50] |
| Improve biofilm structure and promote aerobic metabolism; improve COD and ammonia removal rate and reduce aeration. | Air | <225 | / | ④ | [45,51] | |
| Improve the production efficiency of probiotics through fermentation, mainly in the lag stage and logarithmic stage of strain growth. | Air | 180~220 | (3.59 ± 1.14) × 107 | ⑥ | [46] | |
| Improve the production efficiency and recovery rate of yeast. | Air | ≈3 × 105 | / | ④ | [52] | |
| Groundwater remediation | Improve the mass transfer efficiency of O3 and the in situ remediation efficiency of organically contaminated groundwater. | O3 | 10~1000 | (1~1000) × 106 | ③, ④ | [53] |
| Surface cleaning | Prevent and remove protein adsorbed on solid surface. | Air | 25~35 | / | ⑦ | [54,55] |
| Remove oil stain on metal surface. | Air | (2~6) × 104 | / | ①, ② | [44] | |
| Agronomy | Improve irrigation water use efficiency, crop yield, and quality. | Air | 124~148 | (6~7) × 108 | ④ | [56] |
| Improve plant growth; purify blue-green algae pollution. | Air | 200~2200 | / | ④ | [57] | |
| Soil environment | Change the redox conditions of submerged paddy soil to reduce methane emission. | O2 | 128~242 | (6~8) × 107 | ④ | [58] |
| Remove metal pollutants from soil. | O2 | <103 | / | ④ | ||
| Improve the availability of oxygen in clay or sandy soil and improve the soil anoxic environment. | O2 | 190~210 | (0.5~1.5) × 109 | ④ | [59] | |
| Marine animals and food | Significantly promote the growth of plants, fish, and mice. | O2 | <200 | / | ④ | [60] |
| Air | / | |||||
| Water pollution treatment | Aeration to improve oxygen mass transfer efficiency. | Air | 102~105 | / | ④ | [61] |
| Disinfect and can effectively remove bacteria and viruses. | O3 | (3~6) × 104 | / | ⑤ | [62] | |
| Flotation to improve the treatment effects of printing and dyeing wastewater. | Air | <6 × 104 | / | ②, ③, ④, ⑤ | [63] | |
| Degradation of organic pollutants | ||||||
Macrobubbles | Microbubbles | Nanobubbles | References | |
|---|---|---|---|---|
| Size | >100 μm | 1–100 μm | <1 μm | [43,65] |
| Specific surface area | Small | Large | Larger | |
| Buoyancy force | Large | Small | Smaller | |
| Existence time | 100–101 s | 101–102 s | >105 s | |
| Rising velocity | >104 μm/s | 101–103 μm/s | <100 μm/s | |
| Mass transfer efficiency | Low | High | Higher | |
| Zeta potential | / | −50 mv–10 mv | <−50 mv | |
| Formation of hydroxyl radicals | / | Yes | Yes | |
| Internal pressure | Low | High | Higher | |
| DO level | <100 mg/L | 100–101 mg/L | >101 mg/L |
| Bubble Type | T | P | Reference |
|---|---|---|---|
| MNBs | >5000 K | / | [91] |
| Air NBs | 3000 K | 5 GPa | [82] |
| Oxygen NBs | 2800 K | 4.5 GPa | |
| MNBs | 500~15,000 K | 100~5000 Pa | [107] |
| MNBs | 2000–6000 K | / | [108] |
| Pollutants | Generation of MNBs | Type of Air Source | Reaction Time (min) | Initial Concentration/(mg/L) | pH | Temperature | Degradation Rate Constant/Degradation Rate/lnc/c0 | References |
|---|---|---|---|---|---|---|---|---|
| Alachlor | Swirling jet-induced cavitation | Air | 100 | 50 | 5.9 | 40 °C | 4.90 × 10−2 min−1 | [118] |
| Rhodamine B | Swirling jet-induced cavitation | Air | 180 | 5 | 5.4 | 40 °C | 62%/5.13 × 10−3 min−1 | [117] |
| Diethyl phthalate | Aeration method | O3 | 30 | 222 | 9 | 25 °C | 98% | [130] |
| Phenol | Dissolved gas release method | O2 | 120 | 18.8 | 2.3 | 35 °C | 83%/2.67 × 10−2 min−1 | [114] |
| Dissolved gas release method | Air | 180 | / | <7 | <50 °C | 30% | [91] | |
| Micro bubble ozonation reactor | O3 + Ga(OH)2 | 40 | 450 | / | 25 °C | 99% | [131] | |
| Methyl orange | Spiral liquid flow-coupled pressurized dissolution | O3 | 30 | 10 | / | 20 °C | 96% | [128] |
| Aeration method | O3 | 30 | 50 | 3~11 | 20 °C | >90% | [127] | |
| Spiral liquid flow-type | O3 | 30 | 10 | / | / | 98% | [53] | |
| Photoresist | Dissolved gas release method | O3 | 9.6 | / | / | 22 °C | 100% | [92] |
| Butylated hydroxytoluene | Aeration method | O3 | 0.5 | <2 | 7 | / | 97% | [129] |
| Dimethyl sulfoxide | Aeration method | O3 | / | / | / | / | 7.0 × 10−4 − 1.9 × 10−3s−1 | [132] |
| P-chlorophenol | Ultrasonic cavitation | Air | 120 | / | / | 38 °C | 0.00899 min−1/−0.83 | [133] |
| P-nitrophenol | Jet cavitation reactor | Air | 90 | 8 | 3.5 | / | 50% | [134] |
| Trichloroethylene | Aeration method | O3 | 20 | 14 | / | / | 100% | [135] |
| Polyvinyl alcohol | Dissolved gas release method | O3 | 120 | / | <7 | <35 °C | 30% | [112] |
| Benzothiophene | Ultrasonic cavitation | Air | 60 | / | 5 | 25 °C | 0.0492 min−1 | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Yang, C.; Sun, P.; Wang, M.; Lin, F.; Fiallos, M.; Khu, S.-T. Generation Mechanism of Hydroxyl Free Radicals in Micro–Nanobubbles Water and Its Prospect in Drinking Water. Processes 2024, 12, 683. https://doi.org/10.3390/pr12040683
Wang T, Yang C, Sun P, Wang M, Lin F, Fiallos M, Khu S-T. Generation Mechanism of Hydroxyl Free Radicals in Micro–Nanobubbles Water and Its Prospect in Drinking Water. Processes. 2024; 12(4):683. https://doi.org/10.3390/pr12040683
Chicago/Turabian StyleWang, Tianzhi, Ci Yang, Peizhe Sun, Mingna Wang, Fawei Lin, Manuel Fiallos, and Soon-Thiam Khu. 2024. "Generation Mechanism of Hydroxyl Free Radicals in Micro–Nanobubbles Water and Its Prospect in Drinking Water" Processes 12, no. 4: 683. https://doi.org/10.3390/pr12040683
APA StyleWang, T., Yang, C., Sun, P., Wang, M., Lin, F., Fiallos, M., & Khu, S.-T. (2024). Generation Mechanism of Hydroxyl Free Radicals in Micro–Nanobubbles Water and Its Prospect in Drinking Water. Processes, 12(4), 683. https://doi.org/10.3390/pr12040683







