Study on the Mechanism of Wellbore Blockage and Scaling Trend Prediction of Keshen Block
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials
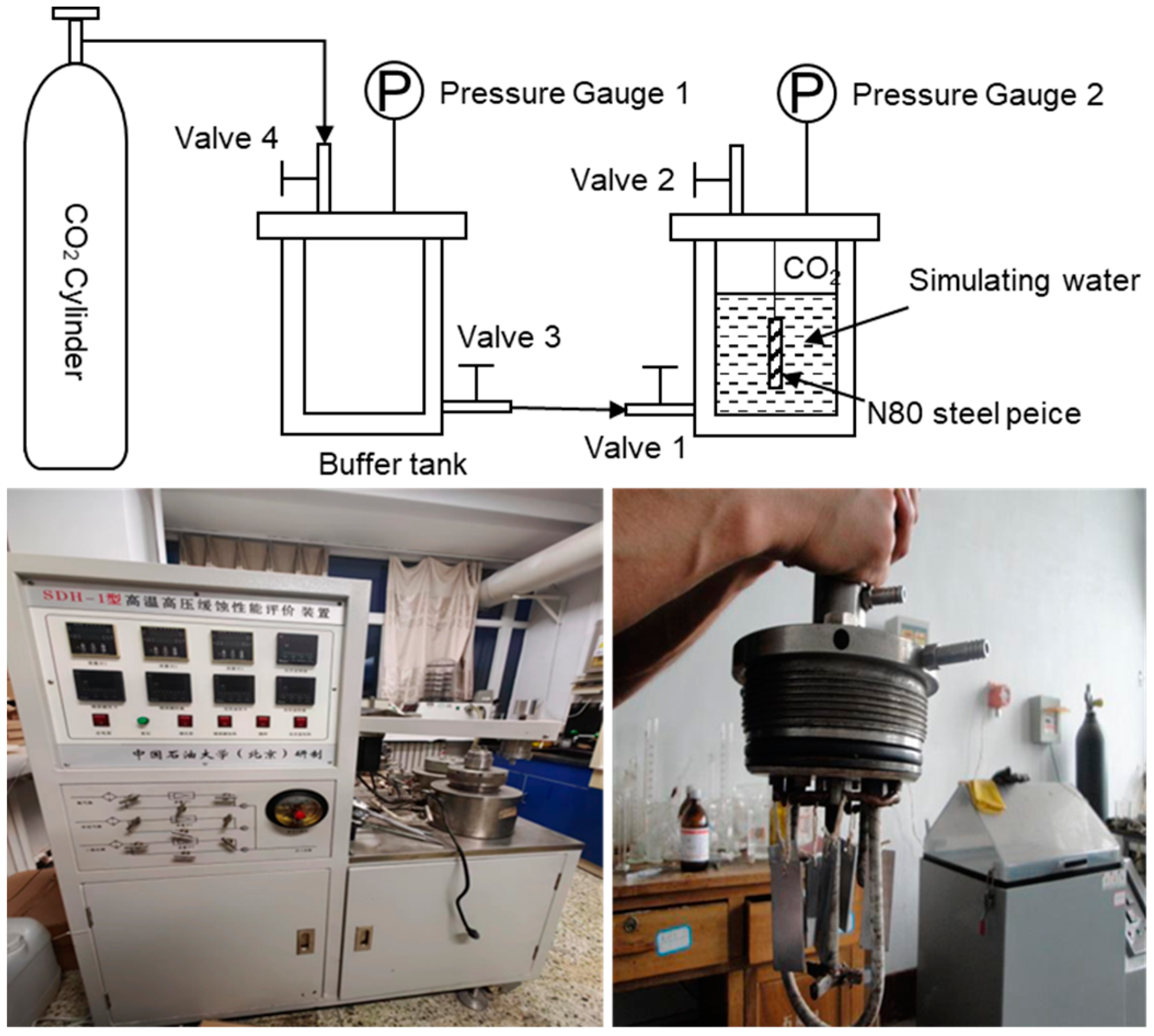
2.2. Experimental Apparatus
2.3. Experimental Method to Simulate the Mechanism
- (1)
- We chose grams of deep block bore from the same N80 steel sheet as for hanging experiments; the experimental device is shown in Figure 1. The N80 steel sheet elemental composition is shown in Table 1. The steel piece was immersed in petroleum ether for 3 h to dissolve the grease protecting the surface of the N80 steel sheet. After drying the surface with filter paper, the N80 steel piece was washed with anhydrous ethanol to remove the grease and water stains adhering to the surface of the piece. We weighed the test piece with an electronic balance of one-thousandth accuracy and record as m0.
- (2)
- The ionic concentration of the simulated water is shown in Table 2. We placed the dried N80 steel sheet into the reaction kettle and added 50 mL of simulated water to the kettle. We placed the treated N80 steel sheet into the experimental reactor, added 50 mL of simulated water, and screwed it down. We opened reactor valve 2, valve 3, and valve 4, opened the CO2 gas cylinder (pay attention to the buffer tank pressure), slowly opened valve 1, and passed in the CO2 gas. After the air in the reactor had been exhausted, we closed valve 2 of the reactor and continued to pass in CO2 gas from valve 1. When the pressure in the reactor reached the experimental pressure, we closed valve 2 and finished pressurizing the reactor. The pressurized reactor was placed in a thermostat for one week, two weeks, four weeks, and eight weeks. After completion of the reaction, the N80 steel sheet was removed, cleaned and dried, and weighed, and recorded as m1, m2, m3, and m4.
- (3)
- We performed a surface micromorphology analysis of the dried N80 steel sheet using SEM and ion concentration analysis of the reaction solution using ion chromatography.
2.4. Wellbore Fouling and Corrosion Trend Predictions
2.5. Synthesis and Testing of Scale Inhibitors
2.6. Scale Inhibitor Scale Inhibition Performance Test
3. Results and Discussion
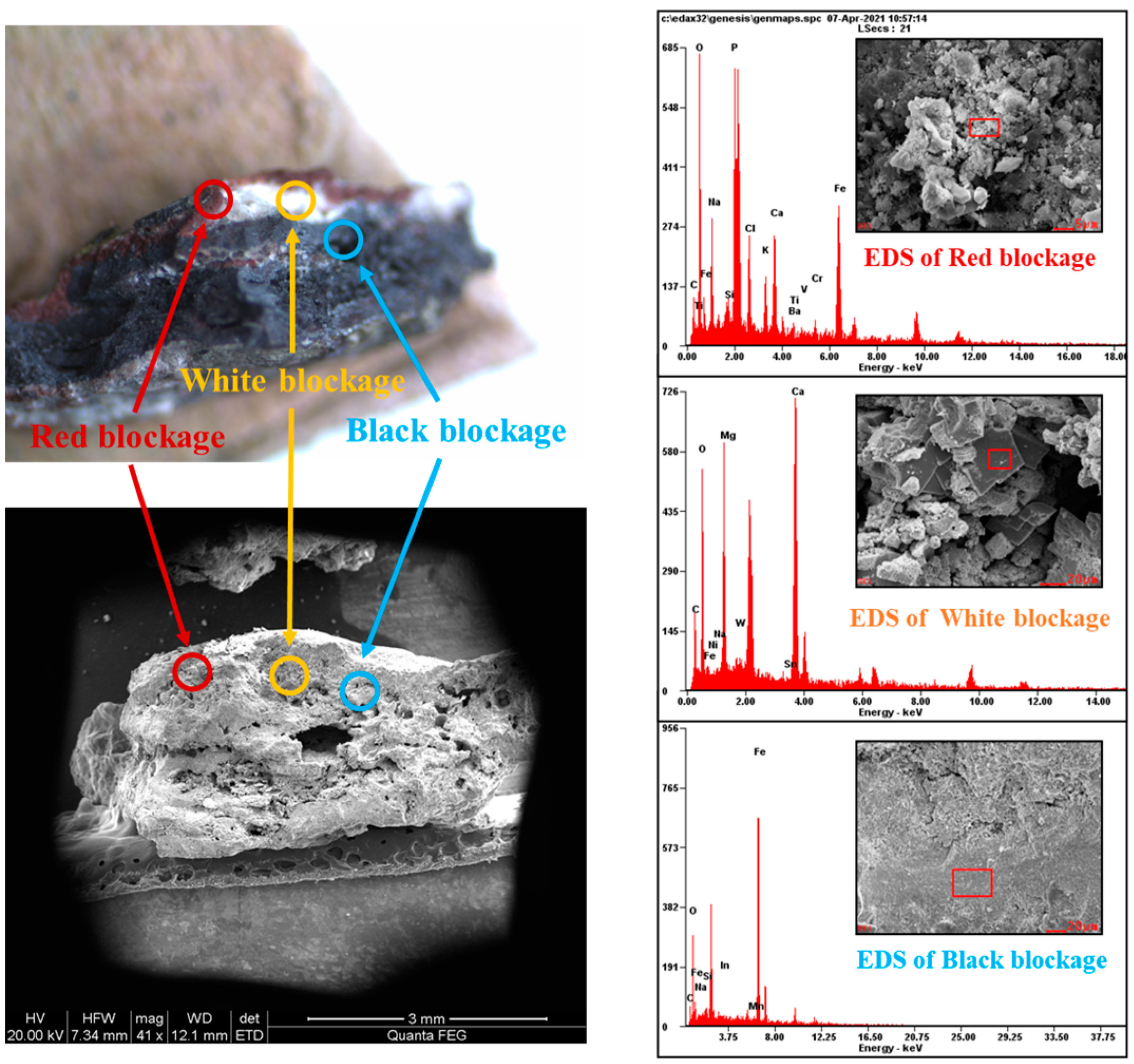
3.1. Analysis of Wellbore Scale Composition
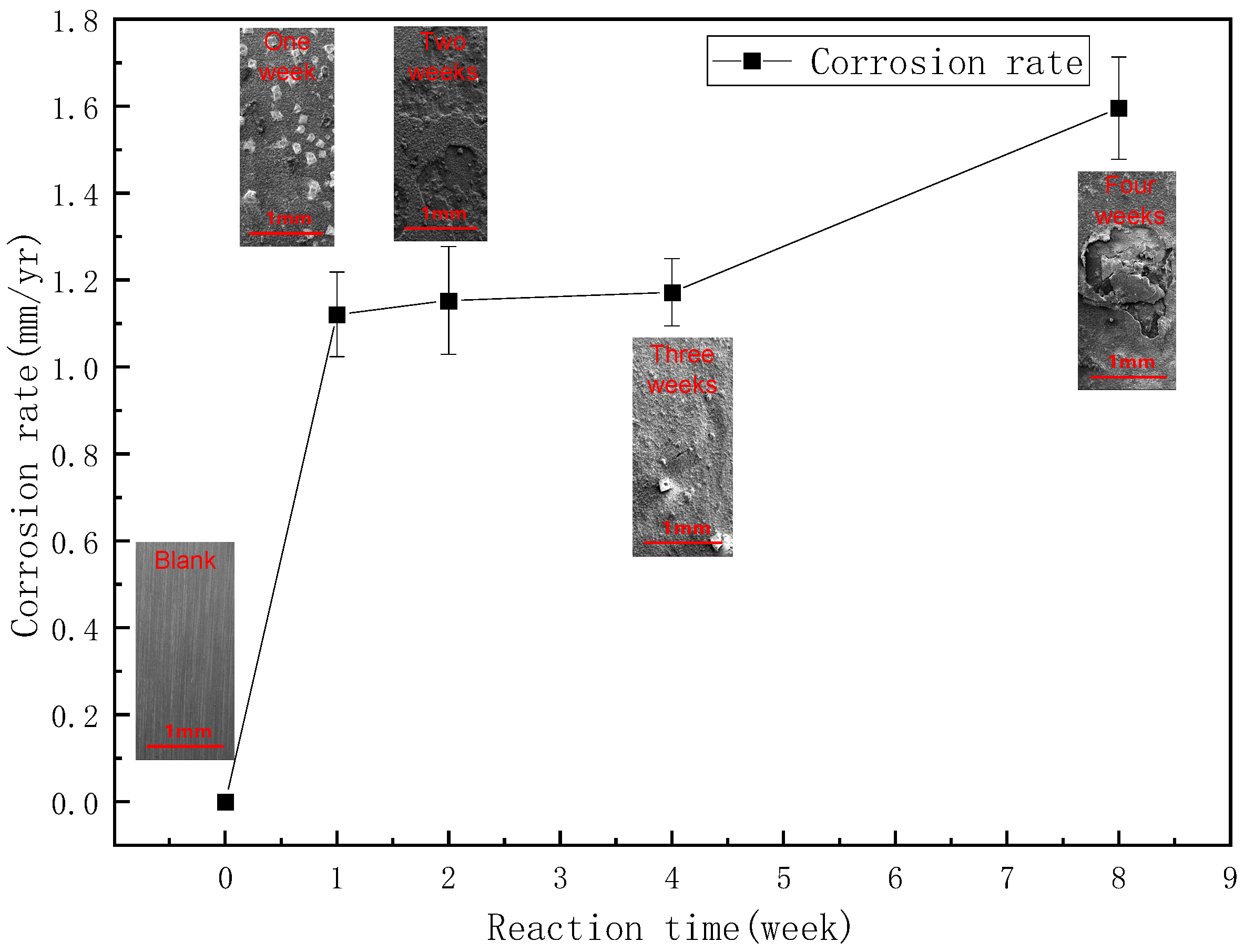
3.2. Study of Wellbore Fouling and Corrosion Patterns in Blocks in Keshen
3.3. Changes in Ion Concentrations in Simulated Aqueous Solutions
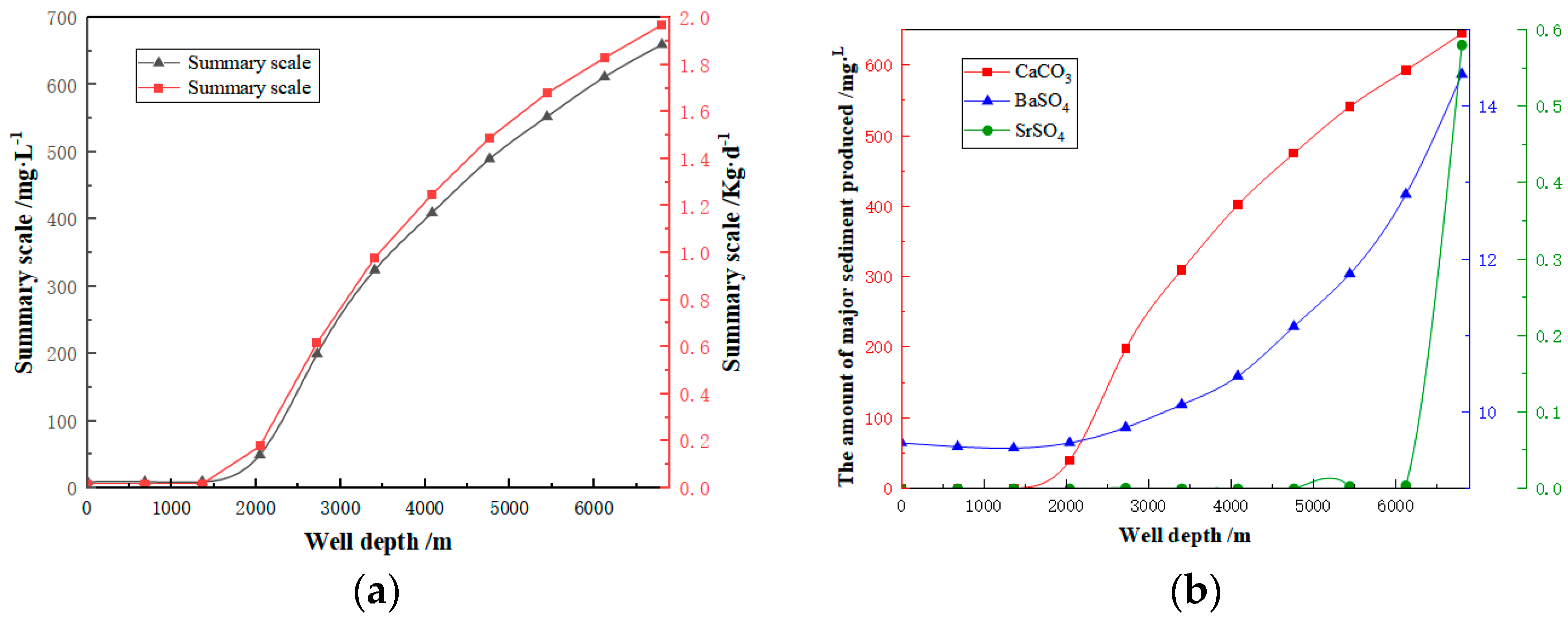
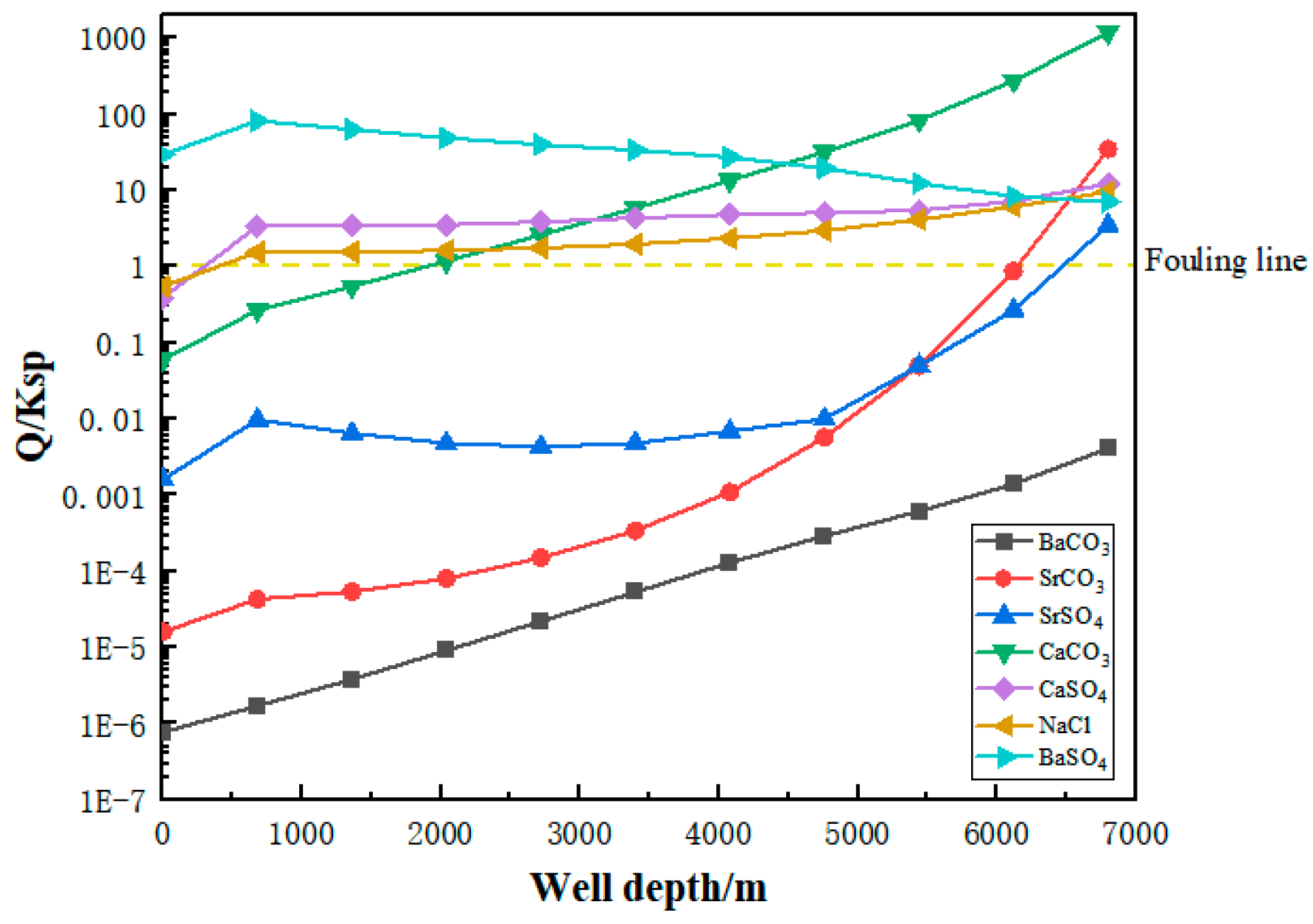
3.4. Gram Deep Wellbore Fouling Prediction
- (1)
- Wellbore fouling prediction results
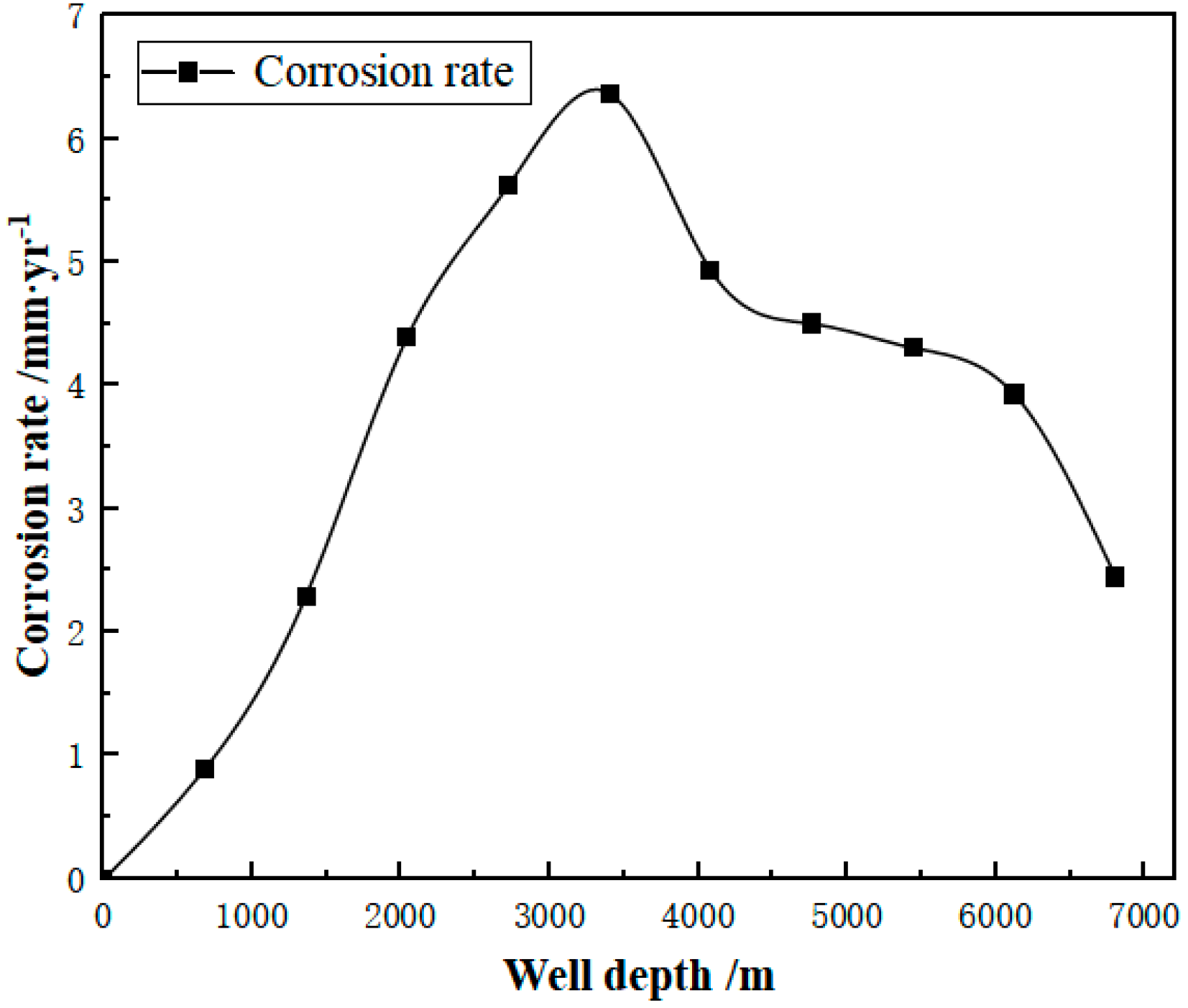
- (2)
- Wellbore corrosion rate prediction
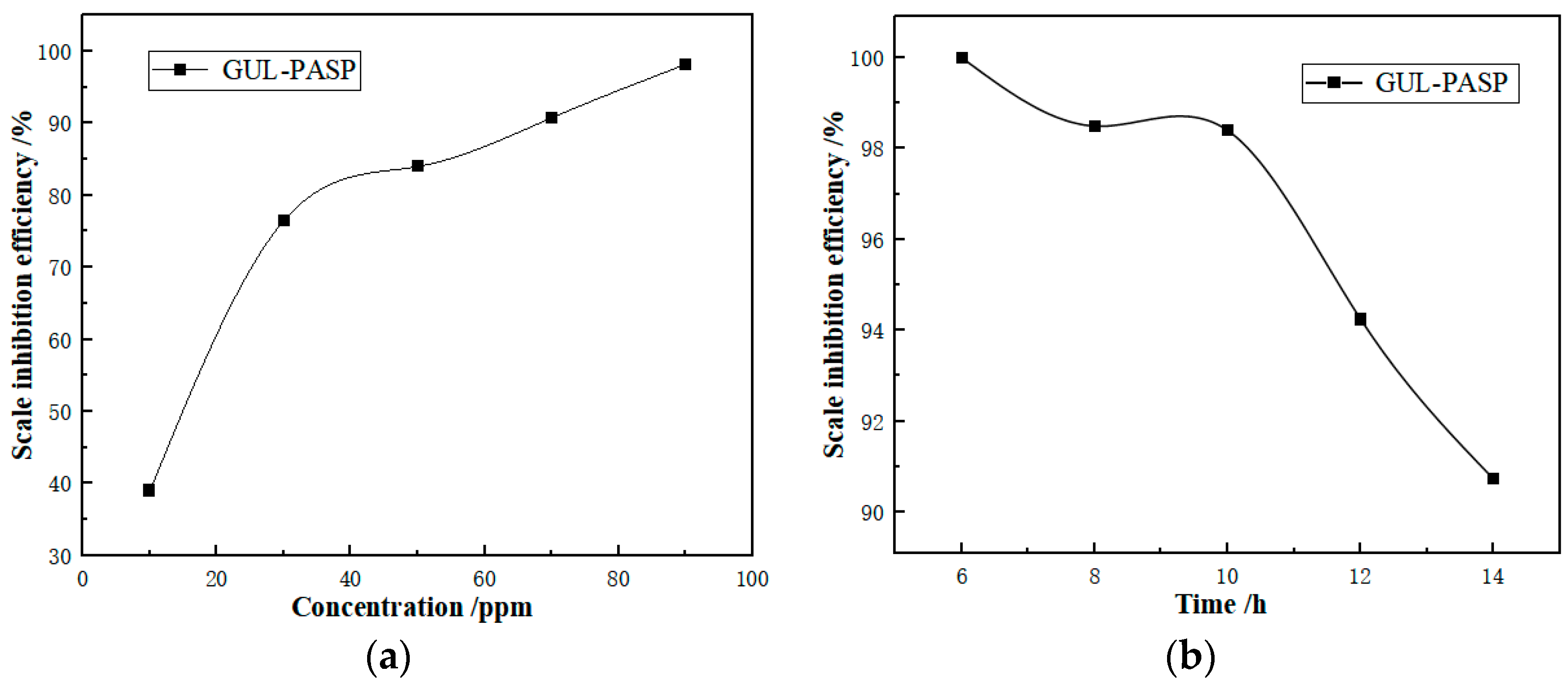
3.5. Scale Inhibitor Performance Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, T.; Sun, X. Understanding and technical countermeasures of ultra-deep and ultra-high pressure gas reservoir development in Keshen gas field of Kuqa foreland basin. Nat. Gas Ind. 2018, 38, 1–9. [Google Scholar]
- Zhao, L. Characteristics of Micro-fractures in Tight Sandstone Reservoirs and Their Influence on Physical Properties—A Case Study of Shilijiahan Zone in Hangjinqi Area. Eval. Dev. Oil Gas Reserv. 2022, 12, 285–291. [Google Scholar]
- Wang, K.; Zhang, R.; Dai, J.; Wang, J.; Zhao, L. Fracture development characteristics of low permeability sandstone reservoir in Keshen 2 gas field of Kuqa depression. Oil Gas Geol. Recovery Ratio 2016, 23, 53–60. [Google Scholar]
- Shen, Y.; Lü, X.; Guo, S.; Song, X.; Zhao, J. Effective evaluation of gas migration in deep and ultra-deep tight sandstone reservoirs of Keshen structural belt, Kuqa depression. J. Nat. Gas Sci. Eng. 2017, 46, 119–131. [Google Scholar] [CrossRef]
- Zeng, L.; Lv, W.; Xu, X.; Tian, H.; Lu, S.; Zhang, M. Development characteristics, formation mechanism and oil and gas significance of bedding fractures between typical tight sandstone and shale. Acta Pet. Sin. 2022, 43, 180–191. [Google Scholar]
- Liu, H.; Cui, Y.; Xia, S.; Liu, X.; Fei, S.; Wang, Z. Occurrence mechanism and main controlling factors of formation water in tight gas reservoirs in He-8 and Shan-1 member—Taking a block in Sulige as an example. Nat. Gas Explor. Dev. 2020, 43, 84–91. [Google Scholar]
- Gui, F.; Cai, M.; Zhang, C. Study on irreducible water saturation of low porosity and permeability reservoirs in Keshen area. Contemp. Chem. Ind. 2021, 50, 178–182. [Google Scholar]
- Yang, B.; Xun, T.; Li, F.; Tian, H.; Yang, L. Numerical Simulation Study on the Influence of Water-rock Interaction on Reservoir Permeability—A Case Study of Upper Paleozoic Sandstone Reservoir in Northeast Ordos Basin. J. Jilin Univ. Earth Sci. Ed. 2019, 49, 526–538. [Google Scholar]
- Du, Z.; Ma, L.; Wang, X.; Chen, Z. Study of Sanding Mechanism and a New Model to Calculate the Sanding Critical Drawdown in Loosened Sandstone. In Proceedings of the SPE Production and Operations Symposium, Oklahoma City, OK, USA, 4–8 April 2009. [Google Scholar]
- Zhang, P.; Kan, A.T.; Tomson, M.B. Oil Field Mineral Scale Control. In Mineral Scales and Deposits; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Amjad, Z.; Demadis, K. Mineral Scales and Deposits; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Vazirian, M.M.; Charpentier, T.V.; de Oliveira Penna, M.; Neville, A. Surface inorganic scale formation in oil and gas industry: As adhesion and deposition processes. J. Pet. Sci. Eng. 2016, 137, 22–32. [Google Scholar] [CrossRef]
- Jiang, T.; Meng, X.; Huang, K.; Zhao, L.; Chen, D.; Wu, H. Mechanism of wellbore plugging and plugging removal technology in Keshen 2 gas field. Pet. Drill. Prod. Technol. 2020, 42, 611–657. [Google Scholar]
- Weng, Z.; Xie, G.; Li, Y. Scaling in oil field and application of chemical scale remover. Chem. Eng. 2020, 34, 61–65. [Google Scholar]
- Revie, R.W. Corrosion and cOrrosion Control: An Introduction to Corrosion Science and Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Zahedzadeh, M.; Karambeigi, M.S.; Roayaei, E.; Emadi, M.A.; Radmehr, M.; Gholamianpour, H.; Ashoori, S.; Shokrollahzadeh, S. Comprehensive management of mineral scale deposition in carbonate oil fields—A case study. Chem. Eng. Res. Des. 2014, 92, 2264–2272. [Google Scholar] [CrossRef]
- Bahadori, A.; Zahedi, G.; Zendehboudi, S. Estimation of Potential Barium Sulfate (Barite) Precipitation in Oilfield Brines Using a Simple Predictive Tool. Environ. Prog. Sustain. Energy 2013, 3, 860–865. [Google Scholar] [CrossRef]
- Xu, T.; Ontoy, Y.; Molling, P.; Spycher, N.; Parini, M.; Pruess, K. Reactive transport modeling of injection well scaling and acidizing at Tiwi field, Philippines. Geothermics 2004, 33, 477–491. [Google Scholar] [CrossRef]
- Ge, X.; Shan, Q.; Zhang, Q.; Wang, T.; Ge, L. Study on scaling trend and compatibility of produced water in tight reservoir. Ind. Water Wastewater 2021, 52, 25–29. [Google Scholar]
- Kamalipour, M.; Abbasi, S.; Dehghani, S.M.; Naseri, A. Experimental and simulation study of scale formation in a carbonate Iranian reservoir. In Proceedings of the Conference of Oil, Washington, PA, USA, 3–14 May 2014. [Google Scholar]
- Liu, Z.; Sun, Y.; Zhou, X.; Wu, T.; Tian, Y.; Wang, Y. Synthesis and scale inhibitor performance of polyaspartic acid. J. Environ. Sci. 2011, 23, S153–S155. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Gu, N.; Li, C. Polyaspartic acid as a green corrosion inhibitor for carbon steel. Mater. Corros. 2011, 4, 362–369. [Google Scholar] [CrossRef]
- Zheng, D.; Ozbayoglu, E.; Miska, S.; Zhang, J. Experimental Study of Anisotropic Strength Properties of Shale. In Proceedings of the 57th U.S. Rock Mechanics/Geomechanics Symposium, Atlanta, GA, USA, 25 June 2023. [Google Scholar] [CrossRef]
- GB/T 16632-2019; Determination of Scale Inhibition Performance of Water Treatment Chemicals—Calcium Carbonate Precipitation Method. Chinese Standard: Beijing, China, 2020.


| Element | C | Si | P | S | Cr | Ni | Mn | Mo | Cu |
|---|---|---|---|---|---|---|---|---|---|
| Content | 0.45 | 0.32 | 0.014 | 0.013 | 0.03 | 0.04 | 1.70 | 0.20 | 0.03 |
| Ionic | Ca2+ | K+ | Mg2+ | Na+ | Sr2+ | Ba2+ | Fe2+ | Cl− | SO42− | HCO3− |
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | 3340.01 | 5660.12 | 1.76 | 60,330.93 | 113.11 | 8.12 | 0 | 104,921.12 | 360.87 | 98.21 |
| Depth/m | Temperature/°C | Pressure/KPa |
|---|---|---|
| 0 | 10 | 2000 |
| 680 | 21.5 | 3100 |
| 1360 | 33 | 4200 |
| 2040 | 44.5 | 5300 |
| 2720 | 56 | 6400 |
| 3400 | 67.5 | 7500 |
| 4080 | 79 | 8600 |
| 4760 | 90.5 | 9700 |
| 5440 | 102 | 10,800 |
| 6120 | 113.5 | 11,900 |
| 6800 | 125 | 13,000 |
| Reaction Time | Blank | One Week | Two Weeks | Four Weeks | Eight Weeks | |
|---|---|---|---|---|---|---|
| Ion Name | ||||||
| Ca2+ | 3340.00 | 3187 | 3152 | 2940.00 | 2302.00 | |
| K+ | 5660 | 5586 | 5493 | 5310 | 3968 | |
| Mg2+ | 1.76 | 1.73 | 1.7 | 1.68 | 1.36 | |
| Na+ | 60,330 | 59,510 | 58,460 | 56,870 | 4168 | |
| Sr2+ | 113 | 108 | 106 | 110 | 103 | |
| Ba2+ | 8.1 | 7.7 | 7.6 | 7.6 | 6.9 | |
| Fe2+ | 0 | 0.13 | 0.19 | 0.22 | 45.79 | |
| Cl− | 104,921 | 103,476 | 102,634 | 100,608 | 99,173.08 | |
| SO42− | 360.87 | 321.5 | 286.32 | 224.42 | 183.24 | |
| HCO3− | 98.21 | 95.724 | 93.106 | 92.598 | 90.361 | |
| Gas Name | N2 | CO2 | C1 | C2 | C3 | iC4 | nC4 | iC5 | nC5 |
|---|---|---|---|---|---|---|---|---|---|
| Percentage % | 3.546 | 2.567 | 93.177 | 0.683 | 0.019 | 0.002 | 0.004 | 0.001 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Zhang, Y.; He, Y.; Yang, Z.; Liang, X.; Wang, X.; Mao, Q. Study on the Mechanism of Wellbore Blockage and Scaling Trend Prediction of Keshen Block. Processes 2024, 12, 782. https://doi.org/10.3390/pr12040782
Zhao L, Zhang Y, He Y, Yang Z, Liang X, Wang X, Mao Q. Study on the Mechanism of Wellbore Blockage and Scaling Trend Prediction of Keshen Block. Processes. 2024; 12(4):782. https://doi.org/10.3390/pr12040782
Chicago/Turabian StyleZhao, Libin, Yongling Zhang, Yuanyuan He, Zihao Yang, Xiao Liang, Xiaopei Wang, and Qi Mao. 2024. "Study on the Mechanism of Wellbore Blockage and Scaling Trend Prediction of Keshen Block" Processes 12, no. 4: 782. https://doi.org/10.3390/pr12040782




