Comparative Review on the Production and Purification of Bioethanol from Biomass: A Focus on Corn
Abstract
:1. Introduction
| Types of Biofuel | Method of Production | Effect of Biofuel on the Environment | Biofuel Blends | References |
|---|---|---|---|---|
| Biodiesel | Transesterification |
| Blended with petroleum-based diesel with different percentages:
| [13,14] |
| Biobutanol | Fermentation of sugars | Sharply decreases the PM emissions | Exists in several percentages of biobutanol blends with gasoline: 12.5% or 16% | [15,16] |
| Biogas | Anaerobic | Reduces global CO2 emissions by 18–20% | Biogas blending offers an option to decarbonize the gas network | [17,18] |
| Bioethanol | Fermentation | Produces fewer emissions of particulates, sulfur dioxide, and air toxics than fossil fuel when burned | Bioethanol-petroleum blends also generally result in lower emissions relative to fuels that do not contain bioethanol | [19] |
2. Bioethanol Sources
2.1. First Generation
2.2. Second Generation
2.3. Third Generation
2.4. Fourth Generation
2.5. Comparison between Different Generations of Biomass
3. Bioethanol
3.1. Global Production
3.2. Characteristics, Limitations, and Advantages
| Fuel Properties | Gasoline | Bioethanol | References |
|---|---|---|---|
| Molecular formula | ~C8H15.6 | C2H6O | |
| Density at 15 °C (kg/m3) | 720–775 | 792 | [78,79] |
| Boiling point at 1.013 bar (°C) | 25–210 | 78.4 | [79,80] |
| Octane number, MON/RON | 85/95 | 89.7/108.6 | [68,69] |
| Heat of vaporization (kJ/kg) | 289 | 854 | [60] |
| Energy density (MJ/kg) | 45 | 26 | [81,82] |
| Composition C/H/O (%mass) | 87.4/12.6/0 | 52.18/13.04/34.7 | [83,84] |
| Molecular weight (kg/kmol) | 98 | 46.070 | [85] |
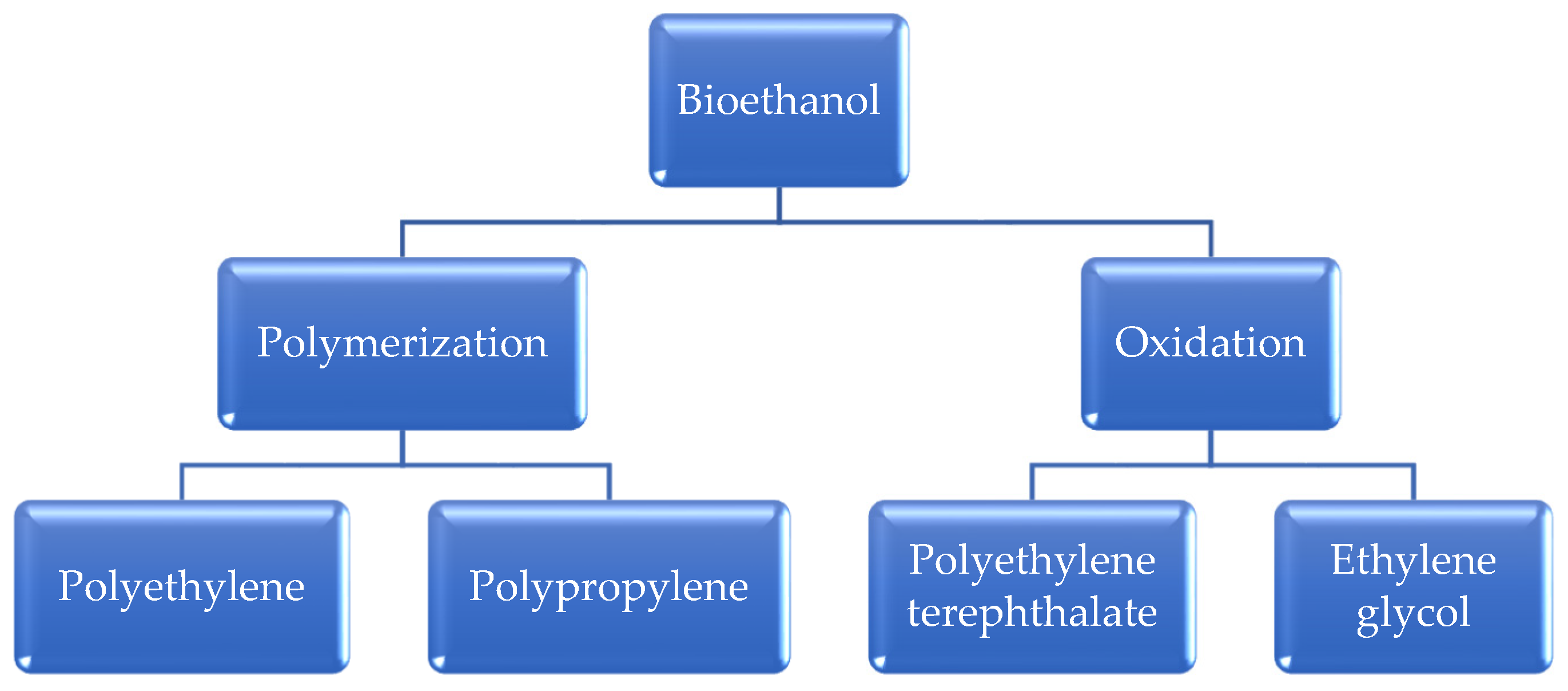
3.3. Diverse Applications
4. Ethanol Production from Different Feedstocks
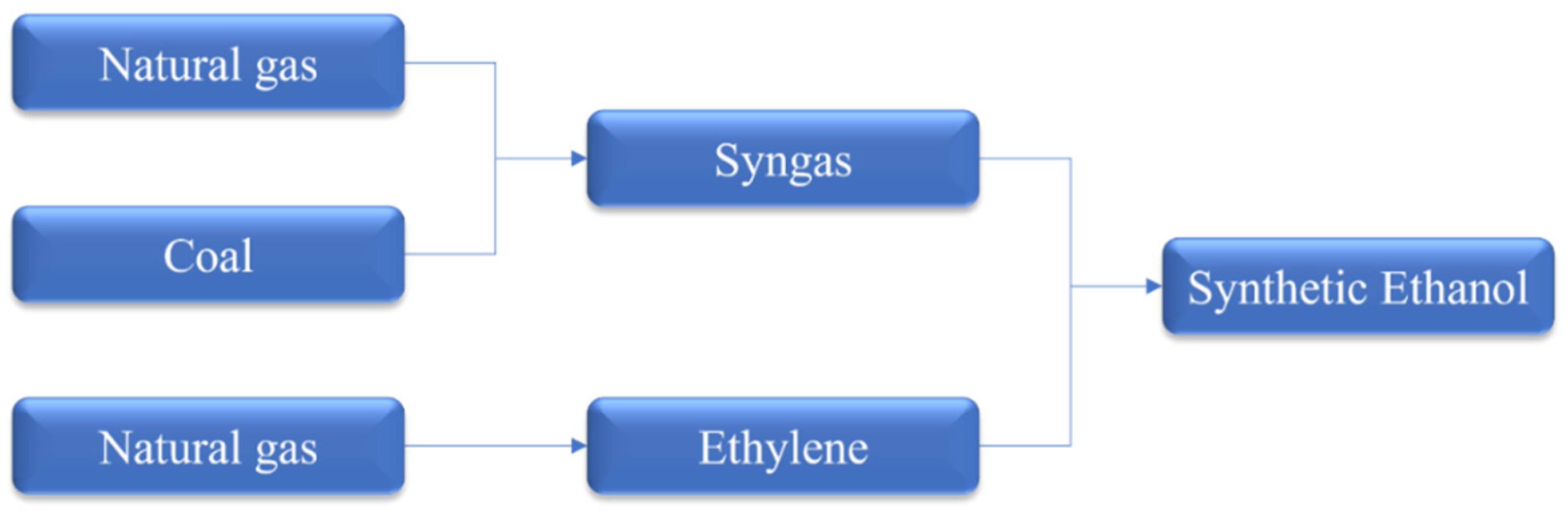
4.1. Production of Synthetic Ethanol from Non-Renewable Resources
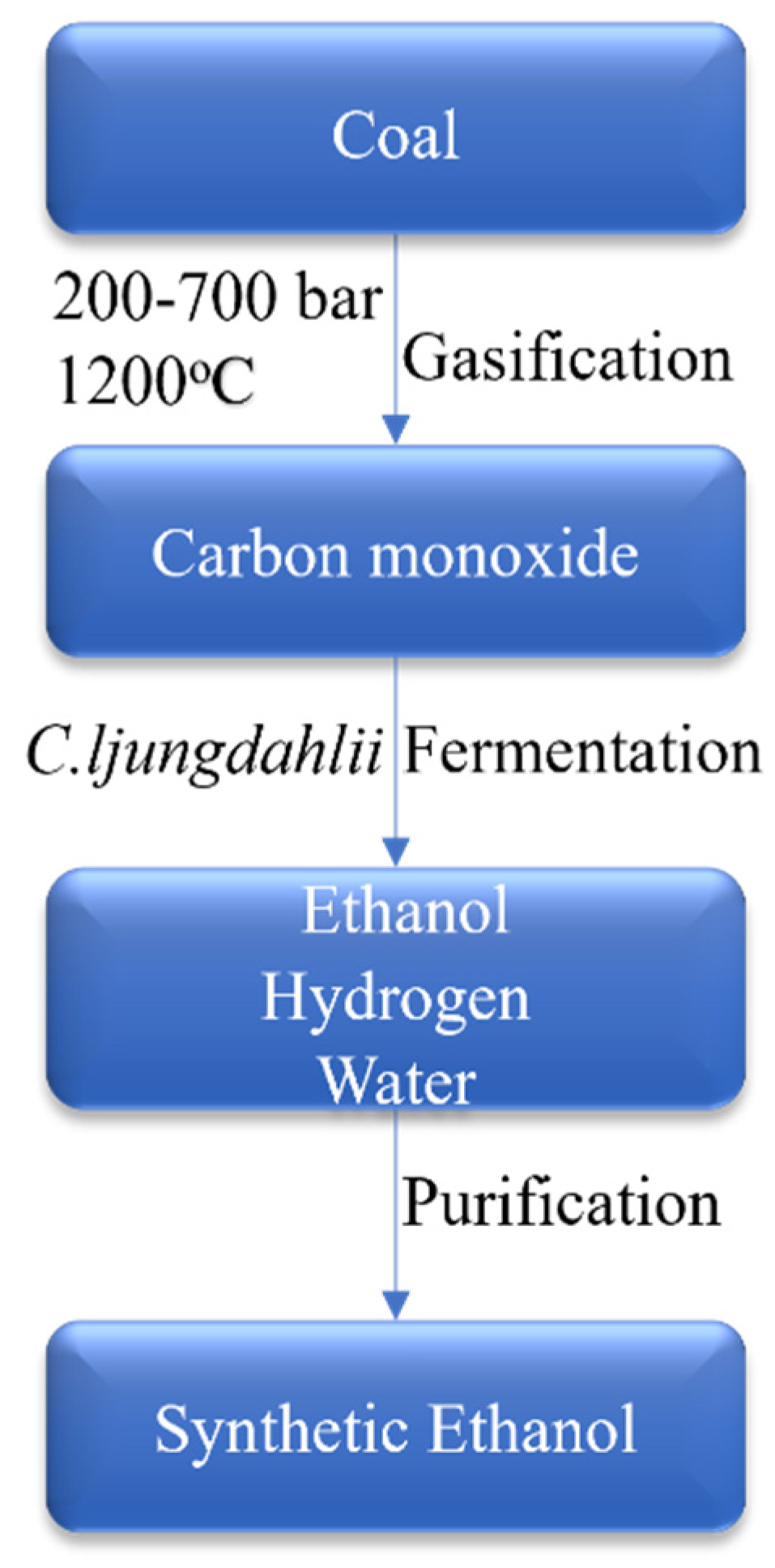
4.1.1. Production of Synthetic Ethanol through Gasification Process
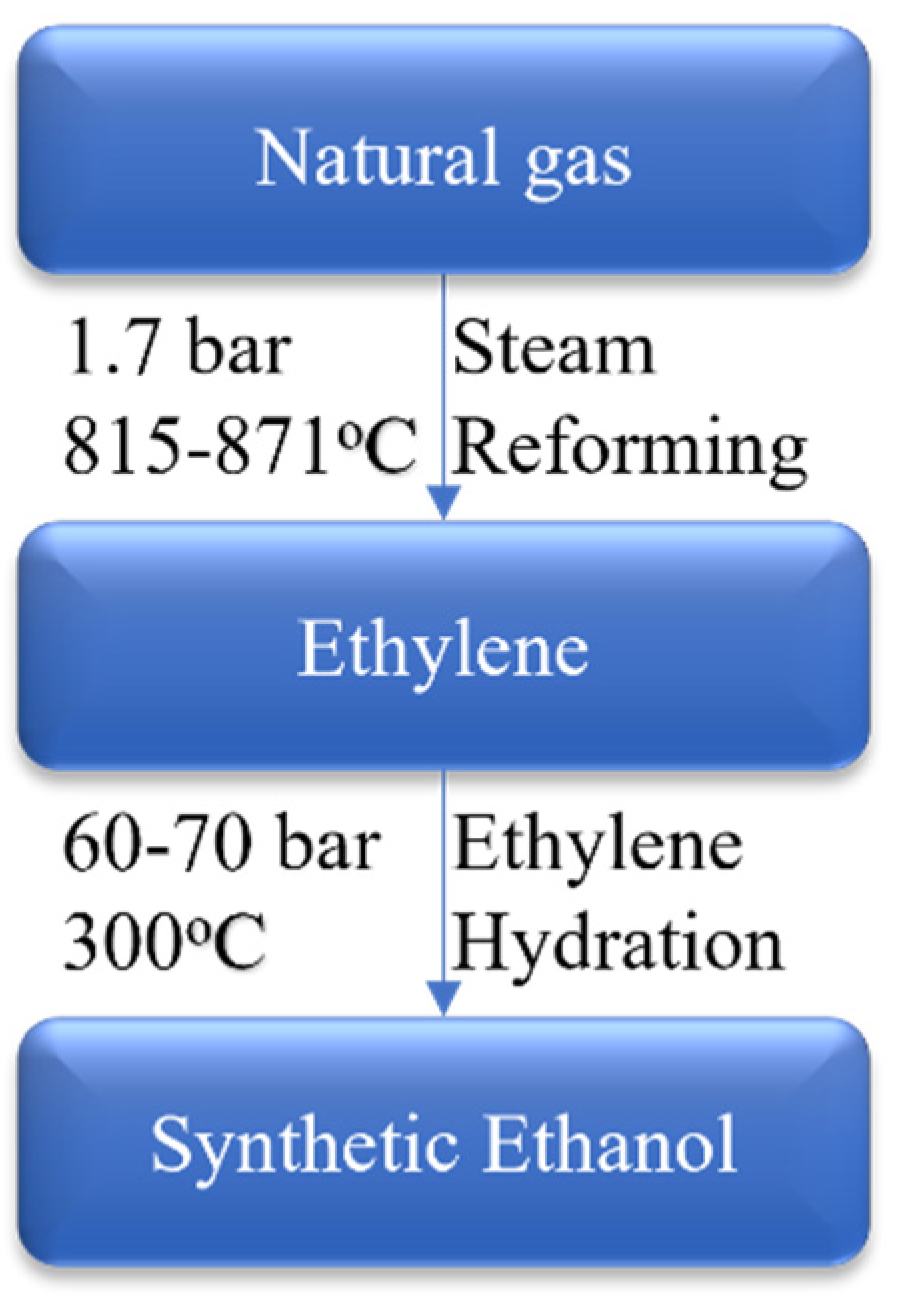
4.1.2. Production of Synthetic Ethanol from Natural Gas
4.1.3. Production of Synthetic Ethanol from Ethylene
4.2. Production of Ethanol from Biomass
5. Bioethanol Production Process
5.1. Dry Milling
5.1.1. Milling
5.1.2. Cooking and Liquefaction
5.1.3. Saccharification
5.1.4. Fermentation
5.1.5. Purification
5.2. Wet Milling
5.3. Advantages and Disadvantages of Wet and Dry Milling
6. Insights and Implications across the Main Generations of Biomass
7. Conclusions
Funding
Conflicts of Interest
References
- Baeyens, J.; Kang, Q.; Appels, L.; Dewil, R.; Lv, Y.; Tan, T. Challenges and Opportunities in Improving the Production of Bio-Ethanol. Prog. Energy Combust. Sci. 2015, 47, 60–88. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; Moreno, A.D. Alcohol Fuels: The Biochemical Route; ITomás-Pejó, E., Moreno, A.D., Riazi, M.R., Chiaramonti, D., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 341–360. [Google Scholar]
- Posada, J.A.; Patel, A.D.; Roes, A.; Blok, K.; Faaij, A.P.C.; Patel, M.K. Potential of Bioethanol as a Chemical Building Block for Biorefineries: Preliminary Sustainability Assessment of 12 Bioethanol-Based Products. Bioresour. Technol. 2013, 135, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Vohra, M.; Manwar, J.; Manmode, R.; Padgilwar, S.; Patil, S. Bioethanol Production: Feedstock and Current Technologies. J. Environ. Chem. Eng. 2014, 2, 573–584. [Google Scholar] [CrossRef]
- Awad, O.I.; Mamat, R.; Ali, O.M.; Sidik, N.A.C.; Yusaf, T.; Kadirgama, K.; Kettner, M. Alcohol and Ether as Alternative Fuels in Spark Ignition Engine: A Review. Renew. Sustain. Energy Rev. 2018, 82, 2586–2605. [Google Scholar] [CrossRef]
- Reijnders, L. Conditions for the Sustainability of Biomass Based Fuel Use. Energy Policy 2006, 34, 863–876. [Google Scholar] [CrossRef]
- Balat, M. An Overview of Biofuels and Policies in the European Union. Energy Sources Part B Econ. Plan. Policy 2007, 2, 167–181. [Google Scholar] [CrossRef]
- Demirbaş, A. Biofuels Sources, Biofuel Policy, Biofuel Economy and Global Biofuel Projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Balat, M. Global Trends on the Processing of Biofuels. Int. J. Green Energy 2008, 5, 212–238. [Google Scholar] [CrossRef]
- Bozbas, K. Biodiesel as an Alternative Motor Fuel: Production and Policies in the European Union. Renew. Sustain. Energy Rev. 2008, 12, 542–552. [Google Scholar] [CrossRef]
- Cheng, J.J.; Timilsina, G.R. Status and Barriers of Advanced Biofuel Technologies: A Review. Renew. Energy 2011, 36, 3541–3549. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Guimarães, P.M.R.; Silva, J.P.A.; Carneiro, L.M.; Roberto, I.C.; Vicente, A.; Domingues, L.; Teixeira, J.A. Technological Trends, Global Market, and Challenges of Bio-Ethanol Production. Biotechnol. Adv. 2010, 28, 817–830. [Google Scholar] [CrossRef]
- Živković, S.B.; Veljković, M.V.; Banković-Ilić, I.B.; Krstić, I.M.; Konstantinović, S.S.; Ilić, S.B.; Avramović, J.M.; Stamenković, O.S.; Veljković, V.B. Technological, Technical, Economic, Environmental, Social, Human Health Risk, Toxicological and Policy Considerations of Biodiesel Production and Use. Renew. Sustain. Energy Rev. 2017, 79, 222–247. [Google Scholar] [CrossRef]
- Mert, G.; Atilla, B. Effects of Temperature and Biodiesel Fraction on Dynamic Viscosities of Commercially Available Diesel Fuels and Its Blends with the Highest Methyl Ester Yield Corn Oil Biodisel Produced by Using KOH. In Exergy for a Better Environment and Improved Sustainability 2; Aloui, F., Dincer, I., Eds.; Green Energy and Technology; Springer International Publishing: Cham, Switzerland, 2018; pp. 83–102. [Google Scholar] [CrossRef]
- Yun, H.; Choi, K.; Lee, C.S. Effects of Biobutanol and Biobutanol–Diesel Blends on Combustion and Emission Characteristics in a Passenger Car Diesel Engine with Pilot Injection Strategies. Energy Convers. Manag. 2016, 111, 79–88. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Mathimani, T.; Varjani, S.; Rene, E.R.; Kumar, G.; Kim, S.-H.; Ponnusamy, V.K.; Yoon, J.-J. Biobutanol as a Promising Liquid Fuel for the Future—Recent Updates and Perspectives. Fuel 2019, 253, 637–646. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Ivančić Šantek, M.; Komes, D.; Novak, S.; Šantek, B. University of Zagreb, Faculty of Food Technology and Biotechnology, Pierottijeva 6, 10000 Zagreb, Croatia. Bioethanol Production from Renewable Raw Materials and Its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Riazi, M.R.; Chiaramonti, D. (Eds.) Data on Biofuels Production, Trade, and Demand. In Biofuels Production and Processing Technology; a CRC title, part of the Taylor & Francis imprint, a member of the Taylor & Francis Group, the academic division of T&F Informa, PLC; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2017; pp. 55–99. [Google Scholar] [CrossRef]
- Rosillo-Calle, F.; Walter, A. Global Market for Bioethanol: Historical Trends and Future Prospects. Energy Sustain. Dev. 2006, 10, 20–32. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A. Promising Evolution of Biofuel Generations. Subject Review. Renew. Energy Focus 2019, 28, 127–139. [Google Scholar] [CrossRef]
- Klein, B.C.; De Mesquita Sampaio, I.L.; Mantelatto, P.E.; Filho, R.M.; Bonomi, A. Beyond Ethanol, Sugar, and Electricity: A Critical Review of Product Diversification in Brazilian Sugarcane Mills. Biofuels Bioprod. Biorefining 2019, 13, 809–821. [Google Scholar] [CrossRef]
- Lee, R.A.; Lavoie, J.-M. From First- to Third-Generation Biofuels: Challenges of Producing a Commodity from a Biomass of Increasing Complexity. Anim. Front. 2013, 3, 6–11. [Google Scholar] [CrossRef]
- Ho, D.P.; Ngo, H.H.; Guo, W. A Mini Review on Renewable Sources for Biofuel. Bioresour. Technol. 2014, 169, 742–749. [Google Scholar] [CrossRef]
- Shokravi, Z.; Shokravi, H.; Chyuan, O.H.; Lau, W.J.; Koloor, S.S.R.; Petrů, M.; Ismail, A.F. Improving ‘Lipid Productivity’ in Microalgae by Bilateral Enhancement of Biomass and Lipid Contents: A Review. Sustainability 2020, 12, 9083. [Google Scholar] [CrossRef]
- Bello, S.; Salim, I.; Feijoo, G.; Moreira, M.T. Inventory Review and Environmental Evaluation of First- and Second-Generation Sugars through Life Cycle Assessment. Environ. Sci. Pollut. Res. 2021, 28, 27345–27361. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, J.; Aila, M.; Bangwal, D.P.; Kaul, S.; Garg, M.O. Algae Based Biorefinery—How to Make Sense? Renew. Sustain. Energy Rev. 2015, 47, 295–307. [Google Scholar] [CrossRef]
- Sebayang, A.H.; Masjuki, H.H.; Ong, H.C.; Dharma, S.; Silitonga, A.S.; Mahlia, T.M.I.; Aditiya, H.B. A Perspective on Bioethanol Production from Biomass as Alternative Fuel for Spark Ignition Engine. RSC Adv. 2016, 6, 14964–14992. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H.; Öz, C. Progress in Bioethanol Processing. Prog. Energy Combust. Sci. 2008, 34, 551–573. [Google Scholar] [CrossRef]
- Devi, A.; Bajar, S.; Sihag, P.; Sheikh, Z.U.D.; Singh, A.; Kaur, J.; Bishnoi, N.R.; Pant, D. A Panoramic View of Technological Landscape for Bioethanol Production from Various Generations of Feedstocks. Bioengineered 2023, 14, 81–112. [Google Scholar] [CrossRef]
- De Lima, L.M.; Bacchi, M.R.P. Global Ethanol Market. In Advances in Sugarcane Biorefinery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 253–277. [Google Scholar] [CrossRef]
- Hoang, T.-D.; Nghiem, N. Recent Developments and Current Status of Commercial Production of Fuel Ethanol. Fermentation 2021, 7, 314. [Google Scholar] [CrossRef]
- Flach, B.; Lieberz, S.; Bolla, S. Biofuels Annual—European Union.Global Agricultural Information Network; Annual Report E42020-0032; US Department of Agriculture Foreign Agricultural Service: Washington, DC, USA, 2020.
- El Hage, M.; Rajha, H.N.; Maache-Rezzoug, Z.; Koubaa, M.; Louka, N. Intensification of Bioethanol Production from Different Lignocellulosic Biomasses, Induced by Various Pretreatment Methods: An Updated Review. Energies 2022, 15, 6912. [Google Scholar] [CrossRef]
- Wietschel, L.; Messmann, L.; Thorenz, A.; Tuma, A. Environmental Benefits of Large-scale Second-generation Bioethanol Production in the EU: An Integrated Supply Chain Network Optimization and Life Cycle Assessment Approach. J. Ind. Ecol. 2021, 25, 677–692. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Muhammad, R.; Mujtaba, G.; Memon, A.; Lee, K.; Memon, S. Influence of Salinity and Nitrogen in Dark on Dunaliella Tertiolecta’s Lipid and Carbohydrate Productivity Influence of Salinity and Nitrogen in Dark on Dunaliella Tertiolecta’s Lipid and Carbohydrate Productivity. Biofuels 2020, 13, 475–481. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from Microalgae: The Potential of Domestication towards Sustainable Biofactories. Microb. Cell Factories 2018, 17, 173. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, L.; Bachheti, R.K.; Garlapati, V.K.; Chandel, A.K. Third-Generation Biorefineries: A Sustainable Platform for Food, Clean Energy, and Nutraceuticals Production. Biomass Convers. Biorefinery 2022, 12, 4215–4230. [Google Scholar] [CrossRef]
- Markel, K.; Belcher, M.S.; Shih, P.M. Defining and Engineering Bioenergy Plant Feedstock Ideotypes. Curr. Opin. Biotechnol. 2020, 62, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, B.; Syed Muhammad, S.A.F.; Shokravi, Z.; Ismail, S.; Kassim, K.A.; Mahmood, A.N.; Aziz, M.M.A. Fourth Generation Biofuel: A Review on Risks and Mitigation Strategies. Renew. Sustain. Energy Rev. 2019, 107, 37–50. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, G.; Cao, X.; Wei, D. Molecular Characterization of CO2 Sequestration and Assimilation in Microalgae and Its Biotechnological Applications. Bioresour. Technol. 2017, 244, 1207–1215. [Google Scholar] [CrossRef]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential Use of Algae for Heavy Metal Bioremediation, a Critical Review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.H.; Azella Zaine, S.N.; Ho, Y.C.; Uemura, Y.; Lam, M.K.; Khoo, K.S.; Kiatkittipong, W.; Cheng, C.K.; Show, P.L.; Lim, J.W. Impact of Various Microalgal-Bacterial Populations on Municipal Wastewater Bioremediation and Its Energy Feasibility for Lipid-Based Biofuel Production. J. Environ. Manag. 2019, 249, 109384. [Google Scholar] [CrossRef]
- Mat Aron, N.S.; Khoo, K.S.; Chew, K.W.; Show, P.L.; Chen, W.; Nguyen, T.H.P. Sustainability of the Four Generations of Biofuels—A Review. Int. J. Energy Res. 2020, 44, 9266–9282. [Google Scholar] [CrossRef]
- Bhuiya, M.M.K.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Azad, A.K.; Hazrat, M.A. Second Generation Biodiesel: Potential Alternative to-Edible Oil-Derived Biodiesel. Energy Procedia 2014, 61, 1969–1972. [Google Scholar] [CrossRef]
- Alam, F.; Mobin, S.; Chowdhury, H. Third Generation Biofuel from Algae. Procedia Eng. 2015, 105, 763–768. [Google Scholar] [CrossRef]
- Dutta, K.; Daverey, A.; Lin, J.-G. Evolution Retrospective for Alternative Fuels: First to Fourth Generation. Renew. Energy 2014, 69, 114–122. [Google Scholar] [CrossRef]
- Don, A.; Osborne, B.; Hastings, A.; Skiba, U.; Carter, M.S.; Drewer, J.; Flessa, H.; Freibauer, A.; Hyvönen, N.; Jones, M.B.; et al. Land-use Change to Bioenergy Production in E Urope: Implications for the Greenhouse Gas Balance and Soil Carbon. GCB Bioenergy 2012, 4, 372–391. [Google Scholar] [CrossRef]
- Salama, E.-S.; Kurade, M.B.; Abou-Shanab, R.A.I.; El-Dalatony, M.M.; Yang, I.-S.; Min, B.; Jeon, B.-H. Recent Progress in Microalgal Biomass Production Coupled with Wastewater Treatment for Biofuel Generation. Renew. Sustain. Energy Rev. 2017, 79, 1189–1211. [Google Scholar] [CrossRef]
- Dias, M.O.S.; Junqueira, T.L.; Cavalett, O.; Cunha, M.P.; Jesus, C.D.F.; Rossell, C.E.V.; Maciel Filho, R.; Bonomi, A. Integrated versus Stand-Alone Second Generation Ethanol Production from Sugarcane Bagasse and Trash. Bioresour. Technol. 2012, 103, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, S.; Jiao, F.; Yuan, Q. Catalytic Dehydration of Bioethanol to Ethylene over TiO2/γ-Al2O3 Catalysts in Microchannel Reactors. Catal. Today 2007, 125, 111–119. [Google Scholar] [CrossRef]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for Oil: Strain Selection, Induction of Lipid Synthesis and Outdoor Mass Cultivation in a Low-cost Photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Mizik, T. Economic Aspects and Sustainability of Ethanol Production—A Systematic Literature Review. Energies 2021, 14, 6137. [Google Scholar] [CrossRef]
- Liu, K. Chemical Composition of Distillers Grains, a Review. J. Agric. Food Chem. 2011, 59, 1508–1526. [Google Scholar] [CrossRef]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef]
- Otto, R.; Ferraz-Almeida, R.; Sanches, G.; Lisboa, I.; Cherubin, M. Nitrogen Fertilizer Consumption and Nitrous Oxide Emissions Associated with Ethanol Production—A National-Scale Comparison between Brazilian Sugarcane and Corn in the United States. J. Clean. Prod. 2022, 350, 131482. [Google Scholar] [CrossRef]
- Szulczyk, K.R.; McCarl, B.A.; Cornforth, G. Market Penetration of Ethanol. Renew. Sustain. Energy Rev. 2010, 14, 394–403. [Google Scholar] [CrossRef]
- Maurya, R.K.; Agarwal, A.K. Experimental Study of Combustion and Emission Characteristics of Ethanol Fuelled Port Injected Homogeneous Charge Compression Ignition(HCCI) Combustion Engine. Appl. Energy 2011, 88, 1169–1180. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, W.; Baek, S.; Lee, K. Experimental Study on the Spray Characteristics of Ethanol Gasoline Blended Fuel in a Direct Injection System. Int. J. Automot. Technol. 2020, 21, 555–561. [Google Scholar] [CrossRef]
- Jiao, J.; Li, J.; Bai, Y. Ethanol as a Vehicle Fuel in China: A Review from the Perspectives of Raw Material Resource, Vehicle, and Infrastructure. J. Clean. Prod. 2018, 180, 832–845. [Google Scholar] [CrossRef]
- Senthil Kumar, M.; Karthic, S.V.; Pradeep, P. Investigations on the Influence of Ethanol and Water Injection Techniques on Engine’s Behavior of a Hydrogen—Biofuel Based Dual Fuel Engine. Int. J. Hydrogen Energy 2018, 43, 21090–21101. [Google Scholar] [CrossRef]
- Lynd, L.R. Overview and evaluation of fuel ethanol from cellulosic biomass: Technology, Economics, the Environment, and Policy. Annu. Rev. Energy Environ. 1996, 21, 403–465. [Google Scholar] [CrossRef]
- Stauffer, E.; Dolan, J.A.; Newman, R. Flammable and Combustible Liquids. In Fire Debris Analysis; Elsevier: Amsterdam, The Netherlands, 2008; pp. 199–233. [Google Scholar] [CrossRef]
- Yusuf, M.; Ibrahim, H. Introduction to Biofuel Production: A Step Towards Sustainable Energy. In Emerging Sustainable Technologies for Biofuel Production; Shah, M., Deka, D., Eds.; Environmental Science and Engineering; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 1–14. [Google Scholar] [CrossRef]
- European Union: Biofuel Mandates in the EU by Member State—2022 | USDA Foreign Agricultural Service. Available online: https://fas.usda.gov/data/european-union-biofuel-mandates-eu-member-state-2022 (accessed on 5 February 2024).
- Advanced Biofuel Policies in Select EU Member States: 2018 Update. International Council on Clean Transportation. Available online: https://theicct.org/publication/advanced-biofuel-policies-in-select-eu-member-states-2018-update/ (accessed on 5 February 2024).
- Dernotte, J.; Mounaim-Rousselle, C.; Halter, F.; Seers, P. Evaluation of Butanol–Gasoline Blends in a Port Fuel-Injection, Spark-Ignition Engine. Oil Gas Sci. Technol. Rev. IFP 2010, 65, 345–351. [Google Scholar] [CrossRef]
- Yates, A.; Bell, A.; Swarts, A. Insights Relating to the Autoignition Characteristics of Alcohol Fuels. Fuel 2010, 89, 83–93. [Google Scholar] [CrossRef]
- Celik, M.B. Experimental Determination of Suitable Ethanol–Gasoline Blend Rate at High Compression Ratio for Gasoline Engine. Appl. Therm. Eng. 2008, 28, 396–404. [Google Scholar] [CrossRef]
- Zhuang, Y.; Hong, G. Primary Investigation to Leveraging Effect of Using Ethanol Fuel on Reducing Gasoline Fuel Consumption. Fuel 2013, 105, 425–431. [Google Scholar] [CrossRef]
- Eyidogan, M.; Ozsezen, A.N.; Canakci, M.; Turkcan, A. Impact of Alcohol–Gasoline Fuel Blends on the Performance and Combustion Characteristics of an SI Engine. Fuel 2010, 89, 2713–2720. [Google Scholar] [CrossRef]
- Çelik, M.B.; Özdalyan, B.; Alkan, F. The Use of Pure Methanol as Fuel at High Compression Ratio in a Single Cylinder Gasoline Engine. Fuel 2011, 90, 1591–1598. [Google Scholar] [CrossRef]
- Koç, M.; Sekmen, Y.; Topgül, T.; Yücesu, H.S. The Effects of Ethanol–Unleaded Gasoline Blends on Engine Performance and Exhaust Emissions in a Spark-Ignition Engine. Renew. Energy 2009, 34, 2101–2106. [Google Scholar] [CrossRef]
- Harijan, K.; Memon, M.; Uqaili, M.A.; Mirza, U.K. Potential Contribution of Ethanol Fuel to the Transport Sector of Pakistan. Renew. Sustain. Energy Rev. 2009, 13, 291–295. [Google Scholar] [CrossRef]
- Hsieh, W.-D.; Chen, R.-H.; Wu, T.-L.; Lin, T.-H. Engine Performance and Pollutant Emission of an SI Engine Using Ethanol–Gasoline Blended Fuels. Atmos. Environ. 2002, 36, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Cho, J.H.; Park, J.; Moon, I. Advances in Diesel–Alcohol Blends and Their Effects on the Performance and Emissions of Diesel Engines. Renew. Sustain. Energy Rev. 2013, 22, 46–72. [Google Scholar] [CrossRef]
- Iodice, P.; Langella, G.; Amoresano, A. Ethanol in Gasoline Fuel Blends: Effect on Fuel Consumption and Engine out Emissions of SI Engines in Cold Operating Conditions. Appl. Therm. Eng. 2018, 130, 1081–1089. [Google Scholar] [CrossRef]
- Rodríguez-Antón, L.M.; Gutiérrez-Martín, F.; Hernández-Campos, M. Physical Properties of Gasoline-ETBE-Isobutanol(in Comparison with Ethanol) Ternary Blends and Their Impact on Regulatory Compliance. Energy 2019, 185, 68–76. [Google Scholar] [CrossRef]
- Ganguly, A.; Chatterjee, P.K.; Dey, A. Studies on Ethanol Production from Water Hyacinth—A Review. Renew. Sustain. Energy Rev. 2012, 16, 966–972. [Google Scholar] [CrossRef]
- Bonenkamp, T.B.; Middelburg, L.M.; Hosli, M.O.; Wolffenbuttel, R.F. From Bioethanol Containing Fuels towards a Fuel Economy That Includes Methanol Derived from Renewable Sources and the Impact on European Union Decision-Making on Transition Pathways. Renew. Sustain. Energy Rev. 2020, 120, 109667. [Google Scholar] [CrossRef]
- Petroleum, V.B. Energy Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 29–57. [Google Scholar] [CrossRef]
- Masum, B.M.; Masjuki, H.H.; Kalam, M.A.; Palash, S.M.; Habibullah, M. Effect of Alcohol–Gasoline Blends Optimization on Fuel Properties, Performance and Emissions of a SI Engine. J. Clean. Prod. 2015, 86, 230–237. [Google Scholar] [CrossRef]
- Qi, J.; Finzel, J.; Robatjazi, H.; Xu, M.; Hoffman, A.S.; Bare, S.R.; Pan, X.; Christopher, P. Selective Methanol Carbonylation to Acetic Acid on Heterogeneous Atomically Dispersed ReO4/SiO2 Catalysts. J. Am. Chem. Soc. 2020, 142, 14178–14189. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-G.; Zhen, H.; Lŭ, X.; Zhang, W.; Yang, J. Physico-Chemical Properties of Ethanol–Diesel Blend Fuel and Its Effect on Performance and Emissions of Diesel Engines. Renew. Energy 2005, 30, 967–976. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Nguyen, D.; Surendra, K.C.; Shrestha, S.; Rajendran, K.; Oechsner, H.; Xie, L.; Khanal, S.K. Anaerobic Biorefinery: Current Status, Challenges, and Opportunities. Bioresour. Technol. 2016, 215, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.R.; Klasson, K.T.; Clausen, E.C.; Gaddy, J.L. Biological Production of Ethanol from Coal Synthesis Gas: Medium Development Studies. Appl. Biochem. Biotechnol. 1993, 39–40, 559–571. [Google Scholar] [CrossRef]
- Hawkins, A.S.; McTernan, P.M.; Lian, H.; Kelly, R.M.; Adams, M.W. Biological Conversion of Carbon Dioxide and Hydrogen into Liquid Fuels and Industrial Chemicals. Curr. Opin. Biotechnol. 2013, 24, 376–384. [Google Scholar] [CrossRef]
- Klasson, K.T.; Lundbäck, K.M.O.; Clausen, E.C.; Gaddy, J.L. Kinetics of Light Limited Growth and Biological Hydrogen Production from Carbon Monoxide and Water by Rhodospirillum Rubrum. J. Biotechnol. 1993, 29, 177–188. [Google Scholar] [CrossRef]
- Geerligs, G.; Aldrich, H.C.; Harder, W.; Diekert, G. Isolation and Characterization of a Carbon Monoxide Utilizing Strain of the Acetogen Peptostreptococcus Productus. Arch. Microbiol. 1987, 148, 305–313. [Google Scholar] [CrossRef]
- Sharak Genthner, B.R.; Bryant, M.P. Additional Characteristics of One-Carbon-Compound Utilization by Eubacterium Limosum and Acetobacterium Woodii. Appl. Environ. Microbiol. 1987, 53, 471–476. [Google Scholar] [CrossRef]
- Grethlein, A.J.; Worden, R.M.; Jain, M.K.; Datta, R. Evidence for Production of N-Butanol from Carbon Monoxide by Butyribacterium Methylotrophicum. J. Ferment. Bioeng. 1991, 72, 58–60. [Google Scholar] [CrossRef]
- Higman, C. Gasification. In Combustion Engineering Issues for Solid Fuel Systems; Elsevier: Amsterdam, The Netherlands, 2008; pp. 423–468. [Google Scholar] [CrossRef]
- Barik, S.; Prieto, S.; Harrison, S.B.; Clausen, E.C.; Gaddy, J.L. Biological Production of Ethanol from Coal Synthesis Gas. In Bioprocessing and Biotreatment of Coal; Wise, D.L., Ed.; Routledge: London, UK, 2017; pp. 131–154. [Google Scholar] [CrossRef]
- Huo, M.; Peng, X.; Zhao, J.; Ma, Q.; Cai, R.; Deng, C.; Liu, B.; Sun, C.; Chen, G. Mixed Solvent of Alcohol and Protic Ionic Liquids for CO Capture: Solvent Screening and Experimental Studies. Int. J. Hydrogen Energy 2023, 48, 33173–33185. [Google Scholar] [CrossRef]
- Stiles, A.B.; Chen, F.; Harrison, J.B.; Hu, X.; Storm, D.A.; Yang, H.X. Catalytic Conversion of Synthesis Gas to Methanol and Other Oxygenated Products. Ind. Eng. Chem. Res. 1991, 30, 811–821. [Google Scholar] [CrossRef]
- Lawal, A.M.; Hart, A.; Daly, H.; Hardacre, C.; Wood, J. Kinetics of Hydrogenation of Acetic Acid over Supported Platinum Catalyst. Energy Fuels 2019, 33, 5551–5560. [Google Scholar] [CrossRef]
- Atiku, F.A.; Pirouzfar, V.; Su, C.-H.; Wei, S.-Y. The Technical and Economic Comparison of Ethylene Production from Natural Gas and Ethane. Int. J. Chem. React. Eng. 2021, 19, 415–425. [Google Scholar] [CrossRef]
- Mohsenzadeh, A.; Zamani, A.; Taherzadeh, M.J. Bioethylene Production from Ethanol: A Review and Techno-economical Evaluation. ChemBioEng Rev. 2017, 4, 75–91. [Google Scholar] [CrossRef]
- Acquarola, C.; Ao, M.; Bhatelia, T.; Prakash, B.; Faka, S.; Pareek, V.; Shah, M.T. Simulations and Optimization of a Reduced CO2 Emission Process for Methanol Production Using Syngas from Bi-Reforming. Energy Fuels 2021, 35, 8844–8856. [Google Scholar] [CrossRef]
- Ghanta, M.; Fahey, D.; Subramaniam, B. Environmental Impacts of Ethylene Production from Diverse Feedstocks and Energy Sources. Appl. Petrochem. Res. 2014, 4, 167–179. [Google Scholar] [CrossRef]
- Harahap, B.M.; Ahring, B.K. Acetate Production from Syngas Produced from Lignocellulosic Biomass Materials along with Gaseous Fermentation of the Syngas: A Review. Microorganisms 2023, 11, 995. [Google Scholar] [CrossRef]
- Guigou, M.; Lareo, C.; Pérez, L.V.; Lluberas, M.E.; Vázquez, D.; Ferrari, M.D. Bioethanol Production from Sweet Sorghum: Evaluation of Post-Harvest Treatments on Sugar Extraction and Fermentation. Biomass Bioenergy 2011, 35, 3058–3062. [Google Scholar] [CrossRef]
- Bertrand, E.; Vandenberghe, L.P.S.; Soccol, C.R.; Sigoillot, J.-C.; Faulds, C. First Generation Bioethanol. In Green Fuels Technology; Soccol, C.R., Brar, S.K., Faulds, C., Ramos, L.P., Eds.; Green Energy and Technology; Springer International Publishing: Cham, Switzerland, 2016; pp. 175–212. [Google Scholar] [CrossRef]
- El Hage, M.; Louka, N.; Rezzoug, S.-A.; Maugard, T.; Sablé, S.; Koubaa, M.; Debs, E.; Maache-Rezzoug, Z. Bioethanol Production from Woody Biomass: Recent Advances on the Effect of Pretreatments on the Bioconversion Process and Energy Yield Aspects. Energies 2023, 16, 5052. [Google Scholar] [CrossRef]
- Rakshit, P.K.; Voolapalli, R.K.; Upadhyayula, S. Acetic Acid Hydrogenation to Ethanol over Supported Pt-Sn Catalyst: Effect of Bronsted Acidity on Product Selectivity. Mol. Catal. 2018, 448, 78–90. [Google Scholar] [CrossRef]
- Jambo, S.A.; Abdulla, R.; Mohd Azhar, S.H.; Marbawi, H.; Gansau, J.A.; Ravindra, P. A Review on Third Generation Bioethanol Feedstock. Renew. Sustain. Energy Rev. 2016, 65, 756–769. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A. Comparison of Dry Versus Wet Milling to Improve Bioethanol or Methane Recovery from Solid Anaerobic Digestate. Bioengineering 2019, 6, 80. [Google Scholar] [CrossRef]
- Huang, H.-J.; Ramaswamy, S.; Tschirner, U.W.; Ramarao, B.V. A Review of Separation Technologies in Current and Future Biorefineries. Sep. Purif. Technol. 2008, 62, 1–21. [Google Scholar] [CrossRef]
- Oyinloye, T.M.; Yoon, W.B. Effect of Freeze-Drying on Quality and Grinding Process of Food Produce: A Review. Processes 2020, 8, 354. [Google Scholar] [CrossRef]
- Kojima, M.; Johnson, T. Potential for Biofuels for Transport in Developing Countries, The International Bank for Reconstruction and Development/The World Bank, Energy Sector Management Assistance Programme Report. 2005. Available online: https://documents1.worldbank.org/curated/zh/214151468163474095/pdf/374860KE40Biof1also0ESM31201PUBLIC1.pdf (accessed on 1 April 2024).
- Khullar, E.; Sall, E.D.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Ethanol Production from Modified and Conventional Dry-Grind Processes Using Different Corn Types. Cereal Chem. 2009, 86, 616–622. [Google Scholar] [CrossRef]
- Paraschiv, G.; Moiceanu, G.; Voicu, G.; Chitoiu, M.; Cardei, P.; Dinca, M.N.; Tudor, P. Optimization Issues of a Hammer Mill Working Process Using Statistical Modelling. Sustainability 2021, 13, 973. [Google Scholar] [CrossRef]
- Kelsall, D.R.; Lyons, T.P. Grain Dry Milling and Cooking Procedures: Extracting Sugars in Preparation for Fermentation. In The Alcohol TextBook, 2nd ed.; Nottingham University Press: Nottingham, UK, 1995; pp. 20–32. [Google Scholar]
- Cristiano, E. Rodrigues Reis. New Technologies in Value Addition to the Thin Stillage from Corn-to-Ethanol Process. Rev. Environ. Sci. Bio/Technol. 2017, 16, 175–206. [Google Scholar] [CrossRef]
- Hargono, H.; Kumoro, A.C.; Jos, B. Comparative Study on the Conventional and Non Thermal Simultaneous Saccharification and Fermentation of Manihot Glaziovii Root Starch. In Proceedings of the International Conference of Chemical and Material engineering (ICCME) 2015: Green Technology for Sustainable Chemical Products and Processes, Semarang, Indonesia, 29–30 September 2015; p. 030013. [Google Scholar] [CrossRef]
- Kwiatkowski, J.R.; McAloon, A.J.; Taylor, F.; Johnston, D.B. Modeling the Process and Costs of Fuel Ethanol Production by the Corn Dry-Grind Process. Ind. Crops Prod. 2006, 23, 288–296. [Google Scholar] [CrossRef]
- Schütze, A. Ethanol. In Handbook of Fuels; Elvers, B., Schütze, A., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 207–243. [Google Scholar] [CrossRef]
- Van Der Maarel, M.J.E.C.; Van Der Veen, B.; Uitdehaag, J.C.M.; Leemhuis, H.; Dijkhuizen, L. Properties and Applications of Starch-Converting Enzymes of the α-Amylase Family. J. Biotechnol. 2002, 94, 137–155. [Google Scholar] [CrossRef]
- Haki, G. Developments in Industrially Important Thermostable Enzymes: A Review. Bioresour. Technol. 2003, 89, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Tharanathan, R.N.; Mahadevamma, S. Grain Legumes—A Boon to Human Nutrition. Trends Food Sci. Technol. 2003, 14, 507–518. [Google Scholar] [CrossRef]
- Bertoldo, C.; Antranikian, G. Starch-Hydrolyzing Enzymes from Thermophilic Archaea and Bacteria. Curr. Opin. Chem. Biol. 2002, 6, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Hadiyanto, H.; Ariyanti, D.; Aini, A.P.; Pinundi, D.S. Batch and Fed-Batch Fermentation System on Ethanol Production from Whey Using Kluyveromyces Marxianus. Int. J. Renew. Energy Dev. 2013, 2, 127–131. [Google Scholar] [CrossRef]
- Thatoi, H.; Dash, P.K.; Mohapatra, S.; Swain, M.R. Bioethanol Production from Tuber Crops Using Fermentation Technology: A Review. Int. J. Sustain. Energy 2016, 35, 443–468. [Google Scholar] [CrossRef]
- Ivanova, V.; Petrova, P.; Hristov, J. Application in the Ethanol Fermentation of Immobilized Yeast Cells in Matrix of Alginate/Magnetic Nanoparticles, on Chitosan-Magnetite Microparticles and Cellulose-Coated Magnetic Nanoparticles. arXiv 2011, arXiv:1105.0619. [Google Scholar] [CrossRef]
- Kida, K.; Morimura, S.; Kume, K.; Suruga, K.; Sonoda, Y. Repeated-Batch Ethanol Fermentation by a Flocculating Yeast, Saccharomyces Cerevisiae IR-2. J. Ferment. Bioeng. 1991, 71, 340–344. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, Y.; Zhang, L.; Zhu, M.; Liang, S.; Huang, Y. Study of Sugarcane Pieces as Yeast Supports for Ethanol Production from Sugarcane Juice and Molasses. J. Ind. Microbiol. Biotechnol. 2008, 35, 1605–1613. [Google Scholar] [CrossRef]
- Morimura, S.; Ling, Z.Y.; Kida, K. Ethanol Production by Repeated-Batch Fermentation at High Temperature in a Molasses Medium Containing a High Concentration of Total Sugar by a Thermotolerant Flocculating Yeast with Improved Salt-Tolerance. J. Ferment. Bioeng. 1997, 83, 271–274. [Google Scholar] [CrossRef]
- Choi, G.-W.; Kang, H.-W.; Moon, S.-K. Repeated-Batch Fermentation Using Flocculent Hybrid, Saccharomyces Cerevisiae CHFY0321 for Efficient Production of Bioethanol. Appl. Microbiol. Biotechnol. 2009, 84, 261–269. [Google Scholar] [CrossRef]
- Matano, Y.; Hasunuma, T.; Kondo, A. Cell Recycle Batch Fermentation of High-Solid Lignocellulose Using a Recombinant Cellulase-Displaying Yeast Strain for High Yield Ethanol Production in Consolidated Bioprocessing. Bioresour. Technol. 2013, 135, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Mohd Azhar, S.H.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Mohd Faik, A.A.; Rodrigues, K.F. Yeasts in Sustainable Bioethanol Production: A Review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Appels, L.; Tan, T.; Dewil, R. Bioethanol from Lignocellulosic Biomass: Current Findings Determine Research Priorities. Sci. World J. 2014, 2014, 298153. [Google Scholar] [CrossRef] [PubMed]
- De Vasconcelos, J.N. Ethanol Fermentation. In Sugarcane; Elsevier: Amsterdam, The Netherlands, 2015; pp. 311–340. [Google Scholar] [CrossRef]
- Sánchez, Ó.J.; Cardona, C.A. Trends in Biotechnological Production of Fuel Ethanol from Different Feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef]
- Brexó, R.P.; Sant’Ana, A.D.S. Microbial Interactions during Sugar Cane Must Fermentation for Bioethanol Production: Does Quorum Sensing Play a Role? Crit. Rev. Biotechnol. 2018, 38, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Deesuth, O.; Laopaiboon, P.; Jaisil, P.; Laopaiboon, L. Optimization of Nitrogen and Metal Ions Supplementation for Very High Gravity Bioethanol Fermentation from Sweet Sorghum Juice Using an Orthogonal Array Design. Energies 2012, 5, 3178–3197. [Google Scholar] [CrossRef]
- Kosaric, N.; Velikonja, J. Liquid and Gaseous Fuels from Biotechnology: Challenge and Opportunities. FEMS Microbiol. Rev. 1995, 16, 111–142. [Google Scholar] [CrossRef]
- Dien, B.S.; Cotta, M.A.; Jeffries, T.W. Bacteria Engineered for Fuel Ethanol Production: Current Status. Appl. Microbiol. Biotechnol. 2003, 63, 258–266. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Tan, T. Optimization of Media Conditions for the Production of Ethanol from Sweet Sorghum Juice by Immobilized Saccharomyces Cerevisiae. Biomass Bioenergy 2009, 33, 521–526. [Google Scholar] [CrossRef]
- Zabed, H.; Faruq, G.; Sahu, J.N.; Azirun, M.S.; Hashim, R.; Nasrulhaq Boyce, A. Bioethanol Production from Fermentable Sugar Juice. Sci. World J. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Limtong, S.; Sringiew, C.; Yongmanitchai, W. Production of Fuel Ethanol at High Temperature from Sugar Cane Juice by a Newly Isolated Kluyveromyces Marxianus. Bioresour. Technol. 2007, 98, 3367–3374. [Google Scholar] [CrossRef] [PubMed]
- Nonklang, S.; Abdel-Banat, B.M.A.; Cha-aim, K.; Moonjai, N.; Hoshida, H.; Limtong, S.; Yamada, M.; Akada, R. High-Temperature Ethanol Fermentation and Transformation with Linear DNA in the Thermotolerant Yeast Kluyveromyces Marxianus DMKU3-1042. Appl. Environ. Microbiol. 2008, 74, 7514–7521. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, N.; Alayoubi, R.; Husson, E.; Jacquard, C.; Büchs, J.; Sarazin, C.; Gosselin, I. Kluyveromyces Marxianus, an Attractive Yeast for Ethanolic Fermentation in the Presence of Imidazolium Ionic Liquids. Int. J. Mol. Sci. 2018, 19, 887. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Oberoi, H.S.; Sandhu, S.K.; Nanda, D.; Kumar, D.; Uppal, S.K. Enhanced Ethanol Production from Sugarcane Juice by Galactose Adaptation of a Newly Isolated Thermotolerant Strain of Pichia Kudriavzevii. Bioresour. Technol. 2011, 102, 5968–5975. [Google Scholar] [CrossRef]
- Silva, G.P.D.; Araújo, E.F.D.; Silva, D.O.; Guimarães, W.V. Ethanolic Fermentation of Sucrose, Sugarcane Juice and Molasses by Escherichia Coli Strain Ko11 and Klebsiella Oxytoca Strain P2. Braz. J. Microbiol. 2005, 36, 395–404. [Google Scholar] [CrossRef]
- Fernández-Sandoval, M.T.; Galíndez-Mayer, J.; Bolívar, F.; Gosset, G.; Ramírez, O.T.; Martinez, A. Xylose–Glucose Co-Fermentation to Ethanol by Escherichia Coli Strain MS04 Using Single- and Two-Stage Continuous Cultures under Micro-Aerated Conditions. Microb. Cell Factories 2019, 18, 145. [Google Scholar] [CrossRef] [PubMed]
- Cazetta, M.L.; Celligoi, M.A.P.C.; Buzato, J.B.; Scarmino, I.S. Fermentation of Molasses by Zymomonas Mobilis: Effects of Temperature and Sugar Concentration on Ethanol Production. Bioresour. Technol. 2007, 98, 2824–2828. [Google Scholar] [CrossRef]
- Wallace-Salinas, V.; Gorwa-Grauslund, M.F. Adaptive Evolution of an Industrial Strain of Saccharomyces Cerevisiae for Combined Tolerance to Inhibitors and Temperature. Biotechnol. Biofuels 2013, 6, 151. [Google Scholar] [CrossRef]
- Lee, W.-C.; Huang, C.-T. Modeling of Ethanol Fermentation Using Zymomonas Mobilis ATCC 10988 Grown on the Media Containing Glucose and Fructose. Biochem. Eng. J. 2000, 4, 217–227. [Google Scholar] [CrossRef]
- Yang, S.; Fei, Q.; Zhang, Y.; Contreras, L.M.; Utturkar, S.M.; Brown, S.D.; Himmel, M.E.; Zhang, M. Zymomonas Mobilis as a Model System for Production of Biofuels and Biochemicals. Microb. Biotechnol. 2016, 9, 699–717. [Google Scholar] [CrossRef]
- Sharma, D.; Saini, A. Saccharification Fermentation and Process Integration. In Lignocellulosic Ethanol Production from a Biorefinery Perspective; Springer Singapore: Singapore, 2020; pp. 111–158. [Google Scholar] [CrossRef]
- Bai, F.W.; Anderson, W.A.; Moo-Young, M. Ethanol Fermentation Technologies from Sugar and Starch Feedstocks. Biotechnol. Adv. 2008, 26, 89–105. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Bashan, Y. Microalgal Heterotrophic and Mixotrophic Culturing for Bio-Refining: From Metabolic Routes to Techno-Economics. In Algal Biorefineries; Prokop, A., Bajpai, R.K., Zappi, M.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 61–131. [Google Scholar] [CrossRef]
- Liu, R.; Shen, F. Impacts of Main Factors on Bioethanol Fermentation from Stalk Juice of Sweet Sorghum by Immobilized Saccharomyces Cerevisiae(CICC 1308). Bioresour. Technol. 2008, 99, 847–854. [Google Scholar] [CrossRef]
- Amutha, R.; Gunasekaran, P. Production of Ethanol from Liquefied Cassava Starch Using Co-Immobilized Cells of Zymomonas Mobilis and Saccharomyces Diastaticus. J. Biosci. Bioeng. 2001, 92, 560–564. [Google Scholar] [CrossRef]
- Yuangsaard, N.; Yongmanitchai, W.; Yamada, M.; Limtong, S. Selection and Characterization of a Newly Isolated Thermotolerant Pichia Kudriavzevii Strain for Ethanol Production at High Temperature from Cassava Starch Hydrolysate. Antonie Van Leeuwenhoek 2013, 103, 577–588. [Google Scholar] [CrossRef]
- Ogbonna, J. Scale up of Fuel Ethanol Production from Sugar Beet Juice Using Loofa Sponge Immobilized Bioreactor. Bioresour. Technol. 2001, 76, 1–8. [Google Scholar] [CrossRef]
- Pappas, K.M.; Kouvelis, V.N.; Saunders, E.; Brettin, T.S.; Bruce, D.; Detter, C.; Balakireva, M.; Han, C.S.; Savvakis, G.; Kyrpides, N.C.; et al. Genome Sequence of the Ethanol-Producing Zymomonas Mobilis Subsp. Mobilis Lectotype Strain ATCC 10988. J. Bacteriol. 2011, 193, 5051–5052. [Google Scholar] [CrossRef]
- Plesu Popescu, A.E.; Pellin, J.L.; Bonet, J.; Llorens, J. Bioethanol Dehydration and Mixing by Heterogeneous Azeotropic Distillation. J. Clean. Prod. 2021, 320, 128810. [Google Scholar] [CrossRef]
- Dale, R.T.; Tyner, W.E.; Dale, R.T.; Tyner, W.E. Economic and technical analysis of ethanol dry milling: Model description. Res. Agric. Appl. Econ. 2006, 1, 1–45. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Yu, J.; Huang, H.; Jin, M. In Situ Pretreatment during Distillation Improves Corn Fiber Conversion and Ethanol Yield in the Dry Mill Process. Green Chem. 2019, 21, 1080–1090. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, N.; Prasad, R. Anhydrous Ethanol: A Renewable Source of Energy. Renew. Sustain. Energy Rev. 2010, 14, 1830–1844. [Google Scholar] [CrossRef]
- Ross, A.; Jones, J.; Kubacki, M.; Bridgeman, T. Classification of Macroalgae as Fuel and Its Thermochemical Behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef]
- Demirbas, M.F. Biofuels from Algae for Sustainable Development. Appl. Energy 2011, 88, 3473–3480. [Google Scholar] [CrossRef]
- Pacheco-Basulto, J.Á.; Hernández-McConville, D.; Barroso-Muñoz, F.O.; Hernández, S.; Segovia-Hernández, J.G.; Castro-Montoya, A.J.; Bonilla-Petriciolet, A. Purification of Bioethanol Using Extractive Batch Distillation: Simulation and Experimental Studies. Chem. Eng. Process. Process Intensif. 2012, 61, 30–35. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Tang, L.; Zhang, H.; Zhang, G.; Yang, X.; Liu, P.; Mao, Z. Establishment and Assessment of a Novel Cleaner Production Process of Corn Grain Fuel Ethanol. Bioresour. Technol. 2013, 148, 453–460. [Google Scholar] [CrossRef]
- Loy, Y.Y.; Lee, X.L.; Rangaiah, G.P. Bioethanol Recovery and Purification Using Extractive Dividing-Wall Column and Pressure Swing Adsorption: An Economic Comparison after Heat Integration and Optimization. Sep. Purif. Technol. 2015, 149, 413–427. [Google Scholar] [CrossRef]
- Brunner, G. Counter-Current Separations. J. Supercrit. Fluids 2009, 47, 574–582. [Google Scholar] [CrossRef]
- Babu, V.; Thapliyal, A.; Patel, G.K. (Eds.) Biofuels Production, 1st ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Ramirez, E.C.; Johnston, D.B.; McAloon, A.J.; Yee, W.; Singh, V. Engineering Process and Cost Model for a Conventional Corn Wet Milling Facility. Ind. Crops Prod. 2008, 27, 91–97. [Google Scholar] [CrossRef]
- Wronkowska, M. Wet-Milling of Cereals: Wet-Milling of Cereals. J. Food Process. Preserv. 2016, 40, 572–580. [Google Scholar] [CrossRef]
- Rausch, K.D.; Eckhoff, S.R. Maize: Wet Milling. In Encyclopedia of Food Grains; Elsevier: Amsterdam, The Netherlands, 2016; pp. 467–481. [Google Scholar] [CrossRef]
- Rose, D.J.; Inglett, G.E.; Liu, S.X. Utilisation of Corn (Zea Mays) Bran and Corn Fiber in the Production of Food Components: Applications of Corn Co-Products. J. Sci. Food Agric. 2010, 90, 915–924. [Google Scholar] [CrossRef]
- Front Matter. In Sustainable Recovery and Reutilization of Cereal Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2019; p. iii. [CrossRef]
- Bothast, R.J.; Schlicher, M.A. Biotechnological Processes for Conversion of Corn into Ethanol. Appl. Microbiol. Biotechnol. 2005, 67, 19–25. [Google Scholar] [CrossRef]
- Halder, P.; Azad, K.; Shah, S.; Sarker, E. Prospects and Technological Advancement of Cellulosic Bioethanol Ecofuel Production. In Advances in Eco-Fuels for a Sustainable Environment; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–236. [Google Scholar] [CrossRef]
- Bioethanol Production from Food Crops; Elsevier: Amsterdam, The Netherlands, 2019. [CrossRef]
- Drapcho, C.M.; Nhuan, N.P.; Walker, T.H. Biofuels Engineering Process Technology; McGraw-Hill Education: New York, NY, USA, 2008. [Google Scholar]
- Mizik, T. Impacts of International Commodity Trade on Conventional Biofuels Production. Sustainability 2020, 12, 2626. [Google Scholar] [CrossRef]
- Ramírez, E.C.; Johnston, D.B.; McAloon, A.J.; Singh, V. Enzymatic Corn Wet Milling: Engineering Process and Cost Model. Biotechnol. Biofuels 2009, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Böttger, C.; Südekum, K.-H. Review: Protein Value of Distillers Dried Grains with Solubles (DDGS) in Animal Nutrition as Affected by the Ethanol Production Process. Anim. Feed Sci. Technol. 2018, 244, 11–17. [Google Scholar] [CrossRef]
- Iram, A.; Cekmecelioglu, D.; Demirci, A. Distillers’ Dried Grains with Solubles (DDGS) and Its Potential as Fermentation Feedstock. Appl. Microbiol. Biotechnol. 2020, 104, 6115–6128. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wang, Y.-W.; Dai, Y.-H.; Song, C.; Zhu, Q.-L.; Qin, H.; Tan, F.-R.; Chen, H.-C.; Dai, L.-C.; Hu, G.-Q.; et al. Current Status and Future Prospective of Bio-Ethanol Industry in China. Renew. Sustain. Energy Rev. 2021, 145, 111079. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Romanelli, T.L.; Ray, C.; Hoekstra, A.Y.; Liska, A.J.; Neale, C.M.U. Water, Energy, and Carbon Footprints of Bioethanol from the U.S. and Brazil. Environ. Sci. Technol. 2018, 52, 14508–14518. [Google Scholar] [CrossRef]
- Macrelli, S.; Mogensen, J.; Zacchi, G. Techno-Economic Evaluation of 2nd Generation Bioethanol Production from Sugar Cane Bagasse and Leaves Integrated with the Sugar-Based Ethanol Process. Biotechnol. Biofuels 2012, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of First and Second Generation Biofuels: A Comprehensive Review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Finkbeiner, M. Indirect Land Use Change—Help beyond the Hype? Biomass Bioenergy 2014, 62, 218–221. [Google Scholar] [CrossRef]
- Hemansi; Gupta, R.; Yadav, G.; Kumar, G.; Yadav, A.; Saini, J.K.; Kuhad, R.C. Second Generation Bioethanol Production: The State of Art. In Sustainable Approaches for Biofuels Production Technologies: From Current Status to Practical Implementation; Srivastava, N., Srivastava, M., Mishra, P.K., Upadhyay, S.N., Ramteke, P.W., Gupta, V.K., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 121–146. [Google Scholar] [CrossRef]
- Dias, M.O.S.; Junqueira, T.L.; Cavalett, O.; Cunha, M.P.; Jesus, C.D.F.; Mantelatto, P.E.; Rossell, C.E.V.; Maciel Filho, R.; Bonomi, A. Cogeneration in Integrated First and Second Generation Ethanol from Sugarcane. Chem. Eng. Res. Des. 2013, 91, 1411–1417. [Google Scholar] [CrossRef]
- Zhou, H.; Zhan, W.; Wang, L.; Guo, L.; Liu, Y. Making Sustainable Biofuels and Sunscreen from Corncobs To Introduce Students to Integrated Biorefinery Concepts and Techniques. J. Chem. Educ. 2018, 95, 1376–1380. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; Da Silva, S.S. The Path Forward for Lignocellulose Biorefineries: Bottlenecks, Solutions, and Perspective on Commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Ayodele, B.V.; Alsaffar, M.A.; Mustapa, S.I. An Overview of Integration Opportunities for Sustainable Bioethanol Production from First- and Second-Generation Sugar-Based Feedstocks. J. Clean. Prod. 2020, 245, 118857. [Google Scholar] [CrossRef]
- Arora, R.; Sharma, N.K.; Kumar, S.; Sani, R.K. Lignocellulosic Ethanol: Feedstocks and Bioprocessing. In Bioethanol Production from Food Crops; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–185. [Google Scholar] [CrossRef]
- Slupska, M.; Bushong, D. Lessons from Commercialization of Cellulosic Ethanol—A POET Perspective. Biofuels Bioprod. Biorefining 2019, 13, 857–859. [Google Scholar] [CrossRef]
- Rajendran, K.; Drielak, E.; Sudarshan Varma, V.; Muthusamy, S.; Kumar, G. Updates on the Pretreatment of Lignocellulosic Feedstocks for Bioenergy Production—A Review. Biomass Convers. Biorefinery 2018, 8, 471–483. [Google Scholar] [CrossRef]
- Erdei, B.; Hancz, D.; Galbe, M.; Zacchi, G. SSF of Steam-Pretreated Wheat Straw with the Addition of Saccharified or Fermented Wheat Meal in Integrated Bioethanol Production. Biotechnol. Biofuels 2013, 6, 169. [Google Scholar] [CrossRef] [PubMed]
- Quintero, J.A.; Moncada, J.; Cardona, C.A. Techno-Economic Analysis of Bioethanol Production from Lignocellulosic Residues in Colombia: A Process Simulation Approach. Bioresour. Technol. 2013, 139, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Cruz, A.J.G.; Costa, C.B.B. Improving Second Generation Bioethanol Production in Sugarcane Biorefineries through Energy Integration. Appl. Therm. Eng. 2016, 109, 819–827. [Google Scholar] [CrossRef]
- Della-Bianca, B.E.; Basso, T.O.; Stambuk, B.U.; Basso, L.C.; Gombert, A.K. What Do We Know about the Yeast Strains from the Brazilian Fuel Ethanol Industry? Appl. Microbiol. Biotechnol. 2013, 97, 979–991. [Google Scholar] [CrossRef]
- Ullah, K.; Ahmad, M.; Sofia; Sharma, V.K.; Lu, P.; Harvey, A.; Zafar, M.; Sultana, S. Assessing the Potential of Algal Biomass Opportunities for Bioenergy Industry: A Review. Fuel 2015, 143, 414–423. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Chakravarthy, M.; Ranjith Kumar, R.; Yogendran, D.; Yuvaraj, D.; Jayamuthunagai, J.; Praveen Kumar, R.; Palani, S. Aquatic Biomass (Algae) as a Future Feed Stock for Bio-Refineries: A Review on Cultivation, Processing and Products. Renew. Sustain. Energy Rev. 2015, 47, 634–653. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Bungay, H.R. Confessions of a Bioenergy Advocate. Trends Biotechnol. 2004, 22, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Sambusiti, C.; Bellucci, M.; Zabaniotou, A.; Beneduce, L.; Monlau, F. Algae as Promising Feedstocks for Fermentative Biohydrogen Production According to a Biorefinery Approach: A Comprehensive Review. Renew. Sustain. Energy Rev. 2015, 44, 20–36. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A. Micro-Algae Cultivation for Biofuels: Cost, Energy Balance, Environmental Impacts and Future Prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Renewable Fuels from Algae: An Answer to Debatable Land Based Fuels. Bioresour. Technol. 2011, 102, 10–16. [Google Scholar] [CrossRef]

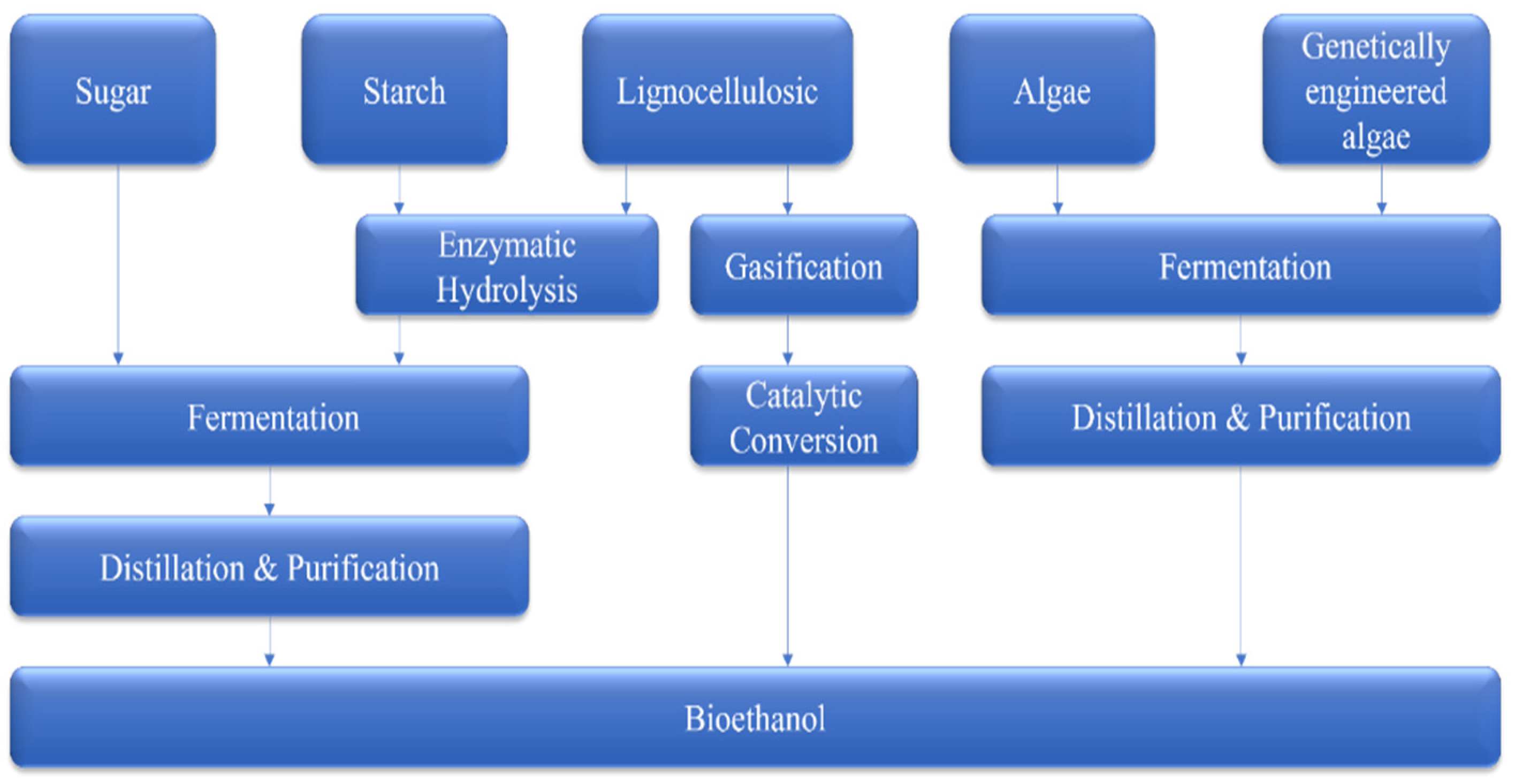

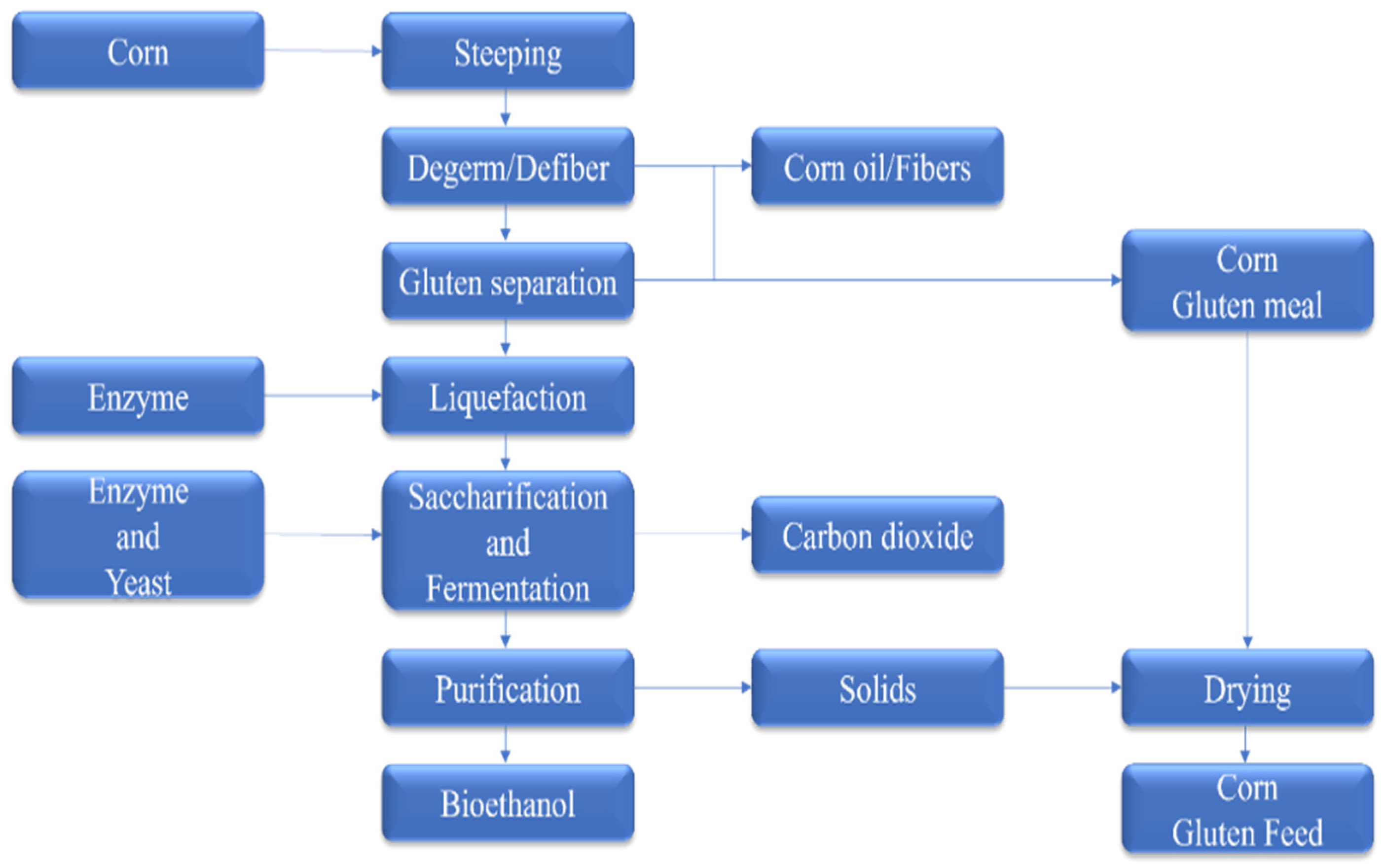
| Country | Number of 1st-Generation Plants | Capacity Used (%) | Feedstocks (×1000 Mt) | References |
|---|---|---|---|---|
| USA | 208 | 80 | Corn: 123465 | [30] |
| Brazil | 360 | 67 | Corn: 5995 Sugarcane: 326630 | [31] |
| EU | 57 | 58 | Corn: 6350 Sugar: 7450 | [31,32] |
| China | 18 | 49 | Corn: 7100 Cassava: 1000 | [31] |
| India | 220 | 85 | Molasses: 6407 | [31] |
| Comparative Elements | First Generation | Second Generation | Third Generation | Fourth Generation | References |
|---|---|---|---|---|---|
| Feedstock sources | Edible crops (sugar beet, sugar can, wheat, corn) | Non-edible crops (wood, grasses, organic waste, agricultural and forestry residues) | Algal biomass (macroalgae, microalgae) | Engineered biomass (engineered crops) | [41,46,47] |
| Land usage for cultivation | Arable land | Arable and marginal lands |
| Non-arable land | [49,50] |
| Conversion technologies |
|
|
| Algal metabolic engineering for enhanced carbon capture, cultivation, harvesting, and conversion processes | [51,52,53] |
| Conversion process | Easily converted to ethanol | Requires more advanced technology | Limited investments and difficulties in process design | Requires an advanced method | [48] |
| Environmental impact | High contribution to the mitigation of CO2 |
| Enhanced CO2-capture ability | High CO2-capture ability | [37,48] |
| Main advantage | Relatively simple conversion process |
| Increased efficiency and sustainability |
| [48,49,50] |
| Main disadvantage | Competition with food supply | Recalcitrant structures of the feedstock |
|
| [26] |
| Production cost | ~0.4–0.5 USD/L | ~0.7–2 USD/L | ~10–20 USD/L | _ | [54] |
| Name of Microorganisms | Carbon Source (g/L) | Nitrogen Source (g/L) | Growth Temperature (°C) | pH | Time (h) | References |
|---|---|---|---|---|---|---|
| S. cerevisiae CICC 1308 | Glucose or sucrose (50) | Peptone (5) | 30 | 5 | 48 | [154] |
| S. diastaticus Y2416 | Maltose (3) and glucose (20) | Yeast extract (5), peptone (5) | 30 | 6 | _ | [155] |
| K. marxianus DMKU 3-1042 | Sugar (50–80) | Ammonium sulfate (0.5) | 35 | 4.5 | 72 | [141] |
| P. kudriavzevii DMKU 3-ET15 | Glucose (20) | Peptone (20) | 40 | 6.5 | 48 | [156] |
| Z. mobilis | Glucose (10) and sucrose (30) | Yeast extract (5) | 30 | 6.5 | 18 | [157] |
| Z. mobilis ATCC 10988 | Glucose (20) | Ammonium sulfate (1) | 30 | 6 | 24–48 | [158] |
| E. coli KO11 and K. oxytoca P2 | Sucrose (20) | Ammonium sulfate (2) | 30 | _ | 24 | [145] |
| Comparative Elements | Wet Milling | Dry Milling | References |
|---|---|---|---|
| Ethanol yield | ~29 kg from 100 kg corn | ~34 kg from 100 kg corn | [179] |
| Investment cost | ~USD 79.3 million | ~USD 51.8 million USD | [160,180] |
| By-products | ~5 kg corn gluten meal ~22 kg corn gluten feed ~3 kg corn germ oil, fiber, feed steep water, and CO2 | ~32 kg distiller’s dried grains with solubles (90% dry content) ~32 kg CO2 | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assaf, J.C.; Mortada, Z.; Rezzoug, S.-A.; Maache-Rezzoug, Z.; Debs, E.; Louka, N. Comparative Review on the Production and Purification of Bioethanol from Biomass: A Focus on Corn. Processes 2024, 12, 1001. https://doi.org/10.3390/pr12051001
Assaf JC, Mortada Z, Rezzoug S-A, Maache-Rezzoug Z, Debs E, Louka N. Comparative Review on the Production and Purification of Bioethanol from Biomass: A Focus on Corn. Processes. 2024; 12(5):1001. https://doi.org/10.3390/pr12051001
Chicago/Turabian StyleAssaf, Jean Claude, Zeinab Mortada, Sid-Ahmed Rezzoug, Zoulikha Maache-Rezzoug, Espérance Debs, and Nicolas Louka. 2024. "Comparative Review on the Production and Purification of Bioethanol from Biomass: A Focus on Corn" Processes 12, no. 5: 1001. https://doi.org/10.3390/pr12051001
APA StyleAssaf, J. C., Mortada, Z., Rezzoug, S.-A., Maache-Rezzoug, Z., Debs, E., & Louka, N. (2024). Comparative Review on the Production and Purification of Bioethanol from Biomass: A Focus on Corn. Processes, 12(5), 1001. https://doi.org/10.3390/pr12051001









