Characterization and Performance Evaluation of Digital Light Processing 3D Printed Functional Anion Exchange Membranes in Electrodialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.3. Analytical Methods
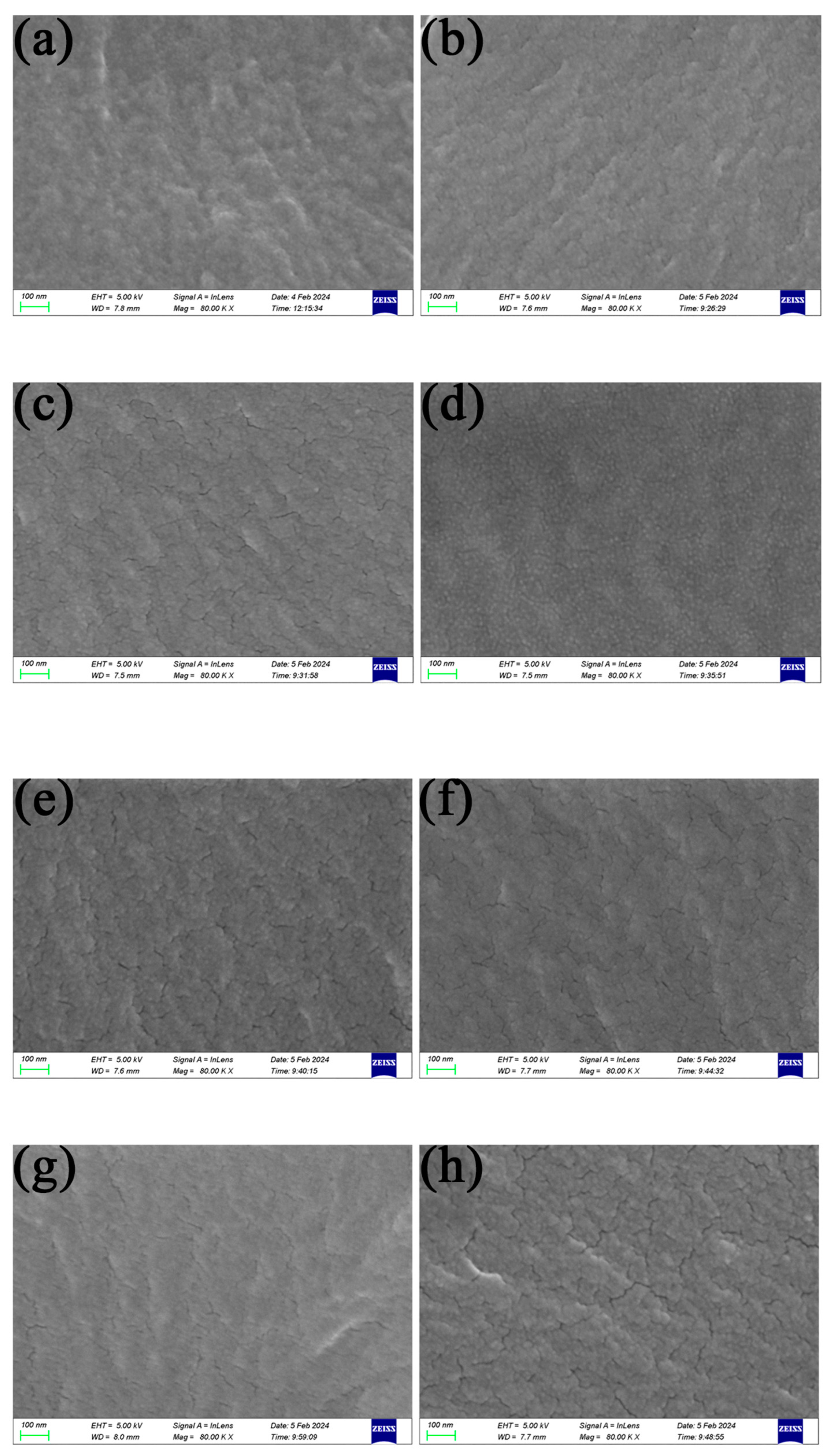
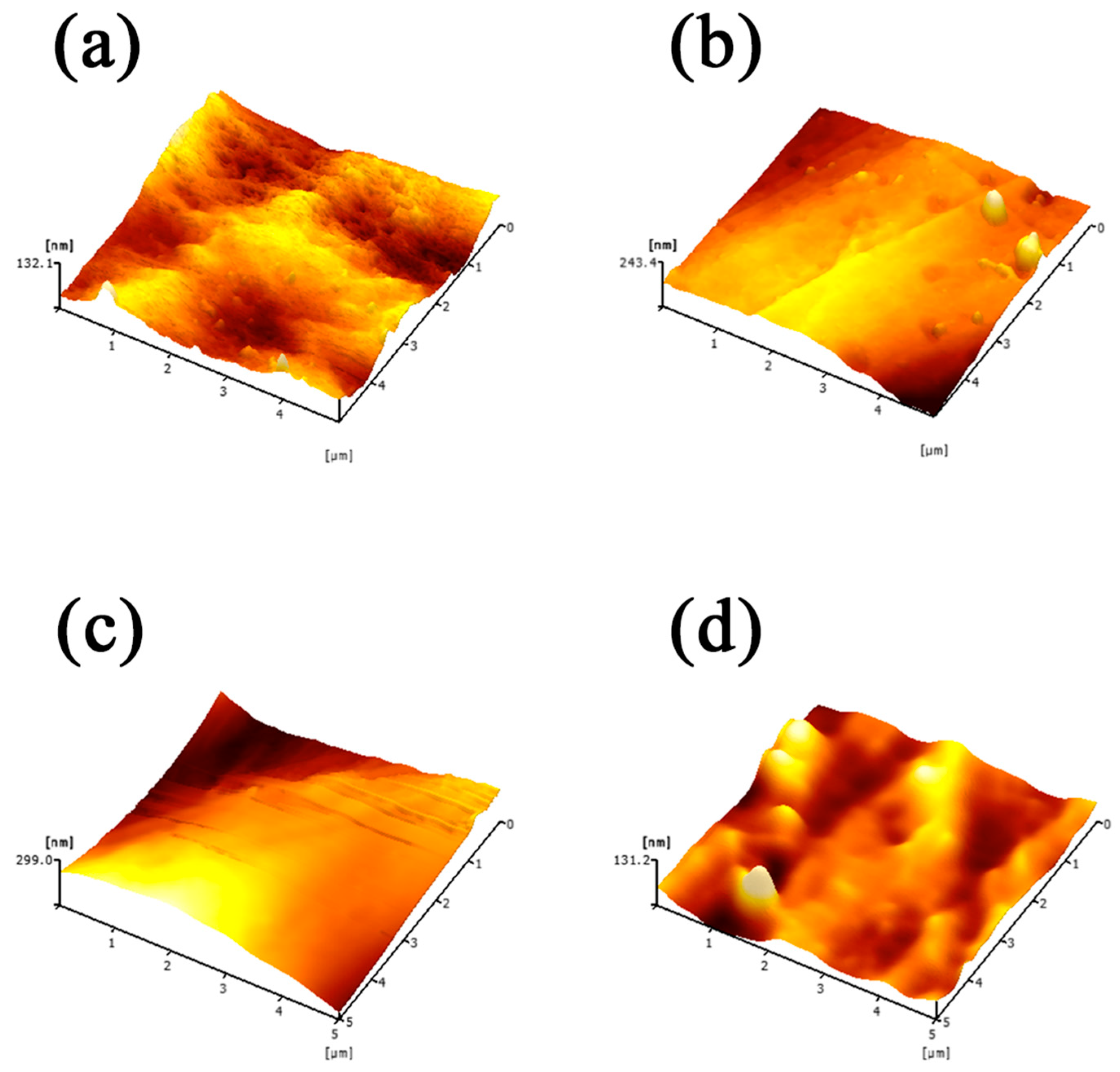
2.3.1. Membrane Structure Analysis
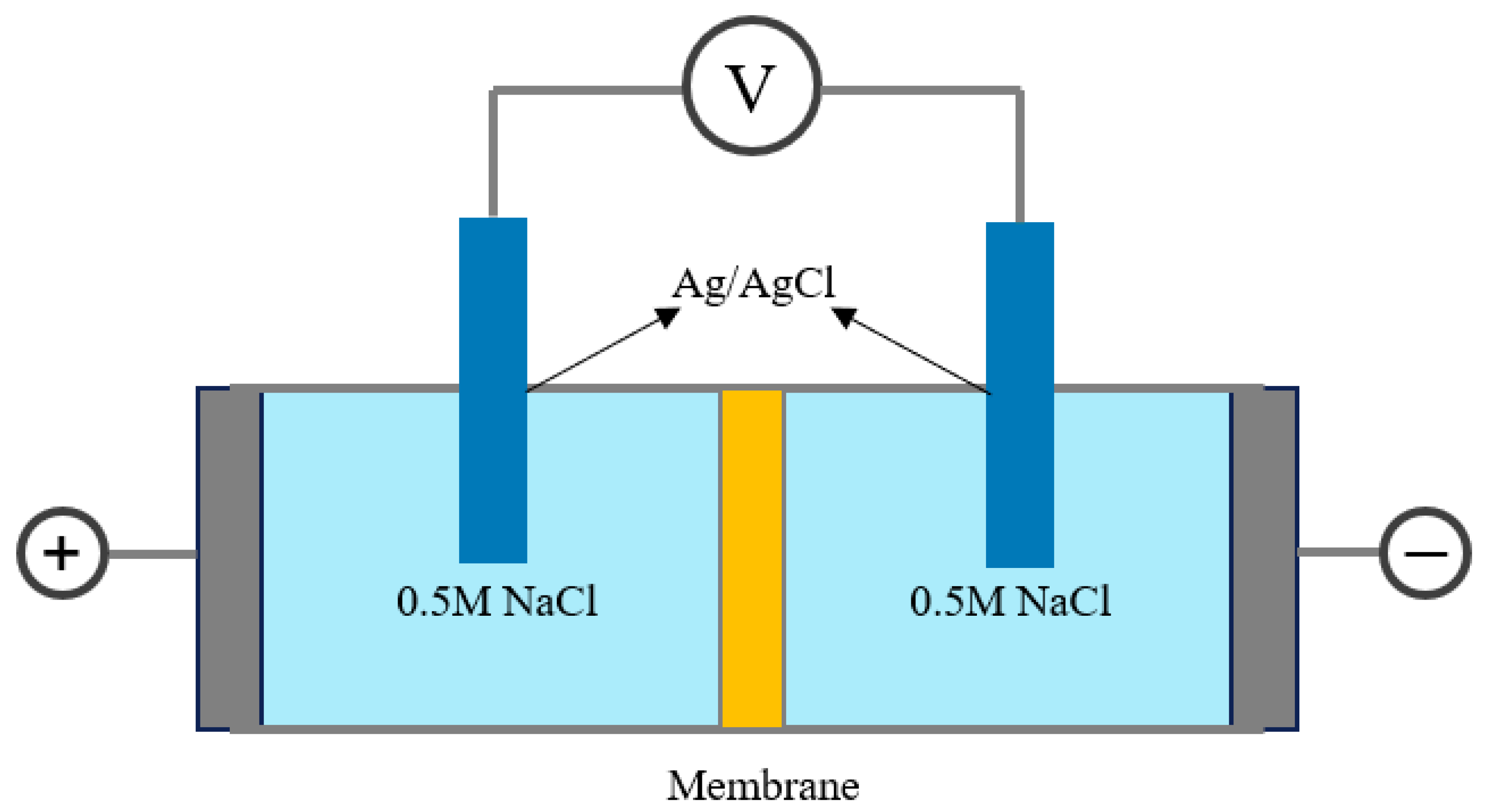
2.3.2. Membrane Performance Analysis
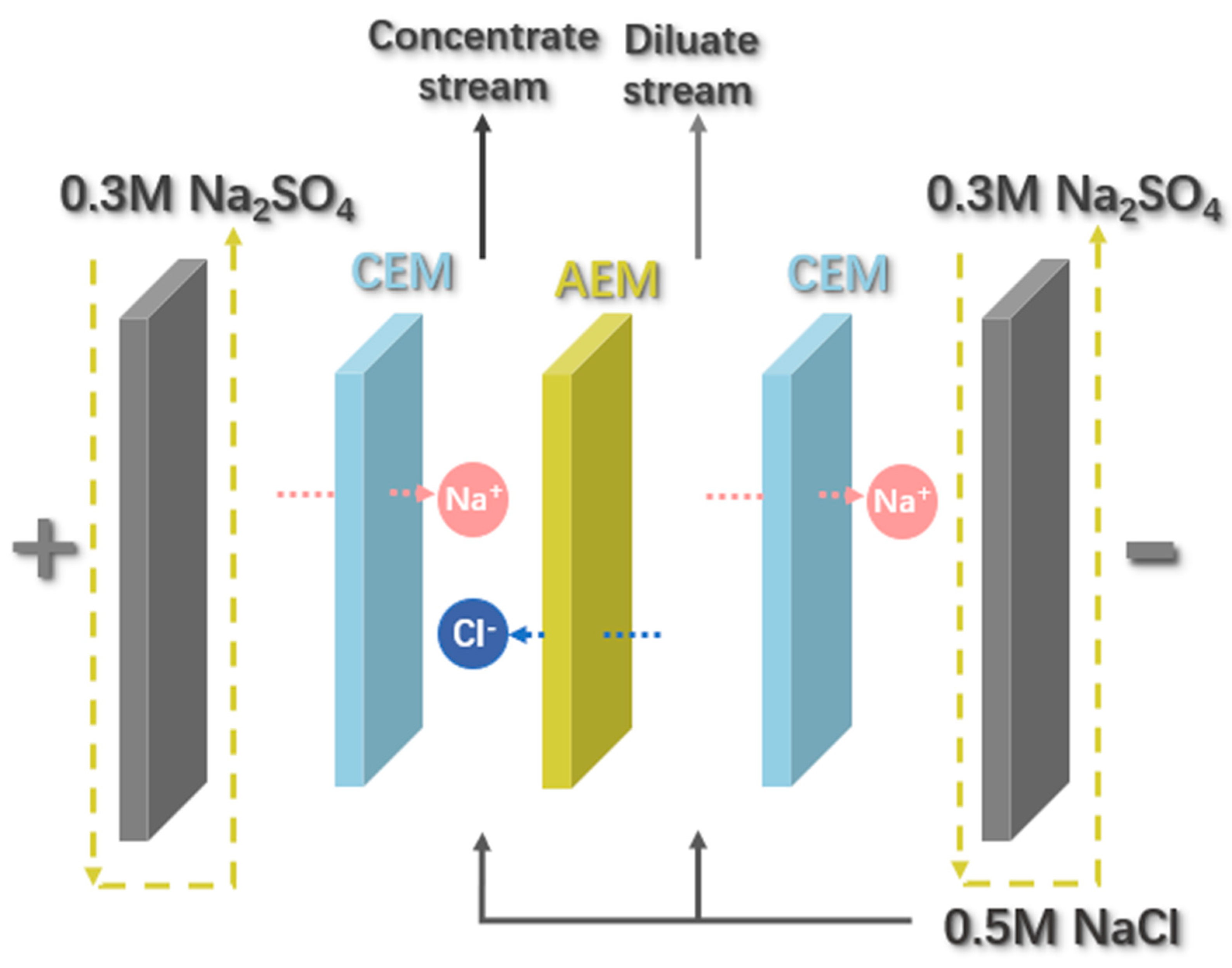
2.3.3. Electrodialysis Performance Analysis
3. Results and Discussion
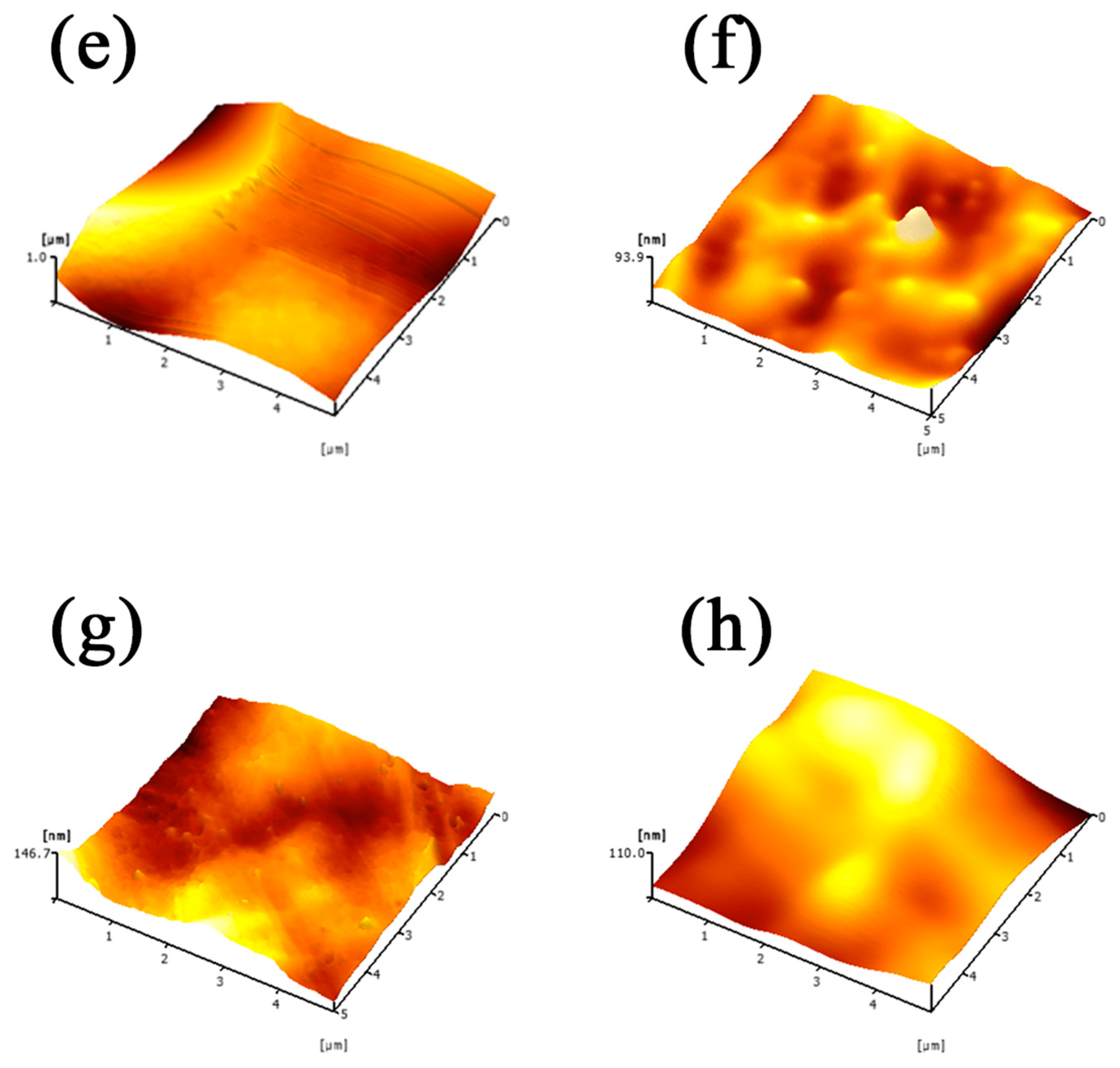
3.1. Analysis of the Membrane Characteristics
3.1.1. Impact of Curing Time
3.1.2. Impact of the Number of Printed Layers
3.1.3. Impact of the PEGDA Molecular Weight
3.1.4. Impact of the VBC content
3.2. Membrane Performance Analysis
3.2.1. Impact of Curing Time
3.2.2. Impact of the Number of Printed Layers
3.2.3. Impact of the PEGDA Molecular Weight
3.2.4. Impact of the VBC Content
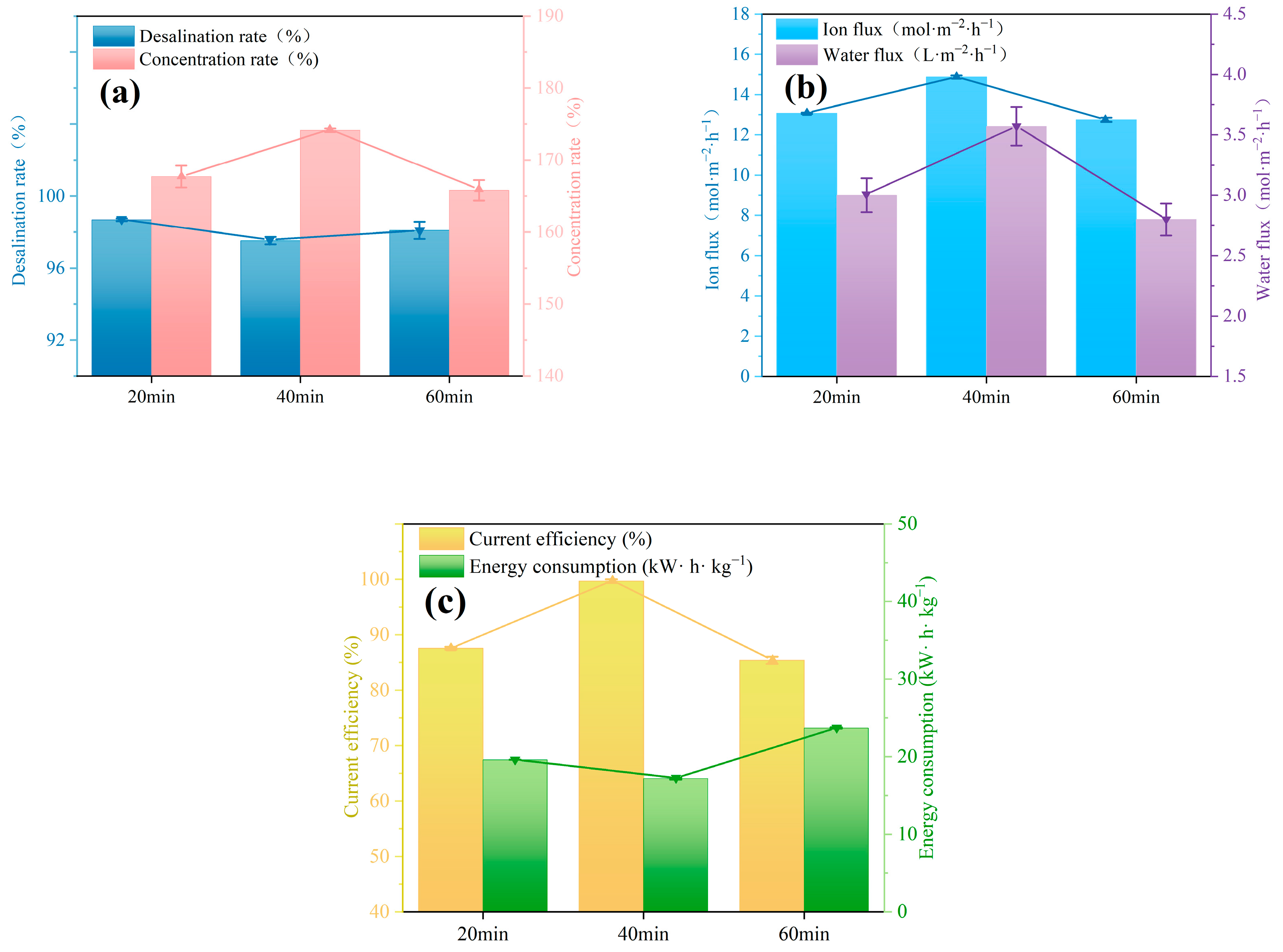
3.3. Electrodialysis Experiments
3.3.1. Impact of Curing Time on Desalination Performance
3.3.2. Impact of the Number of Printed Layers on Desalination Performance
3.3.3. Impact of the PEGDA Molecular Weight on Desalination Performance
3.3.4. Impact of the VBC Content on Desalination Performance
3.3.5. Impact of the Current Density on Desalination Performance
3.4. Ion Selectivity under Various Current Density
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion Exchange Membranes: New Developments and Applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Zha, Y.; Disabb-Miller, M.L.; Johnson, Z.D.; Hickner, M.A.; Tew, G.N. Metal-Cation-Based Anion Exchange Membranes. J. Am. Chem. Soc. 2012, 134, 4493–4496. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. Flat-Sheet Membrane for Power Generation and Desalination Based on Salinity Gradient. In Membrane-Based Salinity Gradient Processes for Water Treatment and Power Generation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 155–174. ISBN 978-0-444-63961-5. [Google Scholar]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, H. Electrodialysis, a Mature Technology with a Multitude of New Applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis Desalination for Water and Wastewater: A Review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Zhang, Y.; Paepen, S.; Pinoy, L.; Meesschaert, B.; Van Der Bruggen, B. Selectrodialysis: Fractionation of Divalent Ions from Monovalent Ions in a Novel Electrodialysis Stack. Sep. Purif. Technol. 2012, 88, 191–201. [Google Scholar] [CrossRef]
- Folaranmi, G.; Bechelany, M.; Sistat, P.; Cretin, M.; Zaviska, F. Towards Electrochemical Water Desalination Techniques: A Review on Capacitive Deionization, Membrane Capacitive Deionization and Flow Capacitive Deionization. Membranes 2020, 10, 96. [Google Scholar] [CrossRef]
- Miesiac, I.; Rukowicz, B. Bipolar Membrane and Water Splitting in Electrodialysis. Electrocatalysis 2022, 13, 101–107. [Google Scholar] [CrossRef]
- Cheng, C.; Shen, H.Y.; Gong, Y.; Chen, W.; Li, P. Auxiliary Functional Group Diffusion Dialysis Membranes for Acid Recovery. J. Polym. Sci. 2022, 60, 3043–3053. [Google Scholar] [CrossRef]
- Moreno, J.; Grasman, S.; Van Engelen, R.; Nijmeijer, K. Upscaling Reverse Electrodialysis. Environ. Sci. Technol. 2018, 52, 10856–10863. [Google Scholar] [CrossRef]
- Velizarov, S.; Reis, M.A.; Crespo, J.G. Integrated Transport and Reaction in an Ion Exchange Membrane Bioreactor. Desalination 2002, 149, 205–210. [Google Scholar] [CrossRef]
- Blagojevic, N.; Müller, M. Simulation of Membrane Fabrication via Solvent Evaporation and Nonsolvent-Induced Phase Separation. ACS Appl. Mater. Interfaces 2023, 15, 57913–57927. [Google Scholar] [CrossRef]
- Getachew, B.A.; Kim, S.-R.; Kim, J.-H. Improved Stability of Self-Healing Hydrogel Pore-Filled Membranes with Ionic Cross-Links. J. Membr. Sci. 2018, 553, 1–9. [Google Scholar] [CrossRef]
- Adamczak, M.; Kamińska, G.; Bohdziewicz, J. Preparation of Polymer Membranes by In Situ Interfacial Polymerization. Int. J. Polym. Sci. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Seo, J.; Kushner, D.I.; Hickner, M.A. 3D Printing of Micropatterned Anion Exchange Membranes. ACS Appl. Mater. Interfaces 2016, 8, 16656–16663. [Google Scholar] [CrossRef] [PubMed]
- Zárybnická, L.; Stránská, E.; Janegová, K.; Vydrová, B. The Effect of 3D Printing Parameters on Electrochemical Properties of Heterogeneous Cation Exchange Membrane. RPJ 2021, 27, 1538–1547. [Google Scholar] [CrossRef]
- Capparelli, C.; Fernandez Pulido, C.R.; Wiencek, R.A.; Hickner, M.A. Resistance and Permselectivity of 3D-Printed Micropatterned Anion-Exchange Membranes. ACS Appl. Mater. Interfaces 2019, 11, 26298–26306. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Shi, Y.; He, S. Method for Preparing Organic Separating Membrane through Three-Dimensional Molding Technology 2016. CN105643931A, 6 August 2016. [Google Scholar]
- Soo, A.; Ali, S.M.; Shon, H.K. 3D Printing for Membrane Desalination: Challenges and Future Prospects. Desalination 2021, 520, 115366. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Thiam, B.G.; El Magri, A.; Vanaei, H.R.; Vaudreuil, S. 3D Printed and Conventional Membranes—A Review. Polymers 2022, 14, 1023. [Google Scholar] [CrossRef] [PubMed]
- Tijing, L.D.; Dizon, J.R.C.; Ibrahim, I.; Nisay, A.R.N.; Shon, H.K.; Advincula, R.C. 3D Printing for Membrane Separation, Desalination and Water Treatment. Appl. Mater. Today 2020, 18, 100486. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Kariz, M.; Kwon, J.H.; Kitek Kuzman, M. Effect of Printing Layer Thickness on Water Absorption and Mechanical Properties of 3D-Printed Wood/PLA Composite Materials. Int. J. Adv. Manuf. Technol. 2019, 102, 2195–2200. [Google Scholar] [CrossRef]
- Yuan, S.; Strobbe, D.; Kruth, J.-P.; Van Puyvelde, P.; Van Der Bruggen, B. Super-Hydrophobic 3D Printed Polysulfone Membranes with a Switchable Wettability by Self-Assembled Candle Soot for Efficient Gravity-Driven Oil/Water Separation. J. Mater. Chem. A 2017, 5, 25401–25409. [Google Scholar] [CrossRef]
- Ponomar, M.; Krasnyuk, E.; Butylskii, D.; Nikonenko, V.; Wang, Y.; Jiang, C.; Xu, T.; Pismenskaya, N. Sessile Drop Method: Critical Analysis and Optimization for Measuring the Contact Angle of an Ion-Exchange Membrane Surface. Membranes 2022, 12, 765. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylin, S.; Bazinet, L. Fouling on Ion-Exchange Membranes: Classification, Characterization and Strategies of Prevention and Control. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef]
- Cavallo, A.; Madaghiele, M.; Masullo, U.; Lionetto, M.G.; Sannino, A. Photo-Crosslinked Poly(Ethylene Glycol) Diacrylate (PEGDA) Hydrogels from Low Molecular Weight Prepolymer: Swelling and Permeation Studies. J. Appl. Polym. Sci. 2017, 134, app.44380. [Google Scholar] [CrossRef]
- S Grewal, M.; Ishibashi, K.; Hara, M.; Ishizaki, Y.; Nagano, S.; Yabu, H. Effect of the Poly(Ethylene Glycol) Diacrylate (PEGDA) Molecular Weight on Ionic Conductivities in Solvent-Free Photo-Cross-Linked Solid Polymer Electrolytes. Langmuir 2023, 39, 10209–10215. [Google Scholar] [CrossRef]
- O’Donnell, K.; Boyd, A.; Meenan, B.J. Controlling Fluid Diffusion and Release through Mixed-Molecular-Weight Poly(Ethylene) Glycol Diacrylate (PEGDA) Hydrogels. Materials 2019, 12, 3381. [Google Scholar] [CrossRef]
- Khan, M.; Luque, R.; Akhtar, S.; Shaheen, A.; Mehmood, A.; Idress, S.; Buzdar, S.; Ur Rehman, A. Design of Anion Exchange Membranes and Electrodialysis Studies for Water Desalination. Materials 2016, 9, 365. [Google Scholar] [CrossRef]
- Borges, F.J.; Roux-de Balmann, H.; Guardani, R. Investigation of the Mass Transfer Processes during the Desalination of Water Containing Phenol and Sodium Chloride by Electrodialysis. J. Membr. Sci. 2008, 325, 130–138. [Google Scholar] [CrossRef]
- Vanoppen, M.; Bakelants, A.F.A.M.; Gaublomme, D.; Schoutteten, K.V.K.M.; Bussche, J.V.; Vanhaecke, L.; Verliefde, A.R.D. Properties Governing the Transport of Trace Organic Contaminants through Ion-Exchange Membranes. Environ. Sci. Technol. 2015, 49, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Chen, Q.; Pan, N.; Yu, X.; Gao, X.; Shen, J.; Gao, C. Amphoteric Blend Ion-Exchange Membranes for Separating Monovalent and Bivalent Anions in Electrodialysis. Sep. Purif. Technol. 2020, 242, 116793. [Google Scholar] [CrossRef]
- Sata, T.; Tagami, Y.; Matsusaki, K. Transport Properties of Anion-Exchange Membranes Having a Hydrophobic Layer on Their Surface in Electrodialysis. J. Phys. Chem. B 1998, 102, 8473–8479. [Google Scholar] [CrossRef]
- Tansel, B.; Sager, J.; Rector, T.; Garland, J.; Strayer, R.F.; Levine, L.; Roberts, M.; Hummerick, M.; Bauer, J. Significance of Hydrated Radius and Hydration Shells on Ionic Permeability during Nanofiltration in Dead End and Cross Flow Modes. Sep. Purif. Technol. 2006, 51, 40–47. [Google Scholar] [CrossRef]




| Variables | No. | VBC Content (wt%) | PEGDA Molecular Weight | Curing Time (min) | Printed Layer |
|---|---|---|---|---|---|
| Curing time | 1 | 15 | 400 | 20 | 50 μm × 1 layer |
| 2 | 15 | 400 | 40 | 50 μm × 1 layer | |
| 3 | 15 | 400 | 60 | 50 μm × 1 layer | |
| Printing layer | 4 | 15 | 400 | 10 × 2 * | 25 μm × 2 layers |
| Printing formula: PEGDA molecular weight | 5 | 15 | 200 | 60 | 50 μm × 1 layer |
| 6 | 15 | 700 | 60 | 50 μm × 1 layer | |
| Printing formula: VBC molecular weight | 7 | 10 | 400 | 60 | 50 μm × 1 layer |
| 8 | 20 | 400 | 60 | 50 μm × 1 layer |
| Curing Time | Membrane Thickness (μm) | Contact Angle (°) | IEC (mmol·g−1) | Water Uptake (%) | Membrane Surface Resistance (Ω·cm2) |
|---|---|---|---|---|---|
| 20 min | 325.7 ± 6.5 | 78.69 | 0.93 | 11.38 | 0.14 ± 0.0098 |
| 40 min | 282.6 ± 23.6 | 63.00 | 1.35 | 14.08 | 0.11 ± 0.0045 |
| 60 min | 240.6 ± 16.0 | 65.00 | 0.94 | 11.42 | 0.17 ± 0.0025 |
| Printing Layers | Membrane Thickness (μm) | Contact Angle (°) | IEC (mmol·g−1) | Water Uptake (%) | Membrane Surface Resistance (Ω·cm2) |
|---|---|---|---|---|---|
| 50 μm × 1 layer (20 min) | 325.7 ± 6.5 | 78.69 | 0.93 | 11.38 | 0.14 ± 0.0098 |
| 25 μm × 2 layers (10 min × 2) | 345.2 ± 9.5 | 67.16 | 1.17 | 13.59 | 0.12 ± 0.0004 |
| PEGDA Molecular Weight | Membrane Thickness (μm) | Contact Angle (°) | IEC (mmol·g−1) | Water Uptake (%) | Membrane Surface Resistance (Ω·cm2) |
|---|---|---|---|---|---|
| PEGDA-200 | 316.6 ± 16.1 | 60.35 | 1.17 | 13.24 | 0.09 ± 0.0040 |
| PEGDA-400 | 240.6 ± 16.0 | 65.00 | 0.94 | 11.42 | 0.17 ± 0.0025 |
| PEGDA-700 | 360.9 ± 5.1 | 57.52 | 1.31 | 15.14 | 0.05 ± 0.0010 |
| VBC Content | Membrane Thickness (μm) | Contact Angle (°) | IEC (mmol·g−1) | Water Uptake (%) | Membrane Surface Resistance (Ω·cm2) |
|---|---|---|---|---|---|
| 10% VBC | 271.3 ± 3.3 | 66.76 | 0.93 | 10.75 | 0.36 ± 0.0106 |
| 15% VBC | 240.6 ± 16.0 | 65.00 | 0.94 | 11.42 | 0.17 ± 0.0025 |
| 20% VBC | 247.5 ± 18.3 | 61.93 | 1.17 | 14.10 | 0.12 ± 0.0070 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Yang, H.; Lv, X.; Zhang, X.; Jegatheesan, V.; Zhou, X.; Zhang, Y. Characterization and Performance Evaluation of Digital Light Processing 3D Printed Functional Anion Exchange Membranes in Electrodialysis. Processes 2024, 12, 1043. https://doi.org/10.3390/pr12061043
Yu X, Yang H, Lv X, Zhang X, Jegatheesan V, Zhou X, Zhang Y. Characterization and Performance Evaluation of Digital Light Processing 3D Printed Functional Anion Exchange Membranes in Electrodialysis. Processes. 2024; 12(6):1043. https://doi.org/10.3390/pr12061043
Chicago/Turabian StyleYu, Xue, Hongyi Yang, Xinran Lv, Xin Zhang, Veeriah Jegatheesan, Xiaobin Zhou, and Yang Zhang. 2024. "Characterization and Performance Evaluation of Digital Light Processing 3D Printed Functional Anion Exchange Membranes in Electrodialysis" Processes 12, no. 6: 1043. https://doi.org/10.3390/pr12061043
APA StyleYu, X., Yang, H., Lv, X., Zhang, X., Jegatheesan, V., Zhou, X., & Zhang, Y. (2024). Characterization and Performance Evaluation of Digital Light Processing 3D Printed Functional Anion Exchange Membranes in Electrodialysis. Processes, 12(6), 1043. https://doi.org/10.3390/pr12061043








