Analysis of the Processes of Paraffin Deposition of Oil from the Kumkol Group of Fields in Kazakhstan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oil Density
2.2. Extraction of Paraffins, Asphaltenes, and Resins
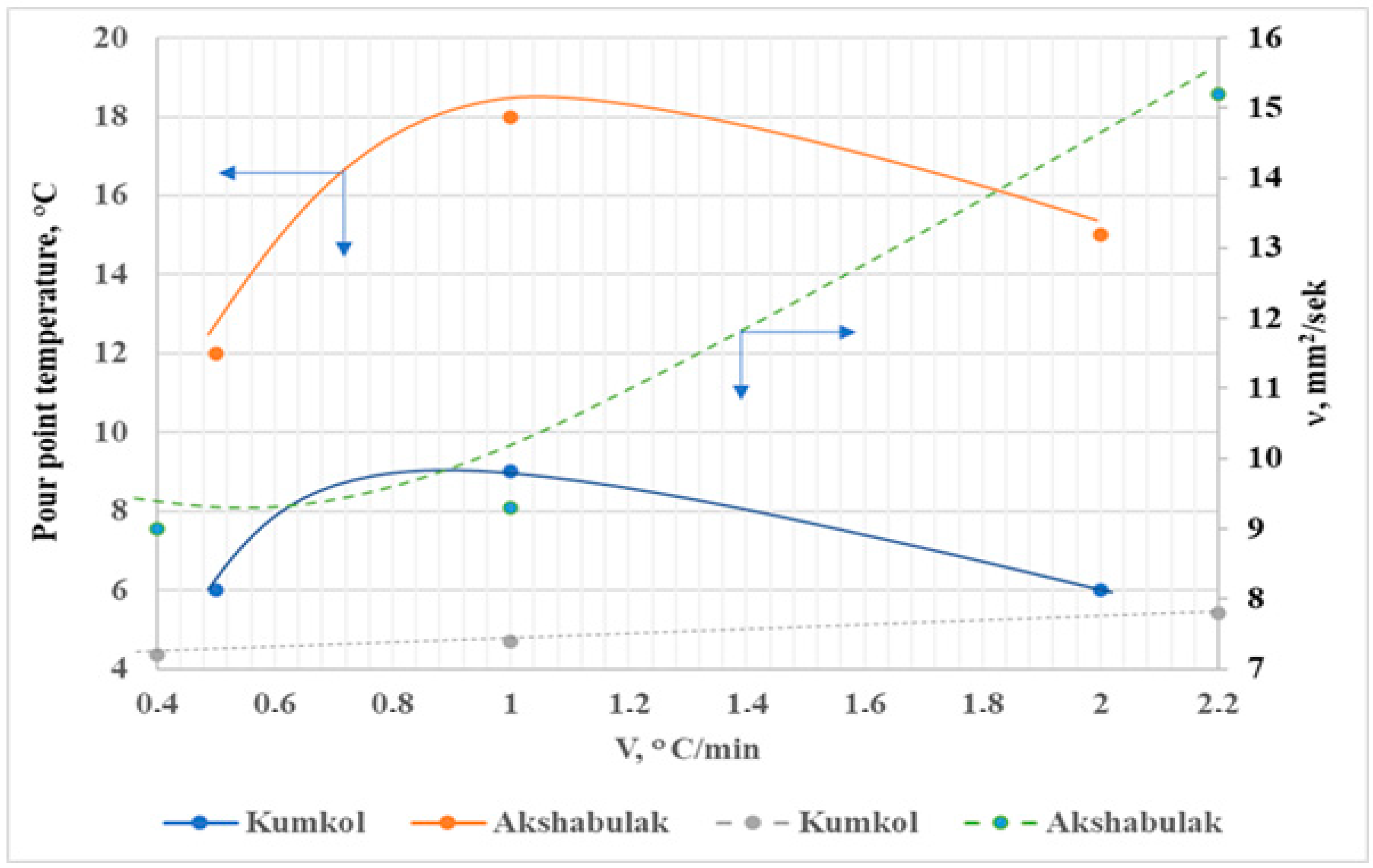
2.3. The Pour Point Temperature
2.4. The Kinematic Viscosity
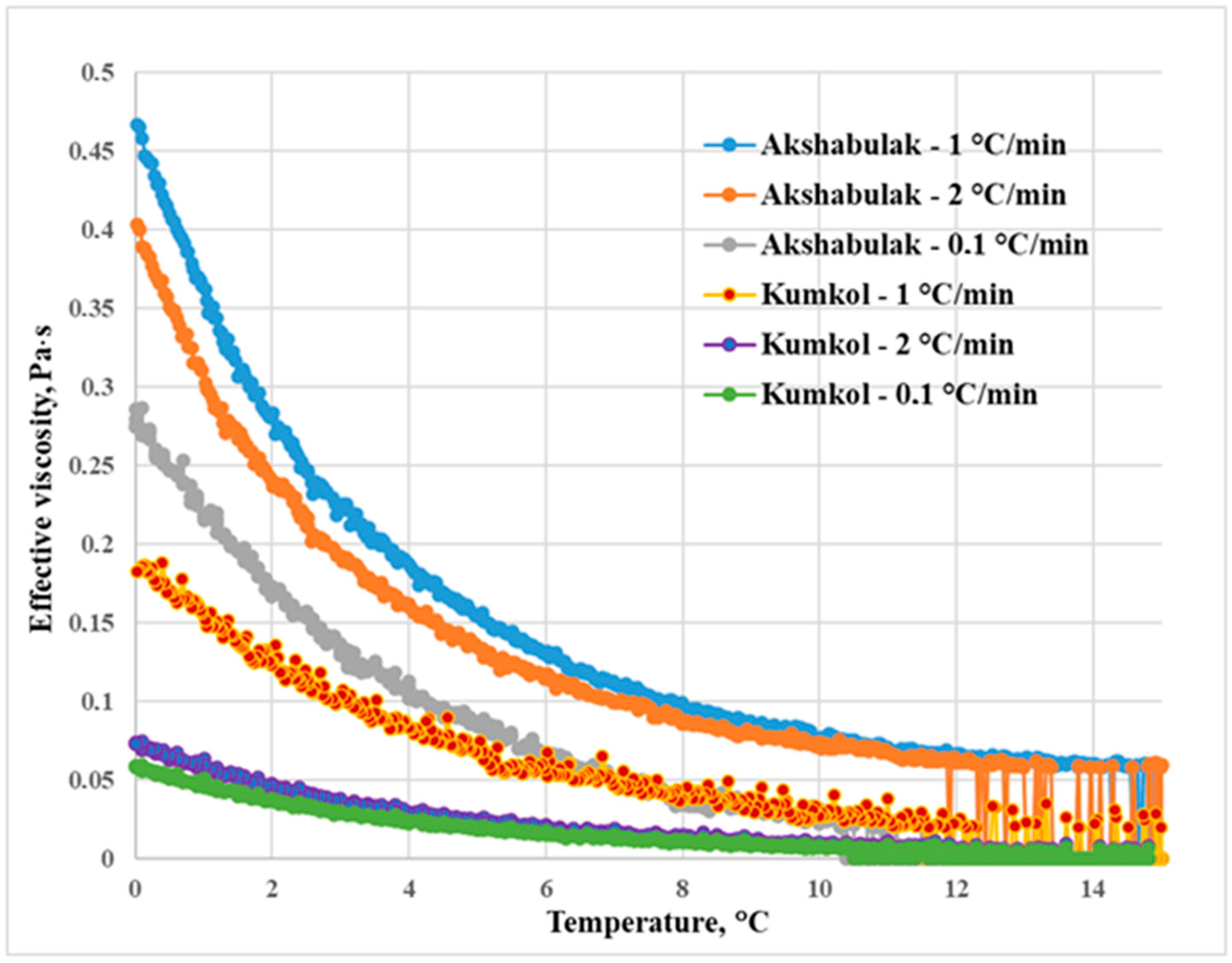
2.5. The Effective Viscosity and Shear Stress
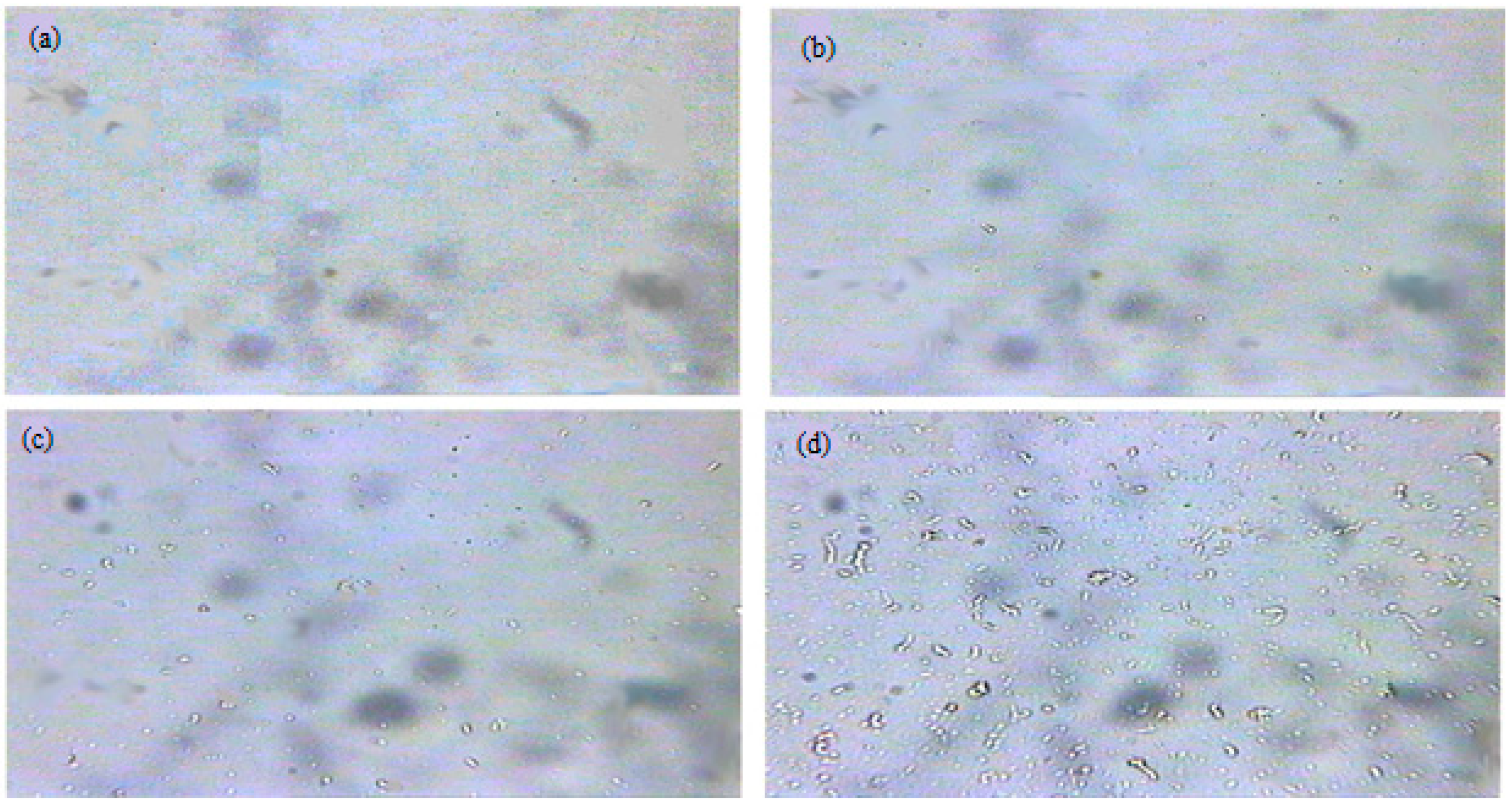
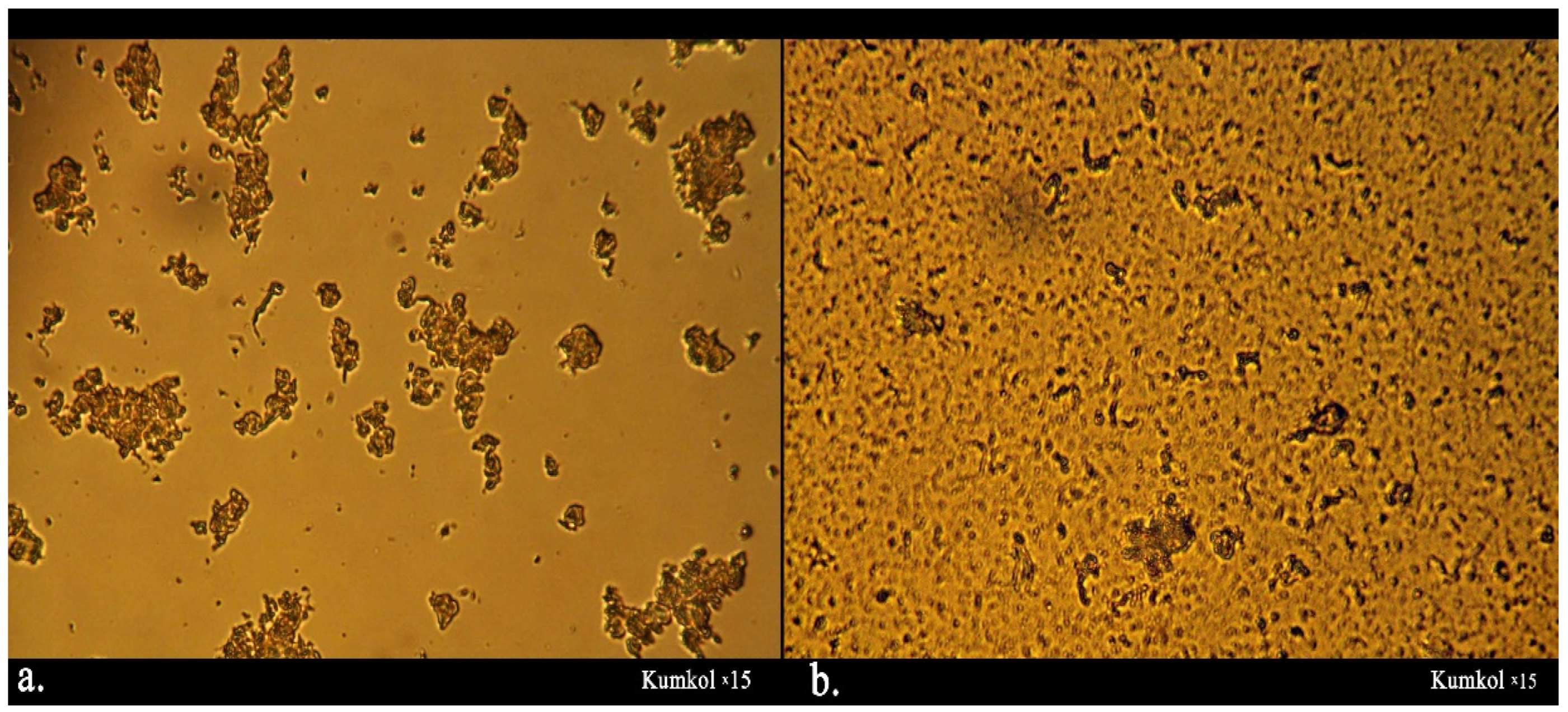
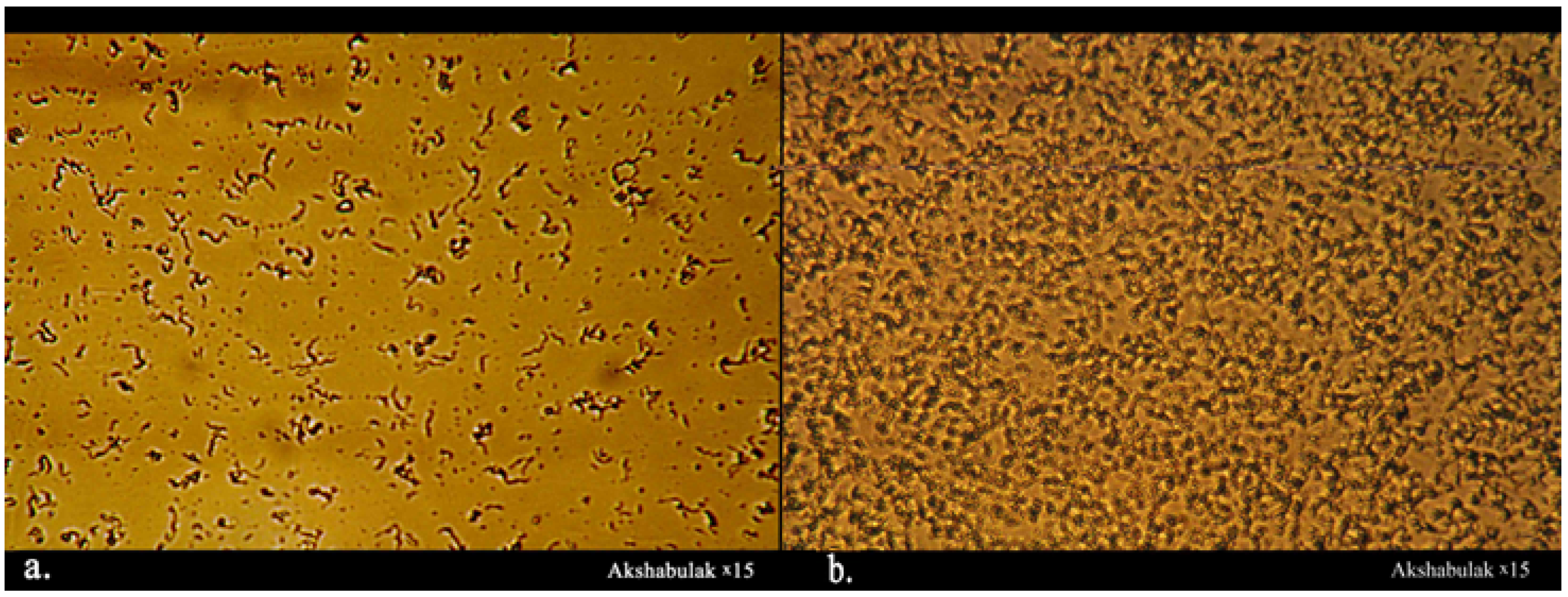
2.6. Oil Microstructure, Paraffin Melting Temperature, and the Onset of Paraffin
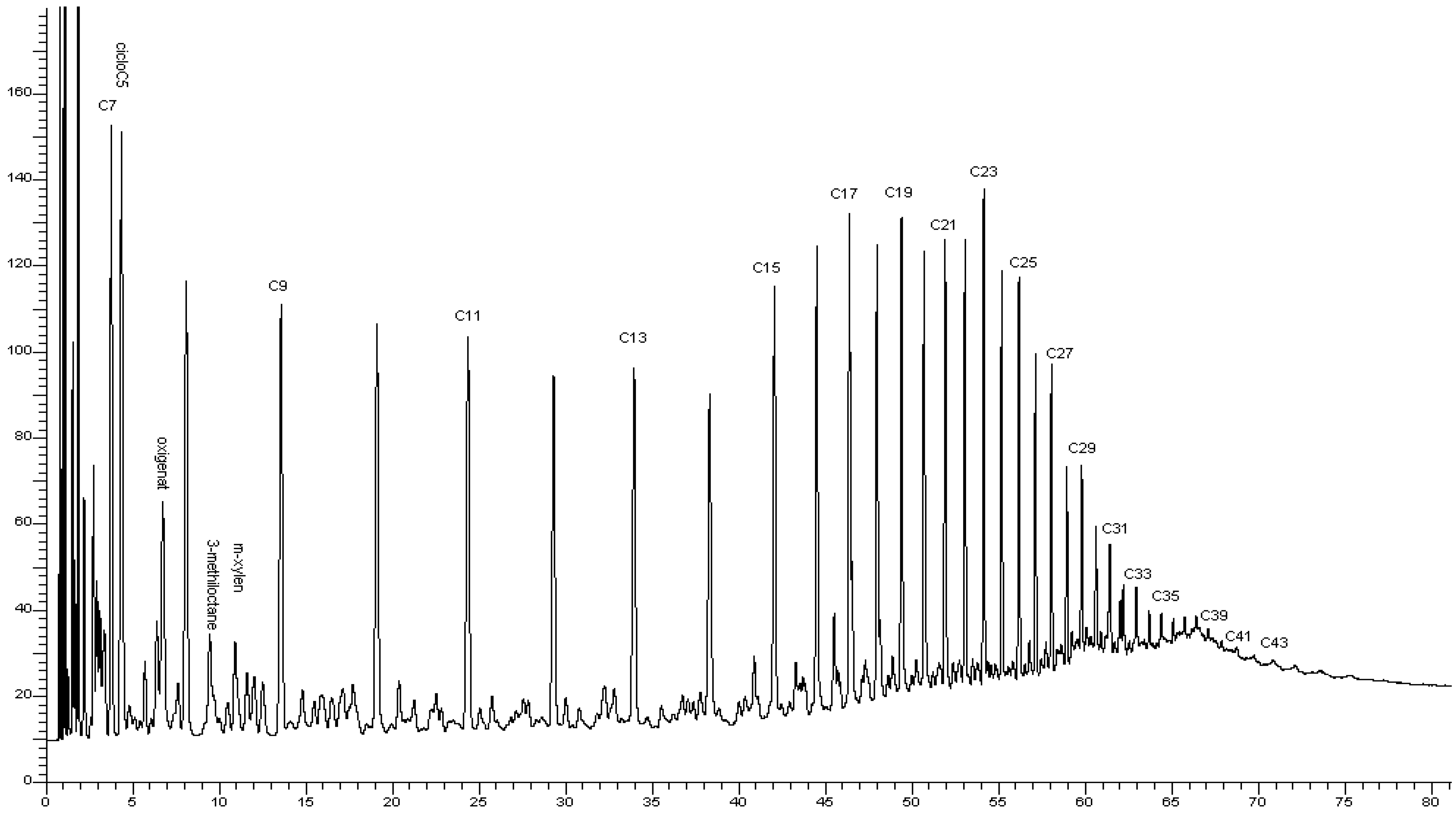
2.7. Gas Chromatographic Analysis
2.8. Inhibition of Asphalt–Resin–Paraffin Deposits
3. Results and Discussion
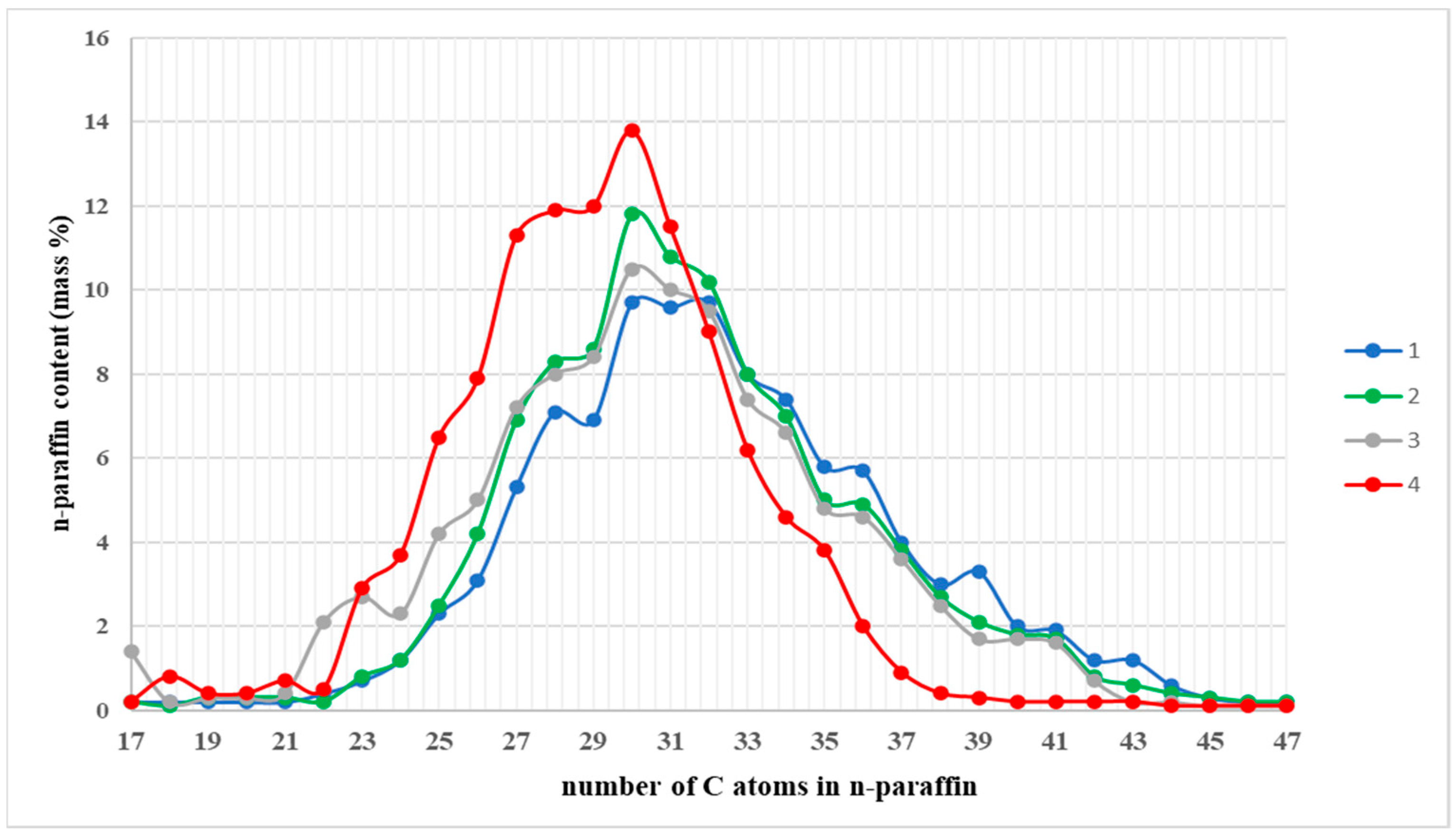
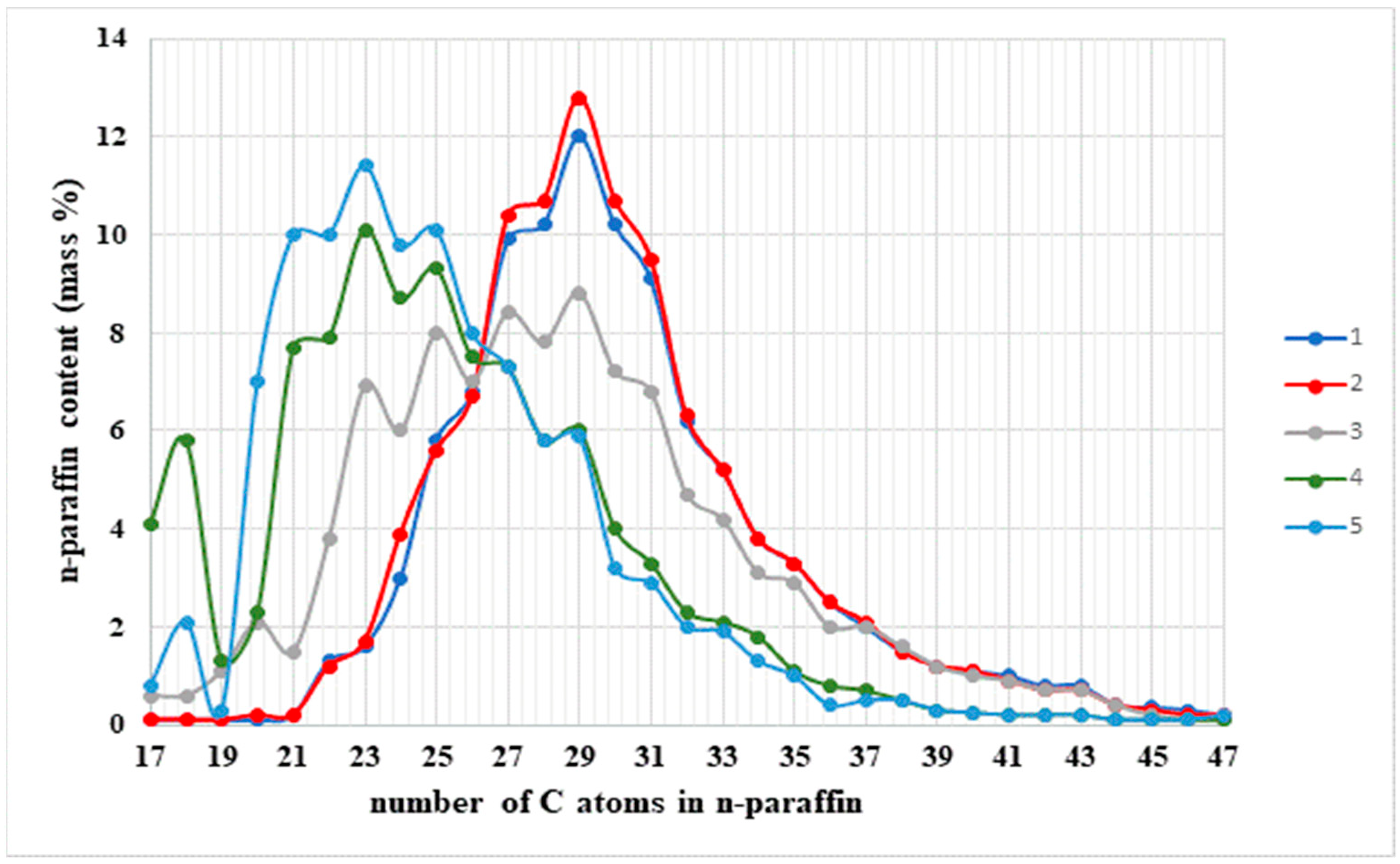
3.1. The Composition, Structure, and Nature of the Components of Normal Alkanes in Oil and Their Influence on the Aggregative Stability of the Paraffinic Oils of the Kumkol Group of Fields
3.2. The Influence of Heat Treatment and Cooling Rate on the Processes of Paraffin Deposition of Oils
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Korshak, A.A.; Nechval, A.M. Pipeline Transport of Oil, Oil Products and Gas; UGNTU: Ufa, Russia, 2005; 516p. [Google Scholar]
- Matzain, A.; Apte, M.S.; Zhang, H.Q.; Volk, M.; Brill, J.P.; Creek, J.L. Investigation of paraffin deposition during multiphase flow in pipelines and wellbores—Part 1: Experiments. J. Energy Resour. Technol. 2002, 124, 180–186. [Google Scholar] [CrossRef]
- Aiyejina, A.; Chakrabarti, D.P.; Pilgrim, A.; Sastry, M. Wax formation in oil pipelines: A critical review. Int. J. Multiphase Flow 2011, 37, 671–694. [Google Scholar] [CrossRef]
- Loskutova, Y.V.; Ryzhova, N.S.; Yudina, N.V.; Beshagina, E.V. The influence of processing conditions on the sedimentation kinetics of highly waxy crude oil. Procedia Chem. 2015, 15, 49–53. [Google Scholar] [CrossRef]
- Terteryan, R.A. Depressor Additives for Crude Oils, Fuels and Lube Oils (Khimiya, Moscow, 1990). Khimiya 1990, 237. (In Russian) [Google Scholar]
- Wang, L.S.; Flamberg, A.; Kikabhai, T. Select the optimum pour point depressant. Hydrocarb. Process. 1999, 78, 59–62. [Google Scholar]
- del Carmen Garcia, L.M.; Carbognani, M.; Orea, A. Urbina. The influence of alkane class-types on crude oil wax crystallization and inhibitors efficiency. J. Pet. Sci. Eng. 2000, 25, 99–105. [Google Scholar] [CrossRef]
- Tumanyan, B.P. Scientific applied aspects of the theory of oil dispersed systems. Technics 2000, 336. [Google Scholar]
- Garcia, M.d.C.; Orea, M.; Carbognani, L.; Urbina, A. The Effect of paraffinic fractions on crude oil wax crystallization. Pet. Sci. Technol. 2001, 19, 189–196. [Google Scholar] [CrossRef]
- Kazakova, L.P. Solid hydrocarbons of oil. Khimiya 1986, 176. [Google Scholar]
- Vignati, E.; Piazza, R.; Visintin, R.F.G.; Lapasin, R.; D’Antona, P.; Lockhart, T.P. Wax crystallization and aggregation in a model crude oil. J. Phys. Condens. Matter 2005, 17, S3651–S3660. [Google Scholar] [CrossRef]
- Alhejaili, A.; Bell, E.; Daraboina, N. Paraffin Deposition in Production Lines: Effect of Operating Parameters on Deposition Characteristics. Energy Fuels 2023, 37, 18642–18651. [Google Scholar] [CrossRef]
- Jennings, D.W.; Weispfennig, K. Effects of shear and temperature on wax deposition: Coldfinger investigation with a Gulf of Mexico crude oil. Energy Fuels 2005, 19, 1376–1386. [Google Scholar] [CrossRef]
- Padula, L.; Gon, S.; Russell, C.; Pohl, E.; Littlehales, I.; Neilson, A. Systematic study of temperature and different types of mixing in paraffin deposition test methods. ACS Omega 2020, 5, 12320–12328. [Google Scholar] [CrossRef] [PubMed]
- Affens, W.A.; Hall, J.M.; Hazlett, R.N. Effect of composition on freezing points of model hydrocarbon fuels. Fuel 1984, 63, 543–547. [Google Scholar] [CrossRef]
- GOST 3900 (ST SEV 6754-89); Crude Oil and Petroleum Products. Methods of Density Determination: Moscow, Russia, 2023.
- GOST 11851; Petroleum. Methods of Determination of Paraffins: Moscow, Russia, 2018.
- ST RK ASTM D 5853; Standart Test Method for Pour. Point of Crude Oils: Astana, Kazakhstan, 2010.
- ASTM D 445; 2011 Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). IDT: Astana, Kazakhstan, 2011.
- GOST 1929; Petroleum Products. Methods of Test for Determination of Dynamic Viscosity by Rotary Viscosimeter. USSR State Committee for Standards: Moscow, Russia, 1987.
- ST RK ASTM D 2887; Standard Method for Determination of Boiling Point Intervals of Oil Fractions by Gas Chromatography. Astana, Kazakhstan, 2019.
- Mussabayeva, B.K.; Orazzhanova, L.K.; Kudaibergenov, S.E.; Kassymbayeva, D.S. Selection of depressor additives for transportation of high-viscositive Kazakhstan oils. Bull. Karaganda Univ. Chem. 2015, 59. [Google Scholar]
- Kucherov, V.G.; Chernoutsan, A.I. Reciprocal influence of crystallization and vitrification processes in complex hydrocarbon systems. I. M. Gubkin Russian State University of Oil and Gas. Khimiya I Tekhnologiya Topl. I Masel 2006, 3, 36–38. [Google Scholar]
- Malkin, A.Y.; Isaev, A.I. Rheology: Concepts, methods, applications. St. Petersburg. ChemTec Publishing: St. Petersburg, Russia, 2005; pp. 164–165. [Google Scholar]
- Mereke, A.; Bauyrzhan, A. Analysis of Kazakhstan Crude Oil Biomarkers by Gas Chromatography in Combination with Mass Spectrometry. Separations 2023, 10, 561. [Google Scholar] [CrossRef]
- Santos, J.S.T.D.; Fernandes, A.C.; Giulietti, M. Study of the paraffin deposit formation using the cold finger methodology for brazilian crude oils. J. Pet. Sci. Eng. 2004, 45, 47–60. [Google Scholar] [CrossRef]
- Bello, O.O.; Teodoriu, C.; Reinicke, K.M. An evaluation of the performance of selected wax inhibitors on paraffin deposition of Nigerian crude oils. Pet. Sci. Technol. 2006, 24, 195–206. [Google Scholar] [CrossRef]
- Soorghali, F.; Zolghadr, A.; Ayatollahi, S. Effect of resins on asphaltene deposition and the changes of surface properties at different pressures: A microstructure study. Energy Fuels 2014, 28, 2415–2421. [Google Scholar] [CrossRef]
- Hammami, A.; Phelps, C.H.; Monger-McClure, T.; Little, T. Asphaltene precipitation from live oils: An experimental investigation of onset conditions and reversibility. Energy Fuels 2000, 14, 14–18. [Google Scholar] [CrossRef]
- Speight, J.G. The chemical and physical structure of petroleum: Effects on recovery operations. J. Pet. Sci. Eng. 1999, 22, 3–15. [Google Scholar] [CrossRef]
- Sedghi, M.; Goual, L. Role of Resins on Asphaltene Stability. Energy Fuels 2009, 24, 2275–2280. [Google Scholar] [CrossRef]
- Pereira, J.C.; López, I.; Salas, R.; Silva, F.; Fernández, C.; Urbina, C.; López, J.C. Resins: The molecules responsible for the stability/instability phenomena of asphaltenes. Energy Fuels 2007, 21, 1317–1321. [Google Scholar] [CrossRef]
- Jennings, D.W.; Weispfennig, K. Experimental solubility data of various n-alkane waxes: Effects of alkane chain length, alkane odd versus even carbon number structures and solvent chemistry on solubility. Fluid Phase Equilibria 2005, 227, 27–35. [Google Scholar] [CrossRef]
- Rehan, M.; Nizami, A.-S.; Taylan, O.; Al-Sasi, B.O.; Demirbas, A. Determination of wax content in crude oil. Pet. Sci. Technol. 2016, 34, 799–804. [Google Scholar] [CrossRef]
- Kok, M.V.; Letofe, J.M.; Clau, P. Comparative methods in the determination of wax content and pour points of crude oils. J. Therm. Anal. Calorim. 2007, 90, 827–831. [Google Scholar] [CrossRef]
- Loskutova, Y.V.; Yudina, N.V. Influence of a permanent magnetic field on structural and mechanical properties of paraffinic oils. Neftekhimiya 2004, 1, 63–67. [Google Scholar]
- Loskutova, Y.V.; Prozorova, I.V.; Yudina, N.V.; Rikkonen, S.V.; Daneker, V.A. Change of rheological properties of high-paraffin oils under the influence of vibrojet magnetic activation (in Russian). Eng. Phys. J. 2004, 77, 27–34. [Google Scholar]
- Lukman, I.; Robin, E.W.; Xiongwei, N. On the effect of wax content on paraffin wax deposition in a batch oscillatory baffled tube apparatus. Chem. Eng. J. 2008, 137, 205–213. [Google Scholar] [CrossRef]
- Mansoori, G.A.; Barnes, H.L.; Webster, G.M. Petroleum waxes. Fuels Lubr. Handb. 2003, 19, 525–558. [Google Scholar]
- Li, B.; Guo, Z.; Zheng, L.; Shi, E.; Qi, B. A comprehensive review of wax deposition in crude oil systems: Mechanisms, influencing factors, prediction and inhibition techniques. Fuel 2024, 357, 129676. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J. A generalized model for predicting non-Newtonian viscosity of waxy crudes as a function of temperature and precipitated wax. Fuel 2003, 82, 1387–1397. [Google Scholar] [CrossRef]


| Oil | ρ, kg/m3 | Asphaltenes, % | Paraffins,% | Resins, % |
|---|---|---|---|---|
| Kumkol | 821.6 | 0.6 | 14.0 | 6.4 |
| Akshabulak | 815.1 | 0.8 | 16.2 | 6.1 |
| Oil | Kinematic Viscosity, mm2/s | FLT, °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 20 °C | 25 °C | 30 °C | 35 °C | 40 °C | 45 °C | 50 °C | 55 °C | 60 °C | ||
| Kumkol | 11.850 | 9.295 | 6.740 | 5.777 | 4.814 | 4.384 | 3.954 | 3.662 | 3.370 | +9 |
| Akshabulak | 11.220 | 8.956 | 6.692 | 5.627 | 4.563 | 4.091 | 3.619 | 3.330 | 3.042 | +12 |
| Oil Sample | t, °C | τ, Pa (D = 5 s−1) | η, Pa·sec (D = 5 s −1) | τ, Pa (D = 10 s−1) | η, Pa·s (D = 10 s−1) | τ0, Pa | Ktek, Pa·s |
|---|---|---|---|---|---|---|---|
| Kumkol | 20 | 0.020 | 0.004 | 0.040 | 0.004 | 0 | 0.004 |
| 15 | 0.035 | 0.007 | 0.204 | 0.020 | 0.031 | 0.018 | |
| 10 | 1.119 | 0.222 | 1.623 | 0.161 | 1.389 | 0.057 | |
| 5 | 6.171 | 1.222 | 7.318 | 0.725 | 5.025 | 0.165 | |
| 0 | 21.852 | 4.327 | 26.833 | 2.657 | 29.350 | 0.409 | |
| Akshabulak | 25 | 0.035 | 0.007 | 0.070 | 0.007 | 0 | 0.007 |
| 20 | 0.140 | 0.028 | 0.228 | 0.023 | 0 | 0.023 | |
| 15 | 2.705 | 0.536 | 3.543 | 0.351 | 3.564 | 0.073 | |
| 10 | 9.547 | 1.890 | 11.054 | 1.094 | 10.650 | 0.143 | |
| 5 | 26.371 | 5.222 | 29.671 | 2.938 | 30.530 | 0.408 |
| Oil | Hydrocarbons and Paraffins (C4–C14), % | Paraffins, % | |||
|---|---|---|---|---|---|
| C4–C8 | C9–C14 | C15–C19 | C20–C29 | C30–C44 | |
| Kumkol | 16.32 | 20.49 | 22.06 | 32.36 | 9.75 |
| Akshabulak | 1.12 | 24.55 | 29.61 | 37.20 | 6.40 |
| Conditions | ARPD Mass, g | Amount of Solid Paraffins in ARPD*, % | Appearance | ||
|---|---|---|---|---|---|
| Toil, °C | Trod, °C | ΔT, °C | |||
| 60 | 6 | 54 | 6.6 | 23.4 | hard, dense, fine grained |
| 50 | 6 | 44 | 4.8 | 19.9 | hard, dense, fine grained |
| 40 | 6 | 34 | 7.8 | 13.7 | hard, loose, coarse grained |
| 30 | 6 | 24 | 7.2 | 11.1 | hard, coarse grained |
| 20 | 6 | 14 | 7.8 | 8.6 | friable, liquid at room temperature |
| Conditions | ARPD Mass, g | Amount of Solid Paraffins in ARPD*, % | Appearance Trod, °C | ||
|---|---|---|---|---|---|
| Toil, °C | Trod, °C | ΔT, °C | |||
| 60 | 12 | 48 | 6.6 | 30.6 | hard, dense, fine grained |
| 50 | 12 | 38 | 7.2 | 20.2 | hard, dense, fine grained |
| 40 | 12 | 28 | 15.0 | 12.4 | hard, dense, coarse grained |
| 30 | 12 | 18 | 50.4 | 9.9 | liquid at room temperature |
| 20 | 12 | 8 | 10.2 | 9.8 | liquid at room temperature |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boranbayeva, L.; Boiko, G.; Sharifullin, A.; Lubchenko, N.; Sarmurzina, R.; Kozhamzharova, A.; Mombekov, S. Analysis of the Processes of Paraffin Deposition of Oil from the Kumkol Group of Fields in Kazakhstan. Processes 2024, 12, 1052. https://doi.org/10.3390/pr12061052
Boranbayeva L, Boiko G, Sharifullin A, Lubchenko N, Sarmurzina R, Kozhamzharova A, Mombekov S. Analysis of the Processes of Paraffin Deposition of Oil from the Kumkol Group of Fields in Kazakhstan. Processes. 2024; 12(6):1052. https://doi.org/10.3390/pr12061052
Chicago/Turabian StyleBoranbayeva, Laura, Galina Boiko, Andrey Sharifullin, Nina Lubchenko, Raushan Sarmurzina, Assel Kozhamzharova, and Serzhan Mombekov. 2024. "Analysis of the Processes of Paraffin Deposition of Oil from the Kumkol Group of Fields in Kazakhstan" Processes 12, no. 6: 1052. https://doi.org/10.3390/pr12061052





