Development of Macro-Encapsulated Phase-Change Material Using Composite of NaCl-Al2O3 with Characteristics of Self-Standing
Abstract
:1. Introduction
2. Experimental
2.1. Preparation
2.2. Characterization
2.2.1. Microstructure and Phase Analysis of Sample

2.2.2. Performance Testing of Samples
3. Results and Discussion
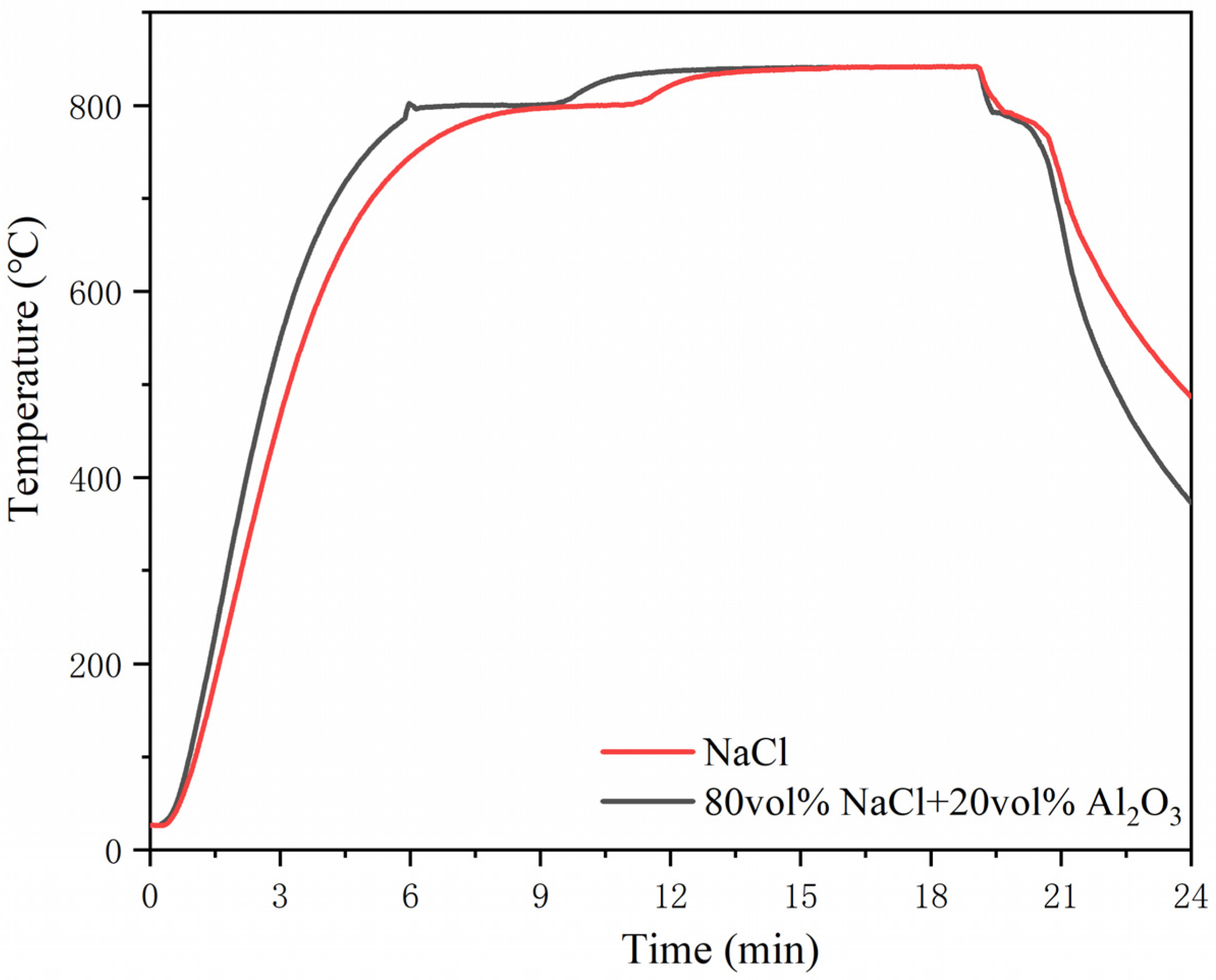
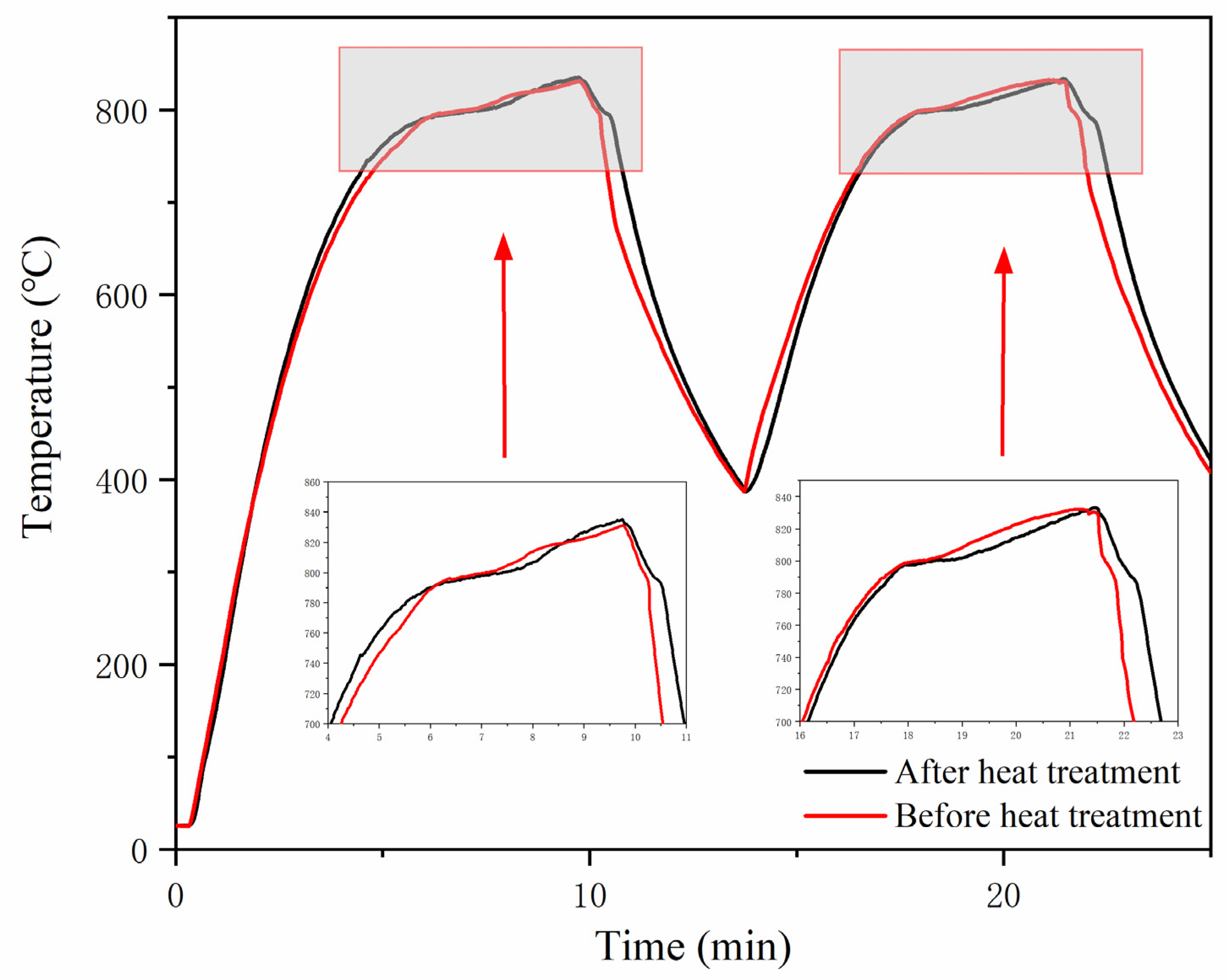
3.1. The Rapid Heat Storage and Release Performance
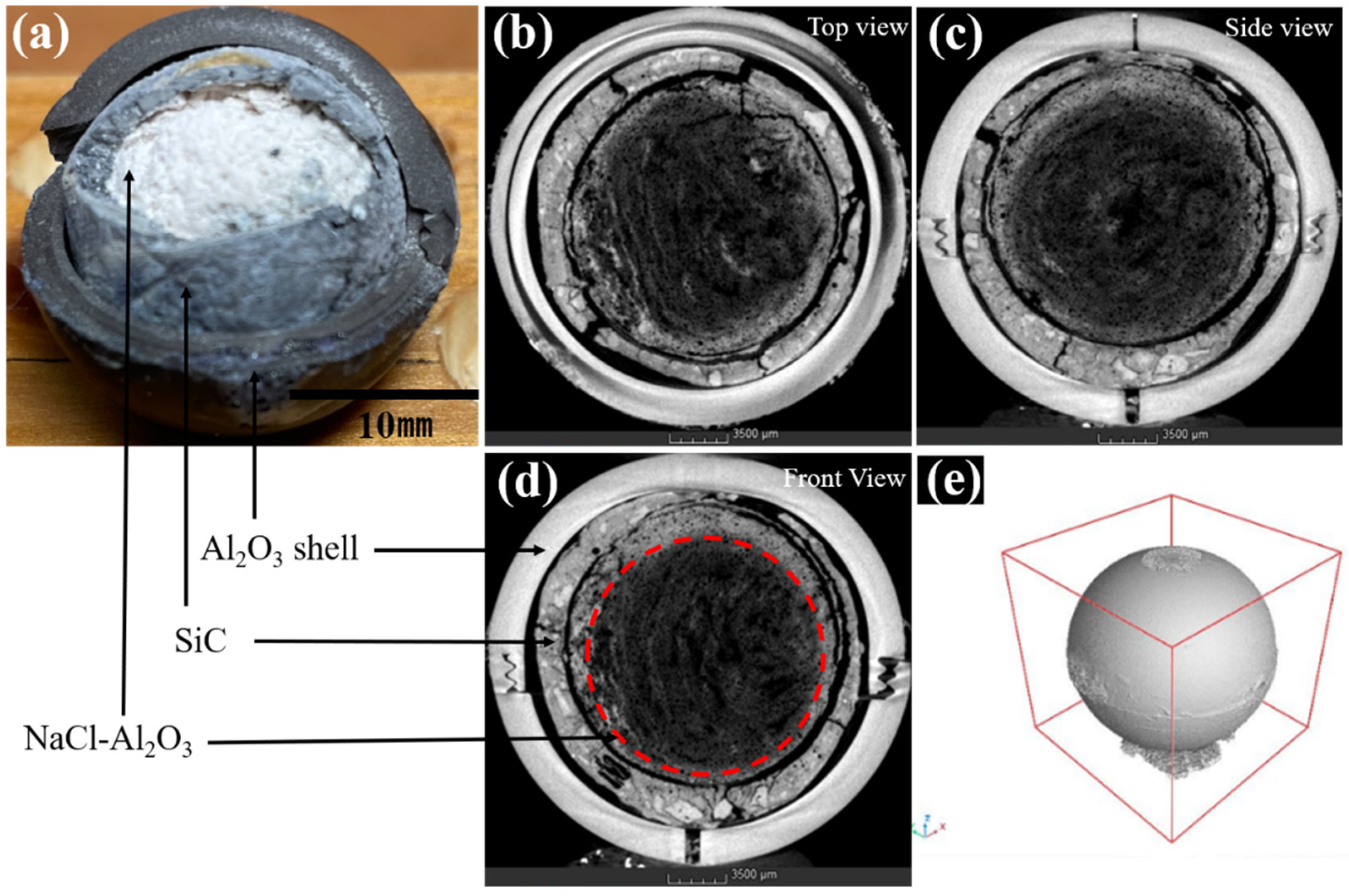
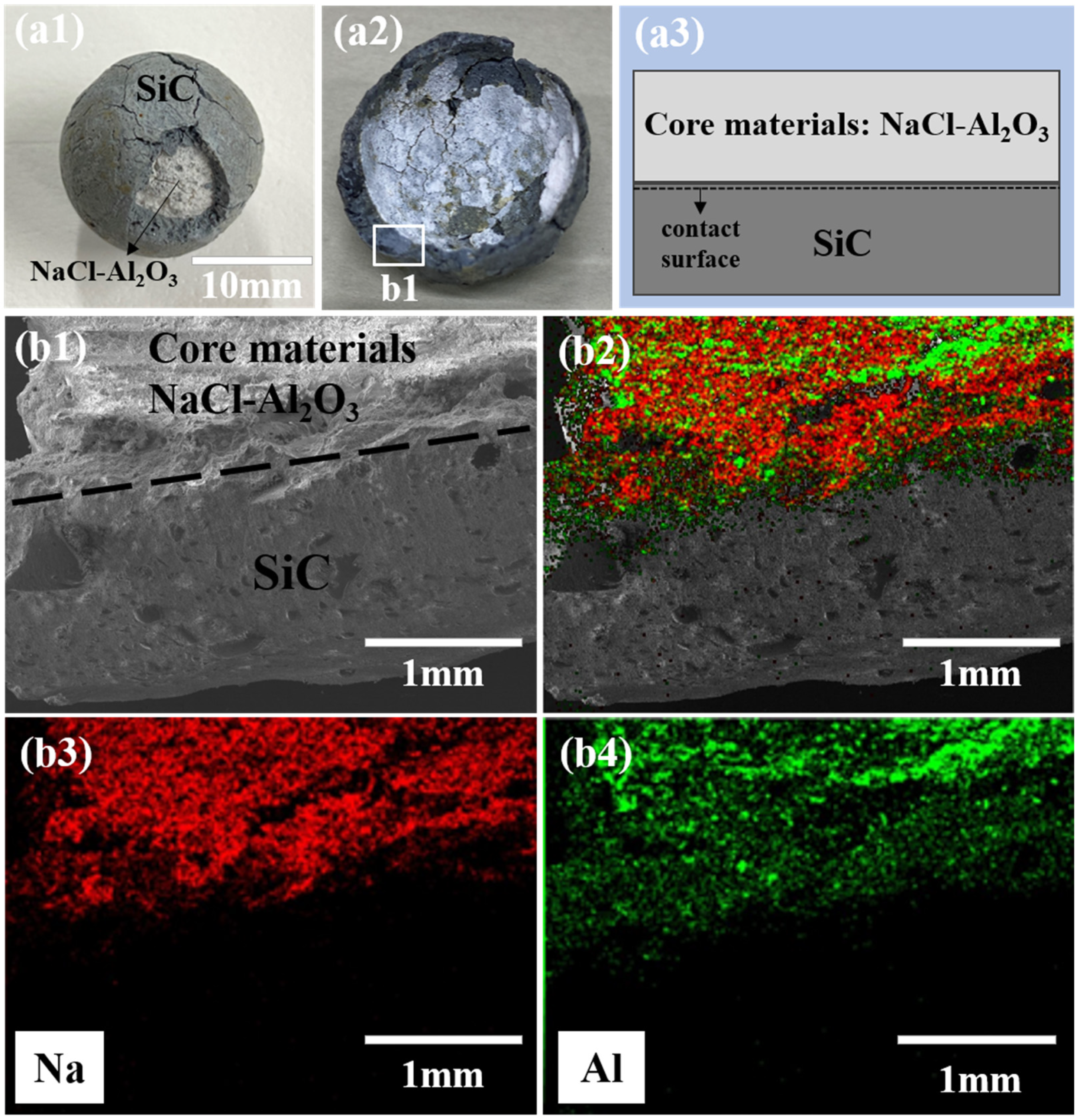
3.2. Leakage Prevention Test and Improvement Mechanism
3.3. Durability Performance after over 1000 h of High-Temperature Treatment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Kannan, N.; Vakeesan, D. Solar energy for future world:-A review. Renew. Sustain. Energy Rev. 2016, 62, 1092–1105. [Google Scholar] [CrossRef]
- Aneke, M.; Wang, M. Energy storage technologies and real life applications—A state of the art review. Appl. Energy 2016, 179, 350–377. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, S.; Yamashita, S.; Kubota, M.; Takeuchi, A.; Gao, P.; Liu, M.; Li, R.; Zhang, C.; Kita, H. Enhanced radiation heat transfer performance of Alumina-Spinel composite sphere with hollow structure for rapidly ultra-high temperature thermal storage/release process. Chem. Eng. J. 2024, 479, 147381. [Google Scholar] [CrossRef]
- Xu, B.; Li, P.; Chan, C. Application of phase change materials for thermal energy storage in concentrated solar thermal power plants: A review to recent developments. Appl. Energy 2015, 160, 286–307. [Google Scholar] [CrossRef]
- Aftab, W.; Usman, A.; Shi, J.; Yuan, K.; Qin, M.; Zou, R. Phase change material-integrated latent heat storage systems for sustainable energy solutions. Energy Environ. Sci. 2021, 14, 4268–4291. [Google Scholar] [CrossRef]
- Kenisarin, M.M. Thermophysical properties of some organic phase change materials for latent heat storage. A review. Sol. Energy 2014, 107, 553–575. [Google Scholar] [CrossRef]
- Sarbu, I.; Dorca, A. Review on heat transfer analysis in thermal energy storage using latent heat storage systems and phase change materials. Int. J. Energy Res. 2019, 43, 29–64. [Google Scholar] [CrossRef]
- Tao, Y.; He, Y.-L. A review of phase change material and performance enhancement method for latent heat storage system. Renew. Sustain. Energy Rev. 2018, 93, 245–259. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, M.H.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.; Sahin, A. A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Da Cunha, J.P.; Eames, P. Thermal energy storage for low and medium temperature applications using phase change materials—A review. Appl. Energy 2016, 177, 227–238. [Google Scholar] [CrossRef]
- Lin, Y.; Alva, G.; Fang, G. Review on thermal performances and applications of thermal energy storage systems with inorganic phase change materials. Energy 2018, 165, 685–708. [Google Scholar] [CrossRef]
- Agarwala, S.; Prabhu, K.N. Review of thermal characterization techniques for salt-based phase change materials. J. Energy Storage 2022, 46, 103865. [Google Scholar] [CrossRef]
- Li, M.-J.; Jin, B.; Ma, Z.; Yuan, F. Experimental and numerical study on the performance of a new high-temperature packed-bed thermal energy storage system with macroencapsulation of molten salt phase change material. Appl. Energy 2018, 221, 1–15. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, C.; Lin, Y.; Fang, G. Thermal properties and applications of microencapsulated PCM for thermal energy storage: A review. Appl. Therm. Eng. 2019, 147, 841–855. [Google Scholar] [CrossRef]
- Wickramaratne, C.; Dhau, J.S.; Kamal, R.; Myers, P.; Goswami, D.; Stefanakos, E. Macro-encapsulation and characterization of chloride based inorganic Phase change materials for high temperature thermal energy storage systems. Appl. Energy 2018, 221, 587–596. [Google Scholar] [CrossRef]
- Yao, X.; Chang, Y.; Gu, H.; Guo, J.; Zou, D. Preparation and thermophysical properties of a novel metallic microencapsulated phase change material/eutectic salt/ceramic composite. Chem. Eng. J. 2023, 477, 146967. [Google Scholar] [CrossRef]
- Sheng, N.; Zhu, H.; Zhang, L.; Zeng, L.; Zhu, C. Macroencapsulation of sodium chloride by a double layer strategy for high temperature phase change thermal storage over 800 °C. J. Energy Storage 2024, 77, 109896. [Google Scholar] [CrossRef]
- Yamashita, S.; Fuhai, B.; Shenghao, L.; Kita, H.; Hong, F. Novel Sodium Chloride/Aluminum Oxide Powder-Composite Structure with High Shape-Retention Performance for the Encapsulation of a High-Temperature Phase-Change Material. Processes 2024, 12, 465. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Gavrilov, A.N.; McCloskey, J.M.; Tolmachev, Y.V.; Sprunt, S.; Lopatina, L.M.; Selinger, J.V. Thermal conductivity and particle agglomeration in alumina nanofluids: Experiment and theory. Phys. Rev. E 2007, 76, 061203. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Qi, S.; Tu, C.; Zhao, H.; Wang, C.; Kou, J. Effect of the particle size of Al2O3 on the properties of filled heat-conductive silicone rubber. J. Appl. Polym. Sci. 2007, 104, 1312–1318. [Google Scholar] [CrossRef]
- Tatar, C.; Özdemir, N. Investigation of thermal conductivity and microstructure of the α-Al2O3 particulate reinforced aluminum composites (Al/Al2O3-MMC) by powder metallurgy method. Phys. B Condens. Matter 2010, 405, 896–899. [Google Scholar] [CrossRef]
- Hu, Y.; Du, G.; Chen, N. A novel approach for Al2O3/epoxy composites with high strength and thermal conductivity. Compos. Sci. Technol. 2016, 124, 36–43. [Google Scholar] [CrossRef]

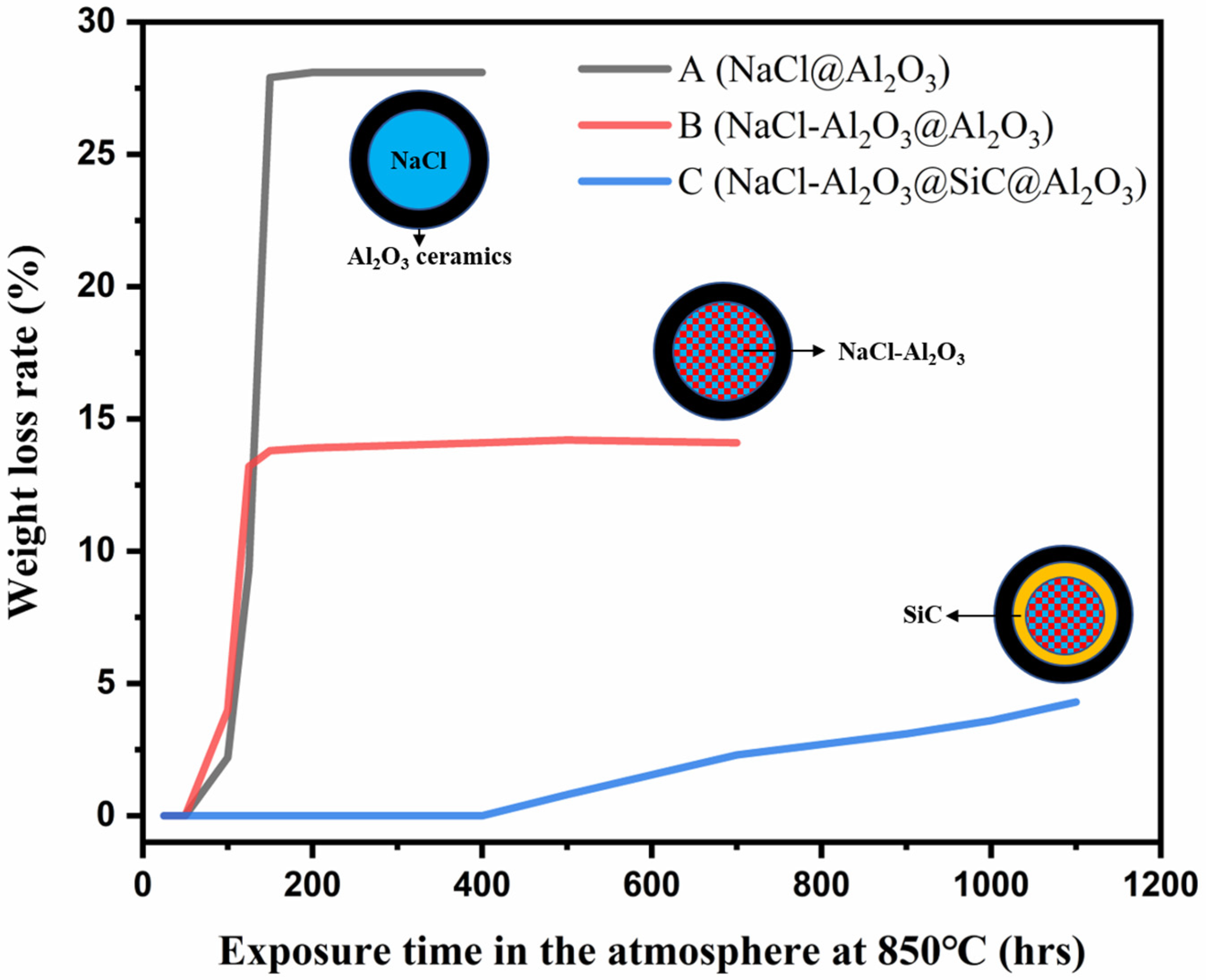


| Sample | 25 h | 100 h | 150 h | 200 h | 400 h | 700 h | 1000 h |
|---|---|---|---|---|---|---|---|
| A | 0 | 2.2 | 27.9 | 28.1 | 28.1 | - | - |
| B | 0 | 4 | 13.8 | 13.9 | 14.1 | 14.1 | - |
| C | 0 | 0 | 0 | 0 | 0 | 2.3 | 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, S.; Zhou, X.; Chen, X.; Li, Z.; Yamashita, S.; Zhang, C.; Kita, H. Development of Macro-Encapsulated Phase-Change Material Using Composite of NaCl-Al2O3 with Characteristics of Self-Standing. Processes 2024, 12, 1123. https://doi.org/10.3390/pr12061123
Liao S, Zhou X, Chen X, Li Z, Yamashita S, Zhang C, Kita H. Development of Macro-Encapsulated Phase-Change Material Using Composite of NaCl-Al2O3 with Characteristics of Self-Standing. Processes. 2024; 12(6):1123. https://doi.org/10.3390/pr12061123
Chicago/Turabian StyleLiao, Shenghao, Xin Zhou, Xiaoyu Chen, Zhuoyu Li, Seiji Yamashita, Chaoyang Zhang, and Hideki Kita. 2024. "Development of Macro-Encapsulated Phase-Change Material Using Composite of NaCl-Al2O3 with Characteristics of Self-Standing" Processes 12, no. 6: 1123. https://doi.org/10.3390/pr12061123
APA StyleLiao, S., Zhou, X., Chen, X., Li, Z., Yamashita, S., Zhang, C., & Kita, H. (2024). Development of Macro-Encapsulated Phase-Change Material Using Composite of NaCl-Al2O3 with Characteristics of Self-Standing. Processes, 12(6), 1123. https://doi.org/10.3390/pr12061123








