Abstract
This study aimed to evaluate the sequential hydrolysis of the biomass from unconventional and versatile Y. lipolytica to recover mannoproteins, carbohydrates, and other compounds as well as to determine the antioxidant activity of ultrafiltered fractions. The crude biomass underwent autolysis, and the resulting supernatant fraction was used for mannoprotein recovery via precipitation with ethanol. The precipitate obtained after autolysis underwent acid hydrolysis, and the resulting supernatant was ultrafiltered, precipitated, and characterized. The process yields were 55.5% and 46.14% for the crude biomass grown in glucose and glycerol, respectively. The mannoprotein with higher carbohydrate content (from crude biomass grown in glycerol) exhibited a higher emulsification index of 47.35% and thermal stability (60% weight loss). In contrast, the mannoprotein with higher protein content (from crude biomass grown in glucose) showed a better surface tension reduction of 44.50 mN/m. The technological properties showed that the crude biomass and the food ingredients are feasible to apply in food processing. The fractionation of the acid hydrolysis portion allowed the evaluation of the antioxidant power synergism among the components present in the hydrolysate, mostly the protein peptide chain. The sequential hydrolysis method is viable for extracting valuable products from Y. lipolytica.
1. Introduction
Yarrowia lipolytica has recently become one of the most widely studied unconventional yeasts. This oleaginous yeast is strictly aerobic and exhibits the capability to produce a wide array of bioproducts, including proteins, sugar alcohols, lipids, amino acids, organic acids, and terpenoids [1,2,3], utilizing various substrates such as hydrophilic compounds like glucose and glycerol, as well as hydrophobic substrates like fatty acids [4,5,6,7]. Furthermore, a dimorphic yeast exhibits different growth forms (round multipolar budding cells and pseudo hyphae) that improve its biotechnological applications. The economic feasibility of Y. lipolytica in the bioprocess is related to its robustness to fermentation conditions, stability, tolerance to inhibitors, high cell density, protein secretion, safety, and others [8].
The yeast cell wall composition, composed mainly of glucans, mannoproteins, and small amounts of chitin, can be affected by different parameters such as growth conditions, culture strain, carbon source, temperature, pH, and aeration [9,10,11]. In general, those components are arranged in layers, consisting of an inner layer composed of cross-linked β-1,3- and β-1,6-linked glucans and chitin providing mechanical strength, and an outer layer consisting of mannoproteins covalently linked by β-1,6 glucan [12,13]. However, the dimorphic Y. lipolytica modulates its cell wall, mainly affecting the protein content. Thus, the protein content in yeast form is 15%, whereas the hyphae form has 6%. Moreover, the other cell wall components are similar with 70% carbohydrate, 5% lipids, and 0.6–0.8% phosphorus [9,14].
The application of Y. lipolytica in the food and feed industries is regulated worldwide by governmental agencies. This microorganism is classified as “safe to use” and received the indication “generally recognized as safe” by the US FDA [15]. Furthermore, the European Food Safety Authority (EFSA) and the International Dairy Federation (IDF) recognize it as a “microorganism with a documented use in food” [16]. The EFSA Panel on Nutrition, Novel Foods, and Food Allergens (NDA) has considered Y. lipolytica yeast as safe biomass for use in food supplements at amounts of up to 3 g/day for children aged 3 to less than 10 years and up to 6 g/day thereafter [17]. Among the biomass highlights, its high protein content (ranging from 30% to 56%) stands out, along with significant levels of essential amino acids (lysine, phenylalanine, valine, tryptophan, isoleucine), B-complex (B1, B2, B6, pantothenic acid, niacin, folic acid, and biotin), E vitamins, and minerals [18], all of which play a fundamental role in maintaining human health.
Another essential food ingredient is mannoprotein, a glycosylated molecule (~90% sugars, mostly mannose) that acts as a structural component in the outermost layer of the yeast cells [19]. Mannoprotein has an amphipathic structure due to the protein bonded with carbohydrates, leading to emulsifying and stabilizing properties [20]. Moreover, mannoproteins show technological features, mainly in the wine industry, due to the reduction of haze formation, prevention of tartaric salt precipitation, increase in the aroma of wine, and interaction with phenolic compounds that improve color stability and reduce the astringency of wine [20,21,22].
Due to the composition, Y. lipolytica yeast biomass can also be used for the extraction of intracellular compounds, which is a strategy already adopted to add value to agro-industrial by-products such as astaxanthin from Xanthophyllomyces dendrorhous, and mannoprotein, β-glucan, and bioactive peptides from the spent brewer’s yeast [23,24,25,26,27]. However, different methodologies (enzymatic, physical, chemical, or mechanical) must be applied to disrupt the yeast cell wall [14]. Mechanical techniques (such as bead milling and ultrasonication) are employed to physically break the cells, although they exhibit low efficiency due to limited penetration power [28,29,30,31]. Physical–chemical methods (such as osmotic shock and thermolysis) involve subjecting the cells to harsh conditions of osmotic pressure or temperature, with thermal degradation of bioactive compounds being a significant drawback [14]. The chemical methods (alkali or acid, organic solvents, deep eutectic solvents, detergents, and ionic liquids) act in the cleavage of cell wall component bonds, which improves the cell wall permeabilization and leakage of intracellular compounds [32,33]. The enzymatic mechanism (autolysis and exogenous enzymes) also has great permeabilization by attacking macro compounds (protein, glucans, lipids) by enzymes without destroying the cell integrity [26]. Moreover, the use of endogenous enzymes (proteinases, glucanases, and chitinases) during autolysis makes the process cheaper than the use of commercial enzymes [34,35]. Moreover, the combination of methods can be a feasible alternative to improve the disruption of cell walls and the release of components such as mannoprotein, β-glucan, other proteins, and bioactive peptides [26,36,37]. For example, autolysis is a feasible first step due to the mild temperature, pH, time conditions, and great penetration power [34]. This method showed promising results when combined with acid shock and pulsed electric fields to extract RNA and mannoprotein [22,38].
After the hydrolysis process, the hydrolyzed solution is rich in dispersed or solubilized components, requiring a recovery and separation process for bioproducts with high added value. The yeast hydrolysates, for example, are composed of different fractions of proteins, carbohydrates, RNA, and bioactive peptides, the fractionation by ultrafiltration being a good downstream process since different membrane sizes can be applied to separate those components [39]. The application of hydrolysis, recovery, and purification processes is crucial as they significantly influence the functional, bioactive, and technological properties of the components, as well as the overall cost of the process [24,40].
Thus, this study aimed to evaluate a two-step process for cell disruption (autolysis and acid hydrolysis) to extract intracellular compounds from unconventional and versatile Y. lipolytica IMUFRJ 50682 cultivated with different carbon sources (glucose and glycerol) as well as to evaluate the recovery of antioxidant activity compounds by ultrafiltration.
2. Materials and Methods
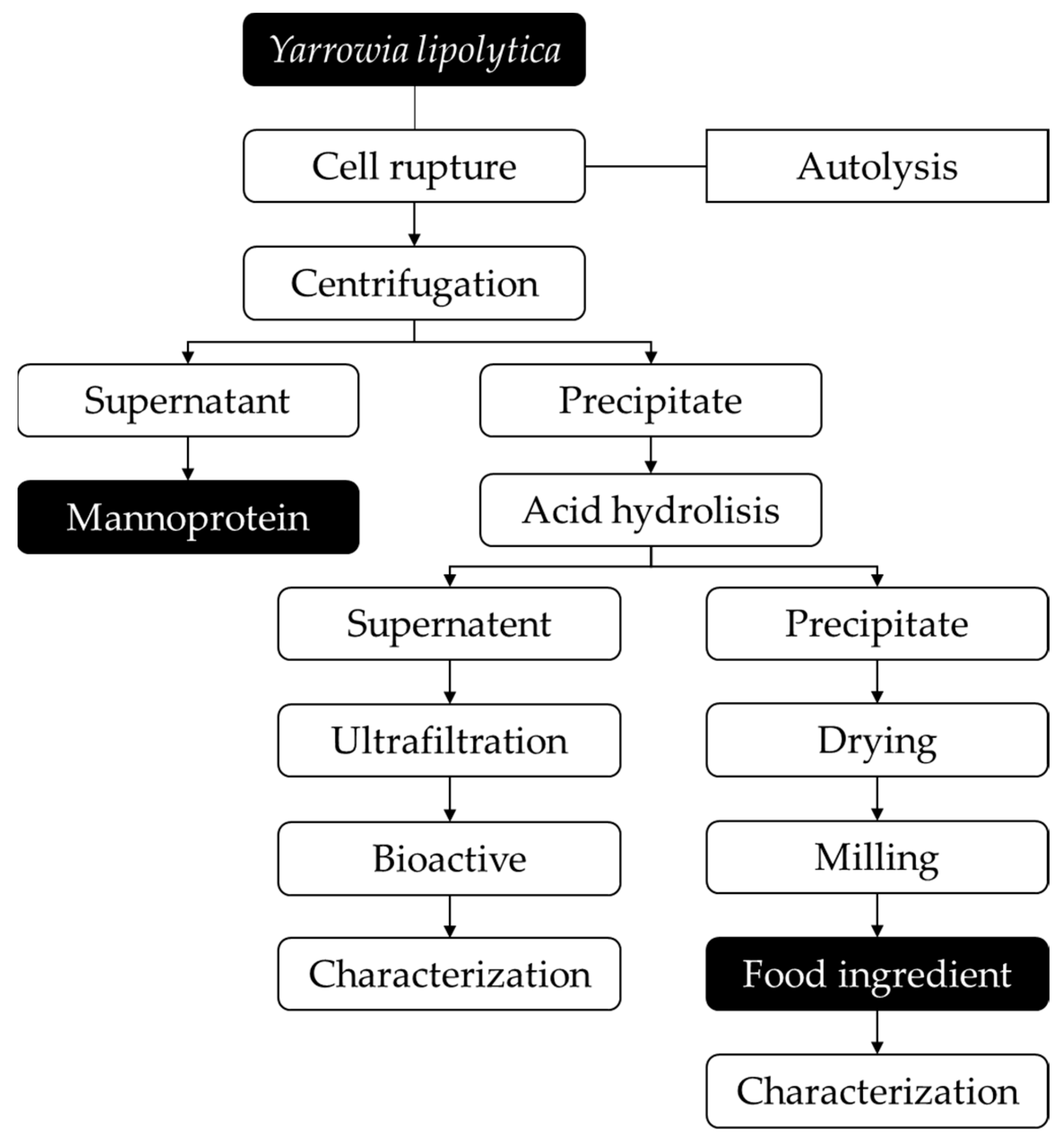
The two-step process for cell disruption (autolysis and acid hydrolysis) to extract intracellular compounds from Y. lipolytica IMUFRJ 50682 cultivated with different carbon sources (glucose and glycerol) was conducted according to Figure 1.
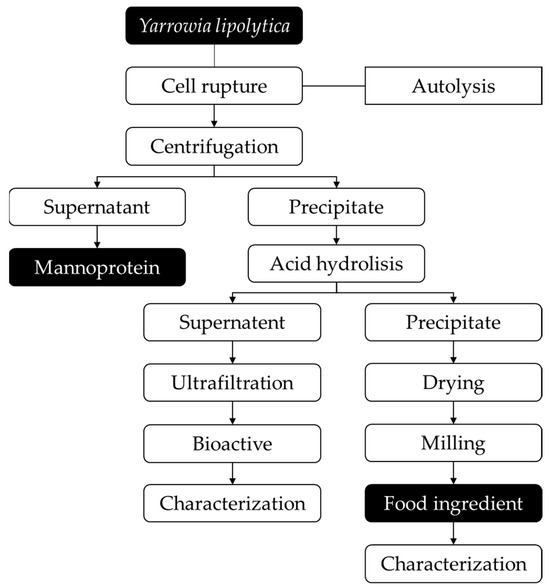
Figure 1.
Flowchart of two-step cell wall disruption of Yarrowia lipolytica IMUFRJ 50682 to obtain food ingredient. The boxes with black backgrounds represent the two main products generated in the process.
2.1. Materials
Yeast extract, peptone, hexadecane, glycerol, bovine serum albumin (BSA), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), potassium persulfate, 2,4,6-tris(2-piridil)-s-triazina (TPTZ), and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich®, St. Louis, MO, USA, EUA. All other chemicals used were of analytical grade and were used as received without any further purification, obtained from Vetec®, Rio de Janeiro, Brazil (glucose), Synth®, Diadema, Brazil (ethanol 96%), and Isofar®, Rio de Janeiro, Brazil (acetone, hydrochloric acid, sulfuric acid, and methanol). The β-glucan Assay kit (K-YBGL) was obtained from Megazyme®, Bray, Ireland. The ultrafiltration membranes were obtained from the company Nadir®, Wiesbaden, Germany (50 kDa UH050 P; 20 kDa UP020 P; 10 kDa UP010 P; 1 kDa NP010 P).
2.2. Microorganism Maintenance and Biomass Production
Y. lipolytica IMUFRJ 50682 stored in stock solution (glycerol 30% v/v, −80 °C) was propagated in YPD (w/v: 1.0% of yeast extract (Sigma-Aldrich®), 2.0% of peptone (Sigma-Aldrich®), 2.0% of glucose (Vetec) medium in 500 mL shaker flasks containing 200 mL (160 rpm, 28 °C, 72 h). After 72 h of cultivation, it was inoculated as 1 g/L of dry weight cells in 1 L shake flasks containing 400 mL of YPD and YPG medium (replacing glucose with glycerol P.A. at the same concentration) at 250 rpm, 28 °C, for 48 h [4,41]. The biomass production was carried out repeatedly to obtain a single batch for application in the assays (without monitoring the fermentation parameters). The biomass was washed with distilled water, centrifuged (4000 rpm, 5 min, 20 °C), and then dried in an oven (60 °C, 18 h). The dry biomass granulometry was standardized to 0.5 mm, and it was kept in screw-capped tubes (25 °C).
2.3. Two-Step Cell Wall Disruption of Yarrowia lipolytica IMUFRJ 50682
A combined method of autolysis followed by acid hydrolysis was used for cell wall rupture. The autolysis was carried out in shaker flasks by adding distilled water to the dry biomass (15%, w/v) under the following conditions: pH 6, 60 °C, 120 rpm, 24 h. The suspension was centrifuged (2.057 g, 5 min, 20 °C), and the supernatant was reserved for mannoprotein extraction (Section 2.4). The precipitate was submitted to the second step (acid hydrolysis) of cell wall rupture. The acid hydrolysis was carried out by adding water in the same concentration as the autolysis (15%, w/v) at pH 2 adjusted with H2SO4 (Isofar®) and kept for 1 h, at 60 °C and 120 rpm. Finally, the suspension was centrifuged (2.057 g, 5 min, 20 °C), and the supernatant was reserved for ultrafiltration separation. The precipitate was dried in an oven (60 °C, 18 h), its granulometry was standardized to 0.5 mm, and the dry mass was characterized as a food ingredient [34,38].
2.4. Mannoprotein Extraction and Characterization
The supernatant from the autolysis process (Section 2.3) was used for mannoprotein extraction. The precipitation was carried out in pre-weighed beakers, where a 3-fold amount of ethanol 96% (Synth®) was added to the volume of the supernatant to precipitate the mannoprotein. The suspension was kept at 4 °C for 18 h; the supernatant was discarded, the precipitate was dried (60 °C, 18 h), and weighed to calculate the mannoprotein extraction yield (EY) [42] according to Equation (1). The dry mannoprotein was diluted in deionized water at a final concentration of 30 mg/mL to determine the surface tension, emulsification index (EI), and carbohydrate and protein content. Moreover, it was evaluated using thermogravimetric analysis and Fourier transform infrared spectroscopy (FTIR):
Wi = Weight of dried mannoprotein
Wf = Weight of dried yeast
2.4.1. Surface Tension (ST) and Emulsification Index (EI)
The ST of 30 mg/mL mannoprotein solution was determined using a Tensiometer K 100 (Kruss®, Hamburg, Germany) by the ring method at room temperature (25 ± 2 °C). The EI was determined by adding 1 mL of hexadecane (Sigma-Aldrich®) to the same amount of sample, vortex-mixing for 2 min, and leaving it to stand for 24 h, then calculated by Equation (2) [4].
He = Emulsified layer height
Ht = Total height of the liquid column
2.4.2. Thermogravimetric Analysis (TGA)
The mannoprotein extracted from Yarrowia lipolytica using a combined method was analyzed through thermogravimetry to evaluate its thermal stability. The sample was weighed (8 mg) on alumina pans. The test was conducted under a nitrogen environment (60 mL/min) at a heating rate of 10 °C/min up to 600 °C using a thermal gravimetric analyzer (TGA 50, Shimadzu®, Kyoto, Japan).
2.4.3. Fourier Transform Infrared Spectroscopy (FTIR)
The chemical characteristics of Y. lipolytica before and after cell rupture and mannoprotein extraction were detected using an FTIR spectrophotometer. Samples of lyophilized yeast biomass and mannoprotein were put on the ATR sampling accessory of an FTIR spectrometer; 64 scans and an analysis window between 4000 and 400 cm−1 with a 4 cm−1 resolution were used. The FTIR graph was generated and the peaks were compared with those in the literature.
2.4.4. Total Carbohydrate and Protein Determination
The sample was diluted in distilled water, and the total carbohydrates were quantified by the phenol–sulfuric acid method (Vetec) using glucose as standard [43]. The soluble protein was determined by the Lowry method using bovine serum albumin (BSA) as the protein standard [44]. For mannoprotein analysis, a 30 mg/mL solution was used.
2.4.5. Determination of Total Glucan, β-Glucan, and α-Glucan
The Beta Glucan Assay kit (K-YBGL, Megazyme®, Wicklow, Ireland) was used to determine total glucan, β-glucan, and α-glucan as soluble fiber [45] using the manufacturer’s recommendations.
2.4.6. Optical Microscopy
Cell morphology of the crude biomass and food ingredient was evaluated by optical microscopy (Nikon Nikon Eclipse E200, Tokyo, Japan) 1000×, in oil immersion, phase contrast mode.
2.5. Technological Properties
2.5.1. Water Holding Capacity (WHC) and the Index of Water Solubility (IWS)
The water holding capacity (WHC) and the index of water solubility (IWS) were carried out using the sample (crude biomass and food ingredient separately) and mixing it in distilled water (5%, w/v) in a pre-weighed centrifuge tube (50 mL). The mixture was vortexed and then kept for 30 min, at 200 rpm, 25 °C. After centrifugation (2.057× g, 25 min, 25 °C), the supernatant was placed in a pre-weighed Petri plate, kept in an oven (70 °C, 18 h), the weight of the dry residue was measured, and the WHC was determined using Equation (3). The dry residue (soluble solids) in the supernatant was determined after drying in an oven at 70 °C for 18 h [28]:
2.5.2. Oil-Binding Capacity
Similarly, for the oil-binding capacity (OBC), the samples (crude biomass and food ingredient separately) were mixed in 5 mL of vegetable oil (5%, w/v). The volume of supernatant collected after centrifugation was measured, and the OBC was calculated using Equation (5):
2.6. Ultrafiltration
The ultrafiltration process was carried out in a stirred dead-end cell with 300 mL using NADIR® membranes of different molecular weight cut-offs—50 kDa UH050 P; 20 kDa UP020 P; 10 kDa UP010 P; 1 kDa NP010 P—used successively to obtain different fractions (F1: >50 kDa; F2: <50 kDa and >20 kDa; F3: <20 kDa and >10 kDa; F4: <10 kDa and > 1 kDa; F5: <1 kDa). The system was operated at 5 bar and 25 °C. The retentate and permeate were collected separately to determine carbohydrates, proteins, and antioxidant activity [46,47].
2.7. Antioxidant Properties
The antioxidant activity was determined by the ABTS, DPPH, and FRAP methods using the samples after acid hydrolysis and fractionated by ultrafiltration. The ABTS method was carried out by reacting 5 mL aqueous ABTS (Sigma-Aldrich) solution (7 mM) and 88 μL potassium persulfate solution (140 mM) to produce the ABTS radical cation (ABTS+) and kept in the dark for 16 h. The ABTS radical was diluted with ethanol to obtain an absorbance of 0.7 ± 0.05 at 734 nm. In the dark, 15 μL of the samples was added to 1500 μL of ABTS radical solution, and after 6 min, the absorbance at 734 nm was measured [48].
The DPPH method was carried out by reacting 45 μL of the sample with 1800 μL of 60 μM 2,2-diphenyl-1-picrylhydrazyl (DPPH, Sigma-Aldrich®) in methanol (Isofar®) solution. After 30-min incubation in the dark, the absorbance was measured at 515 nm. The results were expressed in μM of Trolox (Sigma-Aldrich®) equivalent [48].
The FRAP method was carried out by preparing the FRAP reagent (1 mL of TPTZ 10 mM (Sigma-Aldrich®), 1 mL of FeCl3 20 mM, and 10 mL acetate buffer (Isofar®) 300 mM pH 3.6) in the dark. The reaction was carried out by mixing 15 μL of the sample with 285 μL of the FRAP reagent. After 30 min of incubation in the dark, the absorbance was measured at 593 nm, and the results were expressed in μM of ascorbic acid equivalent [49].
2.8. Proximal Composition
The proximate composition of the samples (crude biomass and food ingredient) was determined by the ash content in a muffle (1150-3P-W3, W-THREE, Piracicaba, Brazil); lipid content by a Goldfish extractor (TE-044-5/50, TECNAL®, Piracicaba, Brazil); moisture content by the gravimetric method using a moisture analyzer oven (M5-Thermo 62L, Belengineering, Italy). Nitrogen content was determined by the micro-Kjeldahl method (TE-040/25), and protein content was calculated using a conversion factor of 5.8. Total carbohydrate content was obtained by difference. The total energy value expressed in kcal/100 g was estimated using conversion values of 4 kcal/g for protein and carbohydrates and 9 kcal/g for lipids. Analyses were performed in triplicate, according to the methodologies described by AOAC International [50].
2.9. Statistical Analyses
All experiments were carried out in triplicate and all analyses were performed at least in triplicate. Results were expressed as average values ± standard deviation. The results for both substrates were submitted to Test T to evaluate the best carbon sources (glucose and glycerol) and other results were submitted to analysis of variance (ANOVA) one way and comparison of means by the Tukey HSD test (p ≤ 0.05) using Statistica 7.0 software (version 8.0, StatSoft®, Tulsa, OK, USA).
3. Results and Discussion
3.1. Obtaining Mannoproteins from Yarrowia lipolytica IMUFRJ 50682 Autolysis
Yarrowia lipolytica IMUFRJ 50682 was cultivated in two different culture media containing glucose and glycerol as carbon sources to assess mannoprotein production and, consequently, the potential of utilizing alternative carbon sources to enhance production and reduce associated costs. Afterwards, Y. lipolytica IMUFRJ 50682 biomass was used as a raw material for the extraction of mannoproteins using an autolysis technique to obtain information about its structure and functionality, since such characteristics affect its application in the food and beverage industry, including acting as stabilizers, emulsifiers, antioxidants, surfactants, among other properties of interest [51].
In autolysis, endogenous enzymes hydrolyze cell wall components, and the endogenous proteolytic activity is the most important one in the process. However, glucanases and chitinases are also important. This process usually takes place by applying yeast cells to mild conditions of temperature (50–55 °C) and pH (5–6) for at least 24 h, without the requirement of expensive equipment [52].
Table 1 shows the characterization of the soluble fraction rich in mannoproteins after autolysis of the crude Y. lipolytica biomass concerning carbohydrate and protein content. The cells that used glycerol as a substrate released more carbohydrates and protein than the ones that utilized glucose. This result could be related to cell wall modulation due to different carbon sources. Cell wall modulation involves the production of cell wall polysaccharides and enzymes responsible for cell wall remodeling, assembly, and degradation. For instance, the expression of the FKS gene is associated with β-1,3-glucan synthesis, with FKS1 expression prevailing under optimal growth conditions, while FKS2 expression is induced in response to various stresses such as glucose limitation and the presence of alternative carbon sources like glycerol [53].

Table 1.
Characterization of the soluble fraction rich in mannoproteins (supernatant) obtained after autolysis of the crude biomass of Yarrowia lipolytica IMUFRJ 50682.
Furthermore, the type of carbon source also significantly influences biosurfactant production by Y. lipolytica. The evaluation of biosurfactant production using glucose and glycerol showed higher production compared to hydrophobic sources (like olive oil). This phenomenon can be attributed to the hydrophobic nature of the surface of Y. lipolytica, which enhances biosurfactant production when utilizing glycerol and glucose as substrates [4].
3.2. Characterization of the Soluble Fraction Rich in Mannoproteins
3.2.1. Composition, Surface Tension, and Emulsification Property
The use and application of an unconventional ingredient obtained from innovative processes require the evaluation of its physicochemical, structural and technological properties to understand its behavior during application in a food matrix, or even to predict how its characteristics can influence the properties of the material to which it will be applied [14]. Thus, the mannoprotein extraction yield, composition, surface tension, and also emulsification index of the final mannoprotein solution were evaluated.
Table 2 presents the characterization of mannoproteins extracted from Y. lipolytica IMUFRJ 50682, where it is observed that the substrate used by the microorganism affected the extractability and the characteristics of the mannoprotein. The extraction yield was higher for mannoprotein extracted from Y. lipolytica cultivated with glycerol, reaching a value of up to 10.13%. These results indicate that almost 50% of mannoprotein from yeast cell walls was extracted since its total value is approximately 20–25% [20].

Table 2.
Characterization of the mannoprotein from Yarrowia lipolytica IMUFRJ 50682.
It is important to note that the protein content was higher for mannoprotein extracted from the cells that used glucose (30.97 g/L) as a carbon source than the ones that used glycerol (19.63 g/L). The higher extraction of mannoprotein from cells that used glycerol as substrate can be explained by the presence of long and highly branched carbohydrate side chains linked to asparagine residues and to the presence of disulfide bridges that are connected to the outer layer of mannoprotein [54].
The total carbohydrates in the mannoprotein solution of 30 mg/mL were 14.6 and 19.6 g/L for Y. lipolytica produced in glucose or glycerol, respectively. The concentrations of carbohydrates and proteins in the mannoprotein solution are important for the emulsion system. The mannoproteins have emulsifying and stabilizing properties due to the amphipathic structure of their highly glycosylated molecule, (~90% sugars, mainly mannose) being located in the outermost layer of the yeast cells acting as structural components [22]. In the emulsion, proteins facilitate the formation of a viscoelastic layer at the oil-water interface, while carbohydrates increase the size of the interfacial layer [55]. The carbohydrates can act by minimizing emulsion stability by improving protein–protein interactions and developing a multilayer cohesive protein film at the interface that prevents foam collapse and enables the formation of a more stable foam [56,57]. The high protein concentration enhances the amphiphilic character of mannoproteins, thereby improving their overall emulsifying properties [58].
The surface tension is an essential parameter for molecules with emulsification power, such as mannoprotein, because it decreases the surface tension of particles, avoiding the formation of larger particles and keeping the emulsion droplets stable for a long time [59]. This property is important in the food industry in the production of mayonnaise, ice cream, sauces, and other foodstuffs [60].
The mannoprotein extracted from Y. lipolytica cultivated in media with glucose showed better results, decreasing the surface tension to 44.50 mN/m, indicating a good emulsifying power and an EI of 37.20%. In comparison, the extracellular biosurfactant from Y. lipolytica IMUFRJ 50682 showed a 67.7% EI and 20.9 mN/m surface tension [4]. Furthermore, an average surface tension of 40 mN/m was found for the extracellular biosurfactant of Y. lipolytica isolated from dairy products in the rural region of Pernambuco (BR) [61]. Using an enzymatic process with β-1,6-glucanase, a mannoprotein from the cell wall of S. cerevisiae reached an extraction index of 11% and retained an EI of 25% for 7 days [19].
The EI test determines whether the biosurfactant has emulsifier properties by calculating the ratio of the height of the stable emulsion layer and the total height of liquid formed after vortexing and leaving it for 24 h. The high stability of the emulsion is evident for mannoprotein with a higher carbohydrate content (19.63 g/L) derived from glycerol as a substrate, as supported by its higher emulsification index of 47.35%.
The EI measures a biosurfactant’s ability to improve contact between oil and water. Thus, a higher index indicates that the interaction between oil and water is strong and stable [62]. Meanwhile, mannoproteins with higher protein content (30.97 g/L), like those obtained using glucose as substrate, show a higher surface tension reduction (44.50 mN/m). These properties are related to amphipathic properties, which give the ability to form hydrophobic and hydrophilic regions in the same molecule [19].
Chemical methods for cell disruption have been documented in various yeast species for the extraction of diverse bioproducts. Examples include Xanthophyllomyces dendrorhous, Sporidiobolus pararoseus, Xanthophyllomyces dendrorhous, and Saccharomyces cerevisiae, where the process yield can reach values exceeding 60%. Nevertheless, the comparison between processes is limited due to the lack of data or detailed information for comparison. Additionally, the processes are specifically developed for the extraction of specific components from the process [36].
3.2.2. FTIR Structural Characterization of Mannoprotein
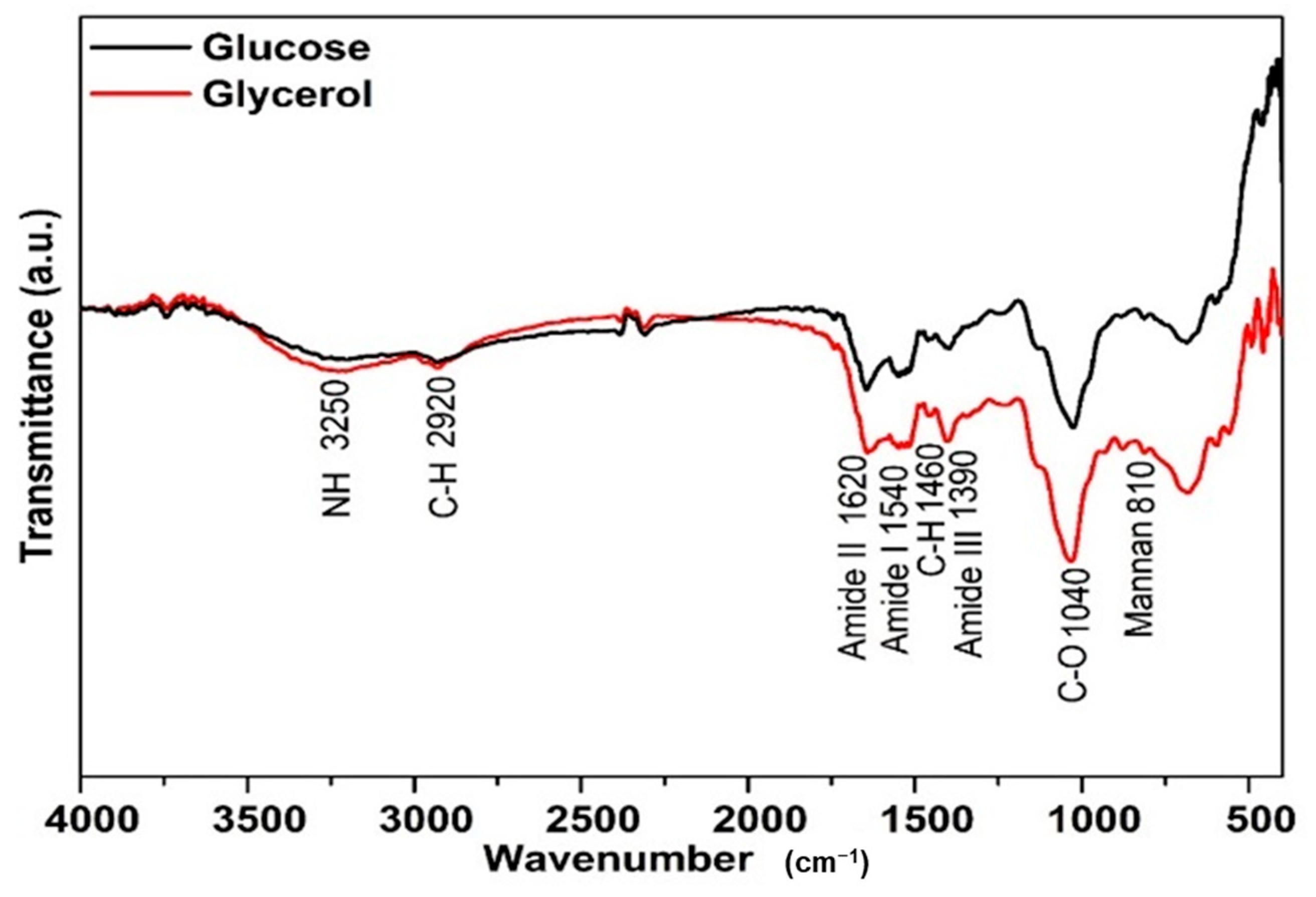
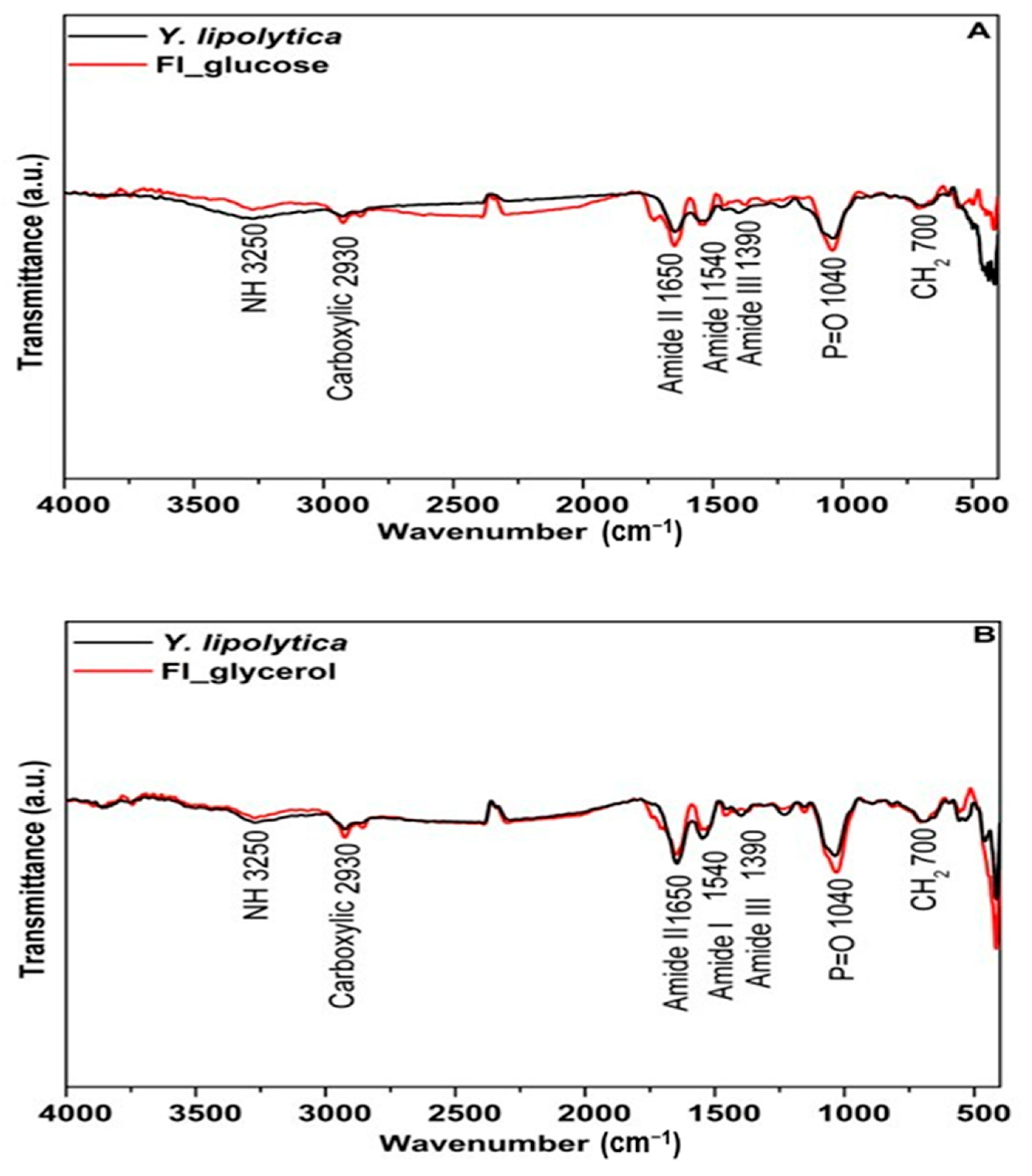
FTIR spectroscopy was performed to elucidate the structural characteristics of the mannoprotein obtained from Y. lipolytica cultivated using different substrates. Figure 2 shows that the mannoprotein obtained from different substrates had similar characteristic peak trends, but different peak intensities. The absorbance peak can indicate the functional groups and chemical bonds of molecules, whereas the peak intensity can demonstrate the concentration of these chemical species [5,63].
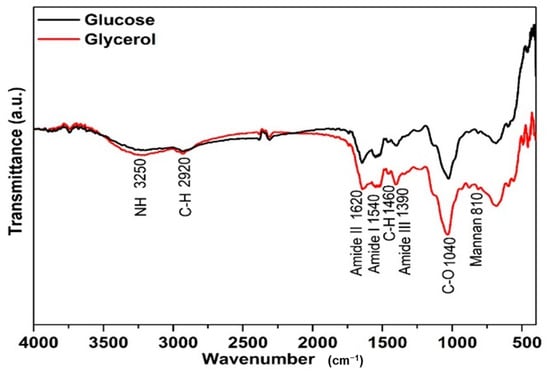
Figure 2.
Characterization of the mannoprotein extracted through autolysis of the crude biomass of Yarrowia lipolytica IMUFRJ 50682 growth in (—) glucose or (—) glycerol.
The mannoproteins from Y. lipolytica IMUFRJ 50682 cultivated in glucose or glycerol substrate had a typical mannoprotein band at 810 cm−1 which is characteristic of mannan. The absorption peaks at 3250 cm−1 are ascribed to an asymmetric stretch of the –NH group, while the 2920 cm−1 is ascribed to the C–H bond of the methyl group. In addition, the peaks 1040 cm−1 (C–O vibration) and 1460 cm−1 (C–H deformation vibrations) indicate the pyranose ring, corresponding to the saccharide fraction of mannoprotein [19].
Amide I and II absorption bands are the most significant vibration bands on the protein skeleton. The peaks at 1620 cm−1 (amide I) and 1540 cm−1 (amide II) indicate acylamino absorption peaks due to the peptide bond’s C–O stretching vibration. Meanwhile, 1390 cm−1 is assigned to the skeletal vibration involving stretching of the C–N bond of amide III [64].
Compared with the cells grown in glycerol, mannoprotein extracted from Y. lipolytica cultivated using glucose as substrate exhibited weaker absorption from 2000 cm−1 to 500 cm−1, probably due to different amino acid composition; the same behavior was observed for mannoprotein from Saccharomyces cerevisiae obtained from different treatments which presented different secondary protein structures due to the amino acid content. Moreover, a higher protein/carbohydrate ratio showed strong absorption in the peaks at 1600–1300 cm−1 [65].
The mannoprotein spectra profile differences are directly related to its emulsification properties. For example, a higher protein/carbohydrate ratio in yeast mannoproteins indicates excellent emulsifying properties because of the high protein content [19]. Moreover, the amino acid composition of yeast mannoprotein, arranged into an amphipathic structure, is related to the emulsification properties due to protein molecular weight. For example, proteins with low molecular weight generate a system with active surfaces. Meanwhile, proteins with higher molecular weight generate more stable and viscous emulsions [60].
3.2.3. Thermogravimetric Characterization of Mannoprotein
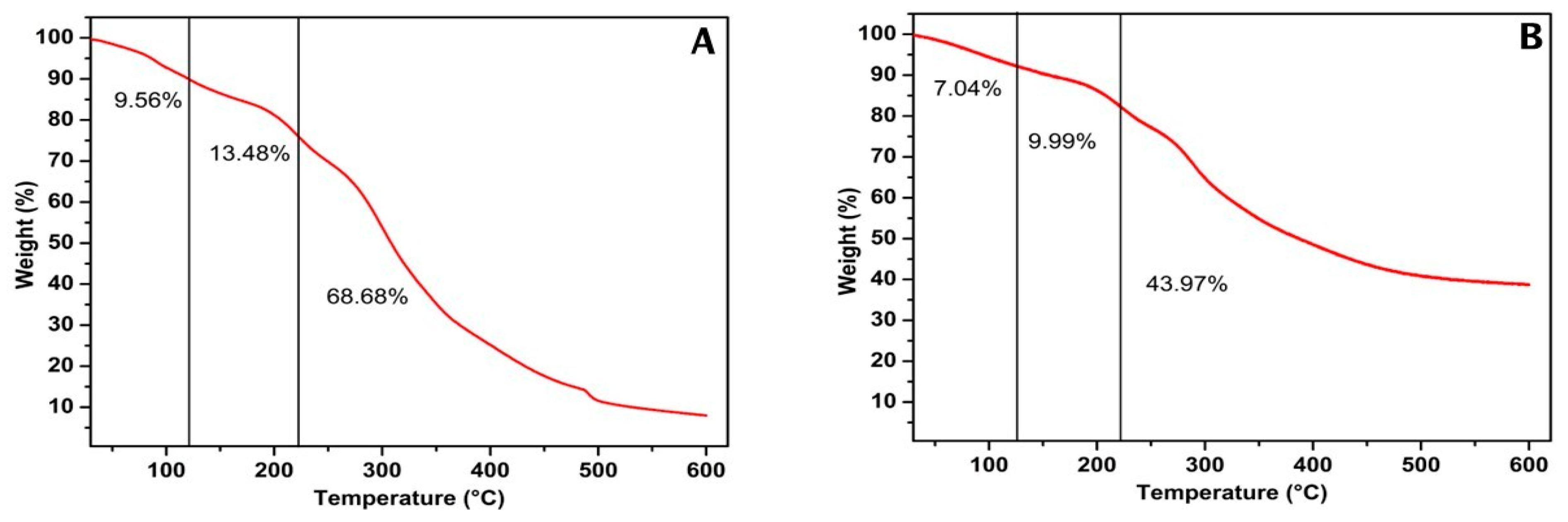
Thermogravimetric measurement was performed to verify the thermal stability of mannoprotein extracted from Y. lipolytica cultivated in glucose and glycerol substrates by determining the weight of the sample subjected to a continuous increase in temperature [66]. Figure 3 presents the different stages of thermal degradation of both mannoproteins. The first weight loss ranged from 7 to 10% for glycerol and glucose, respectively, and was associated with water evaporation up to 100 °C. The second weight loss ranged from 9 to 13% for glycerol and glucose, respectively, and was related to the start of carbohydrate degradation up to 200 °C. The final weight loss ranged from 43 to 68% for glycerol and glucose, respectively. This degradation stage started at 220 °C and is attributed to the degradation of structural water and the cleavage of protein, polysaccharides, and lipids [67]. These results indicate that mannoprotein from Y. lipolytica cultivated in glycerol was more thermally stable than that obtained in glucose. This is related to their different chemical compositions, primarily due to the carbohydrate and protein content. The thermal degradation of macroelement carbohydrates, proteins, and lipids occurs simultaneously at 164–497, 209–309, and 200–635 °C, respectively [68].
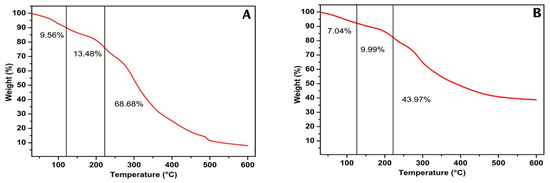
Figure 3.
Thermogravimetric analysis of mannoprotein extracted through autolysis of the rude biomass of Yarrowia lipolytica IMUFRJ 50682 growth in glucose (A) and glycerol (B).
3.3. Characterization of Crude Biomass and Food Ingredient
3.3.1. Chemical Composition and Technological Properties of Crude Biomass and Food Ingredient
The mannoprotein extraction process mentioned in Section 3.1 results primarily from a soluble fraction rich in mannoprotein (a solution containing the components released during autolysis), in addition to another two fractions, a solid fraction after acid hydrolysis (food ingredient), and a liquid fraction obtained as supernatant of the acid hydrolysis step, that was submitted to a concentration process, being denoted as “ultrafiltered bioactive” (Section 3.4).
The process yield shows that Y. lipolytica cultivated in media containing glucose and submitted to sequential hydrolysis (autolysis followed by acid hydrolysis) presented the highest yield, reaching 55%, which was better than the one grown in glycerol (46%) (Table 3). Once again, the substrate used for biomass growth significantly influenced the cell wall characteristics, consequently, in the extraction process and material composition. The substrate is directly related to the cell wall composition. Mannan, glucan, and chitin are the main Y. lipolytica cell wall carbohydrates [9]. Moreover, using glucose as a carbon source promotes a yeast cell wall with approximately 70% carbohydrates, 7% amino sugars, 15% protein, 5% lipids, and 0.8% phosphorus [9]. Thus, the carbon source is directly related to the cell wall composition [69]. For example, glycerol increased the carbohydrate content in Saccharomyces cerevisiae var. boulardii’s cell wall by 58% after cultivation in a medium containing 3% glycerol. Moreover, this strain had a higher content of mannoprotein compared with brewer’s yeasts S. cerevisiae R9, which are mainly composed of β-glucans [70].

Table 3.
Technological properties of Yarrowia lipolytica IMUFRJ 50682 biomass cultivated in glucose and glycerol as substrate and the properties of the food ingredient.
After acid hydrolysis, the partial composition and technological features of the food ingredient were evaluated since even after extraction this solid fraction must have high nutritional value due to the carbohydrate (β-glucan, manana, and chitin) and protein content [17,71], and the feasible properties to be applied in foodstuffs as emulsifier, stabilizer, preservatives, among others [14].
Table 3 shows the technological properties of the crude biomass of Y. lipolytica and the food ingredient produced in both substrates. Notably, the crude biomass exhibited a higher water-holding capacity (WHC) compared to other treatments applied (p ≤ 0.05). This could be associated with the higher temperature applied in both hydrolysis processes, which can affect the stability of the polypeptide secondary and tertiary structures, increasing the surface hydrophobicity of the protein present in the cells [28].
WHC represents the amount of water that a food product can absorb per unit of weight [28] and is crucial for defining product yield, texture, and storage quality. For instance, cookies and crackers require flour with low WHC to achieve a crispy texture, while buns necessitate high water holding capacity to maintain softness [72].
The water solubility index (WSI) indicates that the crude biomass cultivated in glucose media exhibited the highest values (29.12%), whereas the food ingredient resulting from the sequential hydrolysis process showed a WSI of 9.55%. Additionally, the WSI values for crude biomass and food ingredients obtained from glycerol media were 20.00% and 11.05%, respectively.
It was observed that the two-step cell wall disruption method may have caused the exposure of hydrophobic groups (nonpolar amino acids) of the protein, decreasing the IWS of the treated biomass compared to the non-treated one [73]. Moreover, as previously mentioned, different substrates directly influence cell wall components, thereby impacting the amount of carbohydrates, protein, and phospholipids present, resulting in the production of distinct materials [70].
Sequential hydrolysis induces the release of cell wall components, thereby influencing the technological properties of the food ingredient produced. The absence of mannoproteins, for instance, in the food ingredient affects crucial properties like gel formation, emulsification, and the capacity to absorb water and oil [19]. Another significant functional ingredient is β-glucan, known for its prebiotic effects and its potential to enhance rheological properties, thickening, and stability of emulsions [14].
The oil-binding capacity (OBC) did not present a statistical difference, regardless of the treatment applied, reaching values of 3.23–4.07%. OHC is a suitable parameter for use in high-oil/fat-content foods. This parameter influences the order of adding the dry ingredients and the mixing time for a uniform oil distribution in the dry mixture [74].
Concerning the moisture content, values ranging from 4 to 5% were verified. A moisture level below 8% is considered safe for 12–24 months of shelf-life of products and improves the texture and stability of yeast biomass as a food ingredient [75]. S. cerevisiae biomass after autolysis or mechanical cell disruption method increased the moisture content compared to the crude one [28].
One of the most critical characteristics of yeasts is the presence of polysaccharides, especially cell wall glucans. The most abundant polysaccharides in yeast cell walls are α-glucans and β-glucans [76]. The last one consists of β-(1 → 3) and (1 → 6) linkages with a substantial structural diversity, corresponding to 29–64% of the cell wall [36]. The content of these carbohydrates, mostly β-glucans, is important to formulate foodstuffs with prebiotic features apart from modulating the sensorial characteristics [77]. Commercially available glucans from S. cerevisiae are utilized for both human and animal applications. Given that Y. lipolytica is also recognized as a Generally Recognized as Safe (GRAS) organism, its application spans across various industries [71].
The total glucan was higher (21.11%) in Y. lipolytica cells cultivated using glycerol as substrate than in those cultivated in glucose (16.43%). The same behavior was followed by β-glucan concentration with 19.31% for cells grown in glycerol, whereas the biomass cultivated in glucose resulted in 14.13%. Nevertheless, the content of α-glucan was higher for the microorganism cultivated in glucose (2.30%). These differences are associated with the carbon source that can activate different metabolic routes to modulate yeast cell walls [78]. A previous study with S. cerevisiae var. boulardii’ showed that the use of glycerol as an alternative carbon source promoted the production of cell wall carbohydrates, mostly β-glucans [70]. It is also known that under stress conditions, yeast can protect and maintain internal homeostasis by synthesizing more β-glucan in the cell wall [79]. After the two-step cell wall disruption of intracellular compounds from Y. lipolytica, a smaller concentration of the total glucan, α-glucan, and β-glucan in the food ingredient produced is detected. The exception is the observed increase of the α-glucan content in the food ingredient produced using glycerol. Additionally, studies have shown that α-1,3-glucan accumulates in the cell wall during the initial growth stages of Aspergillus nidulans and is degraded under carbon starvation, suggesting that the growth phase also influences α-1,3-glucan production [80].
The ash content varied from 0.66 to 5.73%; due to the sequential hydrolysis process the food ingredient from both substrates presented the lowest values. The main mineral compositions of yeast include phosphorus, potassium, calcium, magnesium, and selenium, accounting for over 10% of the cell dry matter [28].
The carbohydrate content was 38.87% for Y. lipolytica grown in glucose, and 32.35% for Y. lipolytica grown in glycerol, whereas the food ingredient from both substrates presented a carbohydrate content of over 50%. The protein content was higher for Y. lipolytica grown in glycerol (48.92%), whereas the food ingredient from Y. lipolytica grown in glucose retained more protein after sequential hydrolysis (37.63%).
The lipid content was 45% higher for Y. lipolytica grown in glycerol than glucose, whereas both food ingredients had approximately 2% lipids. The intracellular lipid content of Y. lipolytica can reach 80% of cell dry weight, triacylglycerols being composed of long-chain fatty acids (16–18 carbon atoms in the chain) the main components [81].
The caloric value of the crude biomass and the food ingredients was approximately 400 kcal.
3.3.2. FTIR Structural Characterization of Yarrowia lipolytica Biomass and Its Respective Food Ingredient
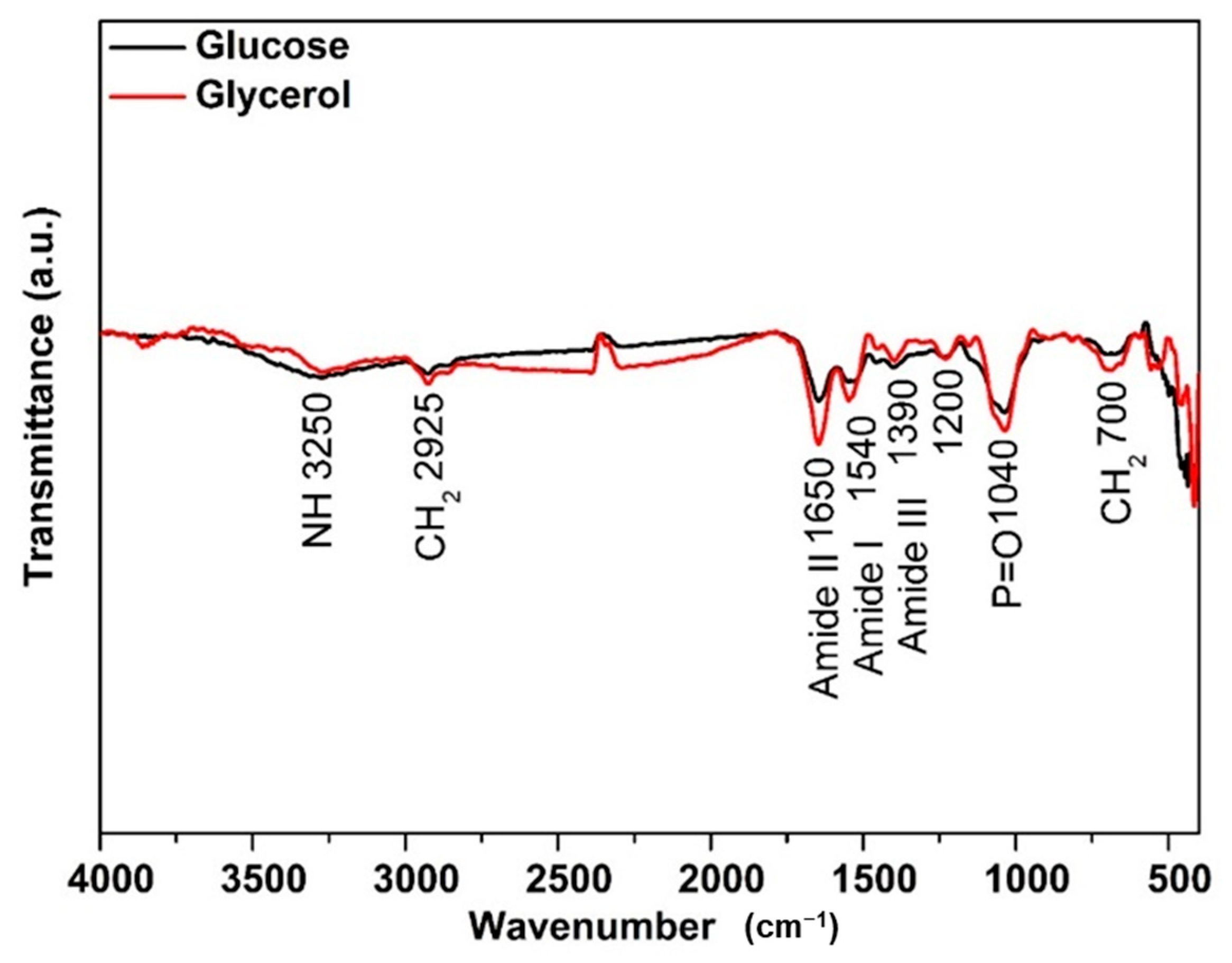
Yarrowia lipolytica IMUFRJ 50682 cultivated with glucose and glycerol as substrate presented similar FTIR spectra characteristic of this yeast (Figure 4). The main differences are at the region corresponding to protein with a higher absorption for the one grown in glycerol at amide I (1540 cm−1) and amide II (1650 cm−1). In addition, the regions corresponding to lipids were at 2925 cm−1 and 725 cm−1, representing stretching of CH2 of acyl chains in fatty acids of triacylglycerols while 1040 cm−1 was P=O stretching (symmetric) of phospholipids in the cell wall [64].
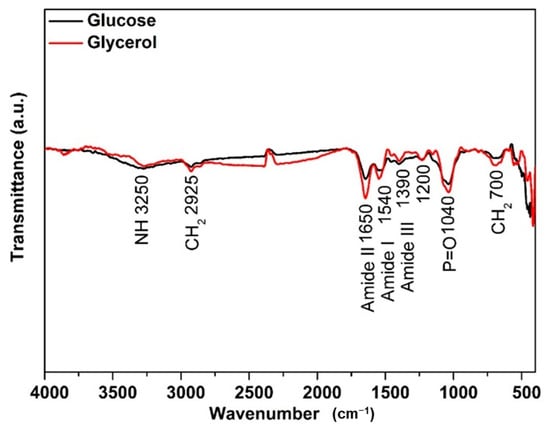
Figure 4.
Characterization of Yarrowia lipolytica IMUFRJ 50682 biomass cultivated with (—) glucose and (—) glycerol.
FTIR spectra of Y. lipolytica cultivated with glucose and glycerol and subjected to sequential hydrolysis are presented in Figure 5. Figure 5A presents a lower absorption for the food ingredient in the regions corresponding to protein (3250 cm−1 and 1390 cm−1) concerning the whole cell. On the other hand, concerning the food ingredient cultivated in glycerol, there were no relevant changes in the spectra of biomass grown on the same substrate. Several factors can contribute to changes in the cell wall and its thickness, with an impact on extractability, including different growth conditions such as temperature, pH, aeration, and mostly the carbon source. The composition of the by-product has already been reported to increase cell wall thickness in the case of S. cerevisiae [82].
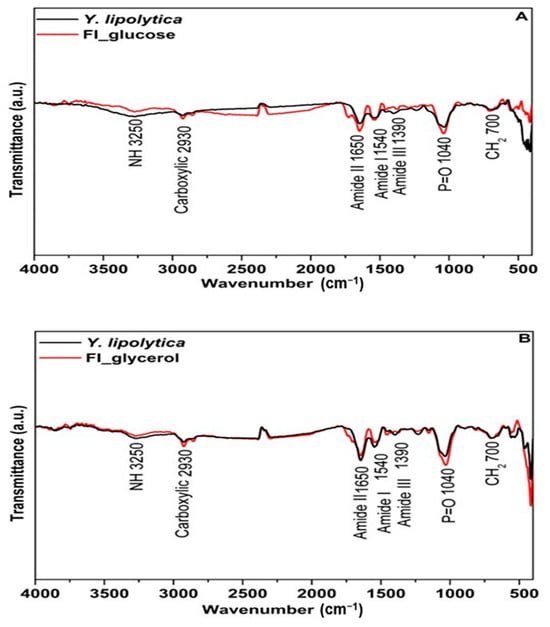
Figure 5.
Characterization of the food ingredient (—) obtained from Yarrowia lipolytica IMUFRJ 50682 cultivated in glucose and glycerol subjected to sequential extraction and the crude biomass (—) cultivated in glucose (A) and glycerol (B).
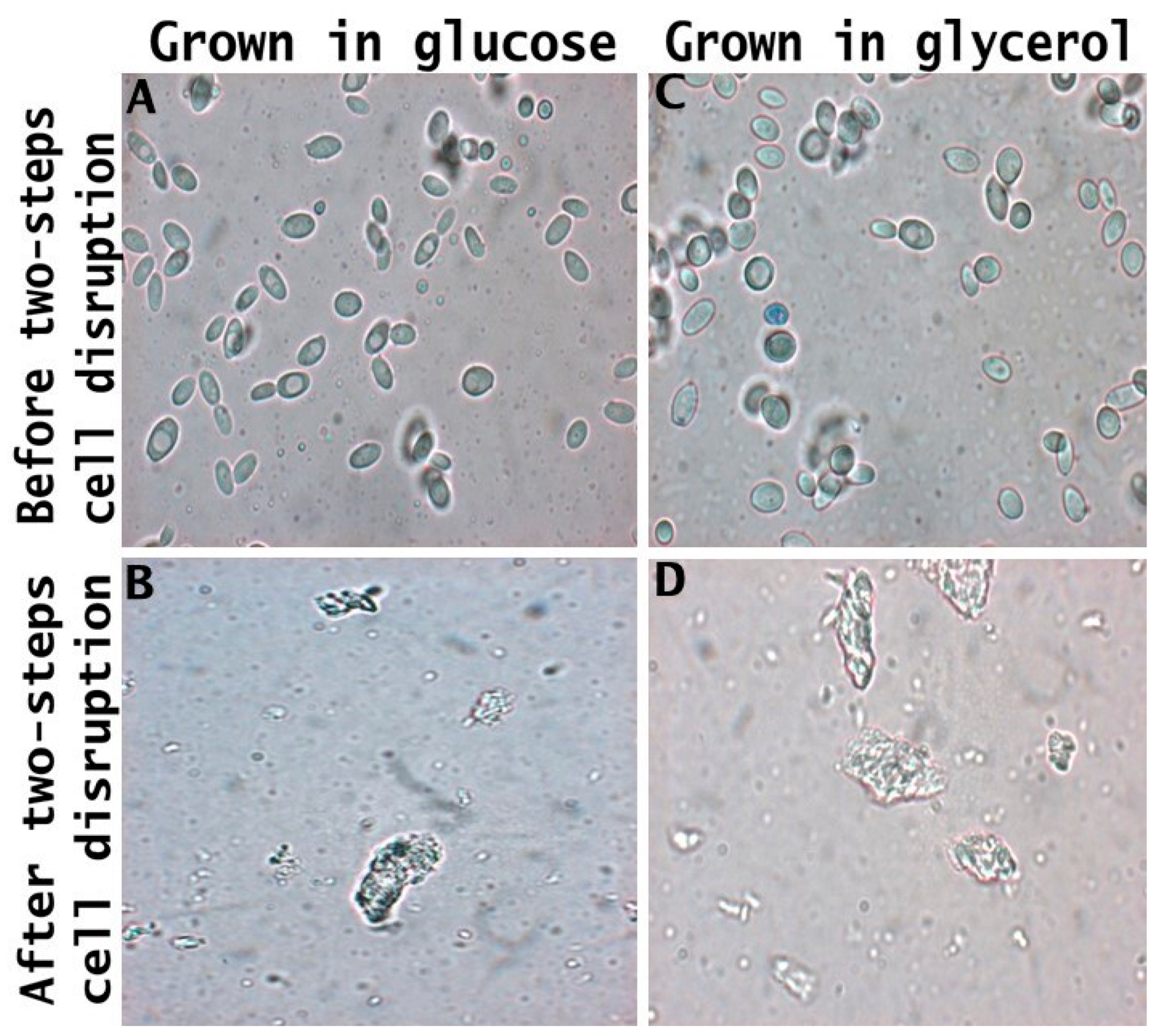
3.3.3. Optical Microscopy of Yarrowia lipolytica Biomass and Its Respective Food Ingredient
Figure 6, obtained from optical microscopy analysis, presents the morphological changes in yeast cell morphology subjected to the different cell disruption processes. Yarrowia lipolytica cultivated in glucose and glycerol (Figure 6A and Figure 6B, respectively) presented similar characteristics around budding cells with large vacuoles within the cytoplasm. After the two-step cell disruption, the cells (Figure 6C,D) were smaller, with a high degree of damage to the roughness of the cell wall surface and aggregates. Cell wall damage was also observed for S. cerevisiae after the autolysis process due to the ethanol toxicity and chemical interaction causing roughness of its cell wall [28]. S. cerevisiae cells treated with pulsed electric fields stored for 7 days at pH 7.0 present a smaller size due to the release of cytoplasm content during autolysis [22].
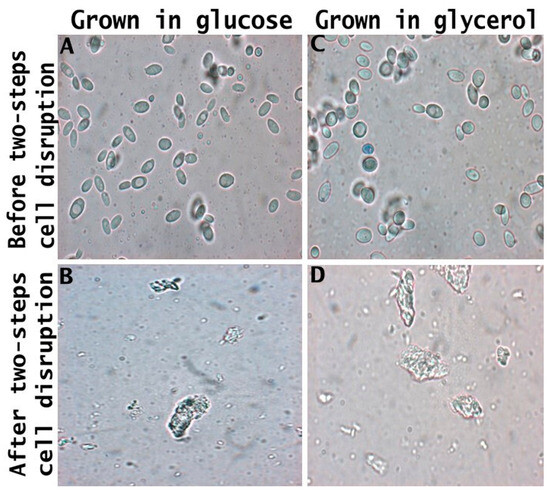
Figure 6.
Microscopy of crude biomass of Y. lipolytica and food ingredient cultivated with glucose (A,B) and glycerol (C,D) subjected to the two-step cell disruption method.
The release of cell wall components, as depicted in Figure 6B,D by the deformation of the cell wall, leads to the production of a food ingredient with distinct characteristics from the crude biomass. This is primarily due to the absence of mannoprotein, which, owing to its amphiphilic nature, serves as an effective stabilizer in food emulsions such as mayonnaise, ice cream, and sauces [83]. Moreover, a lower protein content may reduce the ability to form gels [84], while a reduced carbohydrate content can diminish the thickening properties [14].
3.4. Ultrafiltration of Y. lipolytica Biomass Subjected to Sequential Hydrolysis
3.4.1. Composition and Antioxidant Activity
The cell wall structure of Yarrowia lipolytica comprises various components, predominantly mannoprotein, glucan, and chitin, which play roles in structural integrity, maintenance, and cell replication and can be utilized as food ingredients due to their bioactivity and technological properties [9,14,85]. Therefore, the characterization of this material and the evaluation of its bioactivity are important for its application in different foodstuffs.
Among the bioactivities exhibited by the components of yeast cell walls, antioxidant activity holds significance, particularly as an alternative to synthetic antioxidants, which may have adverse effects depending on the dosage, as antioxidants aim to mitigate oxidative reactions that could lead to changes in flavor, aroma, and other essential properties of foods and beverages [26,86].
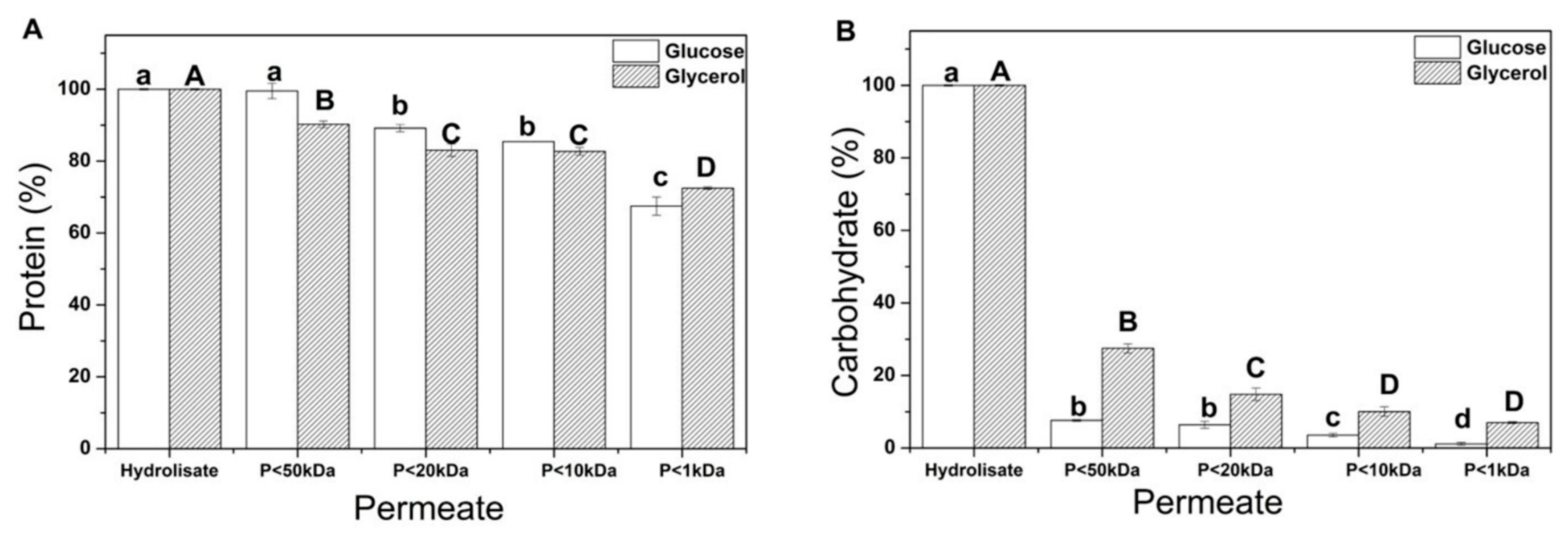
In this sense, Y. lipolytica biomass after the sequential hydrolysis was sequentially fractionated by ultrafiltration membranes to identify the characteristics and bioactivities of the fractions resulting from the concentration process. The ultrafiltration process was performed through four membranes resulting in five fractions (F1: >50 kDa; F2: <50 kDa and >20 kDa; F3: <20 kDa and >10 kDa; F4: <10 kDa and > 1 kDa; F5: <1 kDa) and was evaluated through protein, and carbohydrate content, in addition to antioxidant activity.
In Figure 7, it is possible to verify that a similar behavior for both components originated from cultivation with glucose and glycerol. Almost 70% of proteins presented in the hydrolysate were quantified in membranes with a molecular weight cut-off of less than 1 kDa (Figure 7A) for both substrates. Yeast hydrolysates are combinations of protein fractions and peptides of varying sizes, each with minor differences in their physical and chemical properties, such as charge and hydrophobicity [87]. The molecular weight reduction is caused by endogenous proteolytic enzymes that cleave peptide bonds and generate peptide mixtures with different sizes and affect amino acid compositions, sequences, and peptide structures; however, the enzyme’s specificity determines the bioactivity of the produced peptides [88].
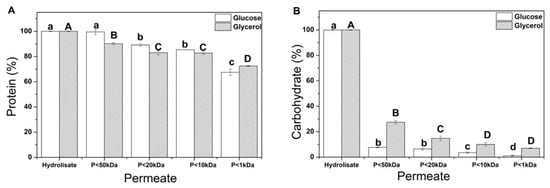
Figure 7.
Soluble fraction ultrafiltered and obtained from Yarrowia lipolytica IMUFRJ 50682 biomass cultivated with glucose (white) and glycerol (black). Protein (A); Carbohydrate (B). “P” refers to the permeate fraction. Different upper/lower case letters denote differences (p ≤ 0.05) between the mean values according to Tukey’s test.
Regarding carbohydrates in Y. lipolytica biomass obtained from sequential hydrolysis (Figure 7B), after fractionation using the membrane with a 50 kDa cut-off, more than 90% of the carbohydrates from acid hydrolysis of crude biomass grown in glucose were retained. Meanwhile, 75% of the carbohydrates from the acid hydrolysis of crude biomass grown in glycerol were retained. This result can be explained by the different molar weights of the carbohydrates present in the hydrolysate. For instance, the average molecular weight of β-glucan is approximately 2000 kDa for high-molecular-weight β-glucan and 300 kDa for low-molecular-weight β-glucan [89]. The other carbohydrate mainly present in yeast cell walls is chitin, which has a molar weight of 1000 kDa [90].
3.4.2. Antioxidant Property of Soluble Fraction Separated by Ultrafiltration
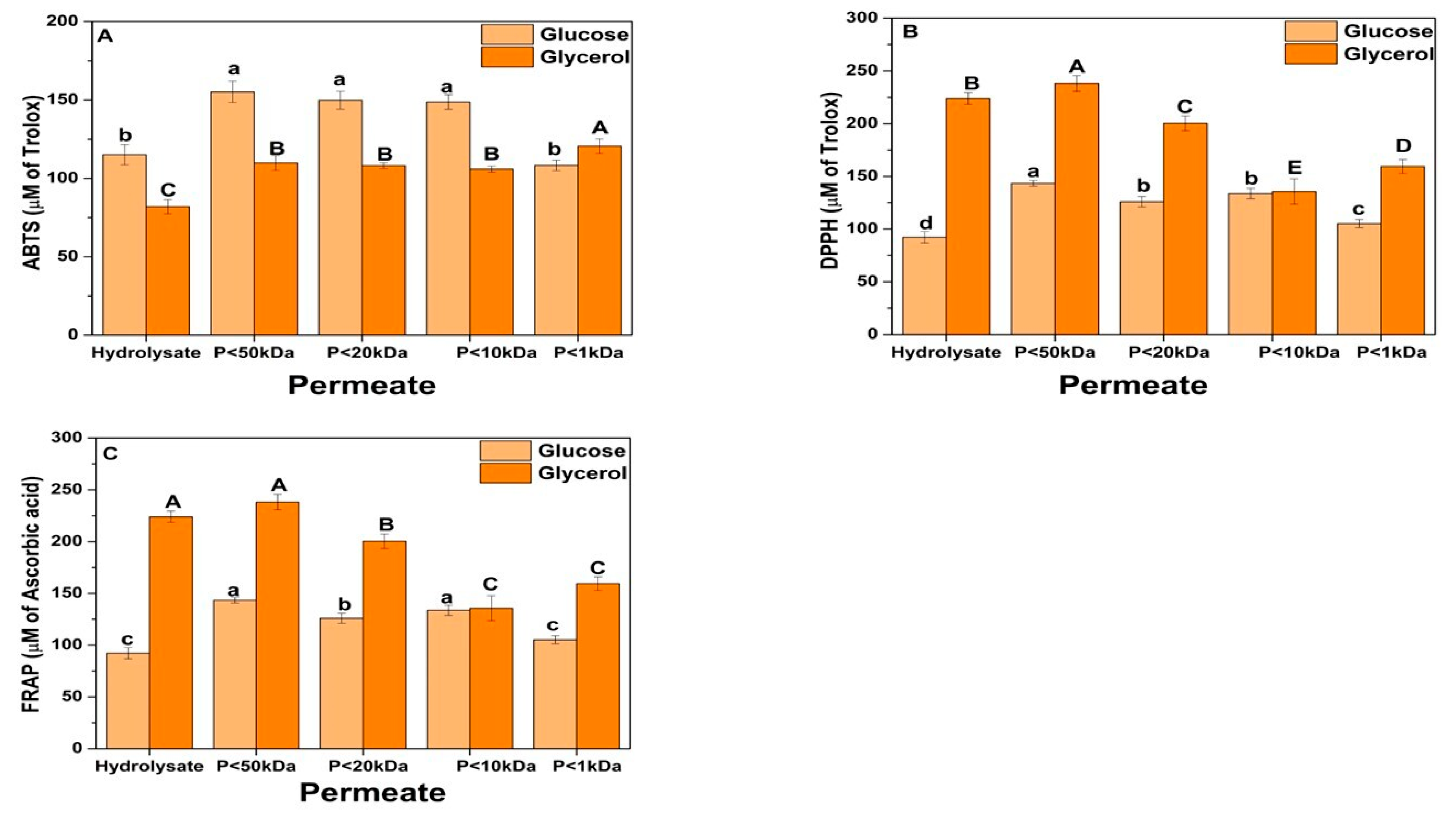
Figure 8 presents the antioxidant capacity of the fractions fractionated by ultrafiltration. Different methods, including ABTS, DPPH, and FRAP, were used to evaluate different antioxidant compounds present in the fractions since these compounds respond to other mechanisms of action and may have synergistic interactions [91]. The ABTS method has a complex reaction mechanism, oxidizing the ABTS by oxidants to its radical cation, ABTS+. However, it is a simple, rapid, sensitive, and reproducible assay able to evaluate hydrophilic and lipophilic antioxidant molecules [92]. DPPH radicals are less reactive than ABTS+ radicals. In the DPPH radical scavenging assay, the antioxidant donates a hydrogen atom, which gives rise to the reduced form with the loss of this violet color [91]. Whereas the FRAP method measures the reduction of ferric ions [48].
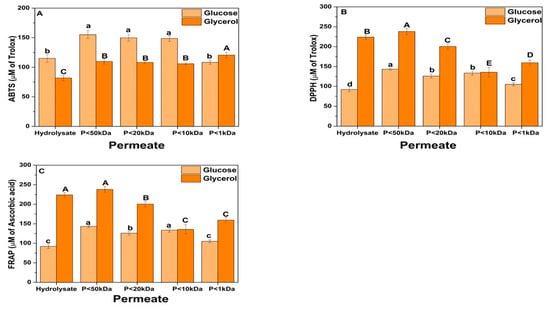
Figure 8.
Antioxidant activity of Yarrowia biomass hydrolyzed by autolysis + acid hydrolysis and fractionated by ultrafiltration. (A) ABTS; (B) DPPH; (C) FRAP. “P” refers to the permeate fraction. Different upper/lower case letters denote differences (p ≤ 0.05) between the mean values according to Tukey’s test.
In general, antioxidant activity (ABTS, DPPH, and FRAP) increased after partial ultrafiltration of the hydrolysates (Fraction < 50 kDa). However, the antioxidant activity appears to decrease with sequential fractionation, likely due to the synergism of components in the acid hydrolysis fraction, which enhance antioxidant potency [93,94]. Antioxidants can chemically reduce each other, acting synergistically to neutralize reactive species. For instance, tryptophan was found to synergize with leucine, lysine, and arginine, thereby enhancing antioxidant potency [95]. Moreover, the mechanism that leads to this synergism in antioxidant activity needs further research. Still, it was shown that the C-terminal tryptophan plays an essential role in the synergism among the peptide chain [96]. Moreover, the bioactivity, primarily due to the antioxidant activity of the higher molecular weight fractions, is related to the synergistic effects of the higher number of amino acid residues when compared to fractions with shorter peptide chains [97].
Hydrophobic and aromatic amino acid residues (leucine, proline, valine, methionine, histidine, and tyrosine) improve the interaction between the peptides and radical species by strengthening the solubility of the peptides in lipids. Moreover, proline residue can act as a proton/hydrogen donor to play its antioxidant role [98]. The antioxidant peptides purified from hydrolysate cardiac arterial bulbs of Skipjack tuna (Katsuwonus pelamis) containing proline–lysine–lysine obtained the highest ABTS scavenger capacity among those isolated with the EC50 value of 0.188 mg/mL [99]. Nevertheless, hydrophilic amino acids (tyrosine, glutamine, threonine, serine, asparagine) can act as antioxidants as was demonstrated with the antioxidant peptides isolated from neutrase hydrolysate of skipjack tuna (Katsuwonus pelamis) milt where the tyrosine–argenine–glutamine–tyrosine sequential isolated presented the highest scavenger capacity for DPPH and ABTS (5.22 ± 0.21, and 6.39 ± 0.21 EC50 mg/mL, respectively) [100].
Furthermore, the lower concentration of carbohydrates in the fractions could promote better antioxidant activity due to bioactive peptides. A study involving protein hydrolysate from S. cerevisiae revealed that antioxidant activity is not solely determined by the concentration of bioactive peptides but predominantly by their physicochemical properties, such as amino acid composition, hydrophobicity, sequence, and molecular weight of the peptides [26]. Moreover, it was shown that some peptides with high antioxidant activity have a molar weight of less than 3 kDa with 2–20 amino acids and the presence of hydrophobic amino acids, for example, proline, valine, tryptophan, and phenylalanine [101]. Yeasts have antioxidant properties due to the main components of their cell wall (β-glucan and mannoprotein). For instance, S. cerevisiae IFST 062013 demonstrated robust antioxidant power through DPPH scavenging activity [102].
The extraction of mannoproteins and other compounds of interest from yeast biomass represents a viable process that optimally utilizes available resources and offers promising applications across various fields.
4. Conclusions
Yarrowia lipolytica emerges as a valuable source for high-value-added products with potential applications across various industries. In this study, Y. lipolytica biomass was efficiently produced using different substrates, with a particular emphasis on glycerol due to its lower cost and comparable or even superior results compared to glucose. The two-step process involving autolysis followed by acid hydrolysis for Y. lipolytica cell disruption proved effective in extracting key components of the cell wall, with a mannoprotein extraction yield exceeding 10% and a biomass yield surpassing 55%.
Due to its distinct composition, the mannoprotein extracted from Y. lipolytica cultivated in the different substrates has different emulsification properties. The mannoprotein from crude biomass grown in glucose decreased the surface tension (44.50 mN/N) better compared to the one that used glycerol (49.21 mN/m). The composition of mannoprotein also influenced the thermal resistance, being better for the mannoprotein (crude biomass grown in glycerol), which has more carbohydrates (60% weight loss) than the mannoprotein (crude biomass grown in glucose) with higher protein content (80% weight loss). The technological properties (water-holding capacity, water solubility index, and oil-binding capacity) demonstrated the potential application of biomass and its components in different foodstuffs. Y. lipolytica biomass and food ingredients have β-glucan content ranging from 12–19%, an important result due to its technological properties and the prebiotic effect when applied in food products. The hydrolysate and soluble fraction obtained by ultrafiltration presented antioxidant properties in both methods but demonstrated a reduction in antioxidant activity in sequential fractionation, indicating a synergistic effect of the components on the general activity of the material. In the future, new disruption methods, or the integration and optimization of existing methods, may be applied to enable higher yields, improved ingredients, and to provide more advantageous processes.
Author Contributions
Conceptualization, B.D.R., A.C.L. and M.A.Z.C.; methodology, R.M.d.S., B.D.R., A.C.L. and M.A.Z.C.; formal analysis, R.M.d.S., B.D.R., A.C.L. and M.A.Z.C.; investigation, R.M.d.S.; data curation, R.M.d.S., B.D.R., A.C.L. and M.A.Z.C.; writing—original draft preparation, R.M.d.S.; writing—review and editing, B.D.R., A.C.L. and M.A.Z.C.; visualization, R.M.d.S., B.D.R., A.C.L. and M.A.Z.C.; supervision, B.D.R., A.C.L. and M.A.Z.C.; project administration, B.D.R., A.C.L. and M.A.Z.C.; funding acquisition, B.D.R., A.C.L. and M.A.Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES—Finance Code 001), Conselho Nacional de Desenvolvimento Científico (CNPq) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Campos, A.L.B.M.A.; Nascimento, F.V.d.; Secchi, A.R.; Coelho, M.A.Z. Phenomenological modeling of polyols, citric acid and bio-oil concurrent production by Yarrowia lipolytica from glycerol. Clean. Chem. Eng. 2023, 5, 100100. [Google Scholar] [CrossRef]
- Silva, J.R.; Souza, C.E.C.; Valoni, E.; Castro, A.M.; Coelho, M.A.Z.; Ribeiro, B.D.; Henriques, C.A.; Langone, M.A.P. Biocatalytic esterification of fatty acids using a low-cost fermented solid from solid-state fermentation with Yarrowia lipolytica. 3 Biotech 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Sales, J.C.S.; de Castro, A.M.; Ribeiro, B.D.; Coelho, M.A.Z. Improved production of biocatalysts by Yarrowia lipolytica using natural sources of the biopolyesters cutin and suberin, and their application in hydrolysis of poly (ethylene terephthalate) (PET). Bioprocess Biosyst. Eng. 2021, 44, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Fontes, G.C.; Amaral, P.F.; Nele, M.; Coelho, M.A. Factorial design to optimize biosurfactant production by Yarrowia lipolytica. J. Biomed. Biotechnol. 2010, 2010, 821306. [Google Scholar] [CrossRef] [PubMed]
- Sales, J.C.S.; Botelho, A.M.; Carvalho, A.S.S.; Giudicelli, L.; Castro, A.M.; Ribeiro, B.D.; Amaral, P.F.F.; Coelho, M.A.Z. Evaluation of Yarrowia lipolytica potential for the biodegradation of poly(ethylene terephthalate) (PET) from mooring lines of Oil & Gas offshore platforms. Clean. Chem. Eng. 2023, 7, 100109. [Google Scholar] [CrossRef]
- Santos, F.; Freitas, K.; Pereira, A.; Fontes, G.; Rocha-Leão, M.H.; Amaral, P. Butter whey and corn steep liquor as sole raw materials to obtain a bioemulsifier from Yarrowia lipolytica for food oil-in-water emulsions. Ciênc. Rural 2021, 51, e20200323. [Google Scholar] [CrossRef]
- Souza, C.E.C.; Farias, M.A.; Ribeiro, B.D.; Coelho, M.A.Z. Adding Value to Agro-industrial Co-products from Canola and Soybean Oil Extraction through Lipase Production Using Yarrowia lipolytica in Solid-State Fermentation. Waste Biomass Valoriz. 2017, 8, 1163–1176. [Google Scholar] [CrossRef]
- Park, Y.-K.; Ledesma-Amaro, R. What makes Yarrowia lipolytica well suited for industry? Trends Biotechnol. 2022, 41, 242–254. [Google Scholar] [CrossRef]
- Vega, R.; Domínguez, A. Cell wall composition of the yeast and mycelial forms of Yarrowia lipolytica. Arch. Microbiol. 1986, 144, 124–130. [Google Scholar] [CrossRef]
- Zainuddin, M.F.; Fai, C.K.; Ariff, A.B.; Rios-Solis, L.; Halim, M. Current Pretreatment/Cell Disruption and Extraction Methods Used to Improve Intracellular Lipid Recovery from Oleaginous Yeasts. Microorganisms 2021, 9, 251. [Google Scholar] [CrossRef]
- Aguilar-Uscanga, B.; François, J.M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef]
- Ferreira, T.; Andrade, L.; Coelho, M.A.; Rocha-Leão, M.H. A new method to obtain β-glucan from Saccharomyces cerevisiae cells. Catal. Sci. Technol. 2011, 1, 1068–1071. [Google Scholar] [CrossRef]
- Ramos-Viana, V.; Møller-Hansen, I.; Kempen, P.; Borodina, I. Modulation of the cell wall protein Ecm33p in yeast Saccharomyces cerevisiae improves the production of small metabolites. FEMS Yeast Res. 2022, 22, foac037. [Google Scholar] [CrossRef]
- Gautério, G.V.; Silvério, S.I.D.C.; Egea, M.B.; Lemes, A.C. β-glucan from brewer’s spent yeast as a techno-functional food ingredient. Front. Food Sci. Technol. 2022, 2, 1074505. [Google Scholar] [CrossRef]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.W.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef]
- Madzak, C. Yarrowia lipolytica Strains and Their Biotechnological Applications: How Natural Biodiversity and Metabolic Engineering Could Contribute to Cell Factories Improvement. J. Fungi 2021, 7, 548. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.-I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Yarrowia lipolytica yeast biomass as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05594. [Google Scholar] [CrossRef]
- Czech, A.; Merska-Kazanowska, M.; Katarzyna, O.; Zieba, G. Effect of the Use of Yarrowia lipolytica or Saccharomyces cerevisiae Yeast with a Probiotic in the Diet of Turkey Hens on Growth Performance and Gut Histology. Ann. Anim. Sci. 2020, 20, 1047–1063. [Google Scholar] [CrossRef]
- Qiao, Y.; Xia, C.; Liu, L.; Tang, L.; Wang, J.; Xu, C.; Wang, J.; Zhang, L.; Ye, X.; Huang, Y.; et al. Structural characterization and emulsifier property of yeast mannoprotein enzymatically prepared with a β-1,6-glucanase. LWT 2022, 168, 113898. [Google Scholar] [CrossRef]
- Assunção-Bicca, S.; Poncet-Legrand, C.; Williams, P.; Mekoue Nguela, J.; Doco, T.; Vernhet, A. Structural characteristics of Saccharomyces cerevisiae mannoproteins: Impact of their polysaccharide part. Carbohydr. Polym. 2022, 277, 118758. [Google Scholar] [CrossRef]
- Gonçalves, F.; Heyraud, A.; de Pinho, M.N.; Rinaudo, M. Characterization of White Wine Mannoproteins. J. Agric. Food Chem. 2002, 50, 6097–6101. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Cebrián, G.; Álvarez, I.; Raso, J. Release of Mannoproteins during Saccharomyces cerevisiae Autolysis Induced by Pulsed Electric Field. Front. Microbiol. 2016, 7, 1435. [Google Scholar] [CrossRef] [PubMed]
- Berzosa, A.; Delso, C.; Sanz, J.; Sánchez-Gimeno, C.; Raso, J. Sequential extraction of compounds of interest from yeast biomass assisted by pulsed electric fields. Front. Bioeng. Biotechnol. 2023, 11, 1197710. [Google Scholar] [CrossRef] [PubMed]
- Ganeva, V.; Angelova, B.; Galutzov, B.; Goltsev, V.; Zhiponova, M. Extraction of Proteins and Other Intracellular Bioactive Compounds From Baker’s Yeasts by Pulsed Electric Field Treatment. Front. Bioeng. Biotechnol. 2020, 8, 552335. [Google Scholar] [CrossRef] [PubMed]
- Storebakken, T.; Sørensen, M.; Bjerkeng, B.; Harris, J.; Monahan, P.; Hiu, S. Stability of astaxanthin from red yeast, Xanthophyllomyces dendrorhous, during feed processing: Effects of enzymatic cell wall disruption and extrusion temperature. Aquaculture 2004, 231, 489–500. [Google Scholar] [CrossRef]
- Marson, G.V.; Machado, M.T.d.C.; Castro, R.J.S.; Hubinger, M.D. Sequential hydrolysis of spent brewer’s yeast improved its physico-chemical characteristics and antioxidant properties: A strategy to transform waste into added-value biomolecules. Process Biochem. 2019, 84, 91–102. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.-P.; Hubinger, M.D. Spent brewer’s yeast as a source of high added value molecules: A systematic review on its characteristics, processing and potential applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar] [CrossRef]
- Bertolo, A.P.; Biz, A.P.; Kempka, A.P.; Rigo, E.; Cavalheiro, D. Yeast (Saccharomyces cerevisiae): Evaluation of cellular disruption processes, chemical composition, functional properties and digestibility. J. Food Sci. Technol. 2019, 56, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Imatoukene, N.; Koubaa, M.; Perdrix, E.; Benali, M.; Vorobiev, E. Combination of cell disruption technologies for lipid recovery from dry and wet biomass of Yarrowia lipolytica and using green solvents. Process Biochem. 2020, 90, 139–147. [Google Scholar] [CrossRef]
- Kosel, J.; Šuštaršič, M.; Petkovšek, M.; Zupanc, M.; Sežun, M.; Dular, M. Application of (super)cavitation for the recycling of process waters in paper producing industry. Ultrason. Sonochem. 2020, 64, 105002. [Google Scholar] [CrossRef]
- Šarc, A.; Kosel, J.; Stopar, D.; Oder, M.; Dular, M. Removal of bacteria Legionella pneumophila, Escherichia coli, and Bacillus subtilis by (super)cavitation. Ultrason. Sonochem. 2018, 42, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Boutros, J.A.; Magee, A.S.; Cox, D. Comparison of structural differences between yeast β-glucan sourced from different strains of saccharomyces cerevisiae and processed using proprietary manufacturing processes. Food Chem. 2022, 367, 130708. [Google Scholar] [CrossRef]
- Schiavone, M.; Vax, A.; Formosa, C.; Martin-Yken, H.; Dague, E.; François, J.M. A combined chemical and enzymatic method to determine quantitatively the polysaccharide components in the cell wall of yeasts. FEMS Yeast Res. 2014, 14, 933–947. [Google Scholar] [CrossRef]
- Alves, E.M.; Souza, J.F.d.; Oliva Neto, P.d. Advances in yeast autolysis technology—A faster and safer new bioprocess. Braz. J. Food Technol. 2021, 24, 1–14. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Aguilar, D.; Cebrián, G.; Álvarez, I.; Raso, J. Factors influencing autolysis of Saccharomyces cerevisiae cells induced by pulsed electric fields. Food Microbiol. 2018, 73, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Gautério, G.V.; da Silva, R.M.; Karraz, F.C.; Coelho, M.A.Z.; Ribeiro, B.D.; Lemes, A.C. Cell disruption and permeabilization methods for obtaining yeast bioproducts. Clean. Chem. Eng. 2023, 6, 100112. [Google Scholar] [CrossRef]
- San Martin, D.; Ibarruri, J.; Iñarra, B.; Luengo, N.; Ferrer, J.; Alvarez-Ossorio, C.; Bald, C.; Gutierrez, M.; Zufía, J. Valorisation of Brewer’s Spent Yeasts’ Hydrolysates as High-Value Bioactive Molecules. Sustainability 2021, 13, 6520. [Google Scholar] [CrossRef]
- Alves, E.M.; Souza, J.F.; Macieja, S.; Oliva-Neto, P. 5′-Ribonucleotides production using 5′-phosphodiesterase from spent malt roots. Braz. J. Food Technol. 2021, 24, e2020246. [Google Scholar] [CrossRef]
- Marson, G.V.; Pereira, D.T.V.; da Costa Machado, M.T.; Di Luccio, M.; Martínez, J.; Belleville, M.-P.; Hubinger, M.D. Ultrafiltration performance of spent brewer’s yeast protein hydrolysate: Impact of pH and membrane material on fouling. J. Food Eng. 2021, 302, 110569. [Google Scholar] [CrossRef]
- Vollet Marson, G.; Belleville, M.-P.; Lacour, S.; Dupas Hubinger, M. Membrane Fractionation of Protein Hydrolysates from By-Products: Recovery of Valuable Compounds from Spent Yeasts. Membranes 2021, 11, 23. [Google Scholar] [CrossRef]
- Silva, L.V.; Tavares, C.; Amaral, P.; Coelho, M.A. Production of Citric Acid by Yarrowia lipolytica in Different Crude Glycerol Concentrations and in Different Nitrogen Sources. Chem. Eng. Trans. 2012, 27, 199–204. [Google Scholar]
- Faustino, M.; Durão, J.; Pereira, C.F.; Oliveira, A.S.; Pereira, J.O.; Pereira, A.M.; Ferreira, C.; Pintado, M.E.; Carvalho, A.P. Comparative Analysis of Mannans Extraction Processes from Spent Yeast Saccharomyces cerevisiae. Foods 2022, 11, 3753. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- McCleary, B.V.; Draga, A. Measurement of β-Glucan in Mushrooms and Mycelial Products. J. AOAC Int. 2016, 99, 364–373. [Google Scholar] [CrossRef]
- Marson, G.V.; Lacour, S.; Hubinger, M.D.; Belleville, M.-P. Serial fractionation of spent brewer’s yeast protein hydrolysate by ultrafiltration: A peptide-rich product with low RNA content. J. Food Eng. 2022, 312, 110737. [Google Scholar] [CrossRef]
- Lemes, A.C.; Molon, F.d.O.; Fagundes, A.d.S.; Egea, M.B.; Di Luccio, M.; Kalil, S.J. Membrane Technology as a Strategy for Improving β-Galactosidase Concentration Processes: The Influence of the pH, Membrane Molecular Weight, Pressure, and Ionic Strength in the Process. Appl. Sci. 2023, 13, 1626. [Google Scholar] [CrossRef]
- Rufino, M.d.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Liu, H.; Li, Y.; Shi, A.; Hu, H.; Sheng, X.; Liu, L.; Wang, Q.; Adhikari, B. Rheological characteristics and chain conformation of mannans obtained from Saccharomyces cerevisiae. Int. J. Biol. Macromol. 2018, 107, 2404–2411. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Limnaios, A.; Aerakis, E.; Andreou, V.; Taoukis, P. Effect of high pressure on the proteolytic activity and autolysis of yeast Saccharomyces cerevisiae. Innov. Food Sci. Emerg. Technol. 2021, 74, 102865. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Bourbon-Melo, N.; Sá-Correia, I. The cell wall and the response and tolerance to stresses of biotechnological relevance in yeasts. Front. Microbiol. 2022, 13, 953479. [Google Scholar] [CrossRef]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Cermeño, M.; Felix, M.; Connolly, A.; Brennan, E.; Coffey, B.; Ryan, E.; FitzGerald, R.J. Role of carbohydrate conjugation on the emulsification and antioxidant properties of intact and hydrolysed whey protein concentrate. Food Hydrocoll. 2019, 88, 170–179. [Google Scholar] [CrossRef]
- Ereifej, K.I.; Rababah, T.M.; Al-Rababah, M.A. Quality Attributes of Halva by Utilization of Proteins, Non-hydrogenated Palm Oil, Emulsifiers, Gum Arabic, Sucrose, and Calcium Chloride. Int. J. Food Prop. 2005, 8, 415–422. [Google Scholar] [CrossRef]
- Herceg, Z.; Režek, A.; Lelas, V.; Krešić, G.; Franetović, M. Effect of carbohydrates on the emulsifying, foaming and freezing properties of whey protein suspensions. J. Food Eng. 2007, 79, 279–286. [Google Scholar] [CrossRef]
- Reis, S.F.; Fernandes, P.A.R.; Martins, V.J.; Gonçalves, S.; Ferreira, L.P.; Gaspar, V.M.; Figueira, D.; Castelo-Branco, D.; Mano, J.F.; Coimbra, M.A.; et al. Brewer’s Spent Yeast Cell Wall Polysaccharides as Vegan and Clean Label Additives for Mayonnaise Formulation. Molecules 2023, 28, 3540. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Costa, A.R.; Pereira, A.S.; Belo, I. Yarrowia lipolytica as a biorefinery platform for effluents and solid wastes valorization-challenges and opportunities. Crit. Rev. Biotechnol. 2022, 42, 163–183. [Google Scholar] [CrossRef]
- Araújo, V.B.d.S.; Melo, A.N.F.d.; Costa, A.G.; Castro-Gomez, R.H.; Madruga, M.S.; Souza, E.L.d.; Magnani, M. Followed extraction of β-glucan and mannoprotein from spent brewer’s yeast (Saccharomyces uvarum) and application of the obtained mannoprotein as a stabilizer in mayonnaise. Innov. Food Sci. Emerg. Technol. 2014, 23, 164–170. [Google Scholar] [CrossRef]
- Silva, J.F.d.; Silva, L.A.R.d.; Barbosa, M.R.; Houllou, L.M.; Malafaia, C.B. Bioemulsifier produced by Yarrowia lipolytica using residual glycerol as a carbon source. J. Environ. Anal. Prog. 2020, 5, 031–037. [Google Scholar] [CrossRef]
- Sumiardi, A.; Soetarto, E.S.; Susilaningsih, D. Screening and characterization of biosurfactant produced by bacterial consortium in degrading polycyclic aromatic hydrocarbon compound. AIP Conf. Proc. 2002, 2002, 1–9. [Google Scholar] [CrossRef]
- Chmielarz, M.; Sampels, S.; Blomqvist, J.; Brandenburg, J.; Wende, F.; Sandgren, M.; Passoth, V. FT-NIR: A tool for rapid intracellular lipid quantification in oleaginous yeasts. Biotechnol. Biofuels 2019, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Shapaval, V.; Brandenburg, J.; Blomqvist, J.; Tafintseva, V.; Passoth, V.; Sandgren, M.; Kohler, A. Biochemical profiling, prediction of total lipid content and fatty acid profile in oleaginous yeasts by FTIR spectroscopy. Biotechnol. Biofuels 2019, 12, 140. [Google Scholar] [CrossRef]
- Wan, M.; Wang, M.; Zhao, Y.; Deng, H.; Tan, C.; Lin, S.; Kong, Y.; Tong, Y.; Meng, X. Extraction of mannoprotein from Saccharomyces cerevisiae and analysis of its chemical composition and molecular structure. Int. J. Biol. Macromol. 2021, 193, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.-W.; Fu, J.-J.; Li, J.-J.; Tang, Y.; Shao, Z.-W.; Zhou, D.-Y.; Song, L. Efficient encapsulation of curcumin into spent brewer’s yeast using a pH-driven method. Food Chem. 2022, 394, 133537. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tian, J.; Zhou, Z.; Pan, Y.; Li, Z. Antioxidant activity and interactions between whey protein and polysaccharides from different parts of Houttuynia cordata. Front. Nutr. 2023, 10, 1020328. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Chu, Y.-S.; Liu, J.-L.; Chang, J.-S. Thermal degradation of carbohydrates, proteins and lipids in microalgae analyzed by evolutionary computation. Energy Convers. Manag. 2018, 160, 209–219. [Google Scholar] [CrossRef]
- Erian, A.M.; Egermeier, M.; Marx, H.; Sauer, M. Insights into the glycerol transport of Yarrowia lipolytica. Yeast 2022, 39, 323–336. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Kieliszek, M.; Błażejak, S. Chemical composition of the cell wall of probiotic and brewer’s yeast in response to cultivation medium with glycerol as a carbon source. Eur. Food Res. Technol. 2013, 237, 489–499. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Esteban, M.; Angulo, C. Yarrowia lipolytica, health benefits for animals. Appl. Microbiol. Biotechnol. 2021, 105, 7577–7592. [Google Scholar] [CrossRef]
- Zlatanović, S.; Kalušević, A.; Micić, D.; Laličić-Petronijević, J.; Tomić, N.; Ostojić, S.; Gorjanović, S. Functionality and Storability of Cookies Fortified at the Industrial Scale with up to 75% of Apple Pomace Flour Produced by Dehydration. Foods 2019, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Zhou, R.; Liu, D.; Xu, X.; Zhou, G. Water-soluble myofibrillar proteins prepared by high-pressure homogenisation: A comparison study on the composition and functionality. Int. J. Food Sci. Technol. 2017, 52, 2334–2342. [Google Scholar] [CrossRef]
- Rios, R.V.; Pessanha, M.D.F.; Almeida, P.F.d.; Viana, C.L.; Lannes, S.C.d.S. Application of fats in some food products. Food Sci. Technol. 2014, 34, 3–15. [Google Scholar] [CrossRef]
- Jach, M.E.; Malm, A. Yarrowia lipolytica as an Alternative and Valuable Source of Nutritional and Bioactive Compounds for Humans. Molecules 2022, 27, 2300. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bassart, Z.; Fabra, M.J.; Martínez-Abad, A.; López-Rubio, A. Compositional differences of β-glucan-rich extracts from three relevant mushrooms obtained through a sequential extraction protocol. Food Chem. 2023, 402, 134207. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.A.; Wyrwisz, J.; Brzeska, M.; Moczkowska, M.; Karp, S.; Wierzbicka, A. Effect of different beta-glucan preparation pretreatments on fortified bread quality. Food Sci. Technol. 2018, 38, 606–611. [Google Scholar] [CrossRef]
- Szczepańska, P.; Rychlicka, M.; Moroz, P.; Janek, T.; Gliszczyńska, A.; Lazar, Z. Elevating Phospholipids Production Yarrowia lipolytica from Crude Glycerol. Int. J. Mol. Sci. 2022, 23, 10737. [Google Scholar] [CrossRef] [PubMed]
- Chotigavin, N.; Sriphochanart, W.; Yaiyen, S.; Kudan, S. Increasing the Production of β-Glucan from Saccharomyces carlsbergensis RU01 by Using Tannic Acid. Appl. Biochem. Biotechnol. 2021, 193, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, A.; Miyazawa, K.; Abe, K. Function and Biosynthesis of Cell Wall α-1,3-Glucan in Fungi. J. Fungi 2017, 3, 63. [Google Scholar] [CrossRef]
- Rakicka, M.; Lazar, Z.; Dulermo, T.; Fickers, P.; Nicaud, J.M. Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions. Biotechnol. Biofuels 2015, 8, 104. [Google Scholar] [CrossRef]
- Pereyra, C.M.; Gil, S.; Cristofolini, A.; Bonci, M.; Makita, M.; Monge, M.P.; Montenegro, M.A.; Cavaglieri, L.R. The production of yeast cell wall using an agroindustrial waste influences the wall thickness and is implicated on the aflatoxin B1 adsorption process. Food Res. Int. 2018, 111, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Snyman, C.; Mekoue Nguela, J.; Sieczkowski, N.; Marangon, M.; Divol, B. Optimised Extraction and Preliminary Characterisation of Mannoproteins from Non-Saccharomyces Wine Yeasts. Foods 2021, 10, 924. [Google Scholar] [CrossRef] [PubMed]
- Pancrazio, G.; Cunha, S.C.; de Pinho, P.G.; Loureiro, M.; Meireles, S.; Ferreira, I.M.P.L.V.O.; Pinho, O. Spent brewer’s yeast extract as an ingredient in cooked hams. Meat Sci. 2016, 121, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.F.; Aranha, J.B.; Vieira, T.M.F.d.S. Replacing synthetic antioxidants in food emulsions with microparticles from green acerola (Malpighia emarginata). Future Foods 2022, 5, 100130. [Google Scholar] [CrossRef]
- Pereira, A.S.; Sant’Ana, G.C.F.; Amaral, P.F.F. Mango agro-industrial wastes for lipase production from Yarrowia lipolytica and the potential of the fermented solid as a biocatalyst. Food Bioprod. Process. 2019, 115, 68–77. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Sun, H.; Du, M.; Qiu, J.; Tang, M.; Sun, X.; Zhu, B. Antioxidative Peptides from Proteolytic Hydrolysates of False Abalone (Volutharpa ampullacea perryi): Characterization, Identification, and Molecular Docking. Mar. Drugs 2019, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chen, D.; Mao, X.; He, J.; Yu, B.; Cheng, L.; Zeng, D. Purified β-glucans of Different Molecular Weights Enhance Growth Performance of LPS-challenged Piglets via Improved Gut Barrier Function and Microbiota. Animals 2019, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Vasilieva, T.; Sigarev, A.; Kosyakov, D.; Ul’yanovskii, N.; Anikeenko, E.; Chuhchin, D.; Ladesov, A.; Hein, A.M.; Miasnikov, V. Formation of low molecular weight oligomers from chitin and chitosan stimulated by plasma-assisted processes. Carbohydr. Polym. 2017, 163, 54–61. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Alcolea, J.F.; Cano, A.; Acosta, M.; Arnao, M.B. Hydrophilic and lipophilic antioxidant activities of grapes. Die Nahr. 2002, 46, 353–356. [Google Scholar] [CrossRef]
- Machová, E.; Bystrický, S. Antioxidant capacities of mannans and glucans are related to their susceptibility of free radical degradation. Int. J. Biol. Macromol. 2013, 61, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.; Rogiewicz, A.; Kiarie, E.G.; Slominski, B.A. Yeast derivatives as a source of bioactive components in animal nutrition: A brief review. Front. Vet. Sci. 2023, 9, 1067383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Y.; Xu, M.; Ding, L.; Zhang, T.; Liu, J. Antioxidant Synergetic Effect Between the Peptides Derived from the Egg White Pentapeptide Trp-Asn-Trp-Ala-Asp. Int. J. Pept. Res. Ther. 2017, 23, 509–518. [Google Scholar] [CrossRef]
- Jia, X.Y.; Zhu, M.F.; Zhang, L.; Ma, T.X.; Li, Y.H.; Sheng, W.S.; Tu, Z.C. Extraction optimization and screening of antioxidant peptides from grass carp meat and synergistic-antagonistic effect. Food Sci. Nutr. 2022, 10, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Girgih, A.T.; Malomo, S.A.; Ju, X.; Aluko, R.E. Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J. Funct. Foods 2013, 5, 219–227. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Zhao, Y.-Q.; Wang, Y.-M.; Zhao, W.-H.; Wang, P.; Chi, C.-F.; Wang, B. Antioxidant peptides from Antarctic Krill (Euphausia superba) hydrolysate: Preparation, identification and cytoprotection on H2O2-induced oxidative stress. J. Funct. Foods 2021, 86, 104701. [Google Scholar] [CrossRef]
- Cai, W.W.; Hu, X.M.; Wang, Y.M.; Chi, C.F.; Wang, B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs: Preparation, Identification, Antioxidant Activity, and Stability against Thermal, pH, and Simulated Gastrointestinal Digestion Treatments. Mar. Drugs 2022, 20, 626. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Li, X.-Y.; Wang, J.; He, Y.; Chi, C.-F.; Wang, B. Antioxidant peptides from protein hydrolysate of skipjack tuna milt: Purification, identification, and cytoprotection on H2O2 damaged human umbilical vein endothelial cells. Process Biochem. 2022, 113, 258–269. [Google Scholar] [CrossRef]
- Tadesse, S.A.; Emire, S.A. Production and processing of antioxidant bioactive peptides: A driving force for the functional food market. Heliyon 2020, 6, e04765. [Google Scholar] [CrossRef]
- Fakruddin, M.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement. Altern. Med. 2017, 17, 64. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).