Exploration and Frontier of Coal Spontaneous Combustion Fire Prevention Materials
Abstract
:1. Introduction
2. Visual Analytics
3. Current Situation of Fire Prevention Materials
3.1. Physically Inhibited Material
3.1.1. Grouting Material
3.1.2. Gas Material
3.1.3. Colloid Material
3.1.4. Foam Material
Two-Phase Foam
Three-Phase Foam
Gel Foam
3.1.5. Physical Inhibitors
3.2. Chemical Inhibited Material
3.2.1. Chemical Inhibitors
| Author | Materials | Effect |
|---|---|---|
| Li Yiheng [68] | Rare earth hydrotalcites | The -OH group in the inhibitor can react with the oxygen-containing functional groups such as -COO- in the coal, effectively preventing the active -COO- functional groups in the coal from continuing to participate in the oxidation process at low temperatures, reducing the possibility of coal oxidation. |
| Zhang Yutao [69] | Zn1Mg2Al1-CO3-LDHs | The starting exothermic temperature of the coal sample was delayed by 30~60 °C; when the addition amount reached 25%, the heat absorbed in the dehydration and desorption stage was three times that of the original coal sample, and the exothermic amount in the oxidation and combustion process was reduced by 5510 J, and the maximum heat-releasing power was also reduced from 32.96 to 23.5 mW/mg. The inhibition rate increased linearly with the addition of 1% Zn1Mg2Al1-CO3-LDHs, and the inhibition rate increased by 1.6%. |
| Deng et al. [70] | Ionic liquid | The ionic liquid with the greatest effect on independent hydrogen bonding, methylene, and carbonyl groups is [Bmim][BF4], and the ionic liquid with the greatest effect on conjugated hydrogen bonding, methyl, and carboxyl groups is [Bmim][I]. Based on the inhibitory effects, the four ionic liquids were ranked in the following order: [Bmim][I] < [Emim][BF4] < [Bmim][NO3] < [Bmim][BF4]. |
| Li et al. [71] | 2,2,6,6-Tetramethyl-1-piperidinoyloxy (TEMPO) | The exothermic peak of the raw coal sample appeared at 383 °C, the exothermic peak of the MgCl2-treated sample was delayed to 396 °C, and the exothermic peaks of CaCl2 and TEMPO were delayed to 438 and 452 °C, respectively, and the inhibition of the reactive functional groups such as C-O, C-H, C=O, and O-H in the coal by TEMPO was more significant, which reduced the concentration of the reactive free radicals. |
| Lv et al. [72] | [BMIM][BF4] | The high content of ionic liquids can induce exothermic reactions, thus reducing their inhibitory effect; with the decrease in O2 concentration, the inhibitory effect of ionic liquids is enhanced, and the inhibitory effect of ionic liquids is significantly elevated when the O2 volume fraction is lower than 10 percent |
| Sandeep Kumar et al. [73] | NaCl, CaCO3, KI, NaNO, KCl | According to the results of the flammability temperature (FT) test, crossing point temperature (CPT) test, and differential thermal analysis (DTA), KCl and CaCO3 are the most effective inhibitors. At 15% weight ratio of CaCO3, CPT is 35 °C higher than that of raw coal, and at 20% weight ratio of KCl, CPT is 29 °C higher than that of raw coal. At a 20% weight ratio, FT of KCl and CaCO3 increased by 15 °C and 40 °C, and DTA increased by 32.01 °C and 31.96 °C compared with raw coal. |
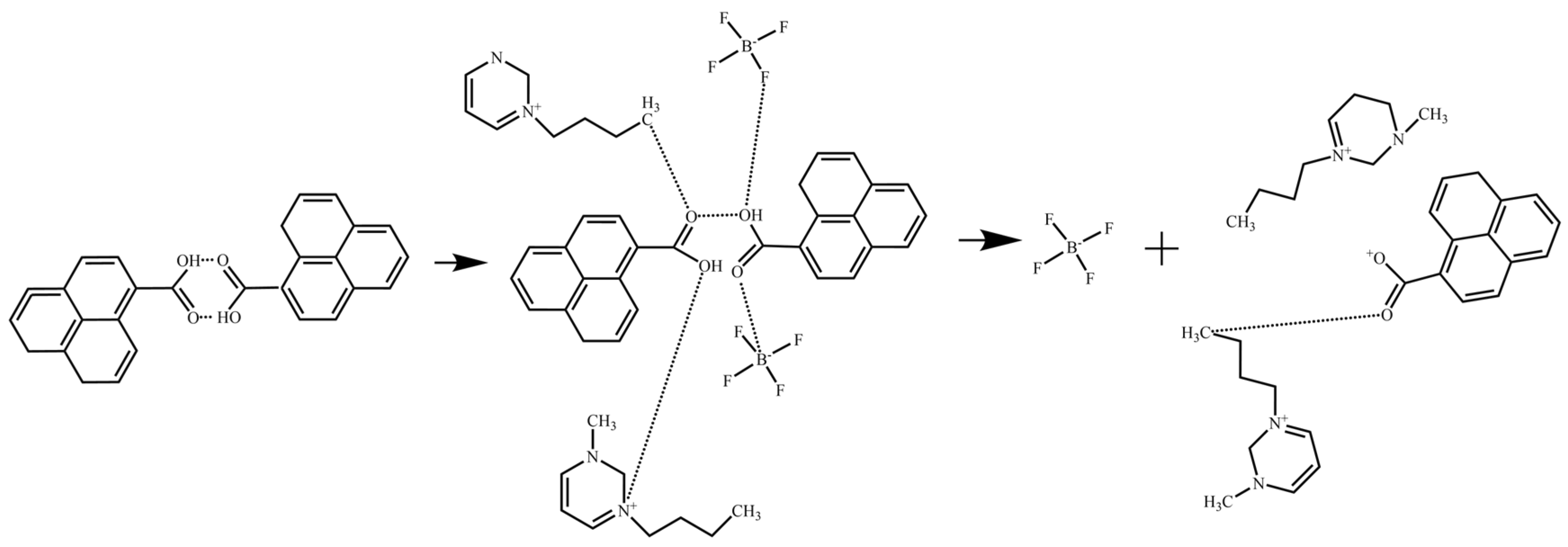
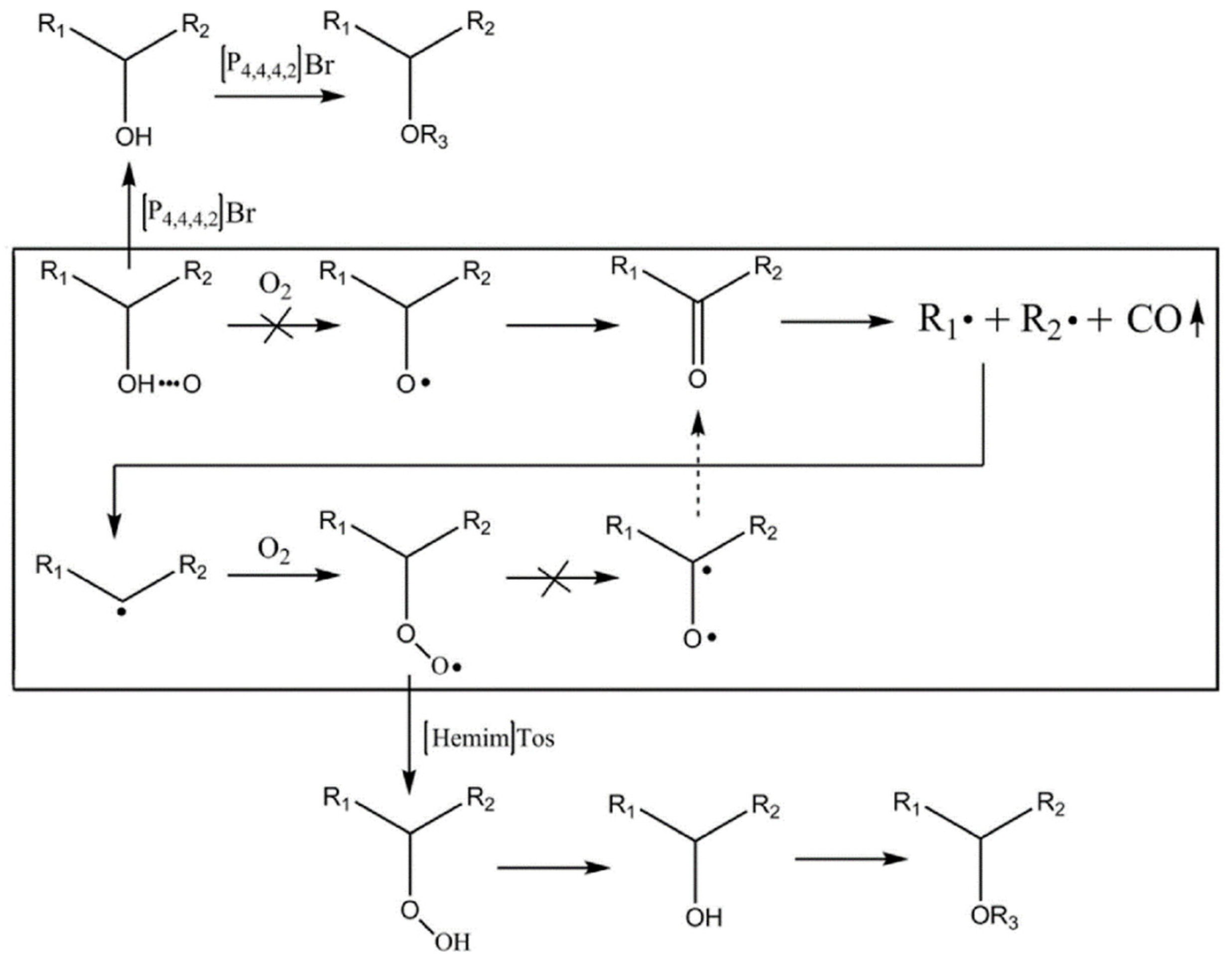

3.2.2. Antioxidant Inhibitor
3.2.3. Microbial Inhibition
3.3. Physicochemical Synergistic Inhibition Material
3.3.1. Composite Foam
3.3.2. Composite Gel
3.3.3. Compound Inhibitor
4. Conclusions
- (1)
- The keyword co-occurrence map of CSC fire-fighting materials was obtained through CiteSpace visual analysis. Through the Atlas network, it is concluded that the development of CSC fire-fighting materials has experienced several stages, such as grouting, inert gas, colloid, retarder, foam, and gel foam. The characterization methods of CSC prevention and control by each inhibitory material were analyzed. Through the keyword network, it is concluded that the current research frontier focuses on the verification of the inhibition mechanism of different inhibition materials by molecular simulation.
- (2)
- The existing CSC fire-fighting materials can be divided into physical inhibition, chemical inhibition, and physicochemical coordination inhibition according to the form of action. These materials reduce the oxidation rate of coal and inhibit CSC by diluting oxygen, isolating oxygen, and hindering chain reaction.
- (3)
- In terms of application, composite inhibitors, environmentally friendly foams, gels, gel foams, etc., not only improve flame retardant efficiency but also show great potential in environmental protection and durability. In addition, the synergistic mechanism of compound antioxidants enhances the flame-retardant effect, reveals the microscopic mechanism of chemical inhibitors, and provides theoretical guidance for the design of high-performance inhibitors. In the future, the use of microbial inhibition of CSC will show environmental friendliness and long-term action, which is a frontier direction worthy of attention, and its management strategy and potential application deserve further exploration.
5. Existing Problems and Development Trends
- (1)
- Affected by factors such as the increasing depth of mining and the complex and dynamic mining environment, CSC prevention and control pose significant challenges. The existing fire prevention materials still fail to fully meet the requirements for effective fire prevention and control. Therefore, developing novel fire prevention materials with prolonged resistance life, enhanced fire prevention efficiency, and simplified manufacturing processes is a pressing issue that needs to be addressed.
- (2)
- The retarding effect of the same coal varies when subjected to the same fire-proof material. Therefore, it is imperative to analyze the alterations in key functional groups, free radicals, reaction pathways, and other factors during the retarding process while comprehensively elucidating the mechanism underlying CSC.
- (3)
- Some materials may decompose into toxic and harmful substances when exposed to heat, which can potentially exert significant ramifications on human health and the ecological environment. Additionally, fire-fighting materials may adhere to coal surfaces and form complex solid impurities, potentially leading to soil and water pollution as well as increased secondary treatment costs. Further research is needed to investigate these potential effects.
- (4)
- Through continuous exploration and innovation, the use of refractory materials with strong popularity and excellent fire protection effect can implement accurate and efficient flame-retardant strategies for different types of coal, greatly enhancing the overall effectiveness of fire prevention measures.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, L.; Tan, J. Financial Development, Industrial Structure and Natural Resource Utilization Efficiency in China. Resour. Policy 2020, 66, 101642. [Google Scholar] [CrossRef]
- Dong, F.; Li, J.; Wang, Y.; Zhang, X.; Zhang, S.; Zhang, S. Drivers of the Decoupling Indicator between the Economic Growth and Energy-Related CO2 in China: A Revisit from the Perspectives of Decomposition and Spatiotemporal Heterogeneity. Sci. Total Environ. 2019, 685, 631–658. [Google Scholar] [CrossRef]
- Li, H.; Long, R.; Chen, H. Economic Transition Policies in Chinese Resource-Based Cities: An Overview of Government Efforts. Energy Policy 2013, 55, 251–260. [Google Scholar] [CrossRef]
- Laryea, A.E.N.; Ren, W.; Guo, Q.; Kang, Z. Spontaneous Coal Combustion, Direct and Indirect Impact on Mining in China: A Prospective Review and Proposal of a Five-Level Comprehensive Mine Safety Management Structure (5L-CMSMS) Coupled with Hazard Zoning and Barrier Systems. Combust. Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Qiao, M.; Ren, T.; Roberts, J.; Yang, X.; Li, Z.; Wu, J. New Insight into Proactive Goaf Inertisation for Spontaneous Combustion Management and Control. Process Saf. Environ. Prot. 2022, 161, 739–757. [Google Scholar] [CrossRef]
- Chu, C.; Muradian, N. Safety and Environmental Implications of Coal Mining. Int. J. Environ. Pollut. 2016, 59, 250–268. [Google Scholar] [CrossRef]
- Kong, B.; Li, Z.; Yang, Y.; Liu, Z.; Yan, D. A Review on the Mechanism, Risk Evaluation, and Prevention of Coal Spontaneous Combustion in China. Environ. Sci. Pollut. Res. 2017, 24, 23453–23470. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, J.; Hao, H.; Fu, G.; Nie, F.; Zhang, W. Root Causes of Coal Mine Accidents: Characteristics of Safety Culture Deficiencies Based on Accident Statistics. Process Saf. Environ. Prot. 2020, 136, 78–91. [Google Scholar] [CrossRef]
- Mahdevari, S.; Shahriar, K.; Esfahanipour, A. Human Health and Safety Risks Management in Underground Coal Mines Using Fuzzy TOPSIS. Sci. Total Environ. 2014, 488, 85–99. [Google Scholar] [CrossRef]
- Eliopoulou, E.; Alissafaki, A.; Papanikolaou, A. Statistical Analysis of Accidents and Review of Safety Level of Passenger Ships. J. Mar. Sci. Eng. 2023, 11, 410. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, K.; Reniers, G.; You, G. Statistical Analysis the Characteristics of Extraordinarily Severe Coal Mine Accidents (ESCMAs) in China from 1950 to 2018. Process Saf. Environ. Prot. 2020, 133, 332–340. [Google Scholar] [CrossRef]
- Zhang, L.; Bian, Y.; Kuai, D. Preparation and Flame Retardant Property of Nano-Aluminum Hydroxide Foam for Preventing Spontaneous Coal Combustion. Fuel 2021, 304, 121494. [Google Scholar] [CrossRef]
- Qin, B.; Jia, Y.; Lu, Y.; Li, Y.; Wang, D.; Chen, C. Micro Fly-Ash Particles Stabilized Pickering Foams and Its Combustion-Retardant Characteristics. Fuel 2015, 154, 174–180. [Google Scholar] [CrossRef]
- Hunter, T.N.; Pugh, R.J.; Franks, G.V.; Jameson, G.J. The Role of Particles in Stabilising Foams and Emulsions. Adv. Colloid Interface Sci. 2008, 137, 57–81. [Google Scholar] [CrossRef]
- Xue, D.; Hu, X.; Cheng, W.; Wu, M.; Shao, Z.; Li, Y.; Zhao, Y.; Zhang, K. Carbon Dioxide Sealing-Based Inhibition of Coal Spontaneous Combustion: A Temperature-Sensitive Micro-Encapsulated Fire-Retardant Foamed Gel. Fuel 2020, 266, 117036. [Google Scholar] [CrossRef]
- Yuan, S.; Sun, B.; Han, G.; Duan, W.; Wang, Z. Application and Prospect of Curtain Grouting Technology in Mine Water Safety Management in China: A Review. Water 2022, 14, 4093. [Google Scholar] [CrossRef]
- Yang, S.; Li, M.; Song, G.; Yang, Y.; Xie, F. Optimization of Face Flexible Bolting and Grouting Technology for Longwall Face Support under Difficult Geological Conditions. Energy Sci. Eng. 2020, 8, 1260–1270. [Google Scholar] [CrossRef]
- Wang, D.; Li, Z.; Qin, B.; Liang, X.; Chen, J. Development of a new green material for preventing mine fires. J. China Univ. Min. Technol. 2004, 33, 81–84. [Google Scholar]
- Wang, Z.P.; Song, X.M.; Xiao, Y.; Wang, H.Q. Research and application of coal bed fire prevention technology based on fly ash resource utilization. China Coal 2010, 36, 99–102. [Google Scholar]
- Xiao, Y.; Ma, T.; Deng, J.; Wang, Z.P.; Song, X.M. Fire suppression technology for coal ash utilization in mines. Min. Saf. Environ. Prot. 2010, 37, 29–31. [Google Scholar]
- Jian, S. Research on the Preparation and Performance of Fire Suppression Materials for Coal Mining. Master’s Thesis, Beijing Institute of Technology, Beijing, China, 2017. [Google Scholar]
- Tang, L.; Qi, Y.; Li, X.; Wang, J. Coal Fire Prevention in Large Areas over Long Term with a Composite Inert Gas-a Case Study in Tangkou Coal Mine, China. Energy Sources Part A-Recovery Util. Environ. Eff. 2019. [Google Scholar] [CrossRef]
- Si, J.; Li, L.; Cheng, G.; Shao, H.; Wang, Y.; Li, Z. Characteristics and Safety of CO2 for the Fire Prevention Technology with Gob-Side Entry Retaining in Goaf. ACS Omega 2021, 6, 18518–18526. [Google Scholar] [CrossRef]
- Ray, S.K.; Singh, R.P. Recent Developments and Practices to Control Fire in Undergound Coal Mines. Fire Technol. 2007, 43, 285–300. [Google Scholar] [CrossRef]
- Ding, C.; Li, Z.; Wang, J.; Lu, B.; Gao, D. Effects of Inert Gas CO2/N2 Injection on Coal Low-Temperature Oxidation Characteristic: Experiments and Simulations. Arab. J. Chem. 2023, 16, 104510. [Google Scholar] [CrossRef]
- Lei, B.; He, B.; Xiao, B.; Du, P.; Wu, B. Comparative Study of Single Inert Gas in Confined Space Inhibiting Open Flame Coal Combustion. Fuel 2020, 265, 116976. [Google Scholar] [CrossRef]
- Cao, N.-F.; Liang, Y.-T. Research on the Control Process of Mining and Combustion Disturbance Zone Based on the Three-Dimensional Dynamic Distribution Model of Void Fraction. Combust. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Lu, Y.; Yan, Z.; Shi, S.; Wang, G.; Li, H.; Niu, H.; Guo, Z.; Wang, P. Delineation and Prevention of the Spontaneous Combustion Dangerous Area of Coal in a Regenerated Roof: A Case Study in the Zhoujing Coal Mine, China. Energy Fuels 2020, 34, 6401–6413. [Google Scholar] [CrossRef]
- Niu, H.; Sun, Q.; Bu, Y.; Yang, Y.; Sun, S.; Li, S.; Tao, M.; Mao, Z. Review and Prospects of Research on Materials to Prevent and Extinguish Mine Fires. Fire Mater. 2023, 47, 739–757. [Google Scholar] [CrossRef]
- Ren, X.; Hu, X.; Xue, D.; Li, Y.; Shao, Z.; Dong, H.; Cheng, W.; Zhao, Y.; Xin, L.; Lu, W. Novel Sodium Silicate/Polymer Composite Gels for the Prevention of Spontaneous Combustion of Coal. J. Hazard. Mater. 2019, 371, 643–654. [Google Scholar] [CrossRef]
- Cheng, W.; Hu, X.; Xie, J.; Zhao, Y. An Intelligent Gel Designed to Control the Spontaneous Combustion of Coal: Fire Prevention and Extinguishing Properties. Fuel 2017, 210, 826–835. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, C.; Gao, Y.; Ji, Y.; Wang, H.; Zhang, Y.; Yang, R. R&D of Colloid Components of Composite Material for Fire Prevention and Extinguishing and an Investigation of Its Performance. Process Saf. Environ. Prot. 2018, 113, 357–368. [Google Scholar]
- Zhao, Z. Research on Temperature Sensitive Expanded Gel Material for Mining and Its Fire Fighting Characteristics. Master’s Thesis, China University of Mining and Technology, Beijing, China, 2023. [Google Scholar]
- Zhou, C.; Tang, Y. A Novel Sodium Carboxymethyl Ellulose/Aluminium Citrate Gel for Extinguishing Spontaneous Fire in Coal Mines. Fire Mater. 2018, 42, 760–769. [Google Scholar] [CrossRef]
- Dong, K.; Wang, J.; Zhang, Y.; Liang, Z.; Shi, Q. Performance of Fire Extinguishing Gel with Strong Stability for Coal Mine. Combust. Sci. Technol. 2022, 194, 1661–1677. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Y.; Yu, S.; Du, J.; Hu, X.; Bai, G.; Wang, Z. Environment-Friendly Dual-Network Hydrogel Dust Suppressant Based on Xanthan Gum, Polyvinyl Alcohol and Acrylic Acid. J. Environ. Manag. 2021, 295, 113139. [Google Scholar] [CrossRef]
- Yan, Q.; Lu, Y.; Wang, Q.; Gu, W.; Wu, F. Research Progress and Development Trend of Coal Spontaneous Combustion Prevention Technology. Combust. Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Yu, M.; Yang, N.; Liu, Z.; Li, H.; Wang, L.; Wu, M.; Li, J.; Yu, Y. Experimental Preparation and Mechanism Analysis of a Neotype Composite Gel for Coal Spontaneous Combustion Prevention and Coal-Fire Extinguishment. Fuel 2023, 339, 127448. [Google Scholar] [CrossRef]
- Kong, B.; Li, J.; Lu, W.; Fu, W.; Song, H.; Liu, J. Research on a Spontaneous Combustion Prevention System in Deep Mine: A Case Study of Dongtan Coal Mine. Combust. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Li, M.; Wang, D.; He, S.; Shao, Z.; Shen, Y. Experimental Study on Foaming Properties of Anion-Cation Compound Foaming Agent to Prevent Coal Spontaneous Combustion. Colloids Surf. A-Physicochem. Eng. Asp. 2019, 581, 123847. [Google Scholar] [CrossRef]
- Tang, Z.; Xu, G.; Yang, S.; Deng, J.; Xu, Q.; Chang, P. Fire-Retardant Foam Designed to Control the Spontaneous Combustion and the Fire of Coal: Flame Retardant and Extinguishing Properties. Powder Technol. 2021, 384, 258–266. [Google Scholar] [CrossRef]
- Xie, H.; Ju, Y.; Ren, S.; Gao, F.; Liu, J.; Zhu, Y. Theoretical and Technological Exploration of Deep in Situ Fluidized Coal Mining. Front. Energy 2019, 13, 603–611. [Google Scholar] [CrossRef]
- Wang, H.; Du, Y.; Wang, D.; Qin, B. Recent Progress in Polymer-Containing Soft Matters for Safe Mining of Coal. Polymers 2019, 11, 1706. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, D.; Wang, Y.; Zhong, X.; Tang, X.; Hu, X. Controlling Coal Fires Using the Three-Phase Foam and Water Mist Techniques in the Anjialing Open Pit Mine, China. Nat. Hazards 2015, 75, 1833–1852. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Du, W.; Dong, H.; Wang, B.; Wang, Y.; Cao, X. Preparation and Characteristic Study of the Hydrogel of Coal Spontaneous Combustion Environmental Protection. Fuel 2024, 360, 130505. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, C.; Guo, J.; Wang, X.; Wu, B. Experimental study on optimization of three-phase foam blowing agent for fire prevention. Coal Technol. 2019, 38, 45–48. [Google Scholar]
- Wang, Z.; Qi, X.; He, S.; Yu, T.; Fan, H.; Sun, L.; Yang, Y. Preparation of three-phase foam from fly ash and its stabilization mechanism. J. China Univ. Pet. (Nat. Sci. Ed.) 2020, 44, 166–176. [Google Scholar]
- Zhang, B.; Qin, Y.; Shen, H.; Meng, H.; Cui, J.; Pan, R. Constructing Fibrous-like Skeleton by Simulated Sea Mud to Improve Three-Phase Foam for Stability and Fire-Extinguishing Performance. J. Dispers. Sci. Technol. 2023, 44, 2311–2321. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Yang, H.; Li, H.; Yu, M.; He, T.; Luo, Z.; Liu, F. A Novel Biomass Thermoresponsive Konjac Glucomannan Composite Gel Developed to Control the Coal Spontaneous Combustion: Fire Prevention and Extinguishing Properties. Fuel 2021, 306, 121757. [Google Scholar] [CrossRef]
- Dickinson, E. Biopolymer-Based Particles as Stabilizing Agents for Emulsions and Foams. Food Hydrocoll. 2017, 68, 219–231. [Google Scholar] [CrossRef]
- Xiong, J.; Zhao, Z.; Sun, W.; Liu, W. Foam Stabilization Mechanism of a Novel Non-Cross-Linked Foam Fracturing Fluid. ACS Omega 2021, 6, 32863–32868. [Google Scholar] [CrossRef]
- Han, C.; Nie, S.; Liu, Z.; Liu, S.; Zhang, H.; Li, J.; Zhang, H.; Wang, Z. A Novel Biomass Sodium Alginate Gel Foam to Inhibit the Spontaneous Combustion of Coal. Fuel 2022, 314, 122779. [Google Scholar] [CrossRef]
- Wu, X.; Nie, S.; Han, C.; Dai, G.; Yang, W.; Fang, C. Preparation of Gel Foam Material for Coal Mine Fire Prevention and Its Performance. China Prod. Saf. Sci. Technol. 2022, 18, 78–84. [Google Scholar]
- Wu, M.; Liang, Y.; Zhao, Y.; Wang, W.; Hu, X.; Tian, F.; He, Z.; Li, Y.; Liu, T. Preparation of New Gel Foam and Evaluation of Its Fire Extinguishing Performance. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 629, 127443. [Google Scholar] [CrossRef]
- Qiao, J.; Hu, X.-M.; Liang, Y.-T.; Zhang, Q.; Wang, W.; Zhao, Y.-Y.; Ju, S.; Tian, F.-C. Preparation and Characterization of PVA-H18 Gel Foam for Preventing Spontaneous Combustion of Coal Based on Interfacial Self-Assembly. Fuel 2022, 327, 125081. [Google Scholar] [CrossRef]
- Xi, X.; Shi, Q. Study of the Preparation and Extinguishment Characteristic of the Novel High-Water-Retaining Foam for Controlling Spontaneous Combustion of Coal. Fuel 2021, 288, 119354. [Google Scholar] [CrossRef]
- He, Y.; Deng, J.; Yi, X.; Xiao, Y.; Deng, Y.; Chen, W. Effect of Rare-Earth-Containing Inhibitors on the Low-Temperature Oxidation Characteristics and Thermodynamic Properties of Coal. Energy 2023, 281, 128316. [Google Scholar] [CrossRef]
- Shu, P.; Zhang, Y.; Deng, J.; Duan, Z.; Zhai, F. Characteristics and Mechanism of Modified Hydrotalcite for Coal Spontaneous Combustion Preventing. Energy 2023, 265, 126353. [Google Scholar] [CrossRef]
- Wei, Z. Experimental Study on the Inhibition of Coal Spontaneous Combustion by Sodium Salt Microencapsulated Retardants. Master’s Thesis, Xi’an University of Science and Technology, Xi’an, China, 2022. [Google Scholar]
- Li, X.; Chen, Y.; Zhang, J.; Wang, R. Study on the effect of chlorine salt composite inhibitor on the spontaneous combustion inhibition of different coal samples. Coal Engineering 2020, 52, 106–110. [Google Scholar]
- Guo, Q.; Ren, W.; Zhu, J.; Shi, J. Study on the Composition and Structure of Foamed Gel for Fire Prevention and Extinguishing in Coal Mines. Process Saf. Environ. Prot. 2019, 128, 176–183. [Google Scholar] [CrossRef]
- Lu, W.; Guo, B.; Qi, G.; Cheng, W.; Yang, W. Experimental Study on the Effect of Preinhibition Temperature on the Spontaneous Combustion of Coal Based on an MgCl2 Solution. Fuel 2020, 265, 117032. [Google Scholar] [CrossRef]
- Cummings, J.; Shah, K.; Atkin, R.; Moghtaderi, B. Physicochemical Interactions of Ionic Liquids with Coal; the Viability of Ionic Liquids for Pre-Treatments in Coal Liquefaction. Fuel 2015, 143, 244–252. [Google Scholar] [CrossRef]
- Raj, J.J.; Magaret, S.; Pranesh, M.; Lethesh, K.C.; Devi, W.C.; Mutalib, M.I.A. Dual Functionalized Imidazolium Ionic Liquids as a Green Solvent for Extractive Desulfurization of Fuel Oil: Toxicology and Mechanistic Studies. J. Clean. Prod. 2019, 213, 989–998. [Google Scholar] [CrossRef]
- Cummings, J.; Tremain, P.; Shah, K.; Heldt, E.; Moghtaderi, B.; Atkin, R.; Kundu, S.; Vuthaluru, H. Modification of Lignites via Low Temperature Ionic Liquid Treatment. Fuel Process. Technol. 2017, 155, 51–58. [Google Scholar] [CrossRef]
- To, T.Q.; Shah, K.; Tremain, P.; Simmons, B.A.; Moghtaderi, B.; Atkin, R. Treatment of Lignite and Thermal Coal with Low Cost Amino Acid Based Ionic Liquid-Water Mixtures. Fuel 2017, 202, 296–306. [Google Scholar] [CrossRef]
- Li, Q.-W.; Xiao, Y.; Zhong, K.-Q.; Shu, C.-M.; Lü, H.-F.; Deng, J.; Wu, S. Overview of Commonly Used Materials for Coal Spontaneous Combustion Prevention. Fuel 2020, 275, 117981. [Google Scholar] [CrossRef]
- Li, Y.-H. Research on the Mechanism of Coal Spontaneous Combustion Retardation by Rare Earth-like Hydrotalcite. Master’s Thesis, Xi’an University of Science and Technology, Xi’an, China, 2018. [Google Scholar]
- Zhang, Y.; Shi, X.; Li, Y.; Wen, H.; Huang, Y.; Li, S.; Liu, Y. Zinc-magnesium-aluminum layered double hydroxide inhibition properties on spontaneous coal combustion. J. Coal 2017, 42, 2892–2899. [Google Scholar]
- Deng, J.; Bai, Z.-J.; Xiao, Y.; Shu, C.-M.; Laiwang, B. Effects of Imidazole Ionic Liquid on Macroparameters and Microstructure of Bituminous Coal during Low-Temperature Oxidation. Fuel 2019, 246, 160–168. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Yang, Y.; Kong, B.; Wang, C. Laboratory Study on the Inhibitory Effect of Free Radical Scavenger on Coal Spontaneous Combustion. Fuel Process. Technol. 2018, 171, 350–360. [Google Scholar] [CrossRef]
- Lü, H.-F.; Xiao, Y.; Deng, J.; Li, D.; Yin, L.; Shu, C.-M. Inhibiting Effects of 1-Butyl-3-Methyl Imidazole Tetrafluoroborate on Coal Spontaneous Combustion under Different Oxygen Concentrations. Energy 2019, 186, 115907. [Google Scholar] [CrossRef]
- Asarapalli, S.K.; Pal, B.K.; Bisoyi, S.K. Assessment of the Role of Chemical Retardants on the Spontaneous Heating Liability of Coal. Min. Metall. Explor. 2024, 41, 287–295. [Google Scholar] [CrossRef]
- Xiao, Y.; Lv, H.; Ren, S.; Deng, J.; Wang, C. Study on the spontaneous combustion inhibition characteristics of coal by imidazolium ionic liquids. J. China Univ. Min. Technol. 2019, 48, 175–181. [Google Scholar]
- Ma, L.; Wang, D.; Kang, W.; Xin, H.; Dou, G. Comparison of the Staged Inhibitory Effects of Two Ionic Liquids on Spontaneous Combustion of Coal Based on in Situ FTIR and Micro-Calorimetric Kinetic Analyses. Process Saf. Environ. Prot. 2019, 121, 326–337. [Google Scholar] [CrossRef]
- Gao, M.; Li, X.; Ren, C.; Wang, Z.; Pan, Y.; Guo, L. Construction of a Multicomponent Molecular Model of Fugu Coal for ReaxFF-MD Pyrolysis Simulation. Energ Fuel 2019, 33, 2848–2858. [Google Scholar] [CrossRef]
- Qu, Z.; Sun, F.; Gao, J.; Pei, T.; Qie, Z.; Wang, L.; Pi, X.; Zhao, G.; Wu, S. A New Insight into the Role of Coal Adsorbed Water in Low-Temperature Oxidation: Enhanced·OH Radical Generation. Combust. Flame 2019, 208, 27–36. [Google Scholar] [CrossRef]
- Dou, G.; Jiang, Z. Sodium Humate as an Effective Inhibitor of Low-Temperature Coal Oxidation. Thermochim. Acta 2019, 673, 53–59. [Google Scholar] [CrossRef]
- Lu, W.; Sun, X.; Gao, L.; Hu, X.; Song, H.; Kong, B. Study on the Characteristics and Mechanism of DL-Malic Acid in Inhibiting Spontaneous Combustion of Lignite and Bituminous Coal. Fuel 2022, 308, 122012. [Google Scholar] [CrossRef]
- Feng, W. Experimental Study on Green Composite Retardant to Inhibit Spontaneous Combustion of Coal. Master’s Thesis, China University of Mining and Technology, Beijing, China, 2019. [Google Scholar]
- Huang, Z.; Liu, X.; Gao, Y.; Zhang, Y.; Li, Z.; Wang, H.; Shi, X. Experimental Study on the Compound System of Proanthocyanidin and Polyethylene Glycol to Prevent Coal Spontaneous Combustion. Fuel 2019, 254, 115610. [Google Scholar] [CrossRef]
- Qin, B.; Dou, G.; Wang, D. Thermal Analysis of Vitamin C Affecting Low-Temperature Oxidation of Coal. J. Wuhan Univ. Technol.-Mat. Sci. Ed. 2016, 31, 519–522. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Yang, Y.; Zhang, X.; Yan, D.; Liu, L. Inhibitive Effects of Antioxidants on Coal Spontaneous Combustion. Energy Fuels 2017, 31, 14180–14190. [Google Scholar] [CrossRef]
- Guo, S.; Yan, Z.; Yuan, S.; Geng, W. Inhibitory Effect and Mechanism of L-Ascorbic Acid Combined with Tea Polyphenols on Coal Spontaneous Combustion. Energy 2021, 229, 120651. [Google Scholar] [CrossRef]
- Taraba, B.; Peter, R.; Slovák, V. Calorimetric Investigation of Chemical Additives Affecting Oxidation of Coal at Low Temperatures. Fuel Process. Technol. 2011, 92, 712–715. [Google Scholar] [CrossRef]
- Slovak, V.; Taraba, B. Urea and CaCl2 as Inhibitors of Coal Low-Temperature Oxidation. J. Therm. Anal. Calorim. 2012, 110, 363–367. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Pi, S.; Yue, Z.; Jia, J. Optimization of Process Conditions for Degradation of Yunnan Zhaotong Lignite by Actinobacteria. Appl. Chem. Eng. 2017, 46, 1683–1687+1691. [Google Scholar]
- Li, J.; Liu, X.; Huang, L.; Yang, X.; Li, T. Optimization of microbial degradation process conditions for lignite from Shengli, Inner Mongolia. Coal Technol. 2017, 36, 266–268. [Google Scholar]
- Kang, H.; Liu, X.; Zhao, S.; Yang, Z.; Yang, Z. Experimental study on the degradation of lignite by four bacteria in Chifeng, Inner Mongolia. Coal Technol. 2019, 38, 130–133. [Google Scholar]
- Mishra, S.; Akcil, A.; Panda, S.; Erust, C. Biodesulphurization of Turkish Lignite by Leptospirillum Ferriphilum: Effect of Ferrous Iron, Span-80 and Ultrasonication. Hydrometallurgy 2018, 176, 166–175. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Xie, P.; Liu, H.; Deng, Q.; Dai, F. Experimental Study on Controlled-Release Inhibitor Foam for Restraining Spontaneous Combustion of Coal. Energy Explor. Exploit. 2020, 38, 1159–1177. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, X.; Yuan, Y.; Qi, G.; Hu, X.; Li, J.; Liang, Y.; Guo, B. Study on the Characteristics and Mechanism of a New Type of Antioxidant Gel Foam for Coal Spontaneous Combustion Prevention. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 628, 127254. [Google Scholar] [CrossRef]
- Yang, W. Research on the Effect of Physical-Chemical Cage Composite Retardant on Coal Spontaneous Combustion. Master’s Thesis, China University of Mining and Technology, Beijing, China, 2017. [Google Scholar]
- Xue, D.; Hu, X.; Cheng, W.; Yu, X.; Wu, M.; Zhao, Y.; Lu, Y.; Pan, R.; Niu, H.; Hu, S. Development of a Novel Composite Inhibitor Modified with Proanthocyanidins and Mixed with Ammonium Polyphosphate. Energy 2020, 213, 118901. [Google Scholar] [CrossRef]
- Huang, Z.; Song, D.; Hu, X.; Zhang, Y.; Gao, Y.; Quan, S.; Yin, Y.; Yang, Y.; Luo, H.; Ji, Y. A Novel Nano-Modified Inhibitor of Tert-Butyl Hydroquinone/Sodium Polyacrylate for Inhibiting Coal Spontaneous Combustion. Energy 2022, 256, 124439. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wang, J.; Wu, Y.; Zhou, C.; Zhang, Z. Synergistic inhibition of coal spontaneous combustion by inorganic salt-based inhibitors and free radical trappers. J. Coal Sci. 2020, 45, 4132–4143. [Google Scholar]
- Jiao, G. Research on Antioxidant Microencapsulated Retardant for Preventing Coal Spontaneous Combustion. Master’s Thesis, China University of Mining and Technology, Beijing, China, 2021. [Google Scholar]
- Huo, Y.; Huo, Y. Efficient Physicochemical Coupling Inhibitor Compounding Method for Low-Temperature Oxidation of Coal and Its Mechanism of Action. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 2023. [Google Scholar]
- Xue, H. Research on Antioxidant-Based Composite Retardant to Inhibit Key Active Groups of Coal Spontaneous Combustion Process. Master’s Thesis, China University of Mining and Technology, Beijing, China, 2019. [Google Scholar]
- Huang, Z.; Yan, L.; Zhang, Y.; Gao, Y.; Liu, X.; Liu, Y.; Li, Z. Research on a New Composite Hydrogel Inhibitor of Tea Polyphenols Modified with Polypropylene and Mixed with Halloysite Nanotubes. Fuel 2019, 253, 527–539. [Google Scholar] [CrossRef]
- Xi, Z.; Jin, B.; Shan, Z. Reaction Mechanisms Involving Peroxy Radical in the Low-Temperature Oxidation of Coal. Fuel 2021, 300, 120943. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, Y.; Liu, B.; Deng, J.; Liu, C.; Yang, J.; Wen, X. Mechanism and performance of halite carrier inorganic salts for retardation of coal spontaneous combustion. J. Eng. Sci. 2021, 43, 1295–1303. [Google Scholar]
- Zhang, Y.; Shu, P.; Zhai, F.; Chen, S.; Wang, K.; Deng, J.; Kang, F.; Li, L. Preparation and Properties of Hydrotalcite Microcapsules for Coal Spontaneous Combustion Prevention. Process. Saf. Environ. Prot. 2021, 152, 536–548. [Google Scholar] [CrossRef]
- Pan, R.; Ma, J.; Fu, D.; Li, C.; Jia, H.; Zheng, L. Experimental Study on the New Environmental Protection Chemical Composite Inhibitor for the Inhibition of Coal Spontaneous Combustion. J. Therm. Anal. Calorim. 2020, 139, 37–45. [Google Scholar] [CrossRef]

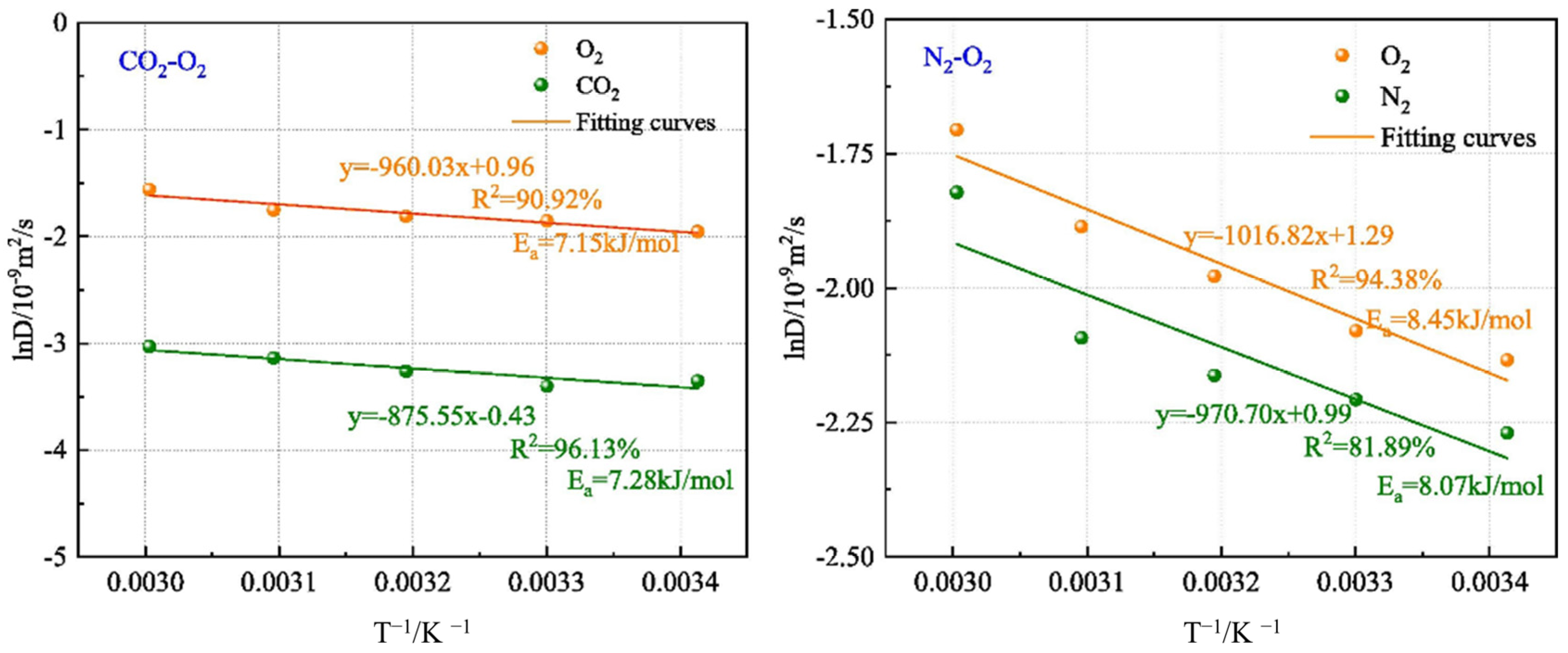
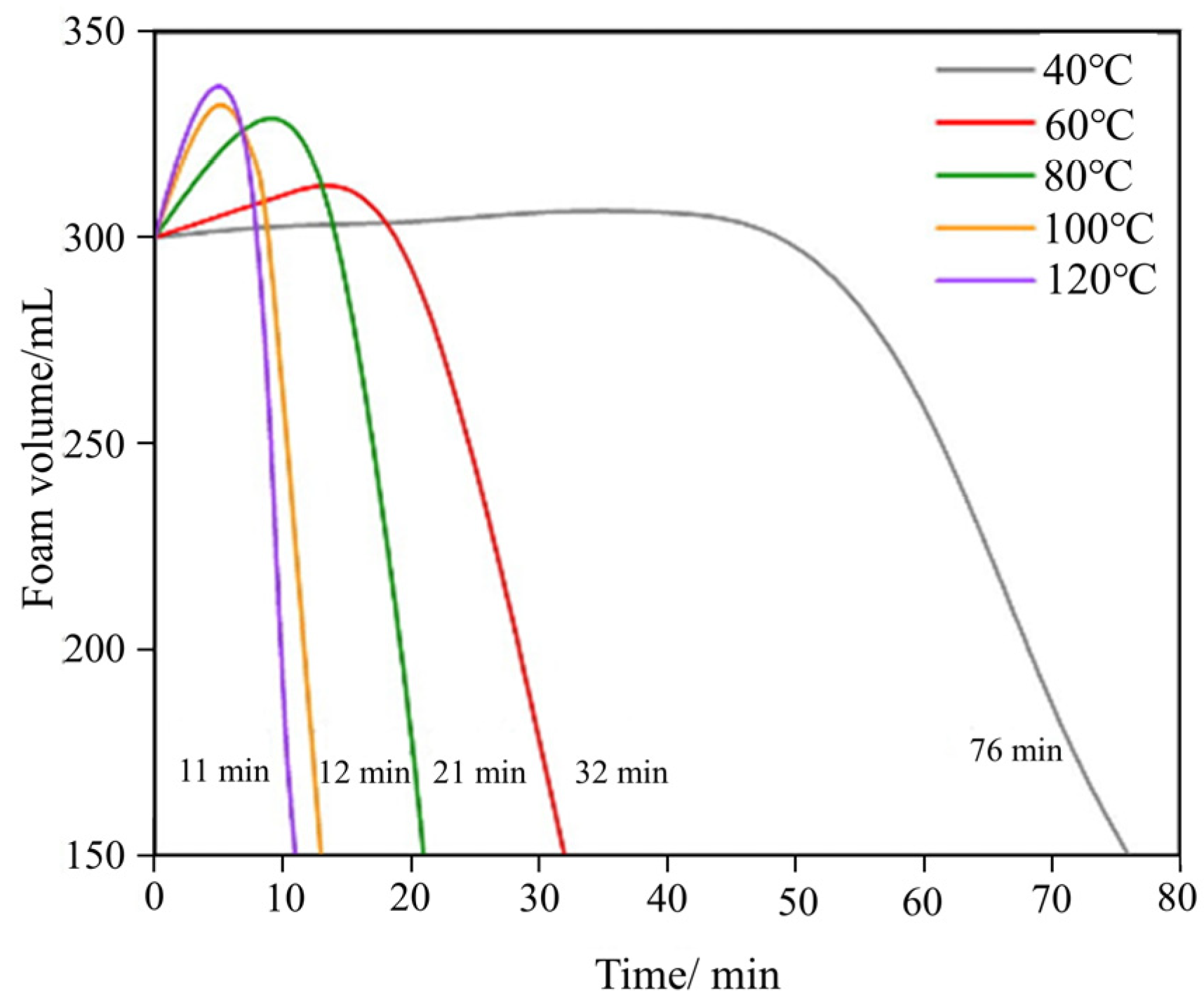
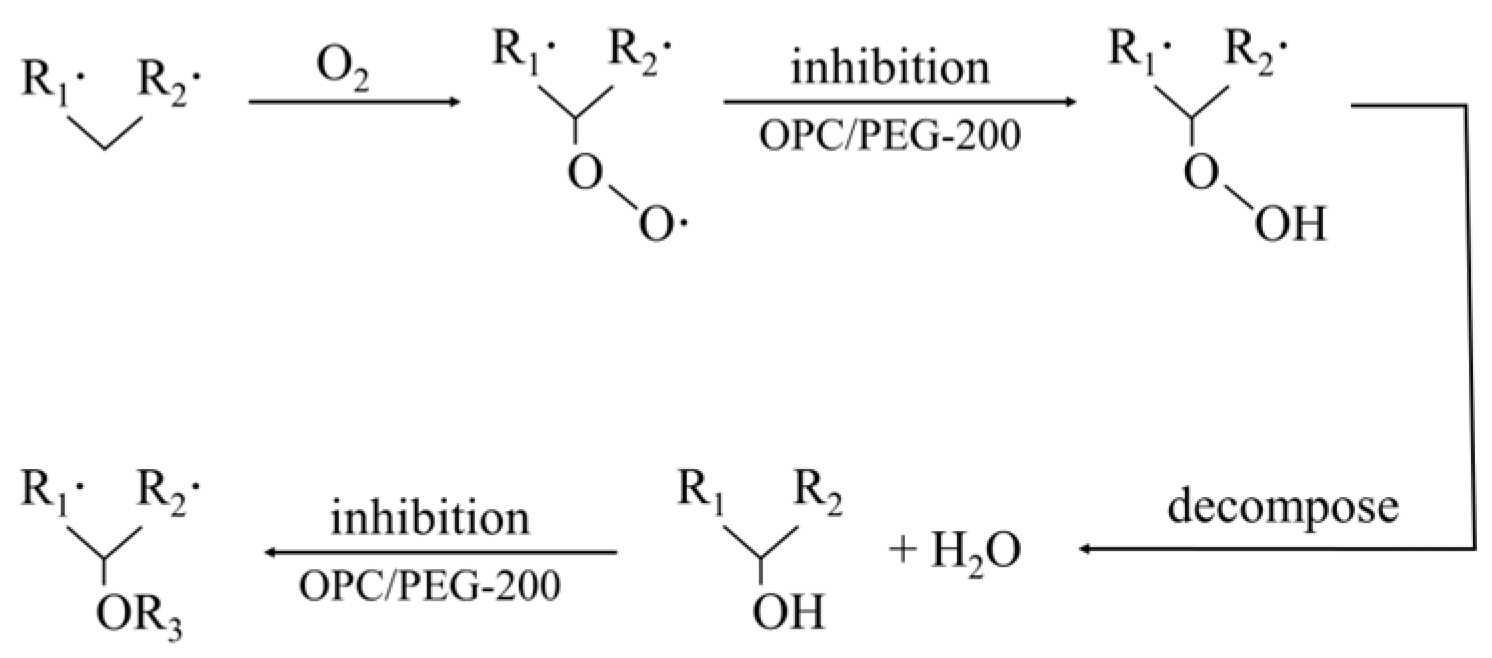

| Category | Highly Effective Suspension JXF1930 | Compound Colloidal Additives FCJ12 |
|---|---|---|
| Performance | It is added into the slurry to form a thickening colloid so that the fly ash in the slurry is suspended, and the problem of pipeline blockage in the long-distance transportation of high-concentration slurry is solved | It is added to the slurry to form a complex colloid so that the slurry gels in a certain time and then loses its fluidity |
| Main role | The slurry containing the JXF1930 suspension agent exhibits excellent water retention, permeability, suspension, and compactness properties. It can be effectively encapsulated within coal for prolonged periods without separation during transportation due to its superior fluidity | The slurry, supplemented with FCJ12 gelling agent, can undergo solidification at the designated location to effectively seal the voids within coal deposits and encapsulate high-temperature coal. Allow its accumulated heat to be fully released to prevent the extinguished fire area from reigniting |
| Use scales | 0.10% | 0.06% |
| Usage | Sprinkle evenly into the slurry in the ground grouting pool according to the proportion used | The ZM-5/1.8G coal mine grouting machine proportionally injects it into the grouting pipeline in the vicinity of the underground grouting site |
| Applicable place | End mining line, eye cutting, gob area, coal field outcrop, surface coal yard, and other large areas of grouting glue to prevent fire | |
| Author | Gel System | Effect |
|---|---|---|
| Huang et al. [32] | Sodium silicate, sodium bicarbonate, and sodium polyacrylate | The optimal ratio of 4%, 5%, and 0.75‰ can increase the oxidation temperature rising zone by 8 m, shorten the length by 20 m, and increase the blocking zone by 28 m |
| Zhao [33] | The swelling gel was prepared with chitosan, sodium carboxymethyl cellulose (CMC), guar gum, and sodium bicarbonate | The optimal formulations were 0.75 wt%, 2.5 wt%, 0.15 wt%, 0.4 wt% and 0.5 wt% acetic acid. The gel viscosity was 5852 mPa·s, the expansion factor was 0.846, and the strength was 319.925 N/m2 after 6 h of storage. The permeability of loose coal was 93.52%. At 200 °C, the CO2 content of coal samples with 1 g of gel is 12,829 ppm, and that of coal samples with 5 g of gel is 23,239 ppm, which is 59.15% and 188.29% higher than that of raw coal, respectively |
| Zhou et al. [34] | CMC, cross-linking agent aluminum citrate (AlCit), and pH regulator δ-gluconate lactone (GDL) | Compared with the raw coal sample, the cross point temperature is increased by 13.9 °C, the activation energy is increased by 16.34%, and the amount of CO gas produced is reduced by 34.5% |
| Dong et al. [35] | CMC, Zirconium Citrate (ZrCit) and GDL | When the ratio is 2.5 wt%, 20 wt%, 2 wt%, the gel can effectively inhibit the spontaneous combustion of floating coal. When the ratio is 3 wt%, 20 wt%, 2 wt%, the gel is suitable for sealing and extinguishing fire sources |
| Wei et al. [36] | Polyvinyl alcohol (PVA), Xanthan gum (XG), and acrylic acid (AA) | Solve the problem of poor mechanical properties of gel materials. The contents of PVA, XG, and AA were 1.5 g, 0.1 g, and 6 g, respectively, and the optimum reaction temperature was 55 °C. Under the optimum conditions, the viscosity was 45 mPa·s, and the surface tension was 30 mN/m |
| Authors | Gel Foam System | Effect |
|---|---|---|
| Han et al. [52] | SA (sodium alginate), CL (L-calcium lactate), CFA (tea saponin, alkyl glycoside), and TA (tannic acid) | Compared to the TA-free foam, the half-life was extended from 0.4 to 30 days, and the strength exhibited a remarkable increase of 72.9%. The concentration of CO decreased significantly from 7556.8 ppm to undetectable levels (0 ppm). SA-CA2+@TA-GF elevated the coal temperature by 60 °C during the rapid oxidation stage, achieving an impressive inhibition rate of 79.6% at 200 °C. |
| Wu et al. [53] | Sodium silicate, polyacrylamide, and film-forming agent | The strength and stability of the sodium silicate gel foam were enhanced. With the optimized formulation, the foaming ratio increased to 3–3.5 times, while the gelation time extended to 480 s. Moreover, the half-life of the gel foam was prolonged to 7 days, and its resistivity reached a remarkable value of 78.35% at 100 °C and 79.6% at 200 °C. |
| Wu et al. [54] | CMC cellulose, compound foaming agent (α-alkenyl sulfonate sodium; fatty alcohol polyoxyethylene ether sodium sulfate), cross-linking agent | The optimized formulations were 0.7 wt%, 0.7 wt%, and 1.1 wt%, exhibiting a foaming time ranging from 4 to 7 min and a gel time spanning from 3 to 10 min. Low-temperature oxygen consumption of the coal sample decreased by 9.74%. |
| Qiao et al. [55] | Superhydrophobic nanoparticles, PVA, sodium bicarbonate, and sodium tetraborate | The gel foam (PGF) without the addition of a foaming agent was prepared with a foaming multiple of 3.29 times, exhibiting a half-life exceeding 13 h and water retention for over 15 h at 100 °C. Furthermore, its inhibitory ability remained effective even under high temperatures up to 400 °C. |
| Xi et al. [56] | Polymer complex (PC) microbial polysaccharide and galactomannan biopolymer, organic boron complex (OBC), homemade anionic surfactant, and non-ionic surfactant foaming agent | The cross-linking time is more than 30 min, and the foam water content is more than 60% after 120 h. The temperature of the burned coal is reduced from about 700 °C to 34.7 °C within 30 min. |
| Inhibitors | Average Inhibition Rate | Inhibitors | Average Inhibition Rate |
|---|---|---|---|
| 5% sodium salt inhibitor | 24.5 | 5% sodium salt microcapsule inhibitor | 41.5 |
| 10% sodium salt inhibitor | 36.7 | 10% sodium salt microcapsule inhibitor | 52.8 |
| 15% sodium salt inhibitor | 46.5 | 15% sodium salt microcapsule inhibitor | 66.6 |
| 20% sodium salt inhibitor | 57.7 | 20% sodium salt microcapsule inhibitor | 60.8 |
| Authors | Composite Inhibitors | Effect |
|---|---|---|
| Wang et al. [96] | MgCl2, CaCl2, N, N-dibenzylhydroxylamine (DBHA), BHT | The release of CO decreased by 88.7% at 128 °C. The inhibition rate reached 82.5% at 100 °C. Reducing hydroxyl, methyl, and methylene groups and carbonyl-containing active components in coal. The relative content of aromatic ketones decreased significantly. The oxygen adsorption process of the coal body is shortened, and the adsorption capacity is reduced. The activation energy was increased by 53.2% during oxygen inhalation |
| Jiao [97] | IOxidant microcapsule compound inhibitor, tea polyphenol, polyethylene glycol 20,000, Pentaerythrityl tetrastearate | When the mass ratio of polyethylene glycol 20,000 and pentaerythritol stearate is 1:1, 1:2, and 1:3, the microcapsule wall material obtained by the combination of polyethylene glycol 20,000 and pentaerythritol stearate has better hydrophobicity. When the wall-material ratio was 1:1 and the core-wall ratio was 1:1, the coating rate of microcapsule material was the highest. When the wall–material ratio was 1:1 and the core–wall ratio was 1:1, the inhibition rate reached 76.8%, which was 11.4% higher than that of single tea polyphenol. The cross point temperature was increased by 43 °C compared with the raw coal |
| Huo [98] | MgCl2, BHT, TPPI, and polyethylene glycol 400 | The optimal quality fractions were 10.26%, 3.15%, 2.09%, and 0.58%, respectively. The optimal quality fraction of P&C was 12% of coal. The cross point temperature of the coal sample was increased to 155.08 °C, 22.48 °C higher than that of the raw coal sample |
| Xue [99] | Melatonin, polyacrylate-alginate sodium | When 6 wt% melatonin was added to the coal, the inhibition effect was the best. When the optimal mass ratio of melatonin and polyacrylic acid–sodium alginate was 1:4, it could effectively inhibit the generation of CO, increase the crossing point temperature of coal oxidation, reduce the oxygen consumption rate at 70 °C, and significantly reduce the risk of CSC |
| Huang et al. [100] | Tea polyphenols, halloysite nanotubes | The maximum heat absorption was 67.15 J/mg, the shortest heat release interval was 120.22 °C to 572.22 °C, and the minimum total heat release was 2536.73 J/mg. Lower the hydroxyl content and reduce the number of aliphatic functional groups converted to carbonyl and carboxyl groups |
| Xi et al. [101] | Compound antioxidant containing superoxide dismutase (SOD) | The results showed that the energy barrier of SOD for eliminating peroxyl radicals was 31.3 kJ/mol, and SOD could automatically eliminate peroxyl radicals at room temperature. |
| Zhang et al. [102] | Rare earth hydrotalcite, halogen salt | The activity of acid functional groups such as -COOH in coal is weakened due to weak hydrogen bonds between -OH and acid functional groups such as -COOH in rare-earth hydrotalc laminates. Mg2+ is complexed with −COO− in coal molecules to form −COOMg−, resulting in reduced C=O activity in −COO−. Compared with the raw coal, the peak temperature of the coal is shifted back by 50–60 °C, the T1 temperature is shifted back by 90–100 °C, and the total heat release is reduced by 19–27 kJ·g−1. |
| Zhang [103] | Polyethylene glycol 6000, LDHs | Increase the inhibition time. The best effect was obtained when the core wall ratio was 1:5. The critical temperature and maximum weight loss temperature were increased by 8.8 °C and 33.6 °C, respectively. The apparent activation energy of water evaporation and gas desorption stage was increased by 13.42 kJ/mol. The thermal decomposition stage and combustion stage increased by 88.64 kJ/mol |
| Pan et al. [104] | Sodium dodecyl sulfate, sodium laurylsulfonate, flame-retardant compound (CaCl2, MgCl2, NaHCO3, (NH4)2HPO4, CO(NH2)2), XG | The higher the concentration of compound inhibitor, the better the effect; the 20% inhibitor solution has the most significant effect. The inhibition rate is 85.92%, and the average inhibition rate is 15 times that of water. The oxidation inhibition effect of coal samples after water treatment can be observed only at 140 °C, and the oxidation rate increases with the increase in temperature. Chloride ions and phosphate elements break down into ions and small molecules, forming stable substances with free radicals, reducing the number of free radicals, and preventing spontaneous combustion. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Niu, G.; Zhu, H.; Chang, T.; Liu, B.; Ren, Y.; Wang, Y.; Song, B. Exploration and Frontier of Coal Spontaneous Combustion Fire Prevention Materials. Processes 2024, 12, 1155. https://doi.org/10.3390/pr12061155
Han D, Niu G, Zhu H, Chang T, Liu B, Ren Y, Wang Y, Song B. Exploration and Frontier of Coal Spontaneous Combustion Fire Prevention Materials. Processes. 2024; 12(6):1155. https://doi.org/10.3390/pr12061155
Chicago/Turabian StyleHan, Dandan, Guchen Niu, Hongqing Zhu, Tianyao Chang, Bing Liu, Yongbo Ren, Yu Wang, and Baolin Song. 2024. "Exploration and Frontier of Coal Spontaneous Combustion Fire Prevention Materials" Processes 12, no. 6: 1155. https://doi.org/10.3390/pr12061155





