Characterization of the Byproducts of Myrciaria dubia and Psidium guajava and Optimization of the Extraction of Their Bioactive Compounds by Ultrasound-Assisted Extraction and Mechanical Agitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Materials and Samples
2.3. Byproduct Characterization
2.3.1. Humidity
2.3.2. pH
2.3.3. Density
2.3.4. Hygroscopicity
2.3.5. Solubility
2.3.6. Water Activity
2.3.7. Carotenoid Content
2.3.8. Morphology and Microstructure
2.4. Optimization of the Extraction of Bioactive Compounds
2.4.1. Ultrasound-Assisted Extraction
2.4.2. Extraction by Mechanical Stirring
2.4.3. Characterization of the Extracts
Antioxidant Capacity (DPPH)
Total Phenolic Content
Ferric Reducing Antioxidant Powder
2.5. Statistical Analysis
3. Results and Discussions
3.1. Byproduct Characterization
3.1.1. Humidity and pH
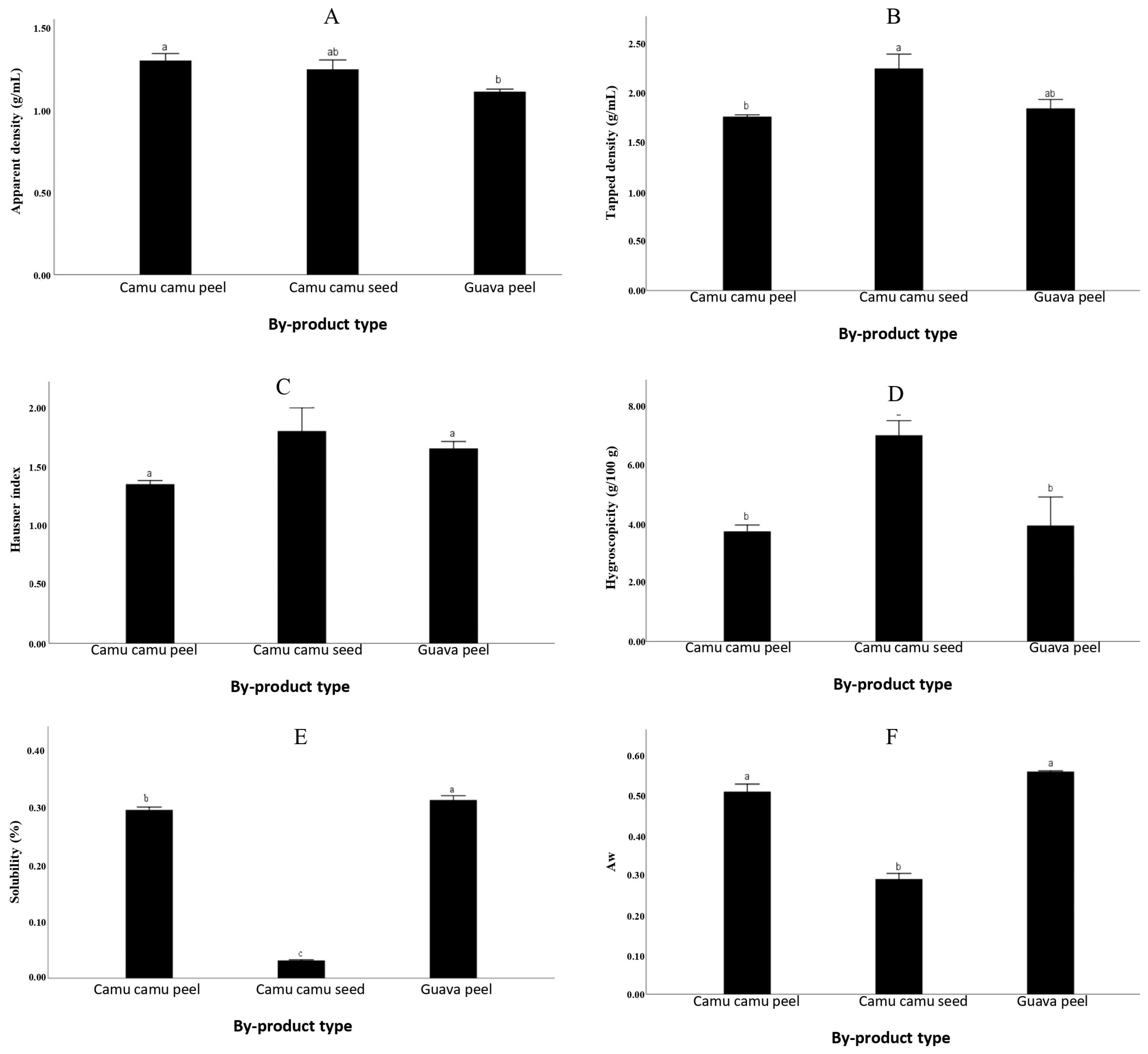
3.1.2. Density, Hausner Index, Hygroscopicity, Solubility and Water Activity
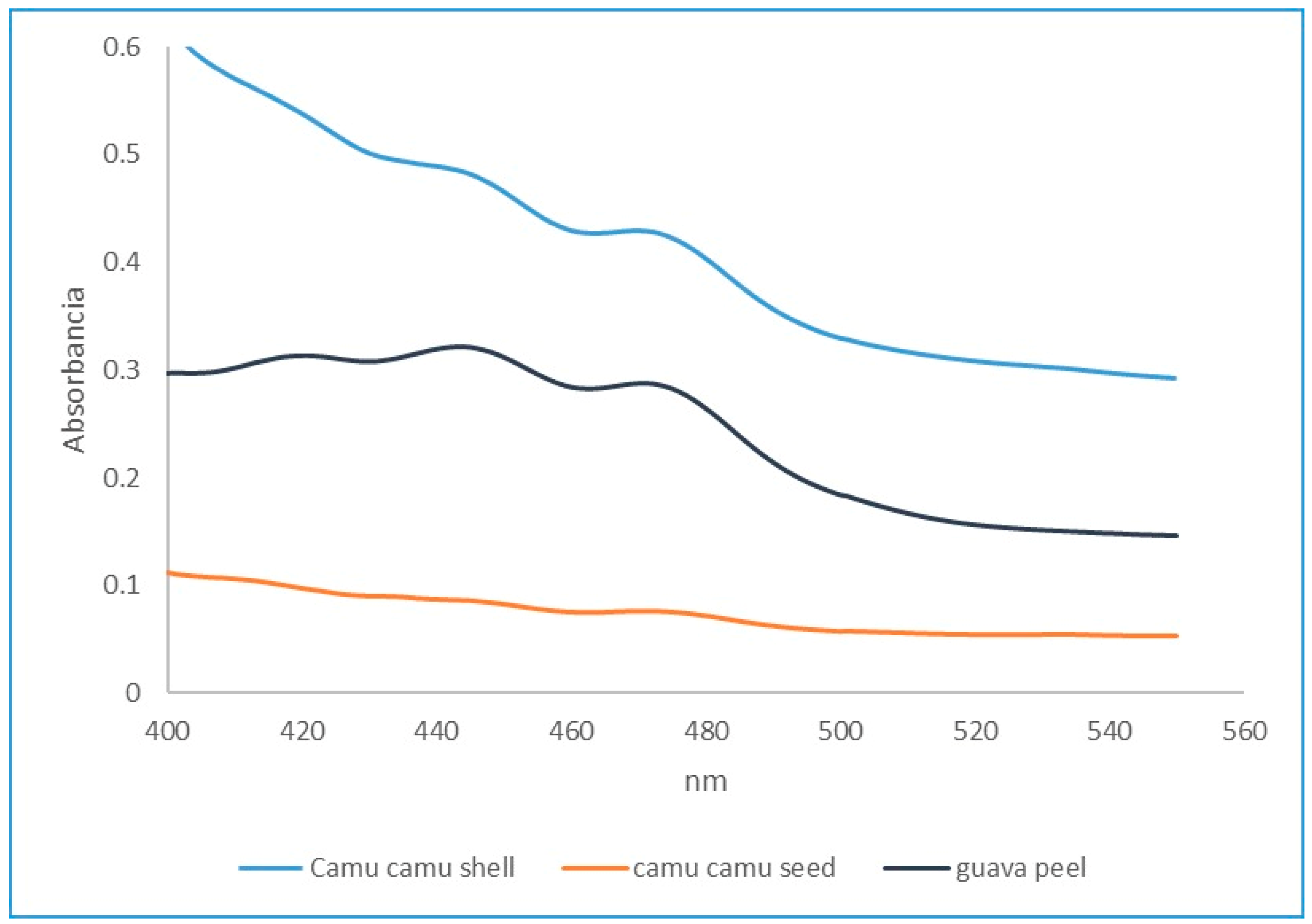
3.1.3. Pigment Content
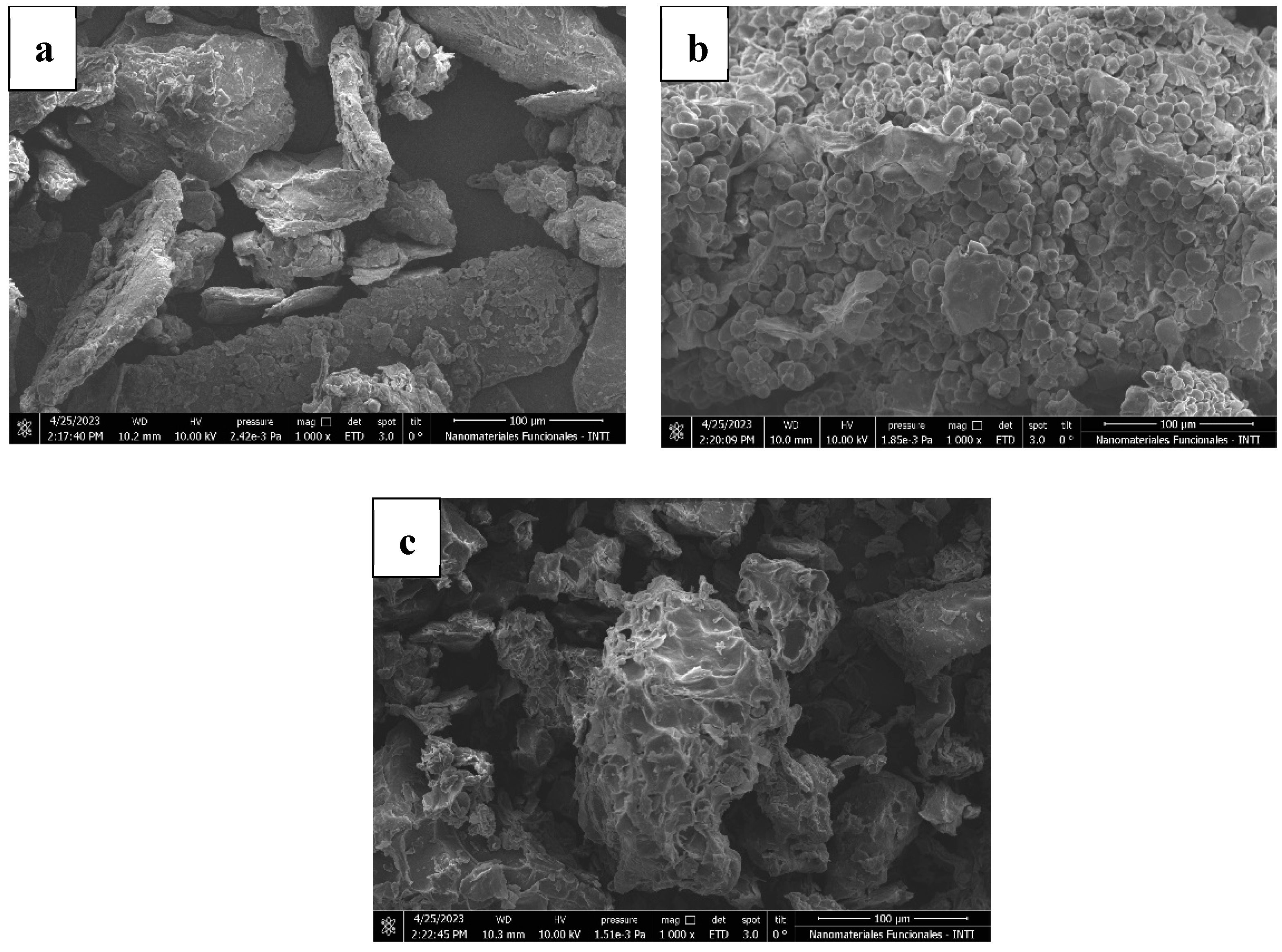
3.1.4. Morphology and Microstructure
3.2. Optimization of Bioactive Extracts
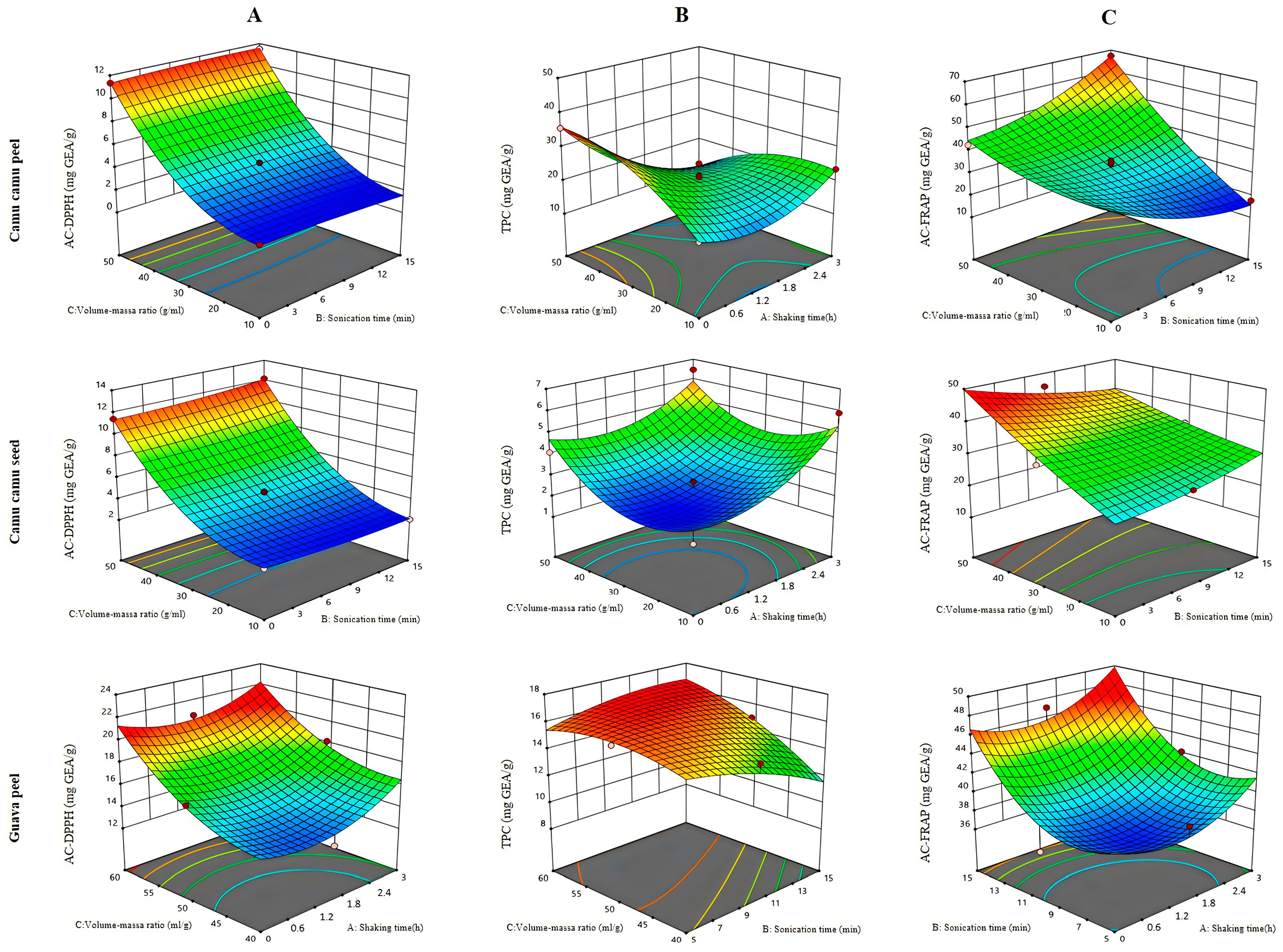
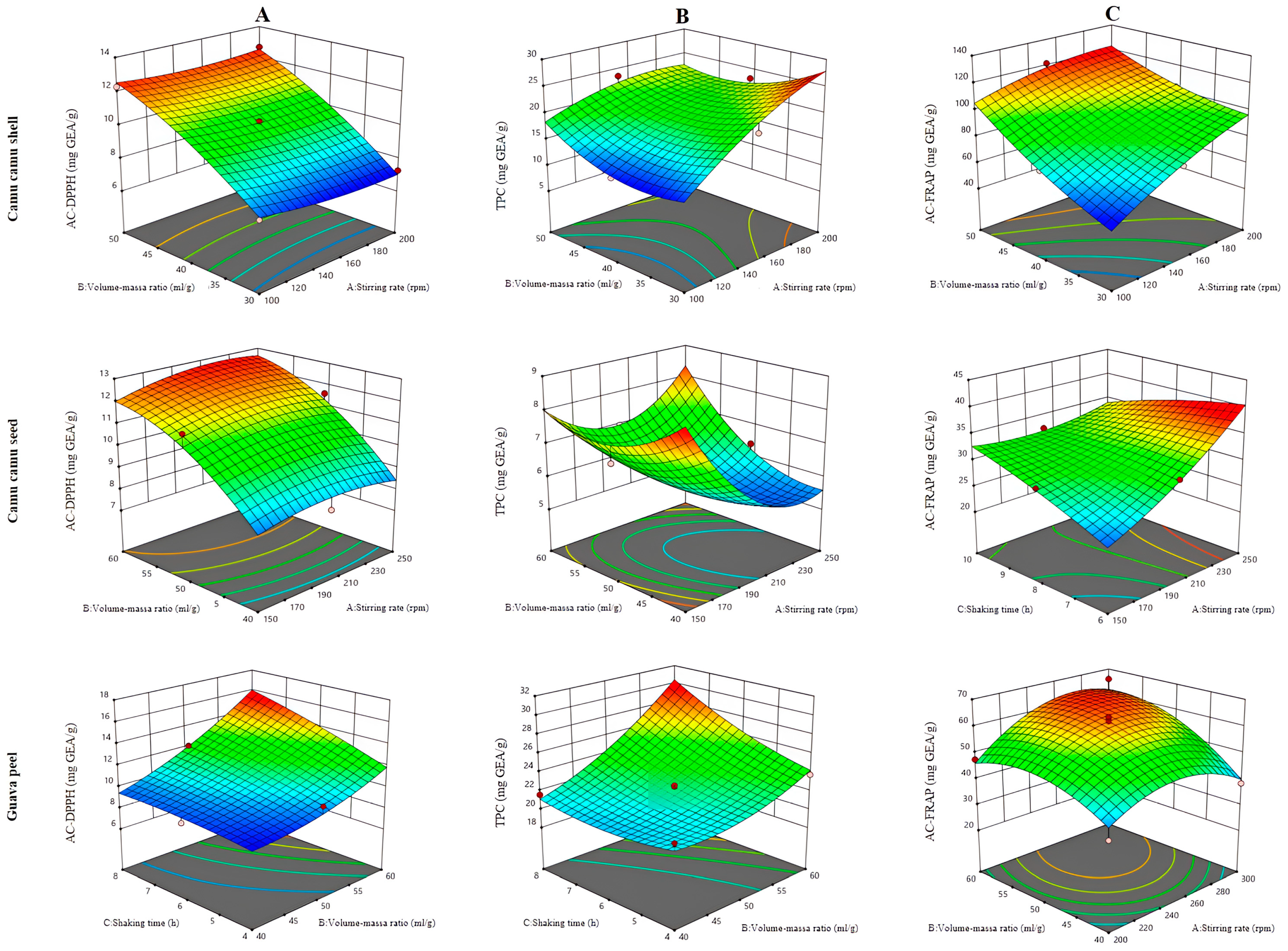
3.2.1. Ultrasound-Assisted Extraction
3.2.2. Extraction by Mechanical Stirring
3.2.3. Optimized Extract Collection Models
3.2.4. Prediction Model Validation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ueda, J.M.; Pedrosa, M.C.; Heleno, S.A.; Carocho, M.; Ferreira, I.C.F.R.; Barros, L. Food Additives from Fruit and Vegetable By-Products and Bio-Residues: A Comprehensive Review Focused on Sustainability. Sustainability 2022, 14, 5212. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Simancas, R.; Labrador-Fernández, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Alañón, M.E. Comprehensive research on mango by-products applications in food industry. Trends Food Sci. Technol. 2021, 118, 179–188. [Google Scholar] [CrossRef]
- De Souza Schmidt Gonçalves, A.E.; Lajolo, F.M.; Genovese, M.I. Chemical composition and antioxidant/antidiabetic potential of brazilian native fruits and commercial frozen pulps. J. Agric. Food Chem. 2010, 58, 4666–4674. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Forbes-Hernández, T.Y.; Afrin, S.; Battino, M. Guava (Psidium guajava L. cv. Red Suprema) Crude Extract Protect Human Dermal Fibroblasts against Cytotoxic Damage Mediated by Oxidative Stress. Plant Foods Hum. Human. Nutr. 2018, 73, 18–24. [Google Scholar] [CrossRef]
- Santos, I.L.; Miranda, L.C.F.; da Cruz Rodrigues, A.M.; da Silva, L.H.M.; Amante, E.R. Camu-camu [Myrciaria dubia (HBK) McVaugh]: A review of properties and proposals of products for integral valorization of raw material. Food Chem. 2022, 372, 131290. [Google Scholar] [CrossRef] [PubMed]
- Chagas, E.G.L.D.; Vanin, F.M.; Garcia, V.A.D.S.; Yoshida, C.M.P.; de Carvalho, R.A. Enrichment of antioxidants compounds in cookies produced with camu-camu (Myrciaria dubia) coproducts powders. LWT 2021, 137, 110472. [Google Scholar] [CrossRef]
- Fidelis, M.; do Carmo, M.A.V.; da Cruz, T.M.; Azevedo, L.; Myoda, T.; Furtado, M.M.; Marques, M.B.; Sant’Ana, A.S.; Genovese, M.I.; Oh, W.Y.; et al. Camu-camu seed (Myrciaria dubia)–From side stream to an antioxidant, antihyperglycemic, antiproliferative, antimicrobial, antihemolytic, anti-inflammatory, and antihypertensive ingredient. Food Chem. 2020, 310, 125909. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.C.; da Silva, V.X.; Pontis, J.A.; Flach, A.; Roberto, S.R. Bioactive compounds and antioxidant activity in pre-harvest camu-camu [Myrciaria dubia (H.B.K.) Mc Vaugh] fruits. Sci. Hortic. 2015, 186, 223–229. [Google Scholar] [CrossRef]
- Darmasiwi, S.; Herawati, O.; Retnaningrum, E. Edible biofilm formation from guava seed waste fermentation. Digit. Press. Phys. Sci. Eng. 2018, 1, 00005. [Google Scholar] [CrossRef]
- Gullón, B.; Muñiz-Mouro, A.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Green approaches for the extraction of antioxidants from eucalyptus leaves. Ind. Crops Prod. 2019, 138, 111473. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Hadidi, M.; Ibarz, A.; Pouramin, S. Optimization of extraction and deamidation of edible protein from evening primrose (Oenothera biennis L.) oil processing by-products and its effect on structural and techno-functional properties. Food Chem. 2021, 334, 127613. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhao, S.; Chen, C.; Ge, F.; Liu, D.; He, X. Optimization of production conditions for antioxidant peptides from walnut protein meal using solid-state fermentation. Food Sci. Biotechnol. 2014, 23, 1941–1949. [Google Scholar] [CrossRef]
- Hadidi, M.; Tan, C.; Assadpour, E.; Jafari, S.M. Oilseed meal proteins: From novel extraction methods to nanocarriers of bioactive compounds. Food Chem. 2024, 438, 137971. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Terra, L.D.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA-J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Palma, A.; Ruiz-Montoya, M.; Díaz, M.J.; Giráldez, I.; Morales, E. Optimization of bioactive compounds by ultrasound extraction and gas chromatography-mass spectrometry in fast-growing leaves. Microchem. J. 2023, 193, 109231. [Google Scholar] [CrossRef]
- Moorthy, I.G.; Maran, J.P.; Ilakya, S.; Anitha, S.L.; Sabarima, S.P.; Priya, B. Ultrasound assisted extraction of pectin from waste Artocarpus heterophyllus fruit peel. Ultrason. Sonochem 2017, 34, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Al Juhaimi, F.; Özcan, M.M.; Uslu, N.; Babiker, E.E.; Ahmed, I.A.M. Bioactive properties and phenolic compounds in bud, sprout, and fruit of Capparis spp. plants. J. Food Process Preserv. 2020, 44, e14357. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Park, G.; Ji, Y.; Lee, Y.G.; Choi, M.; Go, S.H.; Son, M.; Jang, Y.P. Design of Experiments-Based Optimization of Flavonoids Extraction from Daphne genkwa Flower Buds and Flavonoids Contents at Different Blooming Stages. Plants 2022, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Das, P.R.; Eun, J.B. A comparative study of ultra-sonication and agitation extraction techniques on bioactive metabolites of green tea extract. Food Chem. 2018, 253, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Zainol, N.; Aziz, N.H.; Baharudin, A.S. Influence of agitation and solvent percentage on the extraction of phytochemical compound from Asystasia gangetica. Food Chem. Adv. 2023, 3, 100538. [Google Scholar] [CrossRef]
- Zamboi, A.; Garofalo, S.F.; Tommasi, T.; Fino, D. Optimization of ultrasounds assisted extraction of polysaccharides from cladodes of Opuntia ficus-indica using response surface methodology. Sustain. Chem. Pharm. 2024, 37, 101348. [Google Scholar] [CrossRef]
- Paneerselvam, P.; Panithasan, M.S.; Venkadesan, G. RSM optimization of ultrasound-assisted melia dubia oil extraction with green solvents and their suitability for diesel engine applications. Renew. Energy 2024, 222, 119925. [Google Scholar] [CrossRef]
- Mariappan, M.; Panithasan, M.S.; Venkadesan, G. Pyrolysis plastic oil production and optimisation followed by maximum possible replacement of diesel with bio-oil/methanol blends in a CRDI engine. J. Clean. Prod. 2021, 312, 127687. [Google Scholar] [CrossRef]
- Principle, A.; Apparatus, B. 49.2.19A AOAC Official Method 994.08 Aflatoxins in Corn, Almonds, Brazil Nuts, Peanuts, and Pistachio Nuts; AOAC: Rockville, MD, USA, 2000; pp. 4–5. Available online: https://www.researchgate.net/publication/292783651_AOAC_2005 (accessed on 12 May 2023).
- Jangam, S.V.; Thorat, B.N. Optimization of Spray Drying of Ginger Extract. Dry. Technol. 2010, 28, 1426–1434. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Corke, H. Production and properties of spray-dried Amaranthus betacyanin pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Santhalakshmy, S.; Bosco, S.J.D.; Francis, S.; Sabeena, M. Effect of inlet temperature on physicochemical properties of spray-dried jamun fruit juice powder. Powder Technol. 2015, 274, 37–43. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Tkacz, K.; Turkiewicz, I.P. Quantitative and qualitative determination of carotenoids and polyphenolics compounds in selected cultivars of Prunus persica L. and their ability to in vitro inhibit lipoxygenase, cholinoesterase, α-amylase, α-glucosidase and pancreatic lipase. Food Chem X 2023, 17, 100619. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Chimal, S.; Navarro-Cortez, R.O.; Bello-Perez, L.A.; Vargas-Torres, A.; Palma-Rodríguez, H.M. Optimal conditions for anthocyanin extract microencapsulation in taro starch: Physicochemical characterization and bioaccessibility in gastrointestinal conditions. Int. J. Biol. Macromol. 2023, 227, 83–92. [Google Scholar] [CrossRef]
- Mu, H.; Song, Z.; Wang, X.; Wang, D.; Zheng, X.; Li, X. Microencapsulation of algae oil by complex coacervation of chitosan and modified starch: Characterization and oxidative stability. Int. J. Biol. Macromol. 2022, 194, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Trejo, M.C.; Wall-Medrano, A.; Gaytán-Martínez, M.; Mendoza, S. Microencapsulation of pomegranate seed oil using a succinylated taro starch: Characterization and bioaccessibility study. Food Biosci. 2021, 41, 100929. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Romanini, E.B.; Silva, E.; Pilau, E.J.; da Costa, S.C.; Madrona, G.S. Camu-camu bioactive compounds extraction by ecofriendly sequential processes (ultrasound assisted extraction and reverse osmosis). Ultrason. Sonochem 2020, 64, 105017. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.L.; Junior, T.K.; Block, J.M.; Granato, D.; Nunes, I.L. Innovative approach for obtaining phenolic compounds from guava (Psidium guajava L.) coproduct using ionic liquid ultrasound-assisted extraction (IL-UAE). Biocatal. Agric. Biotechnol. 2021, 38, 102196. [Google Scholar] [CrossRef]
- Wang, Z.; Mei, X.; Chen, X.; Rao, S.; Ju, T.; Li, J.; Yang, Z. Extraction and recovery of bioactive soluble phenolic compounds from brocade orange (Citrus sinensis) peels: Effect of different extraction methods thereon. LWT 2023, 173, 114337. [Google Scholar] [CrossRef]
- Gualberto, N.C.; de Oliveira, C.S.; Nogueira, J.P.; de Jesus, M.S.; Araujo, H.C.S.; Rajan, M.; Neta, M.T.S.L.; Narain, N. Bioactive compounds and antioxidant activities in the agro-industrial residues of acerola (Malpighia emarginata L.), guava (Psidium guajava L.), genipap (Genipa americana L.) and umbu (Spondias tuberosa L.) fruits assisted by ultrasonic or shaker extraction. Food Res. Int. 2021, 147, 110538. [Google Scholar] [CrossRef] [PubMed]
- De Carrasco, R.R.; René, C.; Zelada, E. Determinación de la capacidad antioxidante y compuestos bioactivos de frutas nativas peruanas. Rev. De. La. Soc. Química Del. Perú 2008, 74, 108–124. Available online: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1810-634X2008000200004&lng=es&nrm=iso&tlng=es (accessed on 13 October 2022).
- da Silva, M.R.; Bragagnolo, F.S.; Carneiro, R.L.; Pereira, I.D.O.C.; Ribeiro, J.A.A.; Rodrigues, C.M.; Jelley, R.E.; Fedrizzi, B.; Funari, C.S. Metabolite characterization of fifteen by-products of the coffee production chain: From farm to factory. Food Chem. 2022, 369, 130753. [Google Scholar] [CrossRef] [PubMed]
- Chaillou, L.L.; Nazareno, M.A. New Method to Determine Antioxidant Activity of Polyphenols. J. Agric. Food Chem. 2006, 54, 8397–8402. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Dos Santos Ferreira, C.I.; Pereyra, A.; Patriarca, A.R.; Mazzobre, M.F.; Polak, T.; Abram, V.; Buera, M.D.P.; Poklar Ulrih, N. Phenolic Compounds in Extracts from Eucalyptus globulus Leaves and Calendula officinalis Flowers. J. Nat. Prod. Resour. 2016, 2, 53–57. Available online: http://www.jacsdirectory.com/jnpr (accessed on 16 May 2023).
- Saura-Calixto, F.; Goñi, I. Antioxidant capacity of the Spanish Mediterranean diet. Food Chem. 2006, 94, 442–447. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols as Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘Antioxidant Power’: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Qasem, M.H.; Barrajón-Catalán, E.; Micol, V.; Mulet, A.; García-Pérez, J.V. Influence of freezing and dehydration of olive leaves (var. Serrana) on extract composition and antioxidant potential. Food Res. Int. 2013, 50, 189–196. [Google Scholar] [CrossRef]
- Casarotti, S.N.; Borgonovi, T.F.; Batista, C.L.F.M.; Penna, A.L.B. Guava, orange and passion fruit by-products: Characterization and its impacts on kinetics of acidification and properties of probiotic fermented products. LWT 2018, 98, 69–76. [Google Scholar] [CrossRef]
- Muniz, C.E.S.; Santiago, Â.M.; Gusmão, T.A.S.; Oliveira, H.M.L.; de Sousa Conrado, L.; de Gusmão, R.P. Solid-state fermentation for single-cell protein enrichment of guava and cashew by-products and inclusion on cereal bars. Biocatal. Agric. Biotechnol. 2020, 25, 101576. [Google Scholar] [CrossRef]
- Grigio, M.L.; Chagas, E.A.; Rathinasabapathi, B.; Chagas, P.C.; da Silva AR, V.; Sobral ST, M.; de Oliveira, R.R. Qualitative evaluation and biocompounds present in different parts of camu-camu (Myrciaria dubia) fruit. Afr. J. Food Sci. 2017, 11, 124–129. [Google Scholar] [CrossRef]
- da Silveira, J.S.; Durand, N.; Lacour, S.; Belleville, M.P.; Perez, A.; Loiseau, G.; Dornier, M. Solid-state fermentation as a sustainable method for coffee pulp treatment and production of an extract rich in chlorogenic acids. Food Bioprod. Process. 2019, 115, 175–184. [Google Scholar] [CrossRef]
- Buljat, A.M.; Jurina, T.; Tušek, A.J.; Valinger, D.; Kljusuric, J.G.; Benkovic, M. Applicability of foam mat drying process for production of instant cocoa powder enriched with lavender extract. Food Technol. Biotechnol. 2019, 57, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Domian, E.; Poszytek, K. Wheat flour flow ability as affected by water activity, storage time and consolidation. Int. Agrophys. 2005, 19. [Google Scholar]
- Geldart, D.; Harnby, N.; Wong, A.C. Fluidization of cohesive powders. Powder Technol. 1984, 37, 25–37. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, G.R.; González-García, R.; Grajales-Lagunes, A.; Ruiz-Cabrera, M.A.; Abud-Archila, M. Spray-drying of cactus pear juice (Opuntia streptacantha): Effect on the physicochemical properties of powder and reconstituted product. Dry. Technol. 2005, 23, 955–973. [Google Scholar] [CrossRef]
- Phoungchandang, S.; Sertwasana, A. Spray-drying of ginger juice and physicochemical properties of ginger powders. ScienceAsia 2010, 36, 40–45. [Google Scholar] [CrossRef]
- Bonazzi, C.; Dumoulin, E. Quality Changes in Food Materials as Influenced by Drying Processes. Mod. Dry. Technol. 2011, 3, 1–20. [Google Scholar] [CrossRef]
- Quek, S.Y.; Chok, N.K.; Swedlund, P. The physicochemical properties of spray-dried watermelon powders. Chem. Eng. Process. Process Intensif. 2007, 46, 386–392. [Google Scholar] [CrossRef]
- Tarone, A.G.; Cazarin, C.B.B.; Junior, M.R.M. Anthocyanins: New techniques and challenges in microencapsulation. Food Res. Int. 2020, 133, 109092. [Google Scholar] [CrossRef] [PubMed]
- Miranda, P.H.S.; Santos, A.C.D.; de Freitas, B.C.B.; dos Santos, A.C.; de Freitas, B.C.B.; Damiani, C. A scientific approach to extraction methods and stability of pigments from Amazonian fruits. Trends Food Sci. Technol. 2021, 113, 335–345. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhao, Q.; Wu, H.; Ma, X.; Xu, W.; Li, L.; Liang, Q.; Wang, S. Metabolomics and transcriptomics analyses reveal the potential molecular mechanisms of flavonoids and carotenoids in guava pulp with different colors. Sci. Hortic. 2022, 305, 111384. [Google Scholar] [CrossRef]
- Fracassetti, D.; Costa, C.; Moulay, L.; Tomás-Barberán, F.A. Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit (Myrciaria dubia). Food Chem. 2013, 139, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.A.; Knights, E.J.; Chocty, M. Morphology of Chickpea Seeds (Cicer arietinum L.): Comparison of desi and kabuli Types. Int. J. Plant Sci. 2011, 172, 632–643. [Google Scholar] [CrossRef]
- Musa, K.H.; Abdullah, A.; Subramaniam, V. Flavonoid profile and antioxidant activity of pink guava. ScienceAsia 2015, 41, 149–154. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Zimmermann, B.F.; Schulze-Kaysers, N.; Schieber, A. Characterization of phenolic and other polar compounds in peel and flesh of pink guava (Psidium guajava L. cv. ‘Criolla’) by ultra-high performance liquid chromatography with diode array and mass spectrometric detection. Food Res. Int. 2017, 100, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.S.; Kumar, P. Optimization of microwave assisted extraction of bioactive compounds from black bean waste and evaluation of its antioxidant and antidiabetic potential in vitro. Food Chem. Adv. 2023, 3, 100543. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef]
- Paz, J.E.W.; Márquez, D.B.M.; Ávila, G.C.G.M.; Cerda, R.E.B.; Aguilar, C.N. Ultrasound-assisted extraction of polyphenols from native plants in the Mexican desert. Ultrason. Sonochem 2015, 22, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Tripathy, P.P. Ultrasound-mediated hydration of finger millet: Effects on antinutrients, techno-functional and bioactive properties, with evaluation of ANN-PSO and RSM optimization methods. Food Chem. 2024, 435, 137516. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.S.; Milutinović, M.; Lazarević, Z.; Mudrić, J.; Matejić, J.; Kitić, D.; Šavikin, K. Heat- and microwave-assisted extraction of bioactive compounds from Gentiana asclepiadea L. underground parts: Optimization and comparative assessment using response surface methodology. J. Appl. Res. Med. Aromat. Plants 2023, 34, 100483. [Google Scholar] [CrossRef]
- Koyu, H.; Demir, S. Subcritical water extraction of red cabbage regarding cholinesterase and tyrosinase inhibitory activity. Microchem. J. 2024, 199, 109953. [Google Scholar] [CrossRef]
- Muhamad, N.; Muhmed, S.A.; Yusoff, M.M.; Gimbun, J. Influence of Solvent Polarity and Conditions on Extraction of Antioxidant, Flavonoids and Phenolic Content from Averrhoa bilimbi. J. Food Sci. Eng. 2014, 4, 255–260. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Mercado-Mercado, G.; Rosa Carrillo, L.D.L.; Wall-Medrano, A.; Díaz, J.A.L.; Álvarez-Parrilla, E. Compuestos polifenólicos y capacidad antioxidante de especias típicas consumidas en México. Nutr. Hosp. 2013, 28, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Pinelo, M.; Sineiro, J.; Núñez, M.J. Mass transfer during continuous solid–liquid extraction of antioxidants from grape byproducts. J. Food Eng. 2006, 77, 57–63. [Google Scholar] [CrossRef]
- Ali, S.; Ahmed, D. Comparing DES-mediated ultrasound- and heat-assisted extraction of bioactive metabolites from Saussurea lappa and optimization by RSM and validation studies. Green. Anal. Chem. 2023, 7, 100080. [Google Scholar] [CrossRef]
- del Socorro Cruz-Cansino, N.; Ramírez-Moreno, E.; León-Rivera, J.E.; Delgado-Olivares, L.; Alanís-García, E.; Ariza-Ortega, J.A.; de Jesús Manríquez-Torres, J.; Jaramillo-Bustos, D.P. Shelf life, physicochemical, microbiological and antioxidant properties of purple cactus pear (Opuntia ficus indica) juice after thermoultrasound treatment. Ultrason. Sonochem 2015, 27, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, K.; Garvín, A.; Ibarz, A.; Augusto, P.E.D. Ascorbic acid stability in fruit juices during thermosonication. Ultrason. Sonochem. 2017, 37, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.L.; Leite, T.S.; Cristianini, M.; Alvim, I.D.; Augusto, P.E.D. Peach juice processed by the ultrasound technology: Changes in its microstructure improve its physical properties and stability. Food Res. Int. 2016, 82, 22–33. [Google Scholar] [CrossRef]
- do Amaral Souza, F.D.C.; Moura, L.G.S.; de Oliveira Bezerra, K.; Aguiar, J.P.L.; Mar, J.M.; Sanches, E.A.; Dos Santos, F.F.; Bakry, A.M.; Paulino, B.N.; Campelo, P.H. Thermosonication applied on camu–camu nectars processing: Effect on bioactive compounds and quality parameters. Food Bioprod. Process. 2019, 116, 212–218. [Google Scholar] [CrossRef]
- Younas, U.; Iqbal, S.; Bashir, R.; Sajjad, N.; Saeed, Z.; Pervaiz, M.; Hassan, F.; Ali, F.; Ibrahim, S.; Batool, F.; et al. An Eco-Friendly Approach for the Extraction of Antioxidant Components from Artemisia Annua Leaves Using Response Surface Methodology. Pol. J. Env. Environ. Stud. 2021, 30, 4827–4833. [Google Scholar] [CrossRef]
- Wong, B.Y.; Tan, C.P.; Ho, C.W. Effect of solid-to-solvent ratio on phenolic content and antioxidant capacities of ‘Dukung Anak’ (Phyllanthus niruri). Int. Food Res. J. 2013, 20, 325–330. [Google Scholar]
- Mason, T.J.; Paniwnyk, L.; Lorimer, J.P. The uses of ultrasound in food technology. Ultrason Sonochem. 1996, 3, S253–S260. [Google Scholar] [CrossRef]
- Cheng, L.H.; Soh, C.Y.; Liew, S.C.; Teh, F.F. Effects of sonication and carbonation on guava juice quality. Food Chem. 2007, 104, 1396–1401. [Google Scholar] [CrossRef]
- Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Optimization Ultrasound-Assisted Deep Eutectic Solvent Extraction of Anthocyanins from Raspberry Using Response Surface Methodology Coupled with Genetic Algorithm. Foods 2020, 9, 1409. [Google Scholar] [CrossRef] [PubMed]
- Sampath, M. Optimization of the Extraction Process of Phenolic Antioxidant from Polyalthia Longifolia (Sonn.) Thawaites. J. Appl. Pharm. Sci. 2013, 3, 148–152. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Garros, L.; Drouet, S.; Renouard, S.; Lainé, E.; Hano, C. Green Ultrasound Assisted Extraction of trans Rosmarinic Acid from Plectranthus scutellarioides (L.) R.Br. Leaves. Plants 2019, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, H.A.H.; Gamal, M.; Naguib, I.A.; Al-Ghobashy, M.A.; Zaazaa, H.E.; Abdelkawy, M. Development of Green and Efficient Extraction Methods of Quercetin from Red Onion Scales Wastes Using Factorial Design for Method Optimization: A Comparative Study. Separations 2021, 8, 137. [Google Scholar] [CrossRef]
- Zahari, N.A.A.R.; Chong, G.H.; Abdullah, L.C.; Chua, B.L. Ultrasonic-Assisted Extraction (UAE) Process on Thymol Concentration from Plectranthus Amboinicus Leaves: Kinetic Modeling and Optimization. Processes 2020, 8, 322. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Liu, H.Y.; Shi, Z.G. Novel mode of liquid-phase microextraction: A magnetic stirrer as the extractant phase holder. J. Sep. Sci. 2016, 39, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Qiao, X.; Guo, Y.; Liu, P. Valorization of pawpaw (Carica papaya L.) leaves as a source of polyphenols by ionic liquid-based microwave-assisted extraction: Comparison with other extraction methods and bioactivity evaluation. Food Chem. X 2024, 22, 101500. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Wang, C.W.; Zhang, J.; Xu, P.P.; Xue, Y.T.; Wang, Q. Effects of different extraction methods on physicochemical characteristics and bioactivities of fig (Ficus carica L.) leaves polysaccharides. Arab. J. Chem. 2023, 16, 105319. [Google Scholar] [CrossRef]

| Shell of M. dubia | Seed of M. dubia | Shell of P. guajava | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Experiments | A | B | C | A | B | C | A | B | C |
| 1 | 1.5 | 0 | 50 | 1.5 | 0 | 50 | 0 | 15 | 50 |
| 2 | 1.5 | 7.5 | 30 | 1.5 | 7.5 | 30 | 3 | 10 | 40 |
| 3 | 0 | 7.5 | 10 | 0 | 7.5 | 10 | 3 | 15 | 50 |
| 4 | 1.5 | 7.5 | 30 | 1.5 | 7.5 | 30 | 0 | 5 | 50 |
| 5 | 1.5 | 7.5 | 30 | 1.5 | 7.5 | 30 | 3 | 10 | 60 |
| 6 | 3 | 15 | 30 | 3 | 15 | 30 | 1.5 | 15 | 60 |
| 7 | 1.5 | 0 | 10 | 1.5 | 0 | 10 | 0 | 10 | 40 |
| 8 | 0 | 7.5 | 50 | 0 | 7.5 | 50 | 0 | 10 | 60 |
| 9 | 0 | 15 | 30 | 0 | 15 | 30 | 1.5 | 5 | 40 |
| 10 | 3 | 0 | 30 | 3 | 0 | 30 | 1.5 | 10 | 50 |
| 11 | 0 | 0 | 30 | 0 | 0 | 30 | 3 | 5 | 50 |
| 12 | 1.5 | 15 | 50 | 1.5 | 15 | 50 | 1.5 | 10 | 50 |
| 13 | 3 | 7.5 | 50 | 3 | 7.5 | 50 | 1.5 | 5 | 60 |
| 14 | 1.5 | 7.5 | 30 | 1.5 | 7.5 | 30 | 1.5 | 10 | 50 |
| 15 | 1.5 | 7.5 | 30 | 1.5 | 7.5 | 30 | 1.5 | 10 | 50 |
| 16 | 3 | 7.5 | 10 | 3 | 7.5 | 10 | 1.5 | 10 | 50 |
| 17 | 1.5 | 15 | 10 | 1.5 | 15 | 10 | 1.5 | 15 | 40 |
| Shell of M. dubia | Seed of M. dubia | Shell of P. guajava | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Experiments | A | B | C | A | B | C | A | B | C |
| 1 | 150 | 50 | 2 | 150 | 40 | 8 | 250 | 50 | 6 |
| 2 | 150 | 50 | 6 | 150 | 60 | 8 | 300 | 40 | 6 |
| 3 | 200 | 30 | 4 | 200 | 40 | 6 | 300 | 60 | 6 |
| 4 | 100 | 50 | 4 | 200 | 50 | 8 | 300 | 50 | 8 |
| 5 | 150 | 40 | 4 | 200 | 50 | 8 | 250 | 50 | 6 |
| 6 | 100 | 40 | 6 | 200 | 50 | 8 | 200 | 50 | 4 |
| 7 | 200 | 40 | 6 | 200 | 60 | 6 | 250 | 40 | 8 |
| 8 | 200 | 40 | 2 | 250 | 50 | 6 | 200 | 60 | 6 |
| 9 | 150 | 40 | 4 | 200 | 40 | 10 | 250 | 60 | 8 |
| 10 | 150 | 40 | 4 | 200 | 50 | 8 | 250 | 60 | 4 |
| 11 | 150 | 30 | 2 | 250 | 60 | 8 | 250 | 40 | 4 |
| 12 | 100 | 40 | 2 | 150 | 50 | 6 | 250 | 50 | 6 |
| 13 | 150 | 30 | 6 | 250 | 40 | 8 | 250 | 50 | 6 |
| 14 | 200 | 50 | 4 | 200 | 50 | 8 | 200 | 50 | 8 |
| 15 | 150 | 40 | 4 | 150 | 50 | 10 | 300 | 50 | 4 |
| 16 | 150 | 40 | 4 | 250 | 50 | 10 | 200 | 40 | 6 |
| 17 | 100 | 30 | 4 | 200 | 60 | 10 | 250 | 50 | 6 |
| Shell of M. dubia | Seed of M. dubia | Shell of P. guajava | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | AC-DPPH (mg GAE/g) | TPC (mg GAE/g) | AC-FRAP (mg GAE/g) | AC-DPPH (mg GAE/g) | TPC (mg GAE/g) | AC-FRAP (mg GAE/g) | AC-DPPH (mg GAE/g) | TPC (mg GAE/g) | AC-FRAP (mg GAE/g) |
| Model | <0.0001 | 0.0103 | 0.0001 | <0.0001 | 0.0386 | 0.0031 | 0.0013 | 0.0037 | 0.0414 |
| Stirring time (A) | 0.4486 | 0.0057 | 0.1062 | 0.5125 | 0.0110 | 0.0007 | 0.8554 | 0.9765 | 0.4049 |
| Sonication time (B) | 0.1638 | 0.0146 | 0.5983 | 0.2286 | 0.3218 | 0.7586 | 0.6995 | 0.0663 | 0.0161 |
| Volume–mass ratio (C) | <0.0001 | 0.2079 | <0.0001 | <0.0001 | 0.0480 | 0.0005 | <0.0001 | 0.0002 | 0.4480 |
| AB | 0.8046 | 0.1517 | 0.7645 | 0.7172 | 0.7052 | 0.8179 | 0.0794 | 0.8836 | 0.8517 |
| AC | 0.4412 | 0.0015 | 0.1887 | 0.4620 | 0.3948 | 0.1456 | 0.4516 | 0.3915 | 0.5180 |
| BC | 0.0305 | 0.1248 | 0.0015 | 0.0057 | 0.4963 | 0.0292 | 0.2601 | 0.0081 | 0.1876 |
| A2 | 0.0292 | 0.0085 | 0.0592 | 0.2206 | 0.0492 | 0.0297 | 0.0075 | 0.0206 | 0.0463 |
| B2 | 0.0689 | 0.0180 | 0.0286 | 0.0452 | 0.8652 | 0.4067 | 0.1544 | 0.1597 | 0.0080 |
| C2 | <0.0001 | 0.0222 | 0.0431 | <0.0001 | 0.0108 | 0.4993 | 0.0066 | 0.0492 | 0.4209 |
| R2 | 0.9998 | 0.9221 | 0.9825 | 0.9992 | 0.8732 | 0.9273 | 0.9441 | 0.9238 | 0.8361 |
| Shell of M. dubia | Seed of M. dubia | Shell of P. guajava | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | AC-DPPH (mg GAE/g) | TPC (mg GAE/g) | AC-FRAP (mg GAE/g) | AC-DPPH (mg GAE/g) | TPC (mg GAE/g) | AC-FRAP (mg GAE/g) | AC-DPPH (mg GAE/g) | TPC (mg GAE/g) | AC-FRAP (mg GAE/g) |
| Model | <0.0001 | 0.0092 | <0.0001 | 0.0014 | 0.0309 | 0.0007 | 0.0004 | 0.0014 | 0.0129 |
| Stirring speed (A) | 0.2483 | 0.0012 | 0.0409 | 0.1185 | 0.3793 | <0.0001 | 0.0150 | 0.9854 | 0.0979 |
| Volume–mass ratio (B) | <0.0001 | 0.0208 | <0.0001 | <0.0001 | 0.2159 | 0.2140 | <0.0001 | <0.0001 | 0.0106 |
| Stirring time (C) | 0.3747 | 0.3761 | 0.0409 | 0.3217 | 0.2242 | 0.0079 | 0.0103 | 0.0039 | 0.0074 |
| AB | 0.3966 | 0.0326 | 0.0026 | 0.6455 | 0.0242 | 0.1229 | 0.3815 | 0.5145 | 0.1997 |
| AC | 0.0104 | 0.3096 | 0.4941 | 0.5927 | 0.1445 | 0.0003 | 0.0940 | 0.3361 | 0.7668 |
| BC | 0.6361 | 0.0852 | 0.0006 | 0.9888 | 0.5049 | 0.2022 | 0.0139 | 0.0176 | 0.0690 |
| A2 | 0.2129 | 0.1018 | 0.0269 | 0.2013 | 0.0050 | 0.0350 | 0.0429 | 0.0371 | 0.0189 |
| B2 | 0.0319 | 0.0187 | 0.0177 | 0.0274 | 0.0364 | 0.6150 | 0.0378 | 0.0604 | 0.0486 |
| C2 | 0.1240 | 0.4949 | 0.0470 | 0.4294 | 0.5168 | 0.2817 | 0.4257 | 0.1872 | 0.1650 |
| R2 | 0.9848 | 0.8989 | 0.9835 | 0.9437 | 0.8513 | 0.9537 | 0.9618 | 0.9434 | 0.8877 |
| UAE | MS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Plant Source * | Optimal Factors ** | Value (mg GAE/g) | Desirability | Optimal Factors ** | Value (mg GAE/g) | Desirability | ||||
| A | B | C | D | E | F | ||||||
| AC-DPPH | CCP | 3 | 11.2 | 50 | 11.8 | 0.99 | 150 | 50 | 2 | 12.8 | 1 |
| CCS | 0.99 | 15 | 50 | 12.1 | 0.99 | 221.5 | 60 | 10 | 12.8 | 0.99 | |
| GP | 0.07 | 10.4 | 59.7 | 20.8 | 1 | 200.06 | 58.9 | 7.96 | 15.5 | 1 | |
| TPC | CCP | 0.1 | 5.5 | 44.4 | 36.6 | 1 | 196.6 | 30.05 | 2.1 | 27.3 | 1 |
| CCS | 3 | 15 | 50 | 6.2 | 0.95 | 249.98 | 59.98 | 7.8 | 8.5 | 1 | |
| GP | 2.8 | 11.1 | 57.1 | 16.8 | 1 | 232.8 | 59.3 | 7.9 | 29.9 | 1 | |
| AC-FRAP | CCP | 1.8 | 14.99 | 49.9 | 67.8 | 0.83 | 194.2 | 49.8 | 2.03 | 122.1 | 1 |
| CCS | 2.9 | 1.4 | 47.9 | 46.3 | 1 | 249.8 | 44.3 | 6.2 | 38.98 | 1 | |
| GP | 2.5 | 14.95 | 40.5 | 47.8 | 1 | 266.5 | 53.6 | 7.02 | 66.04 | 1 | |
| Plant Source * | Optimal Factors ** | AC-DPPH (mg GAE/g) | TPC (mg GAE/g) | AC-FRAP (mg GAE/g) | Compound Desirability | |||
|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||
| Predicted values | CCP | 0 | 15 | 50 | 11.67 | 31.11 | 59.50 | 0.88 |
| CCS | 3 | 4.42 | 50 | 11.24 | 5.73 | 45.82 | 0.91 | |
| GP | 0 | 15 | 60 | 22.26 | 16.73 | 46.19 | 0.97 | |
| Observed values | CCP | 0 | 15 | 50 | 11.19 ± 0.19 b | 26.15± 01 a | 67.57 ± 1.37 a | - |
| CCS | 3 | 4,5 | 50 | 11.14 ± 0.005 b | 7.67 ± 0.378 c | 44.67 ± 4.46 b | - | |
| GP | 0 | 15 | 60 | 20.06 ± 0.06 a | 14.78 ± 0.02 b | 49.9 ± 0.77 b | - | |
| Plant Source * | Optimal Factors ** | AC-DPPH (mg GAE/g) | TPC (mg GAE/g) | AC-FRAP (mg GAE/g) | Compound Desirability | |||
|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||
| Predicted values | CCP | 172.24 | 50 | 2 | 12.59 | 22.97 | 121.65 | 0.89 |
| CCS | 250 | 60 | 6.39 | 12.47 | 8.41 | 35.53 | 0.90 | |
| GP | 256.26 | 60 | 7.72 | 14.93 | 29.66 | 61.17 | 0.92 | |
| Observed values | CCP | 172 | 50 | 2 | 13.16 ± 0.16 b | 27.92 ± 2.07 a | 126.93 ± 2.53 a | - |
| CCS | 250 | 60 | 6.4 | 9.96 ±0.09 c | 10.58 ± 0.22 c | 28.21 ± 0.47 c | - | |
| GP | 256 | 60 | 6.7 | 14.28 ± 0.07 a | 20.68 ± 0.09 b | 67 ± 0.13 b | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carranza Carranza, L.C.; Chavez, S.G.; dos Santos Ferreira, C. Characterization of the Byproducts of Myrciaria dubia and Psidium guajava and Optimization of the Extraction of Their Bioactive Compounds by Ultrasound-Assisted Extraction and Mechanical Agitation. Processes 2024, 12, 1228. https://doi.org/10.3390/pr12061228
Carranza Carranza LC, Chavez SG, dos Santos Ferreira C. Characterization of the Byproducts of Myrciaria dubia and Psidium guajava and Optimization of the Extraction of Their Bioactive Compounds by Ultrasound-Assisted Extraction and Mechanical Agitation. Processes. 2024; 12(6):1228. https://doi.org/10.3390/pr12061228
Chicago/Turabian StyleCarranza Carranza, Luz C., Segundo G. Chavez, and Cristina dos Santos Ferreira. 2024. "Characterization of the Byproducts of Myrciaria dubia and Psidium guajava and Optimization of the Extraction of Their Bioactive Compounds by Ultrasound-Assisted Extraction and Mechanical Agitation" Processes 12, no. 6: 1228. https://doi.org/10.3390/pr12061228






