Effects of Ti/Al Ratio on Formation of Ti-Al Intermetallics/TiB2 Composites by SHS from Ti-Al-B Powder Mixtures
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
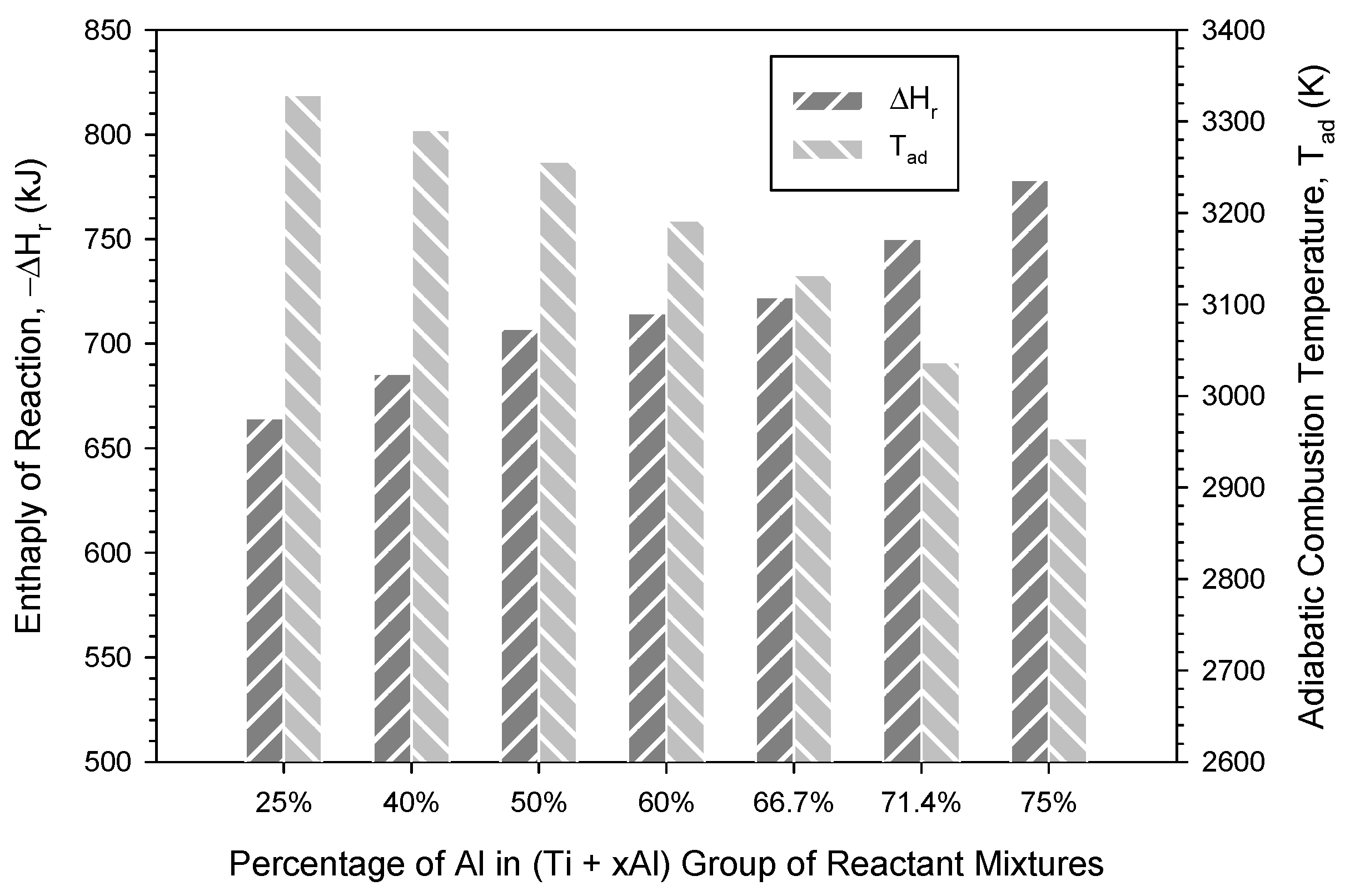
3.1. Analysis of Combustion Exothermicity
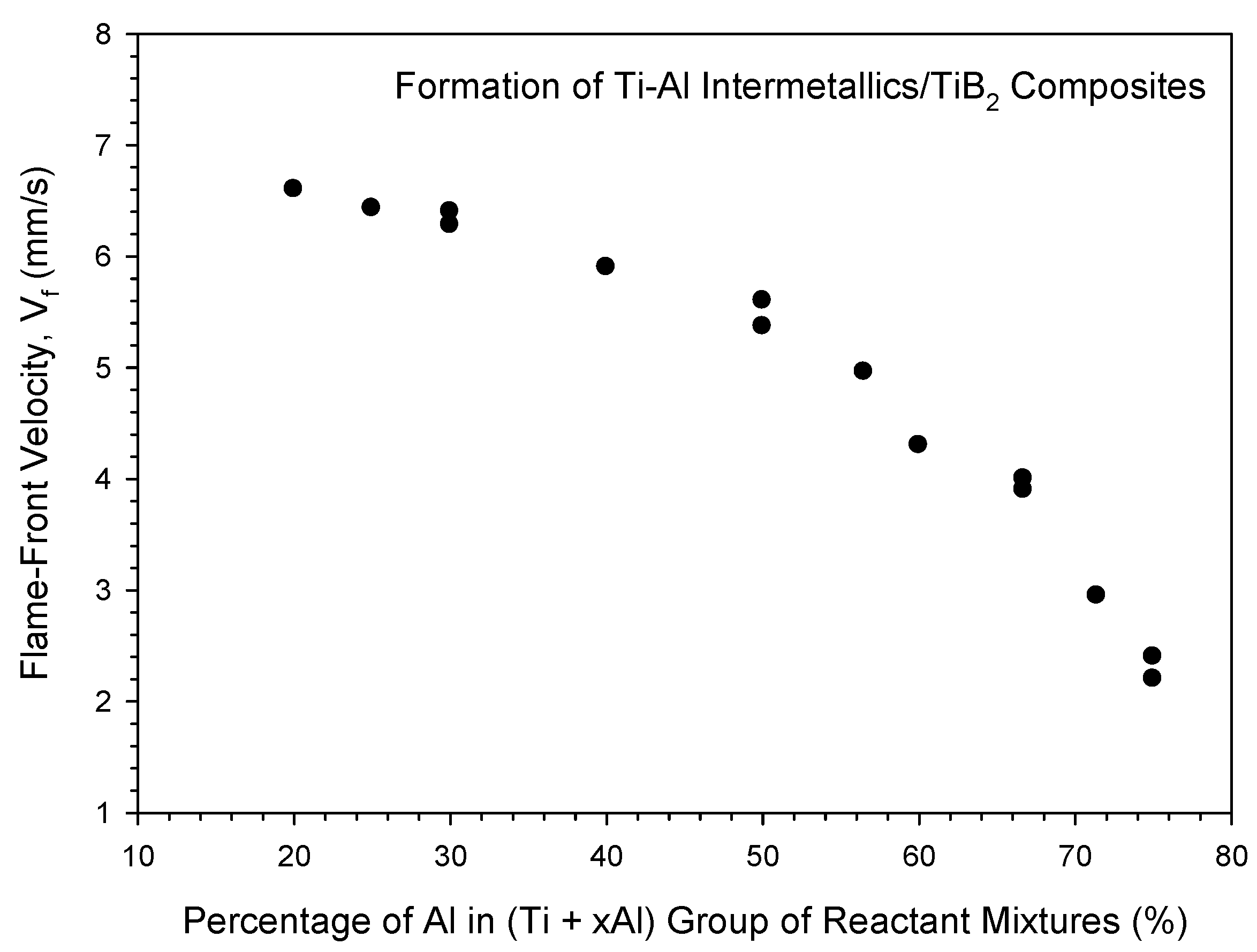
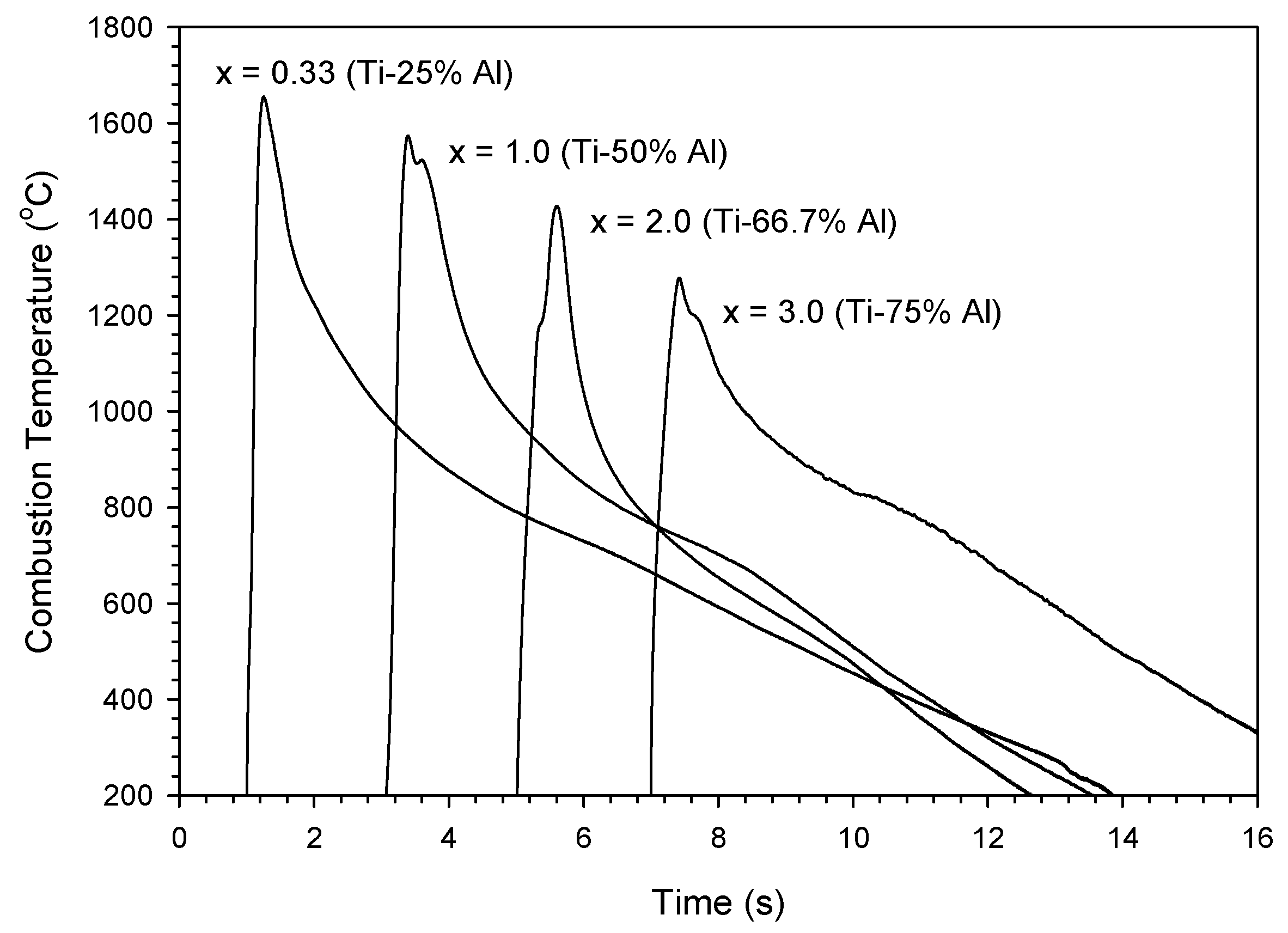
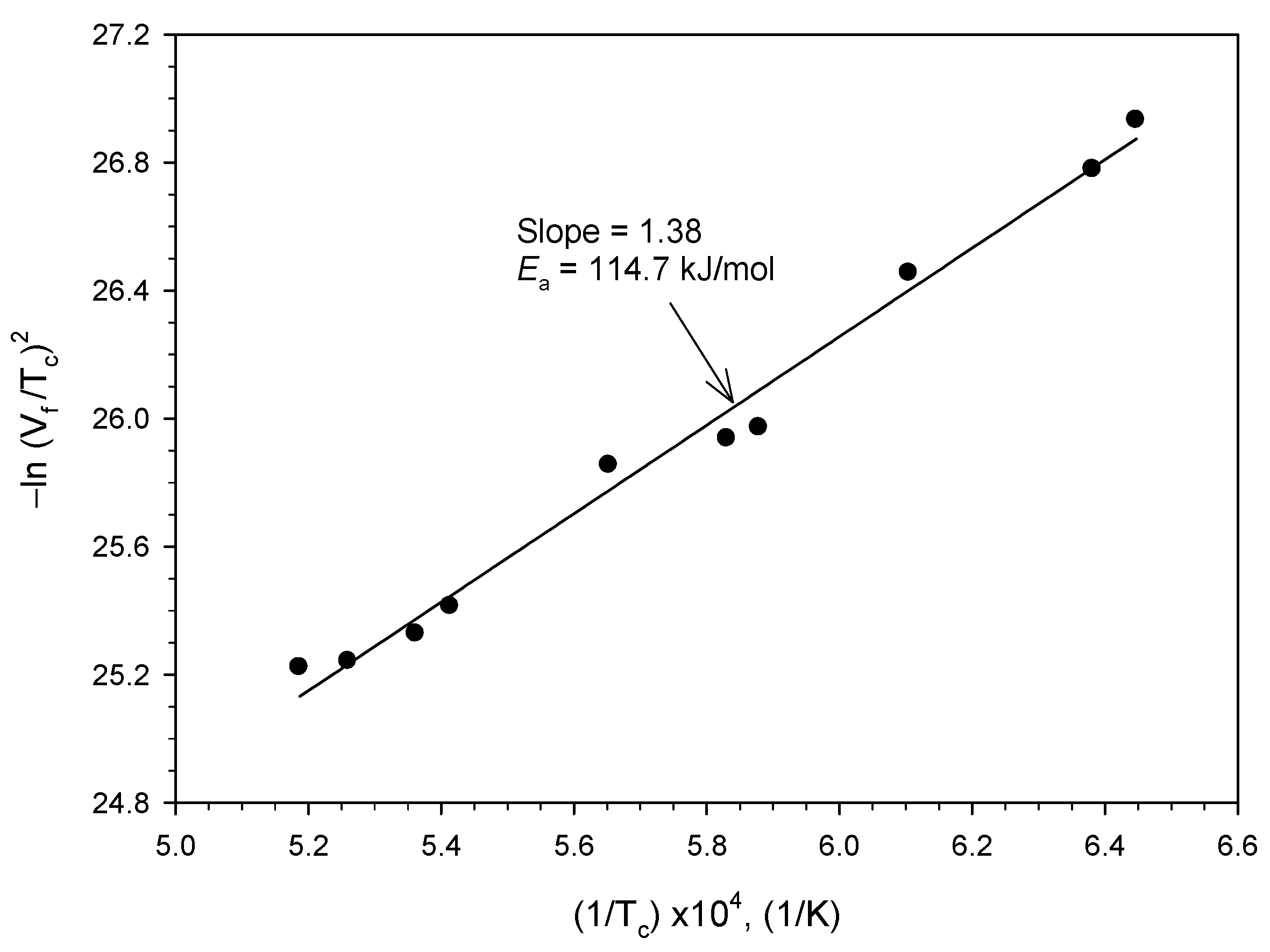
3.2. Self-Propagating Combustion Wave Kinetics
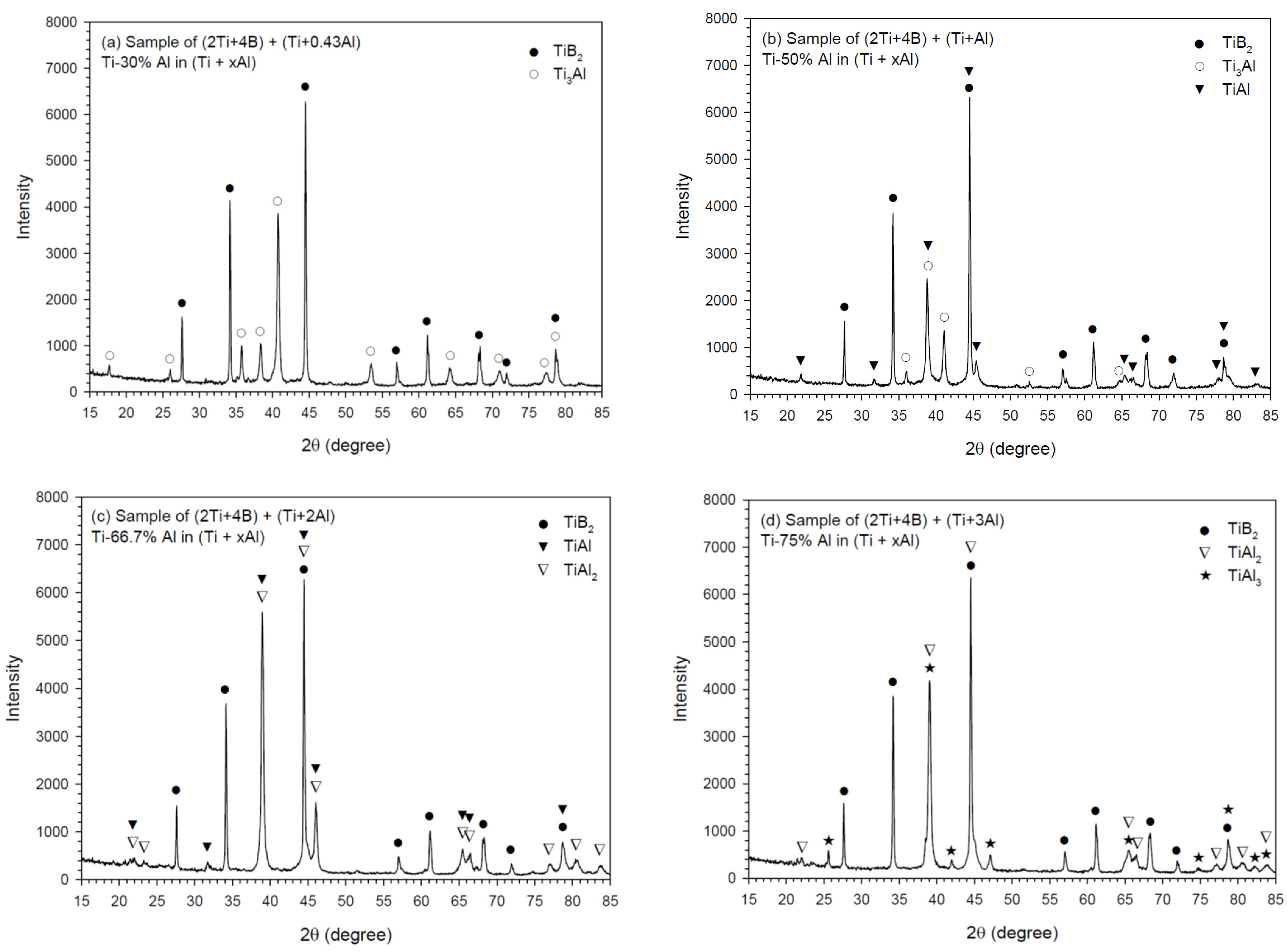
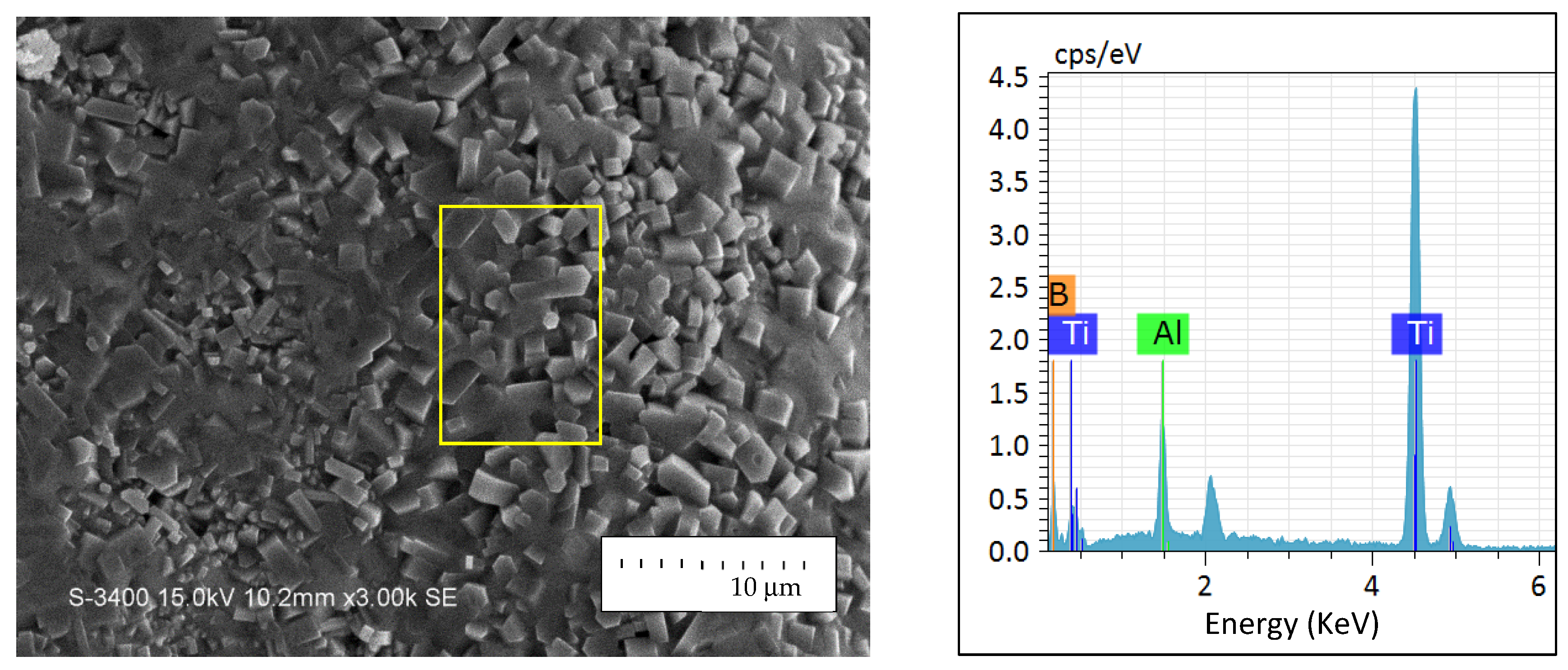
3.3. Phase Composition and Microstructure of Synthesized Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Genc, O.; Unal, R. Development of gamma titanium aluminide (γ-TiAl) alloys: A review. J. Alloys Compd. 2022, 929, 167262. [Google Scholar] [CrossRef]
- Liu, P.; Xie, J.; Wang, A. Recent research progress in TiAl matrix composites: A review. J. Mater. Sci. 2022, 57, 16147–16174. [Google Scholar] [CrossRef]
- Avdeeva, V.; Bazhina, A.; Antipov, M.; Stolin, A.; Bazhin, P. Relationship between structure and properties of intermetallic materials based on γ-TiAl hardened in situ with Ti3Al. Metals 2023, 13, 1002. [Google Scholar] [CrossRef]
- Musi, M.; Graf, G.; Clemens, H.; Spoerk-Erdely, P. Alloying elements in intermetallic γ-TiAl based alloys–a review on their influence on phase equilibria and phase transformations. Adv. Eng. Mater. 2023, 26, 2300610. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Wang, Y.; Ma, T.; Zhu, D.; Xing, Q.; Feng, H.; Chen, R. High-temperature oxidation behavior of ceramic particles-reinforced TiAl composites with multilayered structure. Ceram. Int. 2024, 50, 2233–2241. [Google Scholar] [CrossRef]
- Wei, Y.; Qiu, F.; Shu, S.; Tong, H.; Yang, H.; Jiang, Q. Microstructure manipulation and strengthening mechanism of TiAl composites reinforced by Cr solid solution and in-situ nanometer-sized TiB2 particles. Mater. Sci. Eng. A 2022, 845, 143214. [Google Scholar]
- Li, W.; Yang, Y.; Li, M.; Liu, J.; Cai, D.; Wei, Q.; Yan, C.; Shi, Y. Enhanced mechanical property with refined microstructure of a novel γ-TiAl/TiB2 metal matrix composite (MMC) processed via hot isostatic press. Mater. Design 2018, 141, 57–66. [Google Scholar] [CrossRef]
- Han, J.; Xiao, S.; Tian, J.; Chen, Y.; Xu, L.; Wang, X.; Jia, Y.; Rahoma, H.K.S.; Du, Z.; Cao, S. Microstructure characterization, mechanical properties and toughening mechanism of TiB2-containing conventional cast TiAl-based alloy. Mater. Sci. Eng. A 2015, 645, 8–19. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Stark, A.; Esikov, M.A.; Paul, J.; Bataev, I.A.; Kashimbetova, A.A.; Mali, V.I.; Lorenz, U.; Pyczak, F. Ceramic-reinforced γ-TiAl-based composites: Synthesis, structure, and properties. Materials 2019, 12, 629. [Google Scholar] [CrossRef]
- Lu, X.; Li, J.; Chen, X.; Qiu, J.; Wang, Y.; Liu, B.; Liu, Y.; Rashad, M.; Pan, F. Mechanical, tribological and electrochemical corrosion properties of in-situ synthesized Al2O3/TiAl composites. Intermetallics 2020, 120, 106758. [Google Scholar] [CrossRef]
- Lu, X.; Li, J.; Chen, X.; Ran, C.; Wang, Y.; Liu, B.; Liu, Y.; Rashad, M.; Pan, F. Grinding mechanism and mechanical properties of the in-situ synthesized Al2O3/TiAl composites. Ceram. Int. 2019, 45, 12113–12121. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, R.; Fang, H.; Liu, Y.; Ding, H.; Su, Y.; Guo, J.; Fu, H. Microstructure evolution and mechanical properties of TiAl binary alloys added with SiC fibers. Intermetallics 2018, 98, 69–78. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, P.; Wang, A.; Xie, J.; Hou, B. Effect of spark plasma sintering temperature on the multi-scale microstructure evolution and mechanical properties of Ti2AlC/TiAl composites with network architecture. J. Mater. Res. Technol. 2023, 25, 6209–6223. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Behn, R.; Klassen, T.; Bormann, R. Mechanical characterization of mechanically alloyed ultrafine-grained Ti5Si3+ 40 vol% γ-TiAl composites. Mater. Sci. Eng. A 2013, 579, 18–25. [Google Scholar] [CrossRef]
- Shu, S.; Xing, B.; Qiu, F.; Jin, S.; Jiang, Q. Comparative study of the compression properties of TiAl matrix composites reinforced with nano-TiB2 and nano-Ti5Si3 particles. Mater. Sci. Eng. A 2013, 560, 596–600. [Google Scholar] [CrossRef]
- Yeh, C.L.; Li, R.F. Formation of TiAl–Ti5Si3 and TiAl–Al2O3 in situ composites by combustion synthesis. Intermetallics 2008, 16, 64–70. [Google Scholar] [CrossRef]
- Levashov, E.A.; Mukasyan, A.S.; Rogachev, A.S.; Shtansky, D.V. Self-propagating high-temperature synthesis of advanced materials and coatings. Int. Mater. Rev. 2017, 62, 203–239. [Google Scholar] [CrossRef]
- Cobbinah, P.V.; Matizamhuka, W.R. Solid-state processing route, mechanical behaviour, and oxidation resistance of TiAl alloys. Adv. Mater. Sci. Eng. 2019, 2019, 1–21. [Google Scholar] [CrossRef]
- Lei, C.; Xu, Q.; Sun, Y.Q. Phase orientation relationships in the TiAl–TiAl2 region. Mater. Sci. Eng. A 2001, 313, 227–236. [Google Scholar] [CrossRef]
- Peng, M.; Shou, H.; Cao, Y. First-principles calculations of structural, elastic and thermodynamic properties of (h, r)-TiAl2. Phys. B Condens. Matter 2019, 561, 29–36. [Google Scholar] [CrossRef]
- Zaki, Z.I.; Ahmed, Y.M.Z.; Abdel-Gawad, S.R. In-situ synthesis of porous magnesia spinel/TiB2 composite by combustion technique. J. Ceram. Soc. Jpn. 2009, 117, 719–723. [Google Scholar] [CrossRef]
- Binnewies, M.; Milke, E. Thermochemical Data of Elements and Compounds; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2002. [Google Scholar]
- Yeh, C.L.; Chen, C. In situ formation of titanium diboride/magnesium titanate composites by magnesiothermic-based combustion synthesis. Processes 2024, 12, 459. [Google Scholar] [CrossRef]
- Varma, A.; Rogachev, A.S.; Mukasyan, A.S.; Hwang, S. Combustion synthesis of advanced materials: Principals and applications. Adv. Chem. Eng. 1998, 24, 79–225. [Google Scholar]
- Holt, J.B.; Kingman, D.D.; Bianchini, G.M. Kinetics of the combustion synthesis of TiB2. Mater. Sci. Eng. 1985, 71, 321–327. [Google Scholar] [CrossRef]
- Yeh, C.L.; Ke, C.Y.; Chen, Y.C. In situ formation of TiB2/TiC and TiB2/TiN reinforced NiAl by self-propagating combustion synthesis. Vacuum 2018, 151, 185–188. [Google Scholar] [CrossRef]
- Kurbatkina, V.V. Titanium Aluminides. In Concise Encyclopedia of Self-Propagating High-Temperature Synthesis; Borovinskaya, I.P., Gromov, A.A., Levashov, E.A., Maksimov, Y.M., Mukasyan, A.S., Rogachev, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 392–393. [Google Scholar]
- Shen, P.; Zou, B.; Jin, S.; Jiang, Q. Reaction mechanism in self-propagating high temperature synthesis of TiC-TiB2/Al composites from an Al-Ti-B4C system. Mater. Sci. Eng. A 2007, 454, 300–309. [Google Scholar] [CrossRef]
- Fjellstedt, J.; Jarfors, A.E. On the precipitation of TiB2 in aluminum melts from the reaction with KBF4 and K2TiF6. Mater. Sci. Eng. A 2005, 413, 527–532. [Google Scholar] [CrossRef]



| x | Ti/Al Atomic Ratio | Ti-Al Intermetallics |
|---|---|---|
| 0.33 | Ti-25% Al | 1/3 Ti3Al |
| 0.43 | Ti-30% Al | 1/3 Ti3Al |
| 0.67 | Ti-40% Al | 1/6 Ti3Al + 1/2 TiAl |
| 1.0 | Ti-50% Al | TiAl |
| 1.5 | Ti-60% Al | 1/2 TiAl + 1/2 TiAl2 |
| 2.0 | Ti-66.7% Al | TiAl2 |
| 2.5 | Ti-71.4% Al | 1/2 TiAl2 + 1/2 TiAl3 |
| 3.0 | Ti-75% Al | TiAl3 |
| Product Species | Heat of Formation ∆Hf (kJ/mol) | Specific Heat, cp (J/mol·K) |
|---|---|---|
| TiB2 | –315.9 | |
| Ti3Al | –97.9 | |
| TiAl | –75.3 | |
| TiAl2 | –90.4 | |
| TiAl3 | –146.4 |
| Al Percentage in Ti + xAl | Major Aluminide | Minor Aluminide |
|---|---|---|
| Ti-25% Al | Ti3Al | ― |
| Ti-30% Al | Ti3Al | ― |
| Ti-40% Al | Ti3Al | TiAl |
| Ti-50% Al | TiAl | Ti3Al |
| Ti-60% Al | TiAl | TiAl2 |
| Ti-66.7% Al | TiAl2, TiAl | ― |
| Ti-71.4% Al | TiAl2 | TiAl3 |
| Ti-75% Al | TiAl3 | TiAl2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-L.; Chan, Y.-C. Effects of Ti/Al Ratio on Formation of Ti-Al Intermetallics/TiB2 Composites by SHS from Ti-Al-B Powder Mixtures. Processes 2024, 12, 1237. https://doi.org/10.3390/pr12061237
Yeh C-L, Chan Y-C. Effects of Ti/Al Ratio on Formation of Ti-Al Intermetallics/TiB2 Composites by SHS from Ti-Al-B Powder Mixtures. Processes. 2024; 12(6):1237. https://doi.org/10.3390/pr12061237
Chicago/Turabian StyleYeh, Chun-Liang, and Yi-Cheng Chan. 2024. "Effects of Ti/Al Ratio on Formation of Ti-Al Intermetallics/TiB2 Composites by SHS from Ti-Al-B Powder Mixtures" Processes 12, no. 6: 1237. https://doi.org/10.3390/pr12061237





