Properties of Ni-B/B Composite Coatings Produced by the Electroless Method under Semi-Technical Line Conditions
Abstract
:1. Introduction
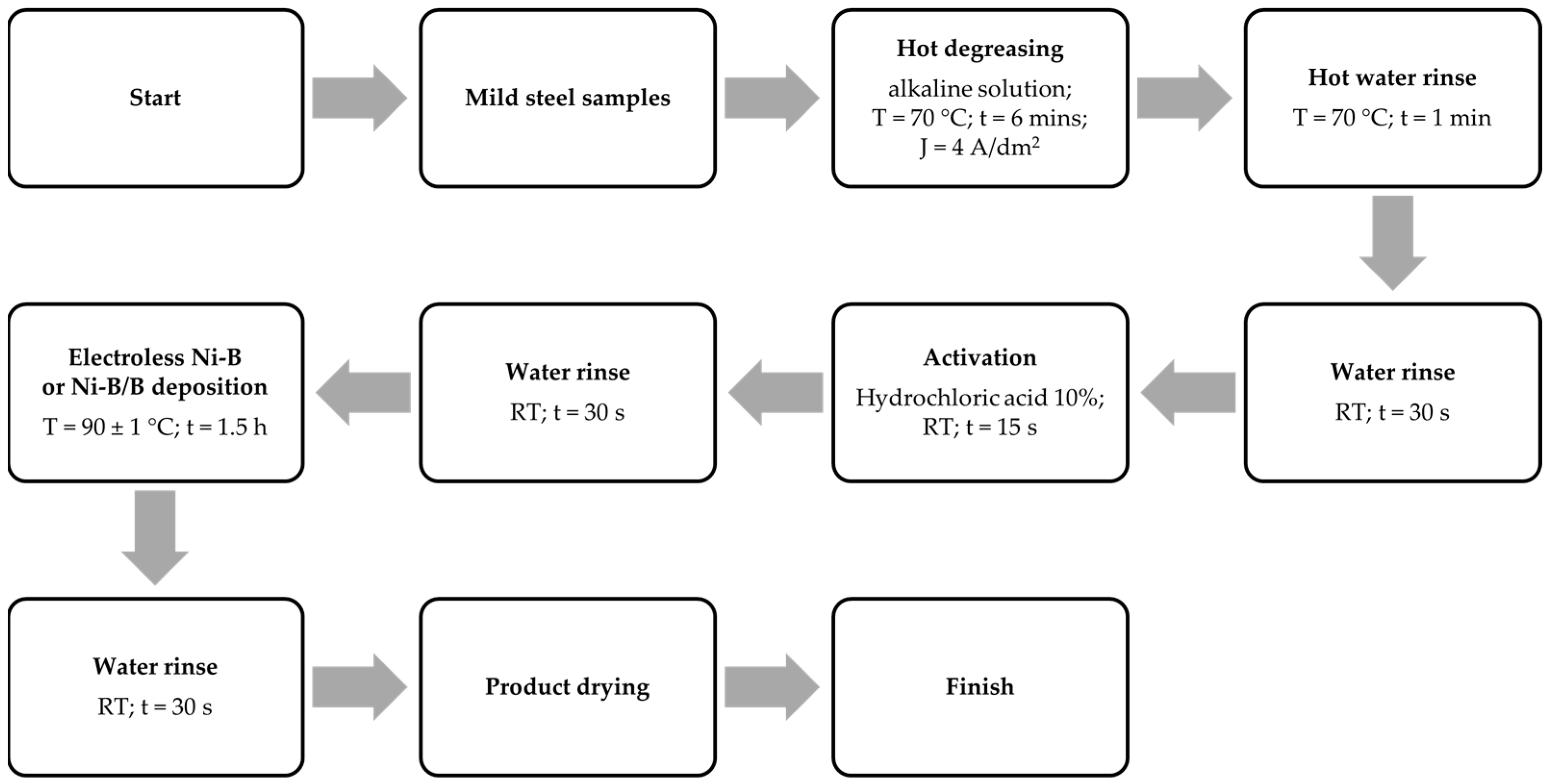
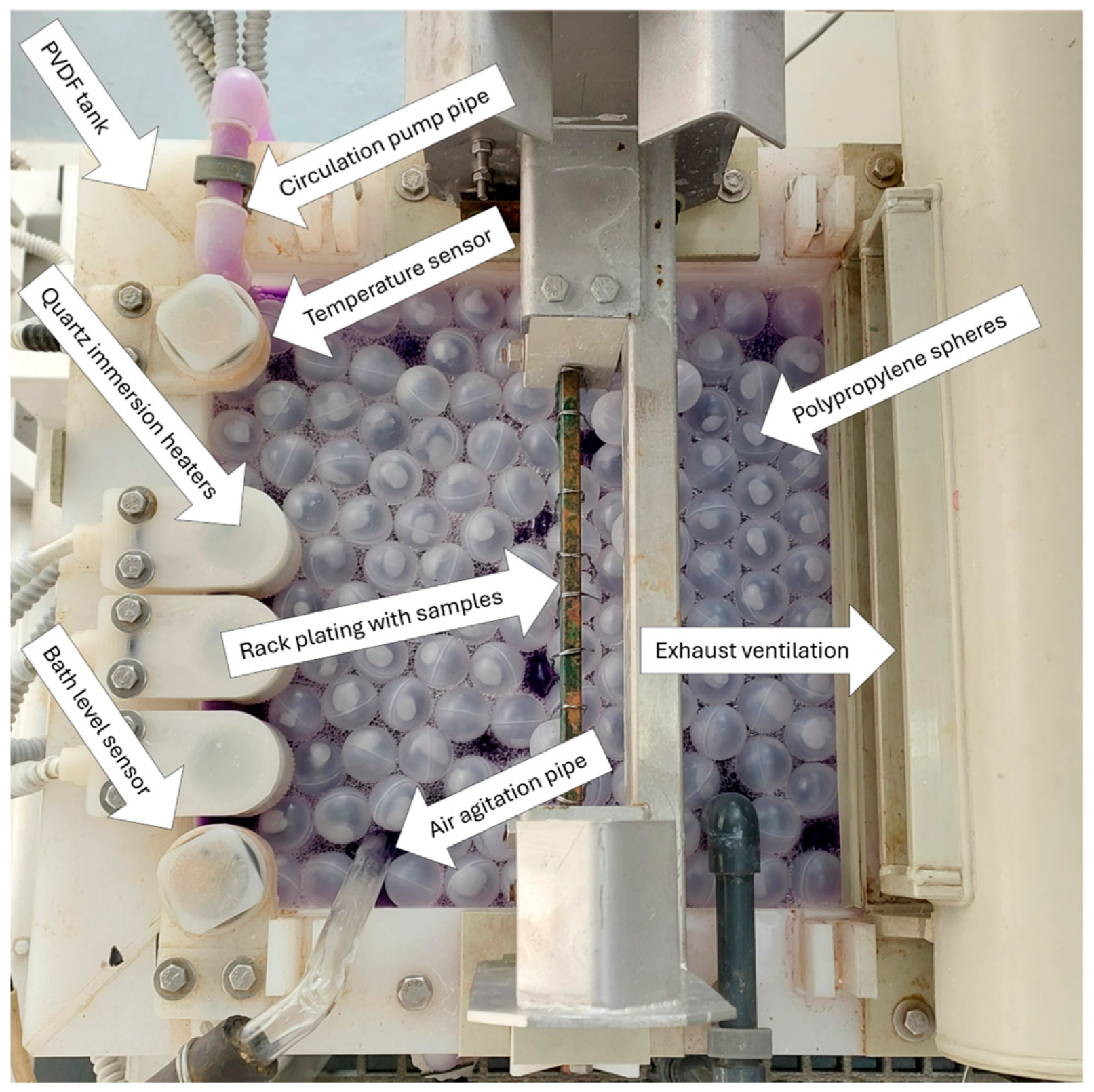
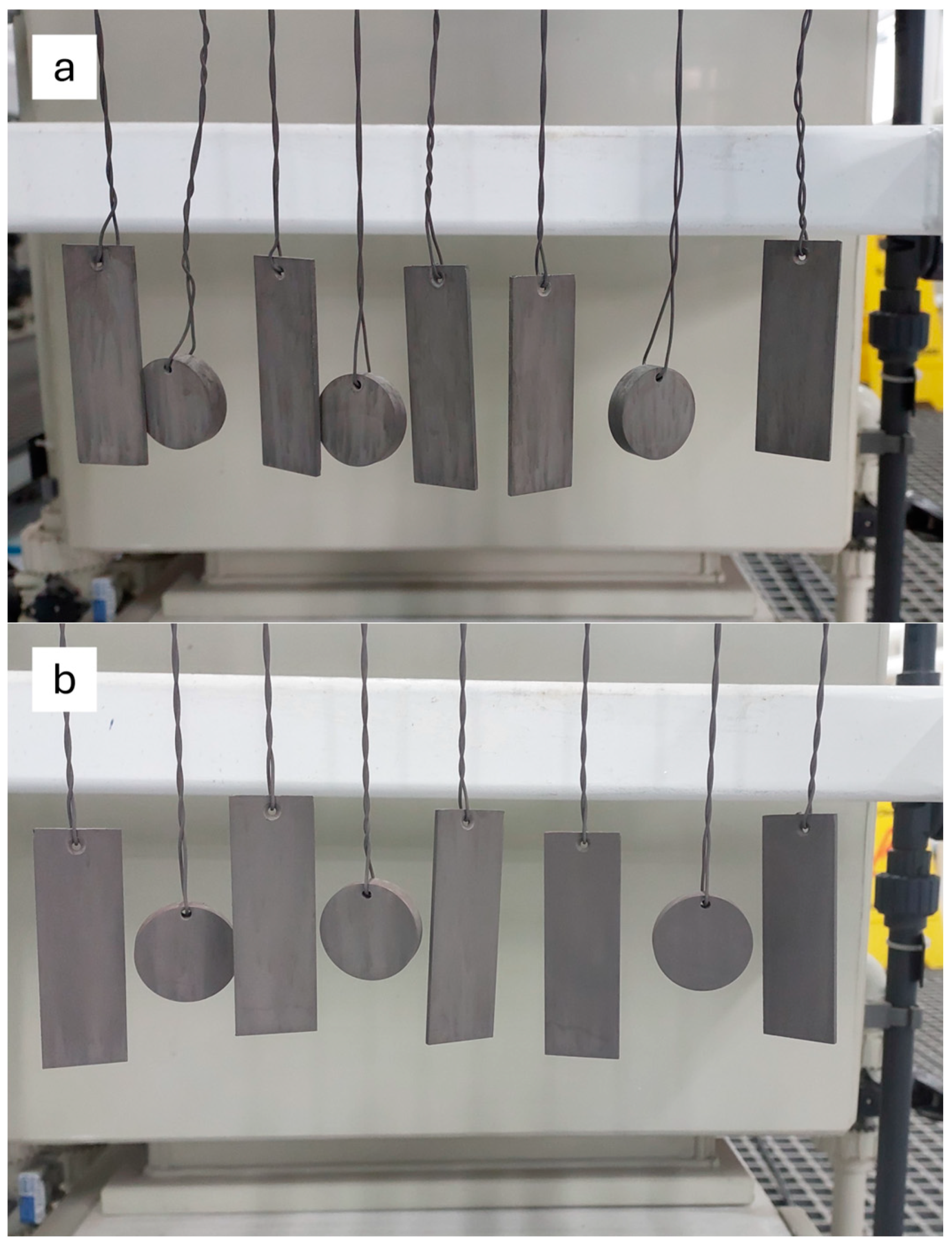
2. Materials and Methods
3. Results and Discussion
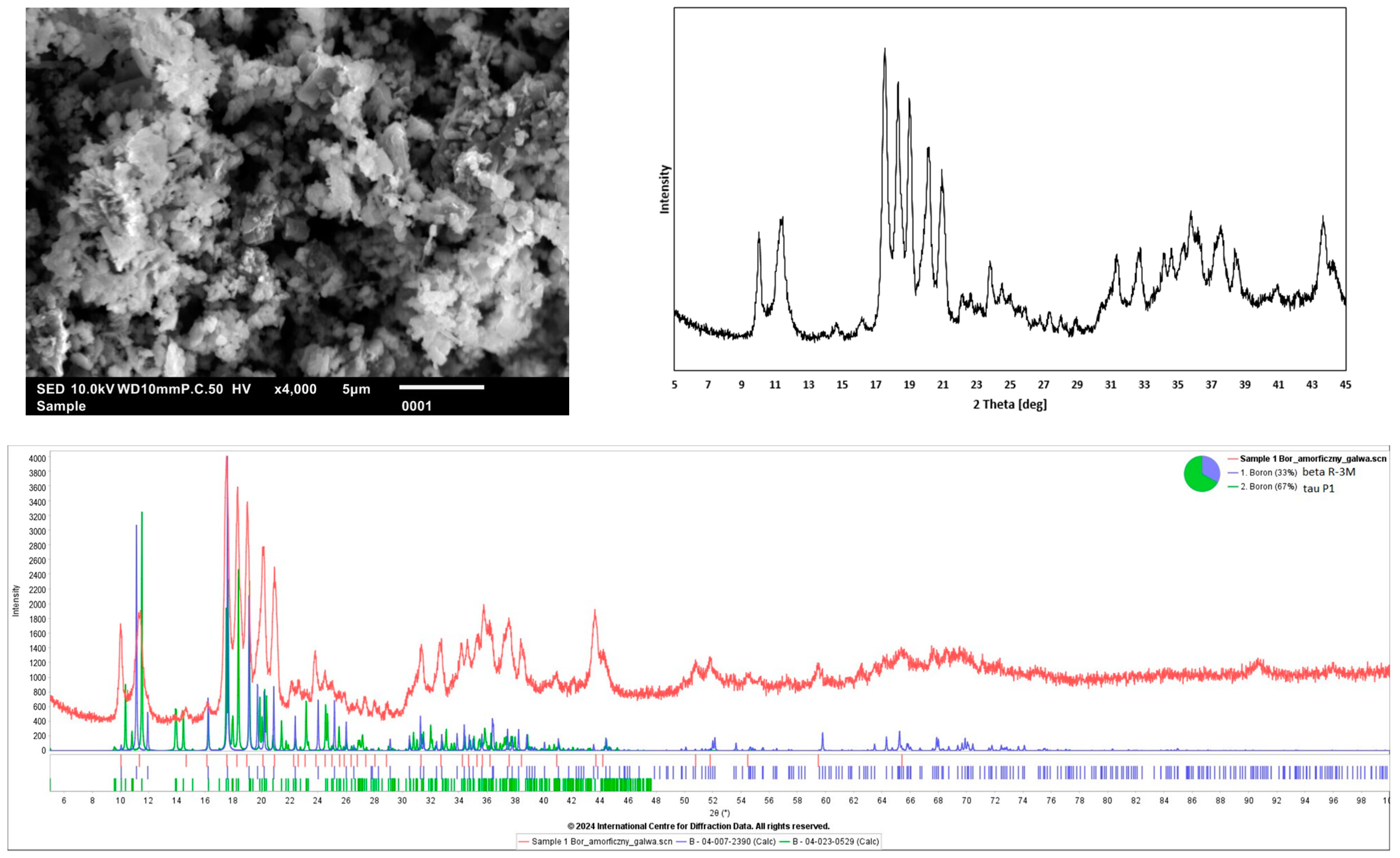
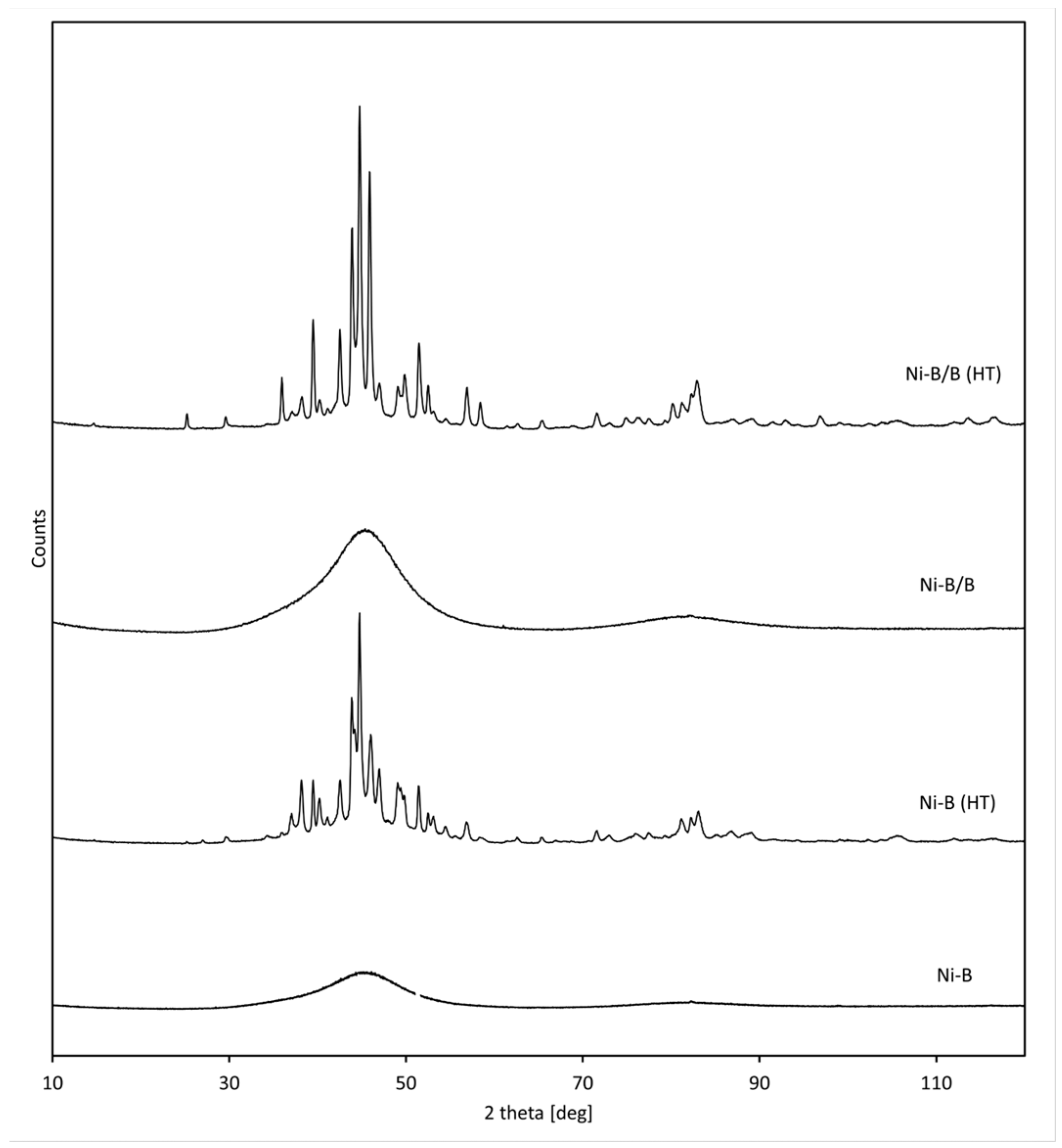
3.1. Dispersion Phase (Boron)
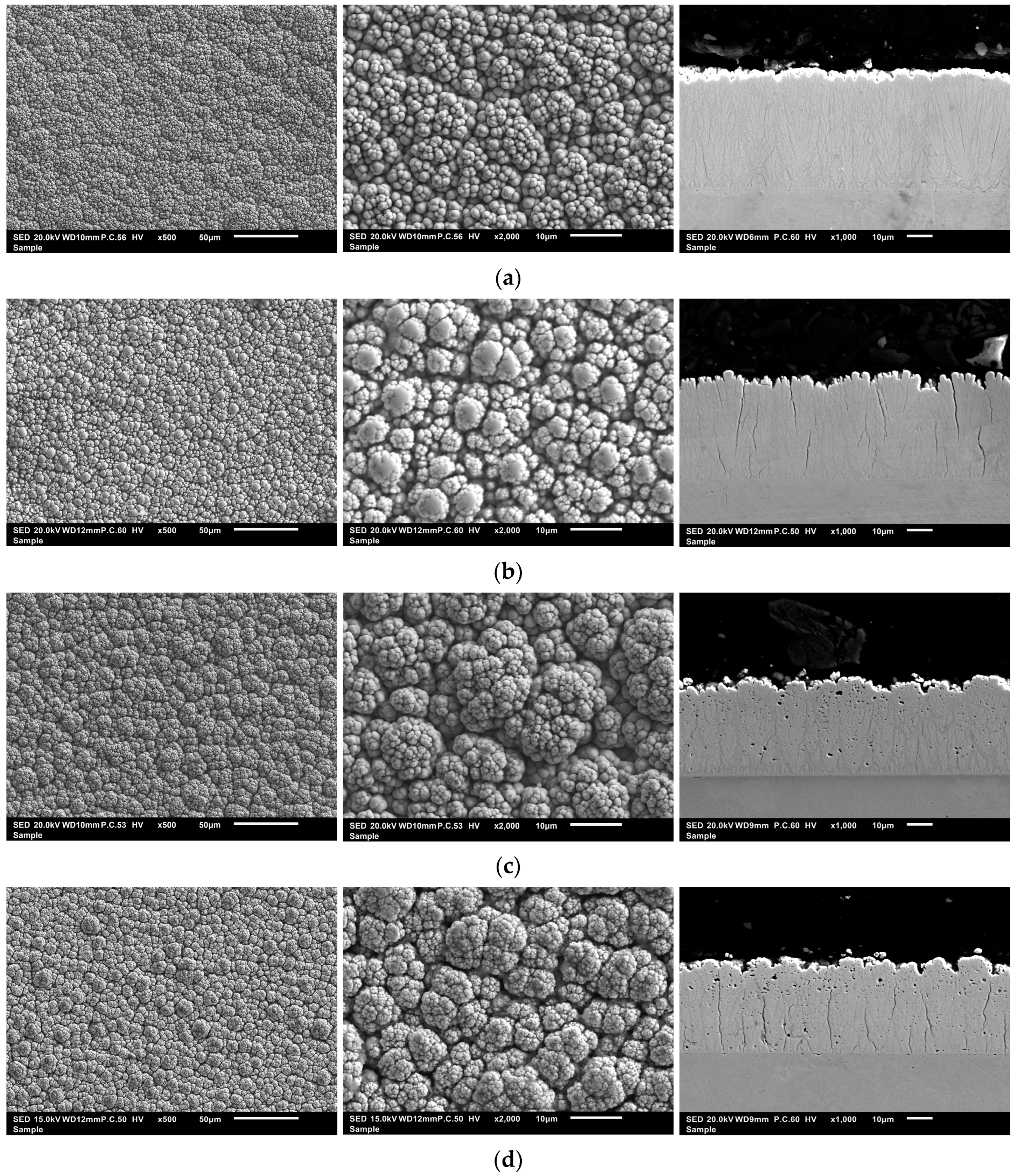
3.2. Morphology and Structure of the Produced Ni-B and Ni-B/B Coatings
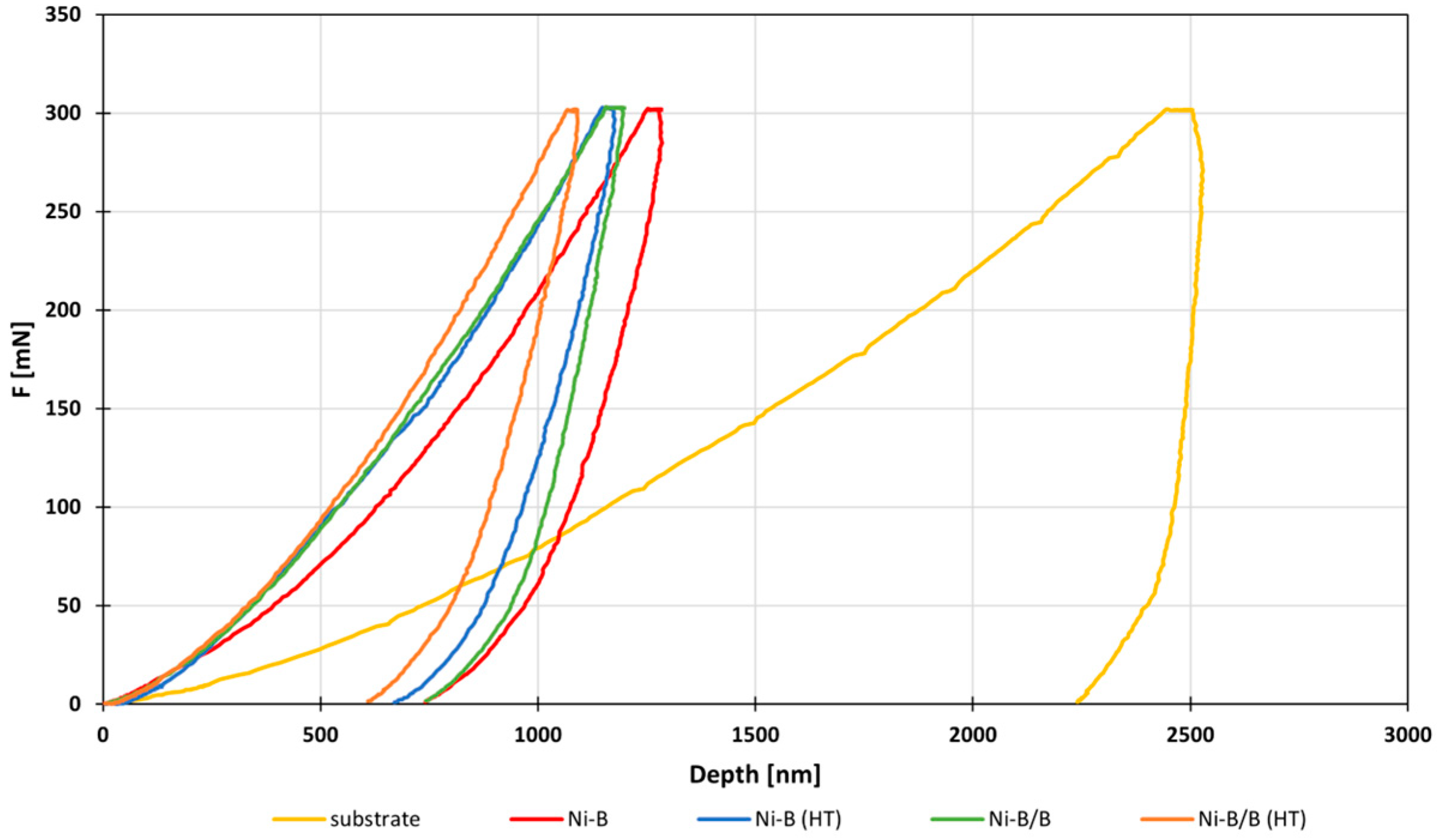
3.3. Mechanical Properties
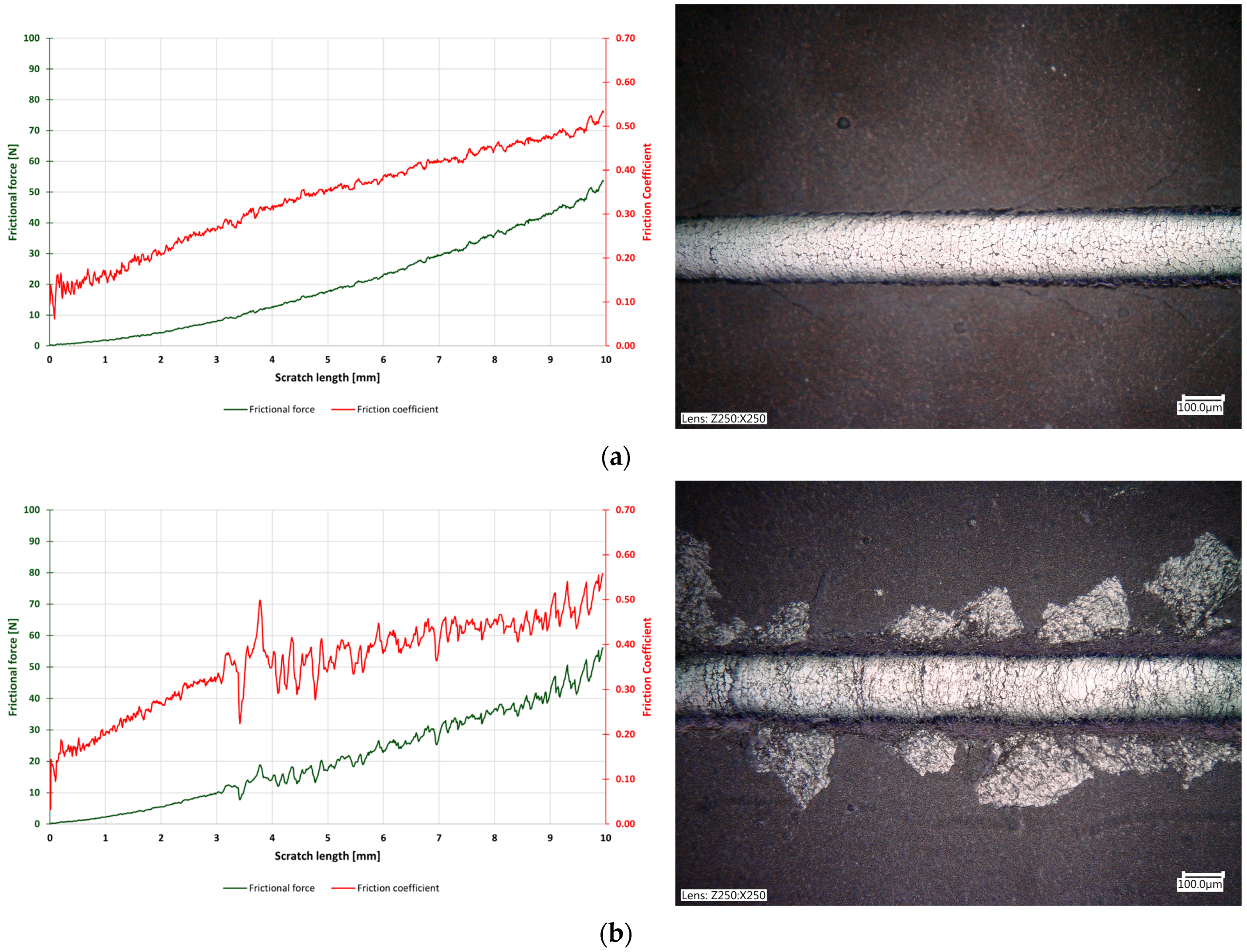
3.4. Scratch Test
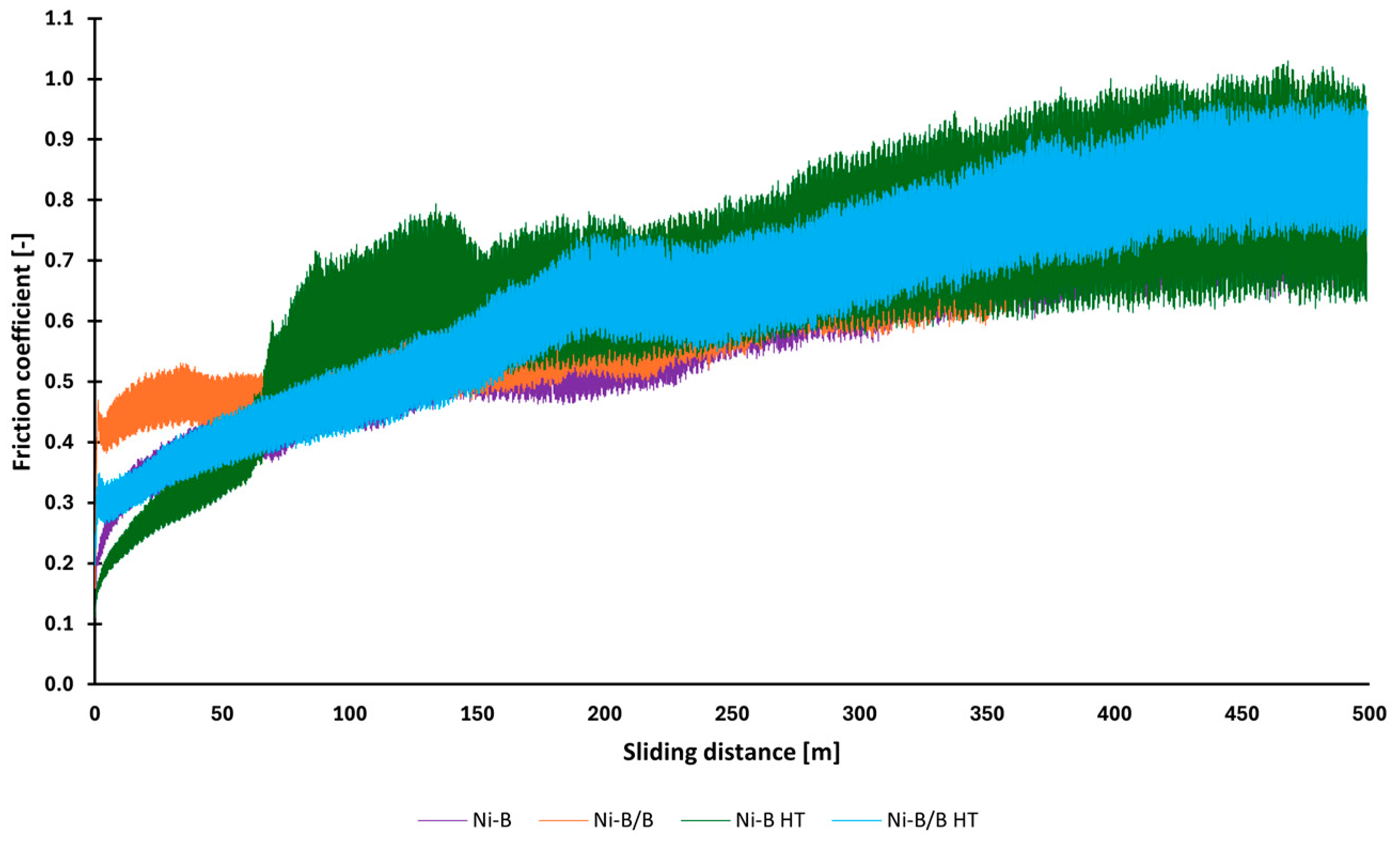
3.5. Tribology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barati, Q.; Hadavi, S.M.M. Electroless Ni-B and composite coatings: A critical review on formation mechanism, properties, applications and future trends. Surf. Interfaces 2020, 21, 100702. [Google Scholar] [CrossRef]
- Bayatli, A.; Sahin, E.F.; Kocabas, M. Effect of boron carbide reinforcement on surface properties of electroless Ni–B and Ni–B–W coatings. Mater. Chem. Phys. 2023, 305, 127899. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, X. Facile fabrication of robust superhydrophobic coating for enhanced corrosion protection on AZ91 magnesium alloy by electroless Ni-B/GO plating. Surf. Coat. Technol. 2023, 455, 129213. [Google Scholar] [CrossRef]
- Gul, H.; Algul, H.; Akyol, A.; Uysal, M.; Alp, A. Evaluation of wear and corrosion behavior of electroless Ni-B-P/CNT composite coatings on aluminum surfaces. Diam. Relat. Mater. 2023, 137, 110075. [Google Scholar] [CrossRef]
- Yan, L.; Yan, S.; He, Y.; He, Y.; Li, H.; Song, R.; Zhou, H.; Cheng, X. Preparation, corrosion resistance and mechanical properties of electroless Ni-B/α-ZrP composite coatings. Colloid. Surf. A 2022, 654, 130132. [Google Scholar] [CrossRef]
- Ekmekci, D.; Bülbül, F. Preparation and characterization of electroless Ni–B/nano-SiO2, Al2O3, TiO2 and CuO composite coatings. Bull. Mater. Sci. 2015, 38, 761–768. [Google Scholar] [CrossRef]
- Salvador-Carulla, L.; Woods, C.; de Miguel, C.; Lukersmith, S. Adaptation of the technology readiness levels for impact assessment in implementation sciences: The TRL-IS checklist. Heliyon 2024, 10, e29930. [Google Scholar] [CrossRef] [PubMed]
- Vitry, V.; Hastir, J.; Megret, A.; Yazdani, S.; Yunacti, M.; Bonin, L. Recent advances in electroless nickel-boron coatings. Surf. Coat. Technol. 2022, 429, 127937. [Google Scholar] [CrossRef]
- Gajewska-Midziałek, A.; Cieślak, G.; Gostomska, M.; Ciciszwili, T.; Skroban, K.; Dąbrowski, A.; Pęśko, E.; Wojda, E.; Głowacki, M.; Kapuścińska, A.; et al. Properties of Ni-B/B Composite Coatings Produced by Chemical Reduction. Coatings 2023, 13, 1535. [Google Scholar] [CrossRef]
- An, X.; Reddy, K.M.; Xie, K.Y.; Hemker, K.J.; Goddard, W.A. New Ground-State Crystal Structure of Elemental Boron. Phys. Rev. Lett. 2017, 117, 159902. [Google Scholar] [CrossRef] [PubMed]
- Ojha, P.K.; Maji, R.; Karmakar, S. Effect of crystallinity on droplet regression and disruptive burning characteristics of nanofuel droplets containing amorphous and crystalline boron nanoparticles. Combust. Flame 2018, 188, 412–427. [Google Scholar] [CrossRef]
- Gültekin, D.; Duru, E.; Akbulut, H. Improved wear behaviors of lead-free electroless Ni-B and Ni-B/CeO2 composite Coatings. Surf. Coat. Technol. 2021, 422, 127525. [Google Scholar] [CrossRef]
- Georgiza, E.; Gouda, V.; Vassiliou, P. Production and properties of composite electroless Ni-B-SiC coatings. Surf. Coat. Technol. 2017, 325, 46–61. [Google Scholar] [CrossRef]
- Baskaran, I.; Sakthi Kumar, R.S.; Sankara Narayanan, T.S.N.; Stepehn, A. Formation of electroless Ni–B coatings using low temperature bath and evaluation of their characteristic properties. Surf. Coat. Technol. 2006, 200, 6888–6894. [Google Scholar] [CrossRef]
- Delaunois, F.; Lienard, P. Heat treatments for electroless nickel–boron plating on aluminium alloys. Surf. Coat. Technol. 2002, 160, 239–248. [Google Scholar] [CrossRef]
- Nemane, V.; Chatterjee, S. Effect of silicon carbide incorporation and heat treatment on tribological properties of electroless Ni–B–W alloy coating. Mater. Chem. Phys. 2024, 311, 128500. [Google Scholar] [CrossRef]
- Chronowska-Przywara, K.; Kot, M. Effect of scratch test parameters on the deformation and fracture of coating-substrate systems. Tribologia 2014, 254, 19–29. [Google Scholar]
- Nemane, V.; Chatterjee, S. Evaluation of microstructural, mechanical, and tribological characteristics of Ni-B-W-SiC electroless composite coatings involving multi-pass scratch test. Mater. Charact. 2021, 180, 111414. [Google Scholar] [CrossRef]
- Vitry, V.; Bonin, L. Increase of boron content in electroless nickel-boron coating by modification of plating conditions. Surf. Coat. Technol. 2017, 311, 164–171. [Google Scholar] [CrossRef]
- Dilek, S.; Algul, H.; Akyol, A.; Alp, A.; Akbulut, H.; Uysal, M. Pulse electro co-deposition of submicron-sized TiC reinforced Ni–W coatings: Tribological and corrosion properties. J. Asian Ceram. Soc. 2021, 9, 673–685. [Google Scholar] [CrossRef]



| Compounds | Quantity [g/dm3] | |
|---|---|---|
| Ni-B | Ni-B/B | |
| NiCl2 * 6H2O | 30 | |
| C2H8N2 | 90 | |
| NaOH | 90 | |
| Pb(NO3)2 | 0.0145 | |
| B | - | 1.0 |
| NaBH4 | 1.6 | |
| Parameter | Ni-B | Ni-B/B |
|---|---|---|
| Temperature [°C] | 90 (±1) | |
| Time [h] | 1.5 | |
| Bath loading [dm2/dm3] | 0.1 | |
| Bath volume [dm3] | 20 | |
| Type of mixing | Static (hydraulic pump) + mechanical (cathodic rail) + compressed air | |
| Coating | Roughness Parameter | |
|---|---|---|
| Ra [µm] | Rz [µm] | |
| Ni-B | 0.55 ± 0.04 | 3.99 ± 0.48 |
| Ni-B HT | 0.60 ± 0.05 | 4.39 ± 0.25 |
| Ni-B/B | 0.85 ± 0.03 | 6.03 ± 0.23 |
| Ni-B/B HT | 0.87 ± 0.05 | 6.57 ± 0.56 |
| Microhardness | Depth [nm] | Elasticity Modulus EIT [GPa] | |||
|---|---|---|---|---|---|
| HIT [MPa] | HM [MPa] | HV | |||
| Ni-B | 8974 (±315) | 5898 (±190) | 847 (±30) | 1274 (±20) | 172 (±7) |
| Ni-B HT | 10,305 (±503) | 6625 (±295) | 973 (±47) | 1203 (±28) | 188 (±9) |
| Ni-B/B | 9358 (±375) | 6623 (±193) | 883 (±35) | 1203 (±17) | 247 (±7) |
| Ni-B/B HT | 13,072 (±1068) | 8081 (±515) | 1234 (±101) | 1087 (±33) | 219 (±10) |
| substrate | 1732 (±48) | 1520 (±41) | 163 (±5) | 2529 (±34) | 234 (±11) |
| Coating | Average Wear Track Width [µm] |
|---|---|
| Ni-B | 338 (±24) |
| Ni-B/B | 303 (±24) |
| Ni-B HT | 307 (±17) |
| Ni-B/B HT | 299 (±24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieślak, G.; Gostomska, M.; Dąbrowski, A.; Ciciszwili-Wyspiańska, T.; Skroban, K.; Mazurek, A.; Wojda, E.; Głowacki, M.; Rygier, T.; Gajewska-Midziałek, A. Properties of Ni-B/B Composite Coatings Produced by the Electroless Method under Semi-Technical Line Conditions. Processes 2024, 12, 1280. https://doi.org/10.3390/pr12061280
Cieślak G, Gostomska M, Dąbrowski A, Ciciszwili-Wyspiańska T, Skroban K, Mazurek A, Wojda E, Głowacki M, Rygier T, Gajewska-Midziałek A. Properties of Ni-B/B Composite Coatings Produced by the Electroless Method under Semi-Technical Line Conditions. Processes. 2024; 12(6):1280. https://doi.org/10.3390/pr12061280
Chicago/Turabian StyleCieślak, Grzegorz, Marta Gostomska, Adrian Dąbrowski, Tinatin Ciciszwili-Wyspiańska, Katarzyna Skroban, Anna Mazurek, Edyta Wojda, Michał Głowacki, Tomasz Rygier, and Anna Gajewska-Midziałek. 2024. "Properties of Ni-B/B Composite Coatings Produced by the Electroless Method under Semi-Technical Line Conditions" Processes 12, no. 6: 1280. https://doi.org/10.3390/pr12061280





