Abstract
This article presents the results of studies on the distribution of rare metals among the products of the alkali sulfate processing of nepheline syenites. In response to the limited reserves of Bayer bauxite in the alumina industrial production region of Kazakhstan, the feasibility of using alternative alumina-containing nonbauxite raw materials was investigated. The most promising nonbauxite raw materials in Kazakhstan are nepheline and kaolinite clays. At present, there is no effective technology for processing nepheline ores. This article describes a proposed complex technology involving nepheline processing with the associated extraction of gallium and vanadium. The technology includes the activation of raw materials, followed by two-stage leaching, where potassium is extracted in the first stage. The sludge and solution obtained from the second stage of the leaching process are utilized for calcium silicate production and two-stage carbonization, respectively. In the first stage, aluminum hydroxide is extracted, and, in the second stage, a concentration of rare metals, such as gallium and vanadium, is obtained. Vanadium is extracted from the solution via crystallization, and gallium is extracted via electrodeposition. Overall, 38.48% of the Ga2O3 and 56.12% of the V2O5 are recovered from raw nepheline syenite. A technological scheme of the developed technology is presented in this article.
1. Introduction
In response to the limited reserves of Bayer bauxite in the alumina industrial production region of Kazakhstan, it is possible to use nonbauxite raw materials, such as the nepheline syenites of the Kubasadyr deposit [1,2].
The products of nepheline processing include alumina, sodium, potassium salts, silica, and rare metals. Sintering the ore with limestone is the conventional processing method for nepheline. The sintering process has significant disadvantages, such as a high energy consumption, high capital costs, and high atmospheric emissions [3,4]. In the sintering technology, the processing of 2 tonnes of nepheline produces about 3 million tonnes of waste sludge, which is not recycled and, when stored in a landfill, is a source of soil, groundwater, and atmospheric pollution [5]. However, a hydrochemical method for processing nepheline syenites is now feasible due to technological advancements [6].
Impurities in the composition of nepheline are very diverse and include Ca, Mg, Fe, Ti, Ga, Li, Rb, Cs, Ba, Sr, and rare earth elements [7].
This paper presents a hydrochemical alkaline sulfate technology that facilitates the wasteless utilization of nepheline to produce aluminum hydroxide, soda, potash, potassium sulfate, construction materials, gallium, and vanadium without polluting the environment. The main product is aluminum hydroxide. The technology is complex because it also recovers other valuable components that are found in nepheline syenites, including rare metals such as rubidium, gallium, and vanadium.
The developed technology includes a two-stage leaching process with the use of an active calcium additive. The advantage and novelty of this technology lie in the use of an alkaline hydrochemical method of processing, which includes pre-activation followed by a change in phase composition [8] and the separation of potassium alkali at the beginning of the process [9]. It allows for increased Al2O3 recovery, as its presence is detrimental to its recovery. The distribution of rare metals in the intermediate products that result from the processing of nepheline has been previously determined, and the methods of their extraction for obtaining V2O5 and metallic gallium have been investigated.
Advancements in modern industry are impossible without the use of rare metals, and the demand for rare metals in the long term will remain stable, with the need increasing every year.
The largest consumers of rare metals are developed countries, such as the USA, Western European countries, Japan, and others [10]. Japan, which does not have its own rare metal raw material resources, has the highest rare metal consumption growth rates, with some increases calculated to be in the tens of percentage points per year [11]. At the same time, the list of countries mining rare metals is short. The country that occupies the first place in terms of the production and export of rare metals is China, which accounts for approximately 50% of the world’s reserves. China currently controls the mining and processing of more than 90% of the total volume of key rare earth elements [12].
The application of rubidium in ion engines, photocells, analytical chemistry, etc., demonstrates the unique properties of this valuable element. The annual world production of Rb is limited to 2–4 tonnes per year [13].
Gallium is widely used in various applications, such as semiconductors [14] and monoclinic gallium oxide (β-Ga2O3) in optoelectronics [15]. Moreover, it is mainly used for the fabrication of integrated circuits, GaAs for high-frequency communication [16] and for fiber optic communication, and LEDs.
Gallium is mainly obtained as a byproduct from the processing of bauxite to produce aluminum and, to a much lesser extent, from the processing of zinc ores. The gallium content in bauxite exceeds 1 million tonnes, and approximately the same amount of Ga is found in zinc deposits [14]. Recycling gallium from industrial waste or recycled electronic components has proven to be a crucial means of expanding the supply of this critical resource, providing a more reliable supply of gallium resources [17].
Most of the gallium in the world is produced in China, and 99.99% pure gallium is produced in Germany, Hungary, Russia, Japan, etc. The largest consumers of 99.99% pure gallium in the world are China, the USA, and Japan [18].
There are several methods of gallium extraction from an aluminate solution: sorption with subsequent cementation using aluminum gallama; extraction of gallium; carbonization followed by cementation with aluminum gallama; mercury cathode electrolysis and cementation with aluminum gallama; flat stationary gallium-covered cathode electrolysis; water-cooled solid cathode electrolysis and cementation with aluminum gallama; and rotating gallium-covered cathode electrolysis [19,20].
Vanadium and its compounds are used in a variety of industrial sectors [18], but the largest volume of vanadium is needed in the metallurgical and chemical industries.
The bulk of the world’s vanadium consumption—approximately 87 percent—comes from the metallurgical industry [21]. Vanadium is mainly used for alloying high-quality structural steels to optimize their performance characteristics. The combination of metamaterials and VO2 is a promising approach for the development of tunable terahertz devices such as switches, filters, modulators, sensors, and optical storage [22] to improve the efficiency of energy storage in zinc-ion batteries [23,24]. The unique properties of vanadium–carbon compounds are complex. These carbides are characterized by refractoriness, a high hardness, and thermal and electrical conductivity [25,26].
Vanadium is characterized by a high chemical activity, which determines the possibility of its application in various industrial sectors, including the chemical industry. Many compounds of this metal, primarily vanadium acid salts (vanadates), vanadium oxides, and carbides, are used in the chemical industry. Specific examples of vanadium include the production of dyes and the production of catalysts [27,28]. Moreover, vanadium is undeniably advantageous in the production of high-quality tools made of various chrome–vanadium steels.
To obtain vanadium, alkaline methods of decomposition are most often used. In such methods, vanadium passes into a solution, as well as aluminum and silicon, which then must be freed using various methods. Single extraction [29], sequential extraction [30], and other chemical extraction methods are also used for vanadium extraction [31,32].
To date, acceptable, continuous technologies and methods for the integrated processing of nepheline with the associated extraction of rare metals have not been developed. Considering its potential benefits, the extension of the process to the treatment of nepheline for the recovery of values such as Al(OH)3, K2SO4 metallic gallium, V2O5, and Rb2SO4 was investigated in this study.
2. Materials and Methods
An X-ray fluorescence analysis of chemical composition was performed using a Venus 200 wave dispersion spectrometer (Panalytical B.V., Almelo, The Netherlands). A chemical analysis was performed using an Optima 2000 DV inductively coupled plasma optical emission spectrometer (Optima, Perkin Elmer, Waltham, MA, USA). A semiquantitative X-ray phase analysis was performed using a D8 Advance diffractometer (BRUKER, Billerica, MA, USA) with copper Cu Kα radiation at an accelerating voltage of 36 kV and a current of 25 mA. An X-ray spectral microanalysis with scanning electron microscopy (SEM) was performed using a JEOL JXA-8230 (Tokyo, Japan) instrument.
Activation was carried out in a solution of 120 g/dm3 NaHCO3 at a liquid-to-solid ratio of 3 and a temperature of 280 °C using an autoclave with a volume of 5 dm3 (Figure 1). The duration of activation was 90 min [9].

Figure 1.
Autoclave volume 5 dm3.
After activation, stage I of the leaching process was carried out in a solution containing 240 g/L Na2O. Then, CaO mixed with CaSO4 was added for the selective extraction of potassium and part of the SiO2 into the solution [33]. The temperature was 280 °C, the duration was 120 min in a thermostat unit with 6 autoclaves rotating through the head, and the working volume was 250 cm3 (Figure 2).
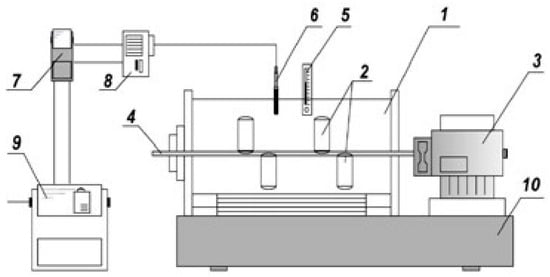
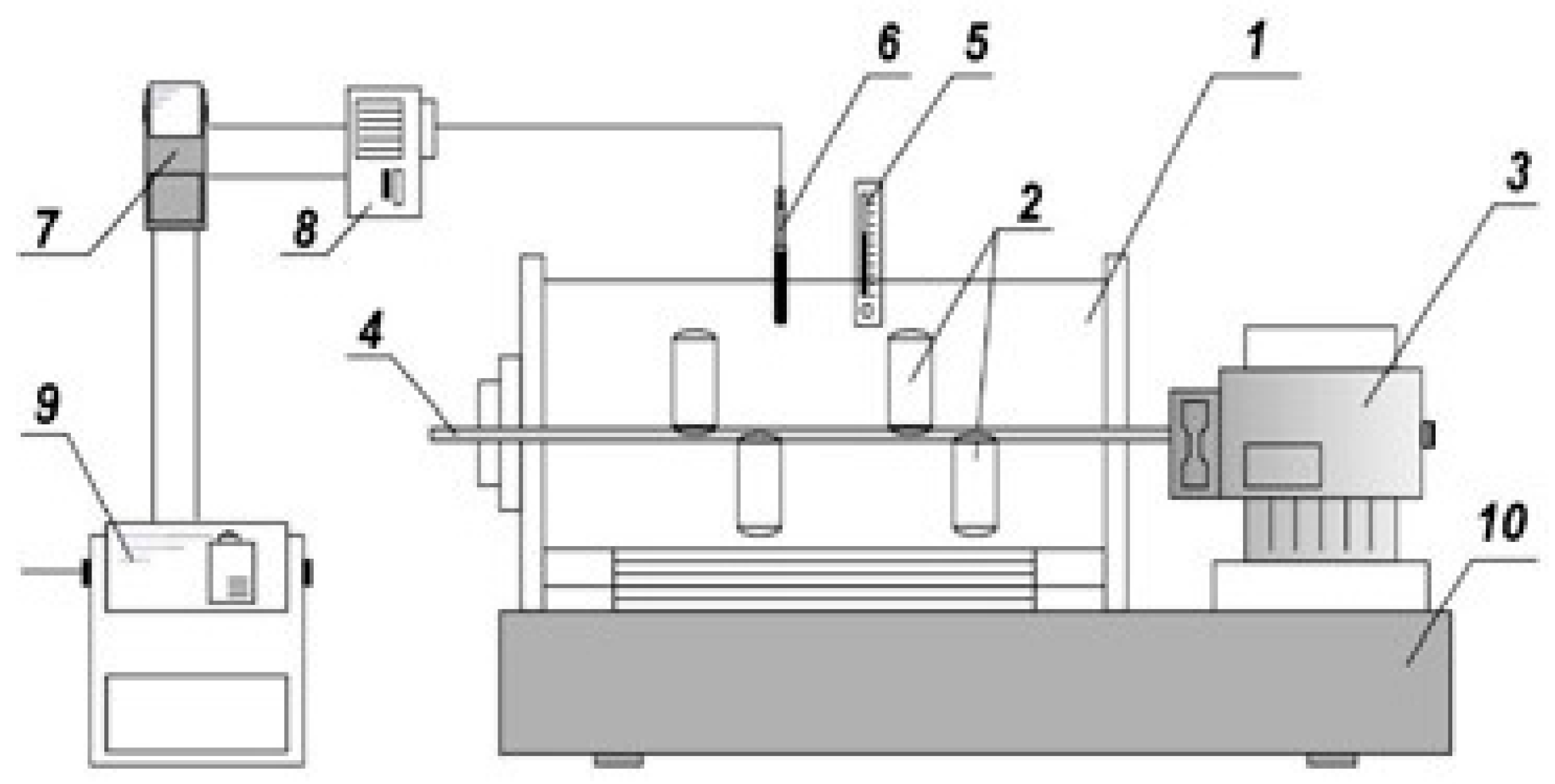
Figure 2.
Thermostat unit plant with autoclaves. 1—thermostat; 2—autoclaves; 3—electric motor; 4—shaft; 5—control thermometer; 6—thermocouple; 7—temperature sensor; 8—starter; 9—gearbox; and 10—frame.
Stage II of the leaching process was carried out in a recycled high-modulus alkaline solution (HMAS) with a caustic modulus, αk, of 30.0, which was determined from the Na2O to Al2O3 ratio, with a Na2O concentration of 240 g/dm3 at 280 °C. Calcium oxide addition was necessary for calcium silicate formation to obtain an aluminous alkaline solution with αk = 10.0.
The synthesis of tricalcium hydroaluminate (TCHA) was carried out at 100 °C for 3–4 h [33], with the addition of CaO from a stoichiometric amount of 100–120%.
TCHA decomposition was carried out in a solution containing NaO2 at 140–160 g/dm3, a temperature of 180 °C, a leaching time of 90 min in an autoclave, and a liquid-to-solid ratio of 4. The carbonization of the solutions was carried out by supplying CO2 gas from a cylinder.
Stage I of the carbonization process of the TCHA decomposition solution was carried out up to a NaO2 concentration of 10–20 g/dm3 for 8–10 h.
Stage II of the carbonization process was conducted at 70 °C for 300 min [34]. After each stage, the pulp was incubated for 1 h without a carbon dioxide supply.
Leaching of the gallium and vanadium concentrates was carried out in a solution containing 280 g/dm3 of Na2O at 90 °C for 60 min with a liquid-to-solid ratio of 2.
Electrodeposition was carried out according to a previously described method [9] under the following conditions: a temperature of 50 °C, a Dk of 50 mA/cm2, and a rotation speed of the gallinated cathode of 2.0 m/s. An electrolyzer with a rotating gallinated surface was used for electrodeposition (Figure 3).

Figure 3.
The electrolyzer used for gallium reduction.
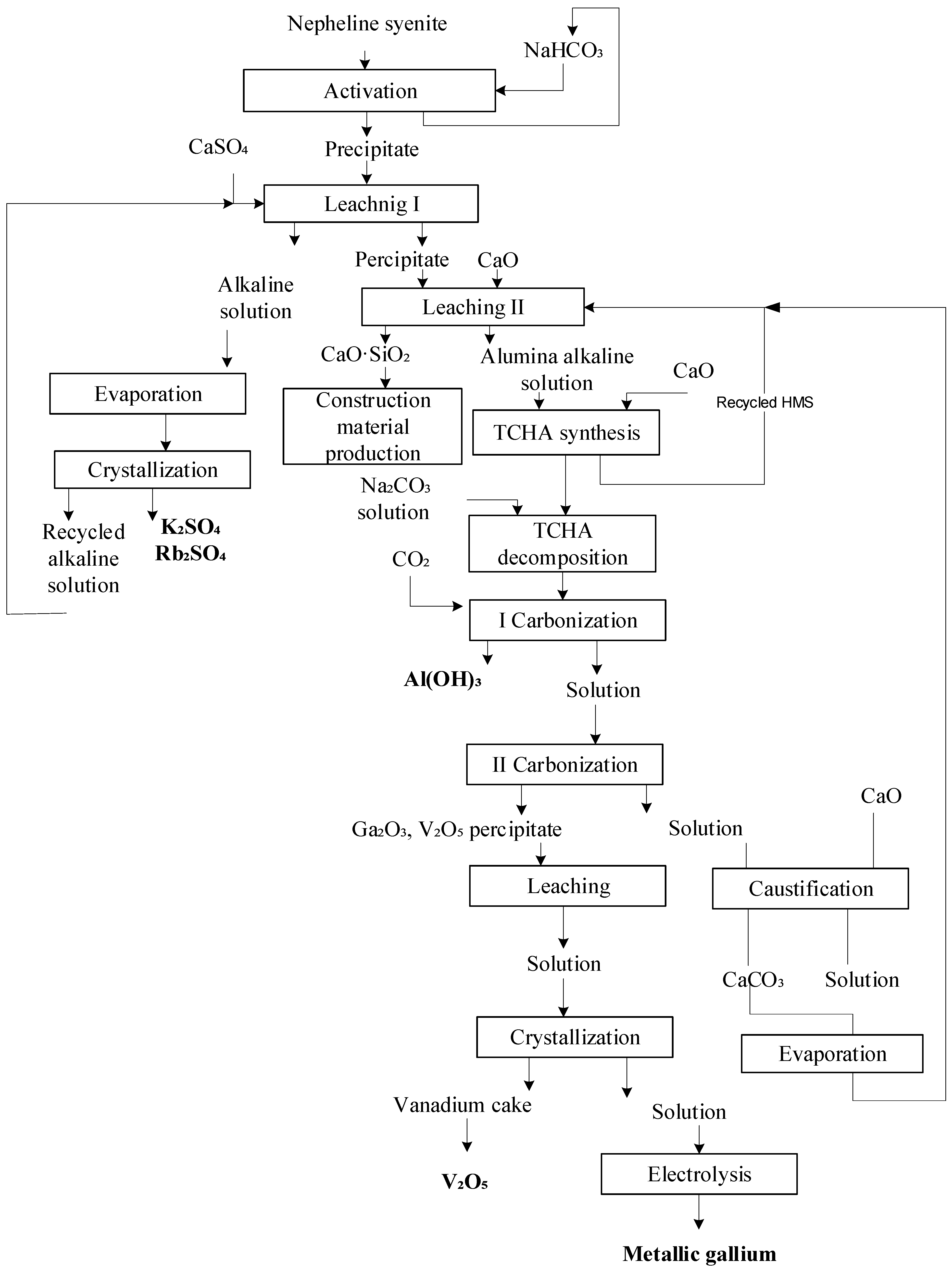
Complex hydrochemical technology was employed to process the nepheline syenites (Figure 4).
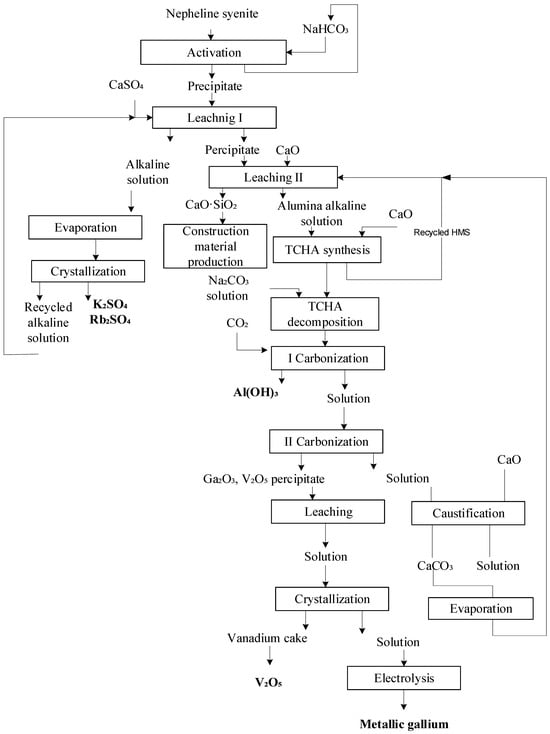
Figure 4.
Technological scheme for processing nepheline syenites, including two-stage leaching.
3. Results and Discussion
Nepheline syenites of the Kubasadyr deposit in the Republic of Kazakhstan served as the initial raw material for this study. The chemical composition in wt % was as follows: Al2O3—17.77; SiO2—48.37; Fe2O3—3.18; CaO—2.89; Na2O—4.24; K2O—7.7; Ga2O3—0.021; and V2O5—0.034.
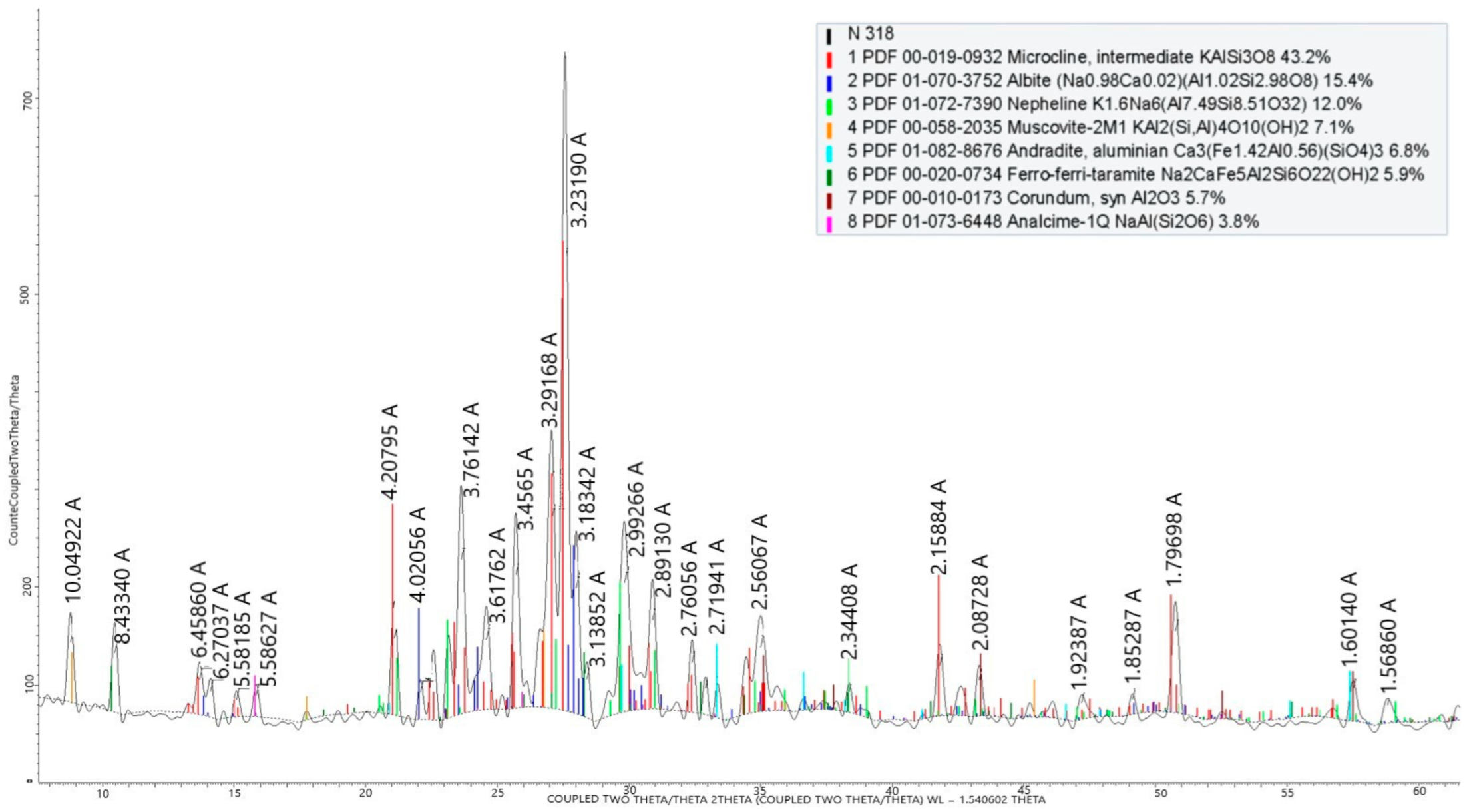
The phase composition of the nepheline syenites is shown in Figure 5.
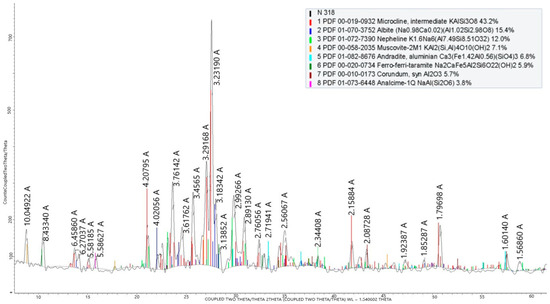
Figure 5.
X-ray phase analysis of nepheline syenites from the Kubasadyr deposit.
The nepheline syenite was subjected to thermochemical activation in a sodium hydrogen carbonate solution. The activation operation facilitates the dispersion of the source material and modifies its mineral composition, which prepares the raw aluminosilicate material for leaching [8]. As a result of activation, we obtained the following ore compositions, shown in mass percentages: Al2O3—17.88; SiO2—47.8; Fe2O3—2.99; CaO—2.76; Na2O—4.16; K2O—7.5; Ga2O3—0.004; and V2O5—0.031.
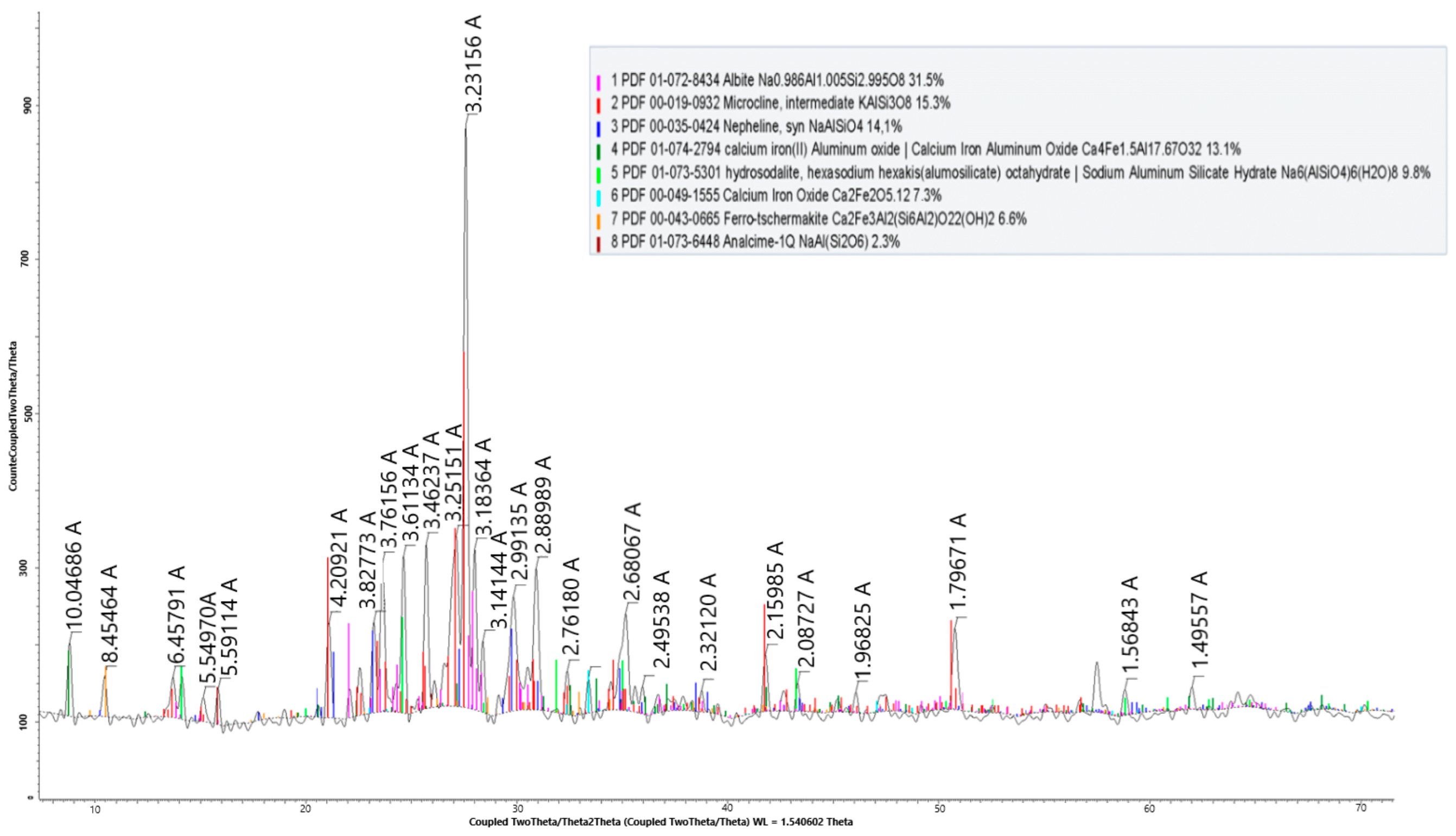
The phase composition of the nepheline after activation is shown in Figure 6.
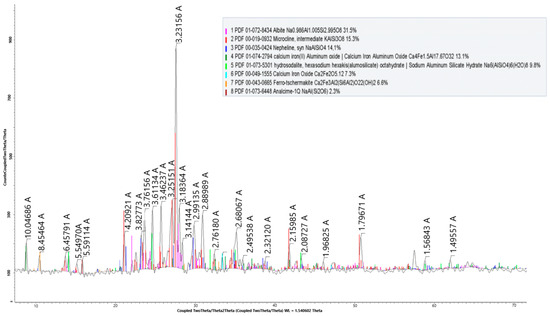
Figure 6.
X-ray radiograph of the nepheline ore after activation.
After stage I of the leaching process, a cake was obtained with the following composition: Al2O3—11.86%; SiO2—21.9%; Fe2O3—1.4%; CaO—17.7%; Na2O—11.73%; K2O—0.89%; Ga2O3—0.167%; and V2O5—0.022%.
The distributions of rare metals obtained by the stage I leaching process are presented in Table 1.

Table 1.
The distribution of rare metals obtained from stage I of the leaching process.
As a result of the stage I leaching process, the recovered components were as follows: K2O—92.06%; Al2O3—6.57%; SiO2—22.84%; Rb2O—89.13%; Ga2O3—7.94%; and V2O5—11.26%.
Approximately 90% of the Rb2O was extracted into the solution as a result of the stage I leaching process. The stage I leaching solution was fed to the leach rack for the extraction of K2SO4. Rb2SO4 was obtained by evaporating the alkaline solution from the stage I leaching process to obtain K2SO4 via crystallization.
Consequently, gallium and vanadium were concentrated in the residue.
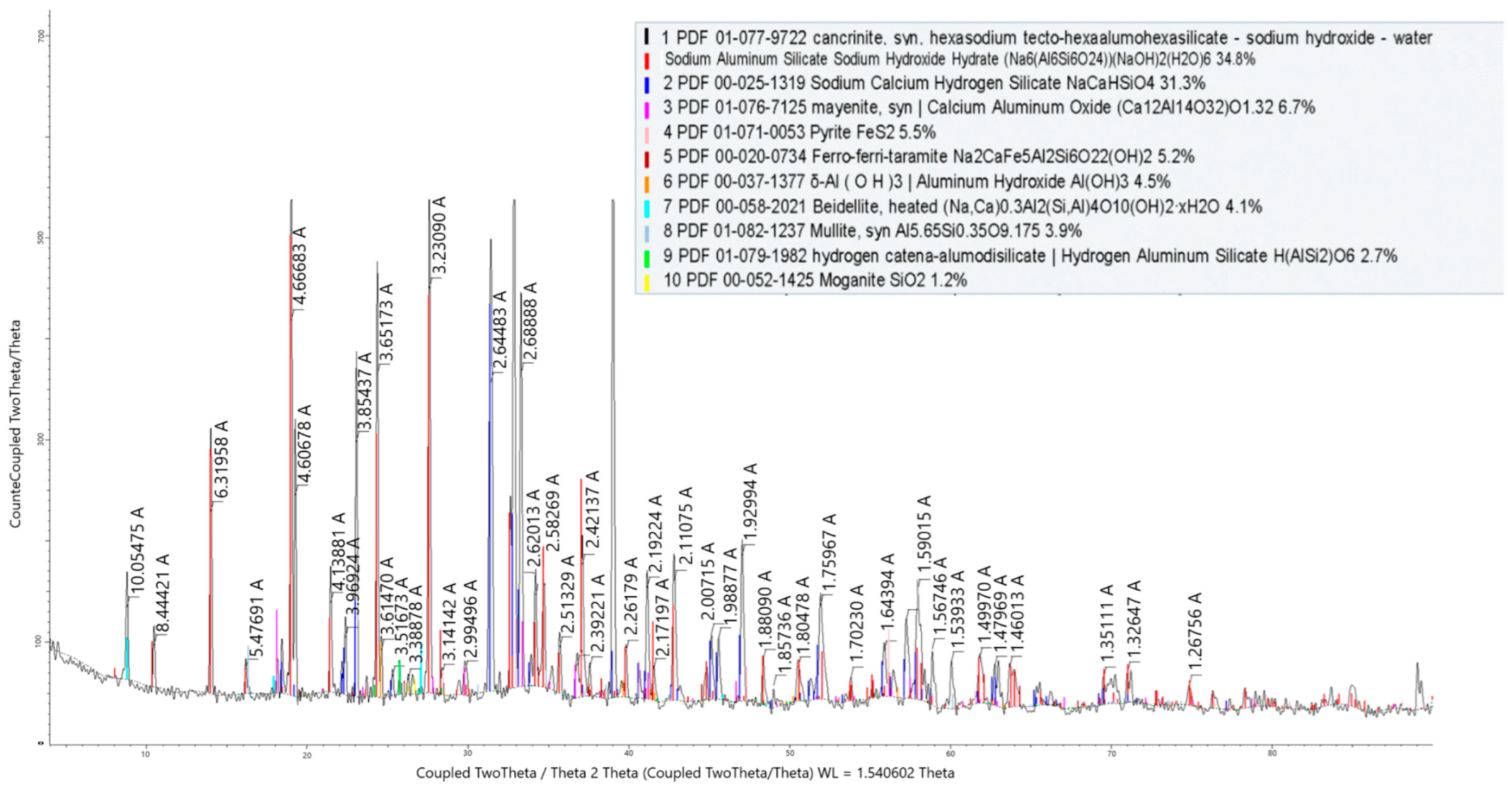
The phase composition of the cake from stage I of the leaching process is shown in Figure 7. According to the X-ray phase analysis, the cake from stage I of the leaching process consisted of cancrinite, sodium calcium hydrogen silicate mayenite, pyrite, ferro-ferri-taramite, aluminum hydroxide, and others.
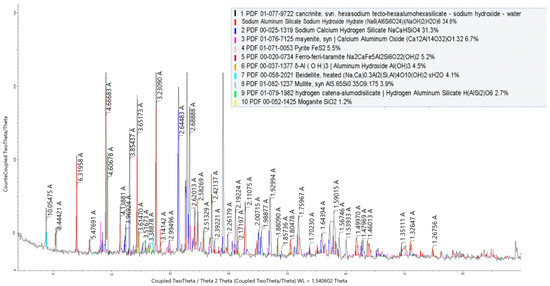
Figure 7.
X-ray radiograph of the cake from stage I of the leaching process.
The distribution of rare metals obtained by the stage II leaching process is presented in Table 2.

Table 2.
The distribution of rare metals obtained from stage II of the leaching process.
As a result of the stage II leaching process, the mass percentages were as follows: Al2O3—2.065%; SiO2—22.87%; Ga2O3—0.002%; V2O5—0.006%; and Rb2O—0.0017%. The Al2O3 yield was 85.72%.
The chemical composition of the stage II leaching solution was as follows (g/dm3): Na2O—260.0; Al2O3—38.87; SiO2—0.1; Ga2O3—0.017; and V2O5—0.0214 (αk = 10.5).
Gallium was extracted into more than 90% of the aluminous alkaline solution, and more than 90% of the gallium was extracted as a result of the stage II leaching process.
The stage II leaching precipitate consists of 8.18% Ga2O3 and is used for construction material production. During the sintering process, 30% of the Ga2O3 is transferred to the sludge and lost [35].
The stage II leaching solution was a medium-modulus solution processed using tricalcium hydroaluminate (TCHA) synthesis.
The aluminous alkaline solution was converted via the synthesis of TCHA to bind the aluminum extracted as a result of the stage I leaching process. Recycled HMS with αk = 30.0 was obtained using the following reaction:
Na2O·AI2O3 +3Ca(OH)2 + 4H2O = 3CaO·AI2O3·6H2O + 2NaOH
The caustic modulus of alkaline solutions (αk) was determined from the ratio αk = Na2O/Al2O3 × 1.645.
The chemical composition of TCHA was as follows (wt.%): Al2O3—23.4; CaO—46.6; Ga2O3—0.0011; and V2O5—0.0059. The recovery of AI2O3 in TCHA was 70.4%. The αk of the obtained HMAS was 30.0. After adjustment for caustic alkali content, the obtained HMAS was recycled as a solution for leaching a new portion of nepheline ore.
As a result of the synthesis of TCHA, 87.56% of the tricalcium hydroaluminate gallium and 81.30% of the vanadium were extracted (Table 3).

Table 3.
The distribution of the rare metals obtained from the synthesis of tricalcium hydroaluminate.
The obtained TCHA was subjected to decomposition in a soda solution for the regeneration of calcium oxide in the form of CaCO3 or Ca(OH)2, as well as for the extraction of Al2O3, Ga2O3, and V2O5 into the aluminous alkaline solution.
TCHA was decomposed with the soda solution to obtain an aluminate solution for carbonization using the following reaction:
3CaO∙Al2O3∙6H2O + Na2CO3 = 3CaCO3 + 2NaAl(OH)4
As a result of TCHA decomposition under the above conditions, the percentage of Al2O3 extracted into the solution was 95.8%.
The chemical composition of the TCHA decomposition solution was as follows (g/dm3): Na2O—140.0; and Al2O3—25.41 (αk = 6.5).
As a result of the decomposition of TCHA, 74.13% of the gallium and 78.103% of the vanadium were extracted into the TCHA decomposition solution (Table 4).

Table 4.
The distribution of the rare metals obtained from the decomposition of TCHA.
To extract aluminum from the TCHA decomposition solution and obtain rare metal concentrates (gallium and vanadium), carbonization was carried out in two stages.
The aluminate solution was carbonized with CO2-containing gas to obtain Al(OH)3, and the Na2CO3 solution was recycled using the following reaction:
2NaAl(OH)4 + CO2 = 2Al(OH)3 + Na2CO3
After the first carbonization step, 90.0% of the AI2O3 precipitated as hydrates. Together, 15.0% Ga2O3 and 9.0% V2O5 were precipitated via coprecipitation.
After the second carbonization stage, a precipitate was obtained with the following composition (wt.%): 27.1% Al2O3, 0.48% Ga2O3, and 15.6% V2O5. This precipitate was a concentrate of gallium and vanadium.
As a result of the second stage of carbonization, 54.894% of the gallium and 63.132% of the vanadium were extracted into the cake (Table 5 and Table 6).

Table 5.
The distribution of the rare metals obtained from the carbonization I process.

Table 6.
The distribution of the rare metals obtained from the carbonization II process.
The method of gallium- and vanadium-containing cake processing with the addition of water in an autoclave at 245 °C for 2 h was obtained from [35]. The Ga2O3 recovery rate was 80%. Processing the cake in an alkaline solution at 90 °C resulted in a recovery rate of 88%.
As a result of the leaching of gallium and vanadium, 48.482% of the gallium-containing cake and 61.66% of the vanadium-containing cake were extracted into tricalcium hydroaluminate (Table 7).

Table 7.
The distribution of the rare metals obtained from the leaching of gallium- and vanadium-containing cakes.
The solution from the second carbonization stage after evaporation was used for the preparation of recycled HMAS.
After the concentrate was leached, the obtained solution was cooled to room temperature, and the vanadium cake was isolated via the crystallization method. The composition of the obtained vanadium cake is as follows (wt.%): 8.7 Al2O3, 0.04 Ga2O3, and 22.1 V2O5. After the extraction of the vanadium cake, a gallium-containing solution was obtained with the following composition (g/dm3): Na2O 169.2; AI2O3 113.5; Ga2O3 2.1; and V2O5 0.5 (αk = 2.3). The solution served as an initial intermediate for the extraction of metallic gallium.As a result of the crystallization process, 9.737% of the gallium and 56.124% of the vanadium were extracted from the vanadium cake (Table 8).

Table 8.
The distribution of rare metals obtained from the crystallization process.
The cake was used to obtain vanadium pentoxide.
A total of 38.487% of the Ga2O3 was extracted from nepheline syenite. In the concentrated leaching solution, the gallium content was 2.1 g/dm3. This content meets the requirements for the efficient electrodeposition of metallic gallium.
In summary, the extraction of 97.8% gallium was obtained in this study, with a yield of 9.22%. The power consumption was 87.7 kWh/kg of gallium.
Thus, the advantages of the proposed hydrochemical alkaline sulfate technology in comparison with the conventional sintering method are as follows:
- An increase in gallium recovery by 30%;
- Exclusion of the energy-intensive sintering process;
- A reduction in waste sludge by 22%.
4. Conclusions
In this study, a complex hydrochemical alkaline sulfate technology involving nepheline processing with the associated extraction of gallium and vanadium was developed. This technology allows for the wasteless utilization of nepheline to produce aluminum hydroxide, soda, potash, potassium sulfate, construction materials, gallium, and vanadium without polluting the environment. The proposed technology includes the activation of raw materials, followed by a two-stage leaching process using an active calcium reagent, where potassium and rubidium are extracted in the first stage. Approximately 90% of Rb2O is extracted into the solution as a result of the first stage of the leaching process. The sludge and solution obtained from the second stage of the leaching process are utilized for calcium silicate production and two-stage carbonization, respectively. Aluminum hydroxide is extracted during the first stage, and a concentration of rare metals such as gallium and vanadium is obtained at the second stage. Vanadium is extracted from the solution via crystallization, and gallium is extracted via electrodeposition. In total, 38.487% of the Ga2O3 and 56.124% of the V2O5 are extracted from nepheline syenite.
Author Contributions
N.A.: project administration, writing—original draft preparation, and writing—review and editing; R.A.: conceptualization, methodology, and writing—review and editing; S.G.: methodology, writing—original draft preparation; A.M.: formal analysis; L.I.: formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP14869579).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kovzalenko, V.A.; Sarsenbai, G.; Sadykov, N.M.-K.; Imangalieva, L.M. Kaolins—Substandard aluminosilicate raw materials. Complex Util. Miner. Raw Mater. 2015, 3, 2–37. [Google Scholar]
- Sadyralieva, U.J.; Tastanov, E.A.; Akhmadiyeva, N.K.; Ruzakhunova, G.S.; Sultangazieva, A.N. Chemical enrichment of nepheline syenites of Sandyk locality of the Republic of Kyrgyzstan. Complex Util. Miner. Raw Mater. 2015, 1, 3–8. [Google Scholar]
- Brough, D.; Jouhara, H. The aluminium industry: A review on state-of-the-art technologies, environmental impacts and possibilities for waste heat recovery. Int. J. Thermofluids 2020, 1–2, 100007. [Google Scholar] [CrossRef]
- Ibn-Mohammeda, T.; Randalld, C.A.; Mustaphae, K.B.; Guo, J.; Walkerg, J.; Berbanod, S.; Kohb, S.C.L.; Wangh, D.; Sinclairh, D.C.; Reaneyh, I.M. Decarbonising ceramic manufacturing: A techno-economic analysis of energy efficient sintering technologies in the functional materials sector. J. Eur. Ceram. Soc. 2019, 39, 5213–5235. [Google Scholar] [CrossRef]
- Seitenov, R.A.; Lipin, V.A.; Akhmedov, S.N.; Medvedev, V.V. Comparative Economic Efficiency of Processing High-Potassium Aluminosilicate Raw Materials into Alumina and Related Products. In Light Metals 2024; The Minerals, Metals & Materials Society; TMS Annual Meeting and Exhibition; Springer: Cham, Switzerland, 2024; pp. 82–89. [Google Scholar]
- Pereira, B.; Rosset, M.; Botelho Junior, A.B. Development of a Hydrometallurgical Process to Obtain High-Purity Alumina Using Bauxite. In Light Metals 2024; The Minerals, Metals & Materials Society; TMS Annual Meeting and Exhibition; Springer: Cham, Switzerland, 2024; pp. 90–98. [Google Scholar]
- Mikhailova, J.A.; Askenov, S.M.; Pakomovsky, Y.A.; Moine, B.N.; Dusseaux, C.; Vaitiva, Y.A.; Voronin, M. Iron in Nepheline: Crystal Chemical Features and Petrological Applications. Minerals 2022, 12, 1257. [Google Scholar] [CrossRef]
- Akhmadiyeva, N.K.; Abdulvaliyev, R.A.; Gladyshev, S.V. Preactivation of nepheline before the enrichment. Complex Use Miner. Resour. 2023, 327, 82–89. [Google Scholar]
- Akhmadiyeva, N.; Gladyshev, S.; Abdulvaliyev, R.; Sukurov, B.; Amanzholova, L. Selective extraction of potassium from raw nepheline materials. Heliyon 2024, 10, e29461. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-L.; Fan, H.-R.; Liu, X.; Meng, J.; Butcher, A.R.; Yann, L.; Yang, K.-F.; Li, X.-C. Global rare earth elements projects: New developments and supply chains. Ore Geol. Rev. 2023, 157, 105428. [Google Scholar] [CrossRef]
- Agency for Natural Resources and Energy. Japan’s New International Resource Strategy to Secure Rare Metals. 2020. Available online: https://www.enecho.meti.go.jp/en/category/special/article/detail_158.html (accessed on 5 February 2024).
- Hurst, C. China’s Rare Earth Elements Industry: What Can the West Learn? Institute of the Analysis of Global Security. 2010.
- Mohammadi, M.R.T.; Koleini, S.M.J.; Javanshir, S.; Abolghasemi, H.; Abdollahy, M. Extraction of rubidium from gold waste: Process optimization. Hydrometallurgy 2015, 151, 25–32. [Google Scholar] [CrossRef]
- Naumov, A.V. About modern condition of the world market of gallium. Izvestiya vuzov. Nonferrous Metall. 2014, 2, 59–64. [Google Scholar]
- Masanobu, Y. Advanced Optical Spectroscopy Techniques for Semiconductors: Raman, Infrared, and Cathodoluminescence Spectroscopy; Springer International Publishing: Tokyo, Japan, 2023. [Google Scholar]
- Chan, W.; Jiao, T.; Chen, P.; Dang, X.; Yan, H.; Yu, H.; Dong, X.; Zhang, Y.; Zhang, B. Preparation of high light-trapping β-Ga2O3 nanorod films via thermal oxidation of GaAs and metal-organic chemical vapor deposition. Mater. Sci. Semicond. Process. 2024, 169, 107912. [Google Scholar] [CrossRef]
- Rudolph, P. Contribution to the development of crystal growth technologies. J. Cryst. Growth 2024, 625, 127456. [Google Scholar] [CrossRef]
- Wang, G.; Chen, C.; Li, J.; Yang, F.; Wang, L.; Lin, X.; Wu, H.; Zhang, J. A clean method for gallium recovery and the coproduction of silica-potassium compound fertilizer and zeolite F from brown corundum fly ash. J. Hazardouz Mater. 2024, 461, 132625. [Google Scholar] [CrossRef] [PubMed]
- Akhmadiyeva, N.; Abdulvaliyev, R.; Gladyshev, S.; Tastanov, Y. Electrochemical extraction of gallium from aluminate solution of Bayer Hydrogarnet process. An. Da Acad. Bras. De Ciencas 2017, 89 (Suppl. 3), 1971–1983. [Google Scholar]
- Market Research: Analysis of the Global Gallium Market (99.99%). Available online: https://www.megaresearch.ru/issledovaniya/metally-i-metalloizdeliya/drugoe/26314 (accessed on 10 February 2024).
- Muthukrishnan, R.M.; Salman, S.S.; Ansari, P.M.Y.; Babu, M.V.G.; Mubina, M.S.K.; Kader, S.M.A. Engineering and analyzing the super exchange interaction of Fe3+-O2−-V5+ on novel multiferroic LaFeO3/BiVO4 nanocomposite. Mater. Lett. 2024, 335, 135433. [Google Scholar] [CrossRef]
- Jing, H.; Kang, J.; Song, C.; Duan, J.; Qu, Z.; Wang, J.; Zhang, B. Bifunctional switchable terahertz metamaterial in the same operating band based on VO2. Opt. Commun. 2024, 552, 130047. [Google Scholar] [CrossRef]
- Tzu-Ho, W.; Jhang-An, C.; Jia-He, S. Interface engineering of heterostructured vanadium oxides for enhanced energy storage in Zinc−Ion batteries. J. Colloid Interface Sci. 2024, 654, 308–316. [Google Scholar]
- Mathew, V.; Sambandam, B.; Kim, S.; Kim, S.; Park, S.; Lee, S.; Alfaruqi, M.H.; Soundharrajan, V.; Islam, S.; Putro, D.Y.; et al. Manganese and Vanadium Oxide Cathodes for Aqueous Rechargeable Zinc-Ion Batteries: A Focused View on Performance, Mechanism, and Developments. ACS Energy Lett. 2020, 5, 2376–2400. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Liu, J.-H.; Gao, Y.; Cao, X.; Zhan, C.; Wang, Y.; Wang, S.; Chou, S.-L.; Dou, S.-X.; et al. Vanadium-based cathodes for aqueous zinc-ion batteries: Mechanism, design strategies and challenges. Energy Storage Mater. 2022, 50, 21–46. [Google Scholar] [CrossRef]
- Mul, D.O.; Bushueva, E.G.; Lazurenko, D.V.; Lozhkina, E.A.; Domarov, E.V. Structure and tribological properties of “carbon steel—VC containing coating” compositions formed by nonvacuum electron-beam surfacing of vanadium-containing powder mixtures. Surf. Coat. Technol. 2023, 474, 130107. [Google Scholar] [CrossRef]
- Cappuyns, V.; Slabbinck, E. Occurrence of Vanadium in Belgian and Europian Alluvial Soils. Appl. Environ. Soil Sci. 2012, 2012, 979501. [Google Scholar] [CrossRef]
- Yang, J.; Tang, Y.; Yang, K.; Rouff, A.A.; Elzinga, E.J.; Huang, J.H. Leaching characteristics of vanadium in mine tailings and soils near a vanadium titanomagnetite mining site. J. Hazard. Mater. 2014, 264, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Gao, X.; Zuo, R.; Song, L.; Jin, C.; Wang, J.; Teng, Y. Vanadium: A Review of Different Extraction Methods to Evaluate Bioavailability and Speciation. Minerals 2022, 12, 642. [Google Scholar] [CrossRef]
- Panther, G.J.; Stewart, R.R.; Teasdale, P.R.; Bennett, W.W.; Welsh, D.T.; Zhao, H. Titanium dioxide-based DGT for measuring dissolved As(V), V(V), Sb(V), Mo(VI) and W(VI) in water. Talanta 2013, 105, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.M.; Alomayri, T.; Zhai, H.; Kareem, A.A. Construction of novel an in situ m-BiVO4/t-BiVO4 isotype heterojunction photocatalyst for improved visible light-dependent degradation of dye. Inorg. Chem. Commun. 2023, 157, 111335. [Google Scholar] [CrossRef]
- Cai, L.; Wang, Z.; Dai, Y.; Fang, C.; Li, Y.; Yang, S.; Wang, J.; Liu, B.; Ding, X.; Zhang, Y.; et al. Facile preparation of polyurethane sponge decorated with polydopamine/BiVO4 for dye photocatalytic degradation under visible light and oil–water separation. Chem. Eng. Sci. 2023, 282, 119213. [Google Scholar]
- Abdulvaliyev, R.; Akhmadiyeva, N.; Gladyshev, S.; Samenova, N.; Kolesnikova, O.; Mankeshova, O. Behavior of calcium compounds under hydrothermal conditions during alkaline leaching of aluminosilicates with the synthesis of fillers for composites. J. Compos. Sci. 2023, 7, 508. [Google Scholar] [CrossRef]
- Abdulvaliyev, R.A.; Akcil, A.; Gladyshev, S.V.; Tastanov, E.A.; Beisembekova, K.O.; Akhmadiyeva, N.K.; Deveci, H. Gallium and vanadium extraction from red mud of Turkish alumina refinery plant: Hydrogarnet process. Hydrometallurgy 2015, 157, 72–77. [Google Scholar] [CrossRef]
- Yatsenko, S.P.; Pasechnik, L.A.; Skachkov, V.M.; Rubinshtein, G.M. Gallium: Technologies of Production and Application of Liquid Alloys; Monography: Moscow, Russia, 2020; 344p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).