Influence Factors of Pressure Swing Adsorption for Oxygen Production by the Orthogonal Method and the Response Surface Method
Abstract
:1. Introduction
2. Principle
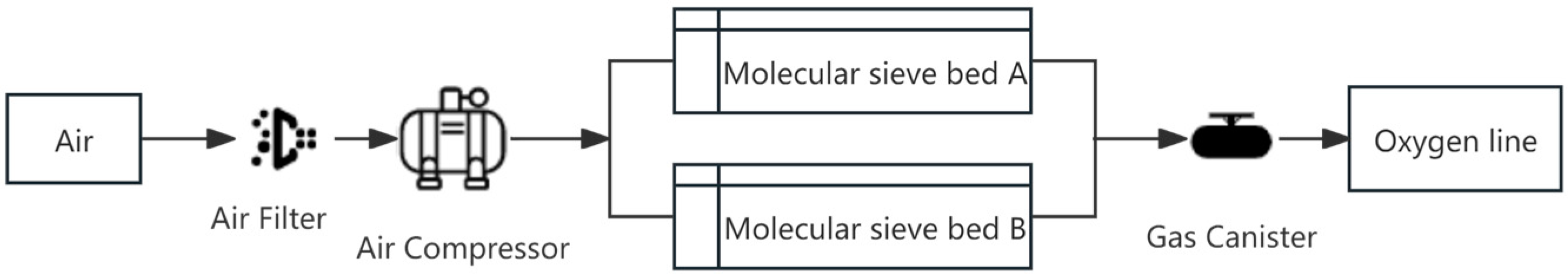
2.1. PSA Oxygen Process Analysis
- (1)
- Pressurised Adsorption: The feed gas is introduced into molecular sieve bed A for pressurisation. During this phase, the molecular sieve adsorbs nitrogen, and the oxygen-enriched product gas is discharged from the top of the adsorption tower;
- (2)
- Equalisation of the Pressure Drop: Upon completion of the adsorption step, the high-pressure gas in molecular sieve bed A is transferred to adsorption tower group B for pressure equalisation, effectively utilising the remaining gas and pressure energy in molecular sieve bed A;
- (3)
- Desorption: Following the equal pressure drop phase, the pressure in the tower decreases, initiating desorption. The nitrogen adsorbed by the molecular sieve is expelled from the bottom of the tower;
- (4)
- Blowback Rinsing: A portion of the product gas from molecular sieve bed B enters molecular sieve bed A through its outlet end to perform blowback rinsing. This step helps release the nitrogen adsorbed in molecular sieve bed A, enhancing the nitrogen adsorption capacity of the molecular sieve for the next cycle;
- (5)
- Pressure Equalisation: The high-pressure gas in molecular sieve bed B is introduced into molecular sieve bed A to equalise the pressure, rapidly increasing the pressure in adsorption tower group A.
2.2. PSA Oxygen Performance Characterisation
- (1)
- Oxygen Concentration: In the PSA oxygen cycle, components of the air are separated according to their adsorption capacity and diffusion rate. Components with weaker adsorption capacity or slower diffusion rates achieve higher concentrations. Conversely, components with higher adsorption capacity or faster diffusion rates are adsorbed by the molecular sieve and expelled as by-product gases during the desorption process. The oxygen concentration refers to the average volume fraction of oxygen. Since the concentration and flow rate of oxygen produced from an experimental setup vary over time, the oxygen concentration is a primary indicator in actual industrial production. Achieving the target concentration is crucial for assessing the unit’s production performance.
- (2)
- Recovery Rate: The recovery rate is the ratio of the oxygen content in the product gas to that in the feed gas. In the PSA oxygen production process, the recovery rate can be enhanced by incorporating additional steps (e.g., the pressure equalisation step) or by increasing the number of beds. Typically, the oxygen recovery rate reflects the energy consumption—an increase in recovery correlates with a decrease in energy usage. Therefore, the recovery rate significantly influences the economic value of the separation process and is a key factor in assessing whether the process is economically efficient.
- (3)
- Production Capacity of the Molecular Sieve: This parameter refers to the flow rate of the product gas or the amount of pure oxygen produced per unit weight of the molecular sieve per unit time. For a given separation process, the product concentration is usually predetermined. Energy consumption is generally proportional to the recovery rate. However, the production capacity per unit of the molecular sieve directly reflects the plant’s production performance. A higher capacity implies that less molecular sieve is required to meet a given product gas flow requirement, thereby reducing the bed size in the production unit. Consequently, the capacity of the molecular sieve is a determining factor for the equipment cost in the separation process.
3. Experiment
3.1. Qualitative Analysis of the Oxygen Production Process on the Effectiveness of Oxygen Production
3.2. Experimental Platform and Experimental Content
- (1)
- Turn on the air compressor and operate it until the output oxygen concentration stabilises. Adjust the oxygen outlet flowmeter and activate the oxygen meter.
- (2)
- With the oxygen outlet flowmeter set to 1 L/min, vary the adsorption cycle duration to 5 s, 6 s, 7 s, 8 s, 9 s, 11 s, and 13 s and record the oxygen concentration.
- (3)
- Maintain the oxygen outlet flowmeter at 1 L/min and adjust the pressure equalisation time to 0 s (no pressure equalisation), 0.3 s, 0.5 s, 0.7 s, 0.9 s, 1.1 s, 1.3 s, and 1.5 s. Record the corresponding oxygen concentration.
- (4)
- Sequentially adjust the outlet flow rate to 2 L/min, 3 L/min, 4 L/min, and 5 L/min. Repeat the experimental procedures outlined in steps (2) and (3) for each flow rate setting, and record the resulting oxygen concentration.
4. Results and Discussion
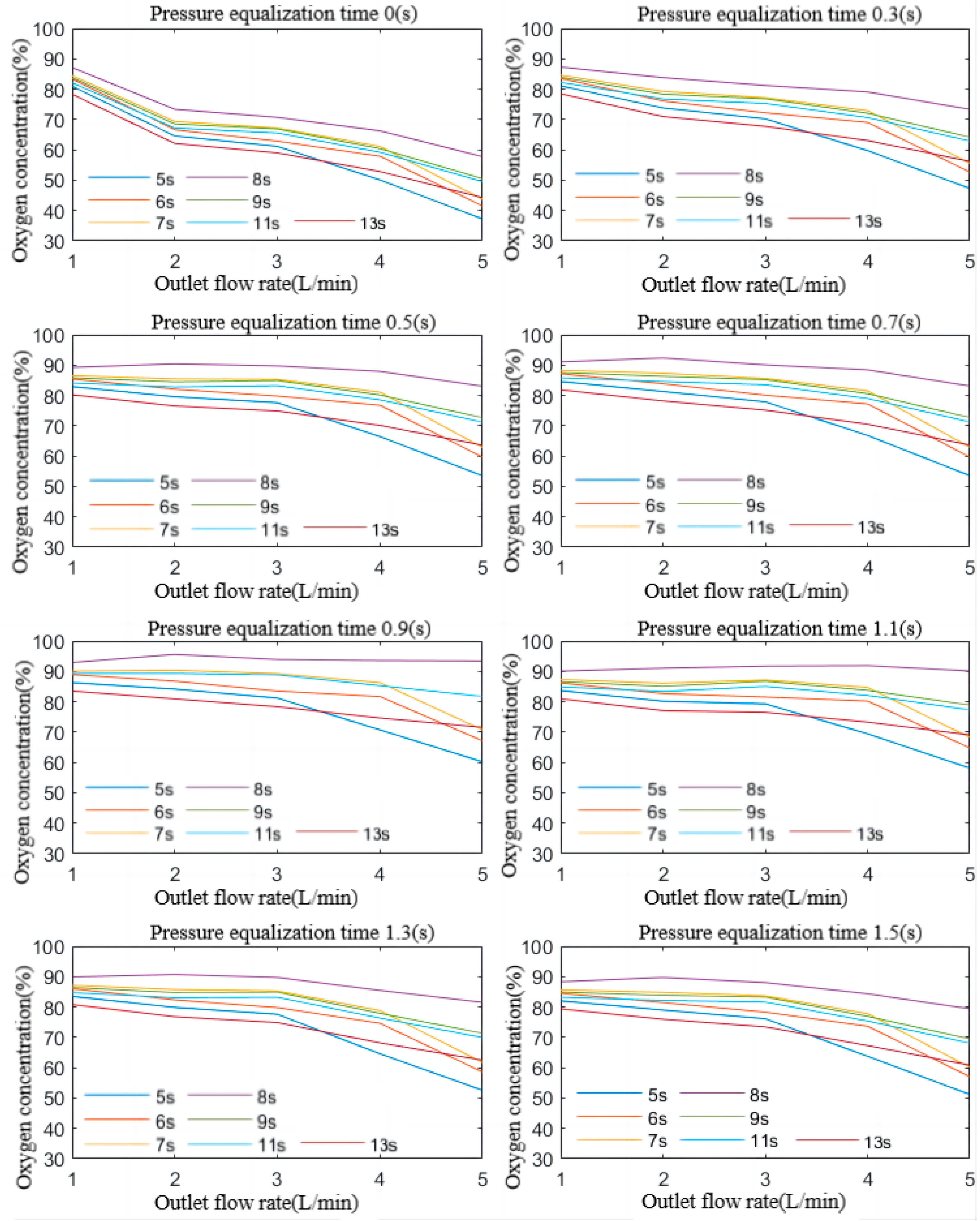
4.1. Influence of the Adsorption Cycle on the Effectiveness of PSA Oxygen Production
4.2. Influence of Pressure Equalisation Time on the Effectiveness of PSA Oxygen Production
4.3. Influence of the Outlet Flow Rate on the Effectiveness of PSA Oxygen Production
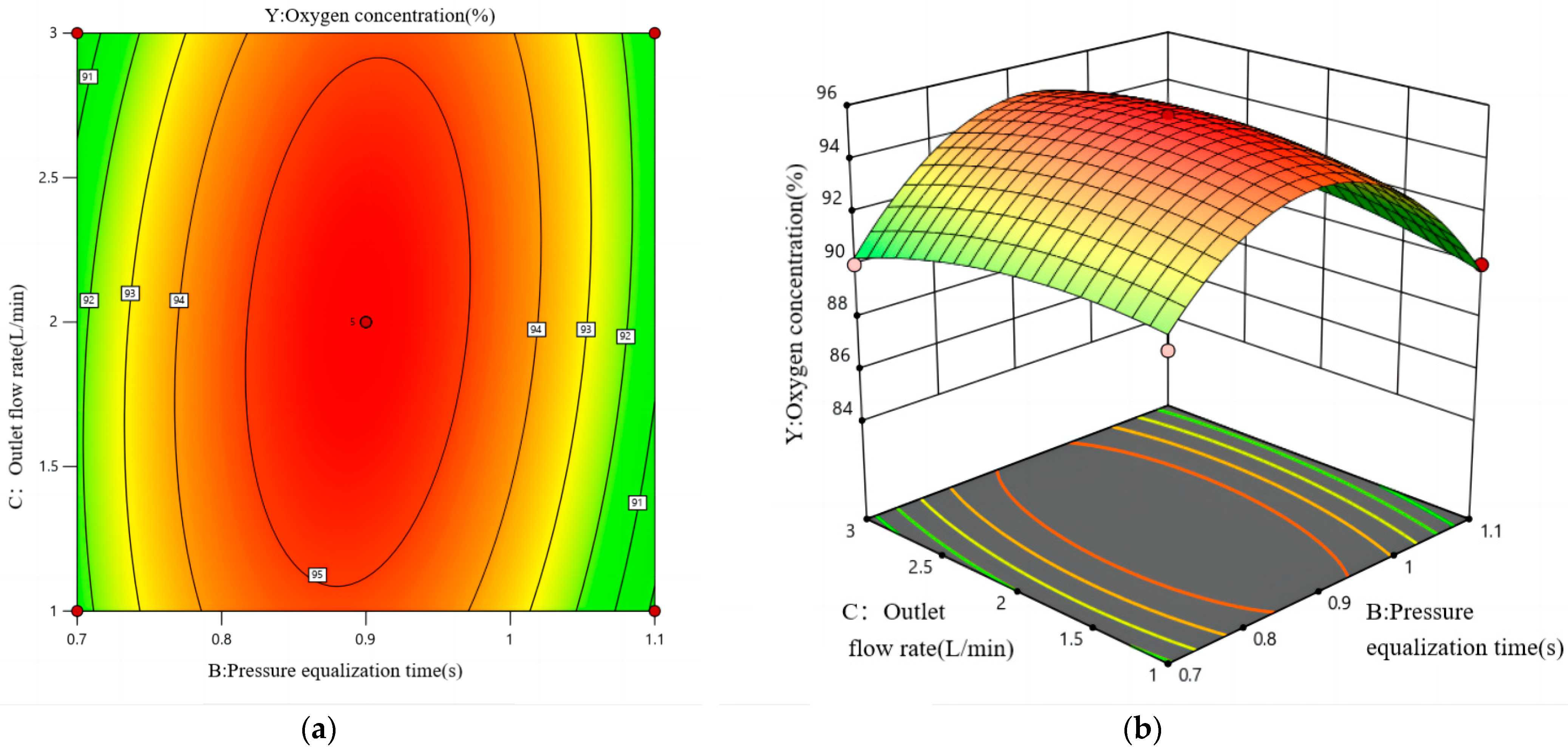
4.4. Response Surface Method Analysis
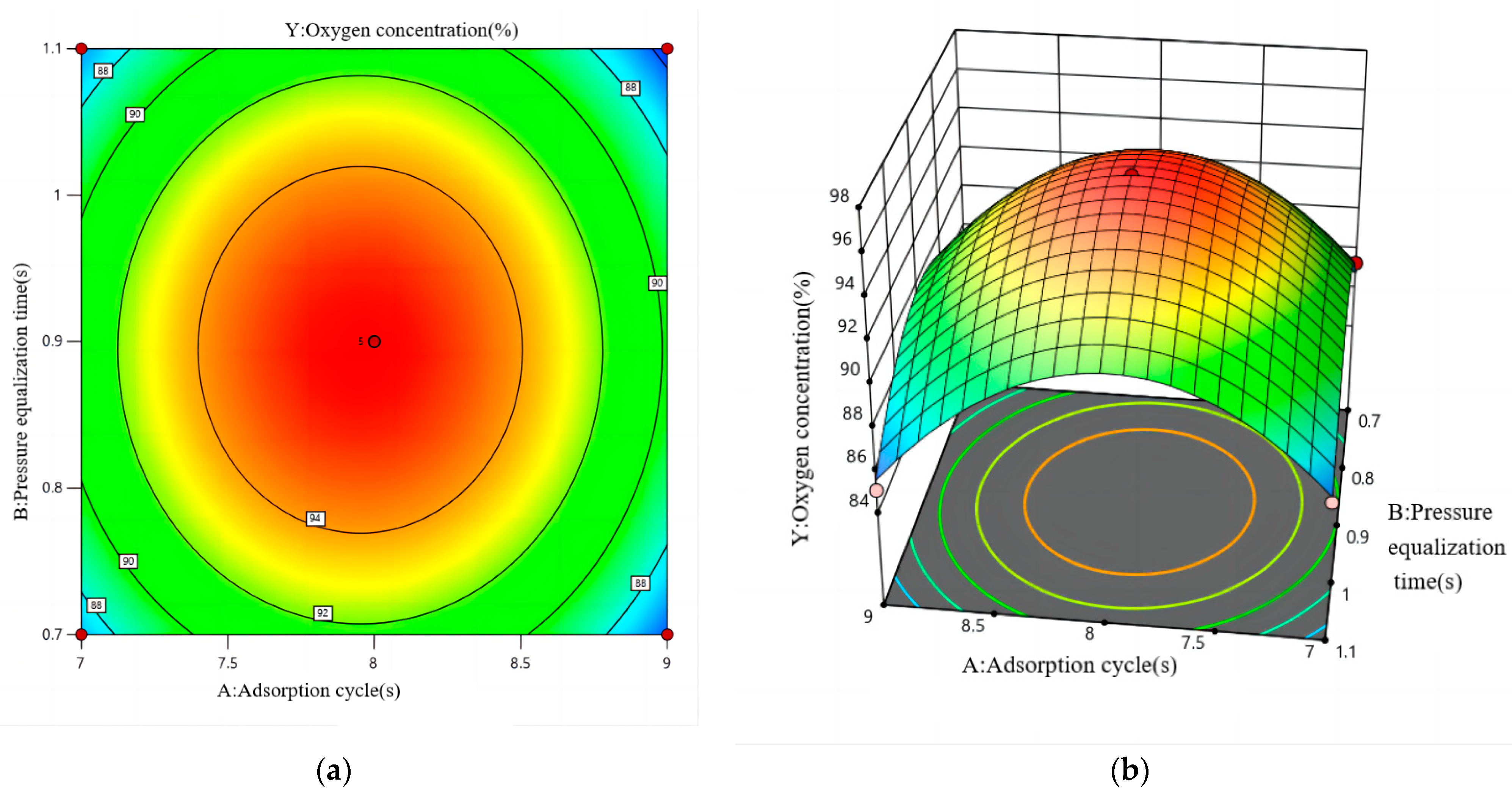
4.4.1. Modelling and Analysis
4.4.2. Analysis of the Effectiveness of PSA Oxygen Generation
5. Conclusions
- (1)
- Three factors—adsorption cycle, pressure equalisation time, and outlet flow rate—have a significant impact on oxygen concentration. Each factor has an optimum value in the oxygen production process. When any two of these factors are held constant as covariates, observing the curve of the third factor on the oxygen concentration reveals a cluster of curves with a similar trend.
- (2)
- The oxygen production concentration in a pressure equalisation process is significantly higher than in a process without pressure equalisation. As the pressure equalisation time increases, the oxygen concentration rises rapidly before decreasing slowly, reaching an optimum point. This optimum point remains stable regardless of changes in the adsorption period. The sensitivity of the oxygen concentration to the equalisation time is higher when the difference between the adsorption cycle and the optimal adsorption cycle is larger at a given outlet flow rate. At a specific pressure equalisation time, the effect curves of the outlet flow rate on the oxygen concentration corresponding to the optimal adsorption cycle differ significantly from those corresponding to non-optimal cycles. The effect curves of the outlet flow rate on the oxygen concentration for different non-optimal cycles show similar characteristics, though the underlying reasons for these patterns vary.
- (3)
- Based on multiple regression analysis of the combined variables, the influence of the factors on oxygen concentration is ranked as follows: adsorption cycle > pressure equalisation time > outlet flow rate. The interaction among these three factors also significantly affects the oxygen concentration. The interaction between the pressure equalisation time and outlet flow rate has the greatest impact, while the interaction between the adsorption cycle and outlet flow rate has the least effect on oxygen concentration.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qadir, S.; Li, D.; Gu, Y.; Yuan, Z.; Zhao, Y.; Wang, S.; Wang, S. Experimental and numerical analysis on the enhanced separation performance of a medical oxygen concentrator through two-bed rapid pressure swing adsorption. Ind. Eng. Chem. Res. 2021, 60, 5903–5913. [Google Scholar] [CrossRef]
- Aljaghoub, H.; Alasad, S.; Alashkar, A.; AlMallahi, M.; Hasan, R.; Obaideen, K.; Alami, A.H. Comparative analysis of various oxygen production techniques using multi-criteria decision-making methods. Int. J. Thermofluids 2023, 17, 100261. [Google Scholar] [CrossRef]
- Bahari, Y.; Agustina, S.; Kurniawan, T. Design and Construction of Portable Oxygen Concentrator using Pressure Swing Adsorption Technology. ASEAN J. Sci. Eng. Mater. 2024, 3, 43–50. [Google Scholar]
- Canevesi, R.; Grande, C.A. Biogas upgrading by pressure swing adsorption using zeolite 4A. Effect of purge on process performance. Sep. Purif. Technol. 2023, 309, 123015. [Google Scholar] [CrossRef]
- Chin, C.; Kamin, Z.; Bahrun, M.H.V.; Bono, A. The production of industrial-grade oxygen from air by pressure swing adsorption. Int. J. Chem. Eng. 2023, 2023, 2308227. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, Y.; Wu, W.; Ma, J.; Song, Z.; Zhang, D.; Zhang, Y. Simulation and analysis of oxygen production process by six-bed pressure swing adsorption process. CIESC J. 2023, 74, 4934. [Google Scholar]
- Lv, A.; Deng, C.; Zhu, M.; Yu, D.; Shi, M.S.; Ma, J.; Gao, W. Analyzing the PSA process of oxygen generation in plateau environment using response surface method. Appl. Chem. Ind. 2018, 47, 1175–1178. [Google Scholar]
- Zhu, X.; Liu, Y.; Yang, R.T. Effects of operating temperature on the performance of small scale rapid cycle pressure swing adsorption air separation process. Adsorption 2021, 27, 205–212. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, Y.; Dong, L.; Sun, X.; Li, S.; Chen, H.; Zhang, J.; Chen, J. Comprehensive performance and numerical analysis of pressure swing adsorption process-based medical oxygen concentrators under various operating conditions. Sep. Sci. Technol. 2023, 58, 2241–2253. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, Y.; Liu, Y.; Li, Z.; Feng, F.; Ma, J.; Li, S. Correlation analysis between working conditions and the performance of a small-scale pressure swing adsorption oxygen production process. Ind. Eng. Chem. Res. 2022, 61, 14962–14970. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, Y.; Liu, Y.; Sun, X.; Shi, J. Experimental performance analysis of vacuum pressure swing adsorption air separation process under plateau special conditions. Sep. Sci. Technol. 2022, 57, 2885–2893. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, Y.; Zhou, S.; Feng, F.; Liu, J.; Ma, J.; Li, S.; Niu, Z. Comparative performance of industrial-scale oxygen production by pressure swing adsorption and vacuum pressure swing adsorption under plateau environment. Adsorption 2023, 1–7. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X. Experimental study of a rotary valve multi-bed rapid cycle pressure swing adsorption process based medical oxygen concentrator. Adsorption 2020, 26, 1267–1274. [Google Scholar] [CrossRef]
- Šulc, R.; Kos, M. Experimental Study of Oxygen Separation in Oxygen-Pressure Swing Adsorption Unit. Chem. Eng. Trans. 2022, 94, 481–486. [Google Scholar]
- Sulc, R.; Kos, M. Experimental Performance of Pilot-Oxygen-Pressure Swing Adsorption Unit. Chem. Eng. Trans. 2023, 100, 223–228. [Google Scholar]
- De Witte, N.; Denayer, J.F.; Van Assche, T.R. Effect of adsorption duration and purge flowrate on pressure swing adsorption performance. Ind. Eng. Chem. Res. 2021, 60, 13684–13691. [Google Scholar] [CrossRef]
- Akulinin, E.; Golubyatnikov, O.; Dvoretsky, D.; Dvoretsky, S. Optimization and analysis of pressure swing adsorption process for oxygen production from air under uncertainty. Chem. Ind. Chem. Eng. Q. 2020, 26, 89–104. [Google Scholar] [CrossRef]
- Beeyani, A.; Singh, K.; Vyas, R.; Kumar, S.; Kumar, S. Parametric studies and simulation of PSA process for oxygen production from air. Pol. J. Chem. Technol. 2010, 12, 18–28. [Google Scholar] [CrossRef]
- Fakhari-Kisomi, B.; Erden, L.; Ebner, A.D.; Ritter, J.A. Equilibrium theory analysis of pressure equalization steps in pressure swing adsorption. Ind. Eng. Chem. Res. 2021, 60, 9928–9939. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, Y.; Zhang, Q.; Cao, X.; Zhao, C. Study on PSA Oxygen producing process under plateau region: Effect of purging flow rate on oxygen concentration and recovery. In Proceedings of the IOP Conference Series: Earth and Environmental Science, 3rd International Conference on Air Pollution and Environmental Engineering, Xi’an, China, 28–29 September 2020; p. 012083. [Google Scholar]
- Liu, Y.; Zhang, Q.; Cao, Y.; Yang, X.; Li, Z.; Liu, W.; Habyarimana, J.B.; Cui, Y.; Wang, H.; Yang, R.T. Effect of intermittent purge on O2 production with rapid pressure swing adsorption technology. Adsorption 2021, 27, 181–189. [Google Scholar] [CrossRef]
- Marcinek, A.; Guderian, J.; Bathen, D. Process intensification of the high-purity nitrogen production in twin-bed pressure swing adsorption plants. Adsorption 2021, 27, 937–952. [Google Scholar] [CrossRef]
- Rumbo Morales, J.Y.; Ortiz-Torres, G.; García, R.O.D.; Cantero, C.A.T.; Rodriguez, M.C.; Sarmiento-Bustos, E.; Oceguera-Contreras, E.; Hernández, A.A.F.; Cerda, J.C.R.; Molina, Y.A. Review of the Pressure Swing Adsorption Process for the Production of Biofuels and Medical Oxygen: Separation and Purification Technology. Adsorpt. Sci. Technol. 2022, 2022. [Google Scholar] [CrossRef]
- Zhang, R.; Shen, Y.; Tang, Z.; Li, W.; Zhang, D. A review of numerical research on the pressure swing adsorption process. Processes 2022, 10, 812. [Google Scholar] [CrossRef]
- Madankar, T.; Deshpande, Y. Development of Portable Oxygen Concentrator-A Review. In Proceedings of the E3S Web of Conferences, 15th International Conference on Materials Processing and Characterization (ICMPC 2023), Newcastle, UK, 5–8 September 2023; p. 01265. [Google Scholar]
- Sharma, P. Prediction-optimization of the effects of di-tert butyl peroxide-biodiesel blends on engine performance and emissions using multi-objective response surface methodology. J. Energy Resour. Technol. 2022, 144, 072301. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Kim, J.; Jin, F.; Jun, M.; Cheong, M.; Yammarino, F.J. Polynomial regression analysis and response surface methodology in leadership research. Leadersh. Q. 2022, 33, 101592. [Google Scholar] [CrossRef]
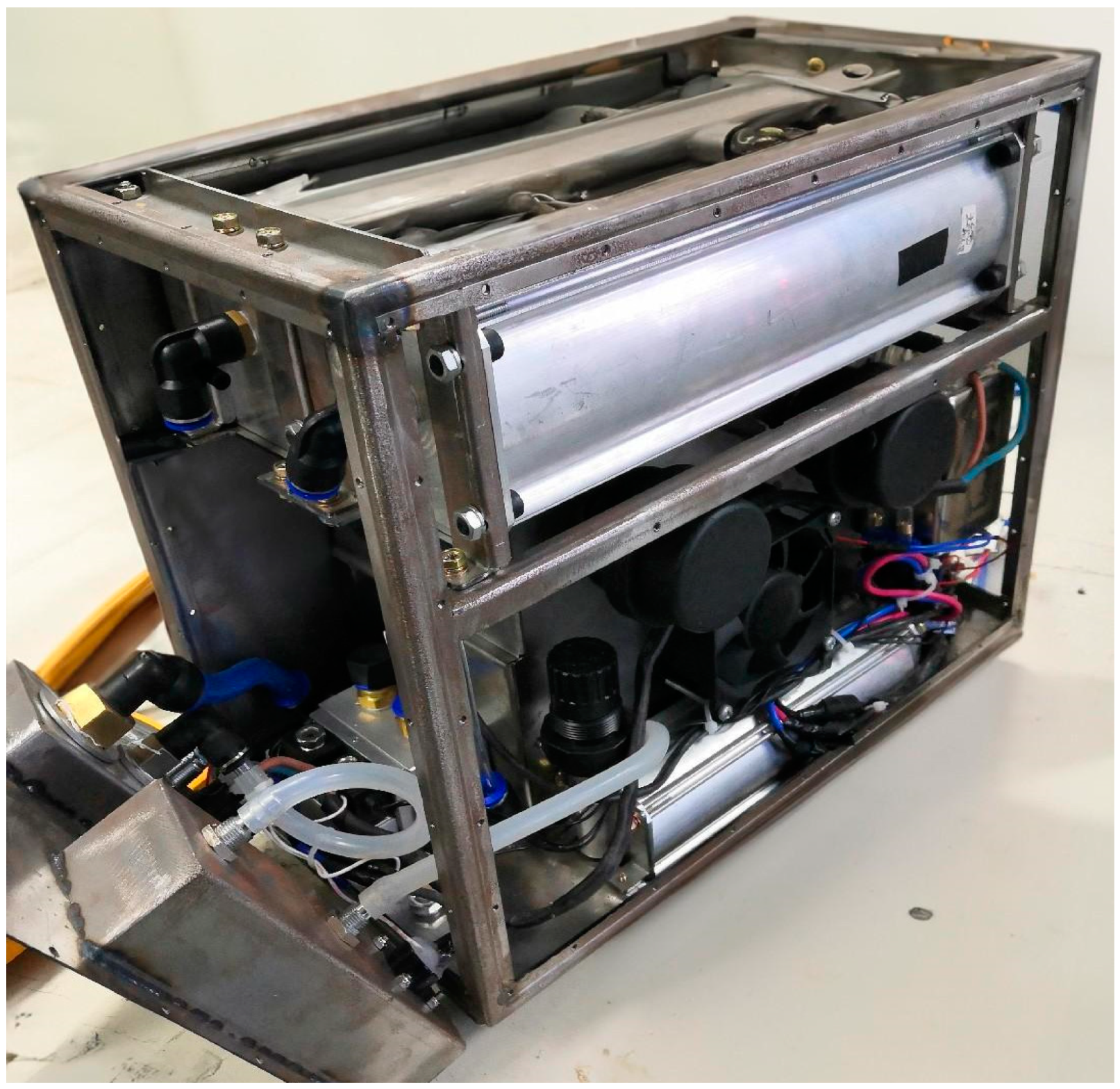



| Material and Instruments | Specification | Manufacture | |
|---|---|---|---|
| 1 | Air compressor | BST300/1.5-80DC26V | Shanghai Ingersoll Rand Compressor. |
| 2 | Molecular sieve | SXSDM | Shanghai EasySorb Molecular Sieve Co. |
| 3 | Cooling fans | DF8038B12H DC12V | MOUSER ELECTRONICS. |
| 4 | Filter | 4100-725 | Hangzhou Cobalt Filter Material Co. |
| 5 | Oxygen concentration sensor | GASboard-7500 | Sifang Optoelectronics Co. |
| 6 | Electronic flow meter | LZM-6TO2(0–9 L/min) | Yuyao Jintai Instrument Co. |
| 7 | Gas storage tank | Ø48X220 | CLW GROUP |
| 8 | Distribution valve | JEL-MP-08--DC12V-1.5 W | Ningbo Jiaerling Pneumatic Machinery Co. |
| 9 | Check valve | DCV1603CVL.01 | Ningbo Jiaerling Pneumatic Machinery Co. |
| 10 | Pressure stabilising valve | NZYR-10XD | Zhejiang Yongsheng Technology Co. |
| Source of Variance | Sum of Square | Degrees of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 211.27 | 9 | 23.47 | 91.15 | <0.0001 |
| X1 | 121.53 | 1 | 121.53 | 471.89 | <0.0001 |
| X2 | 68.13 | 1 | 68.13 | 264.53 | <0.0001 |
| X3 | 3.06 | 1 | 3.06 | 11.88 | 0.0107 |
| X1X2 | 0.0001 | 1 | 0.0001 | 0.0004 | 0.03848 |
| X1X3 | 0.0289 | 1 | 0.0289 | 0.1122 | 0.006 |
| X2X3 | 1.72 | 1 | 1.72 | 6.66 | 0.0264 |
| Residual | 1.8 | 7 | 0.2575 | ||
| Lack of fit | 1.8 | 3 | 0.6 | ||
| Pure error | 0.06 | 4 | 0.08 | ||
| Sum | 213.08 | 16 | |||
| R2adj = 0.9807 R2 = 0.9915 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, D.; Huang, F. Influence Factors of Pressure Swing Adsorption for Oxygen Production by the Orthogonal Method and the Response Surface Method. Processes 2024, 12, 1306. https://doi.org/10.3390/pr12071306
Jiang D, Huang F. Influence Factors of Pressure Swing Adsorption for Oxygen Production by the Orthogonal Method and the Response Surface Method. Processes. 2024; 12(7):1306. https://doi.org/10.3390/pr12071306
Chicago/Turabian StyleJiang, Dongsheng, and Fengzhen Huang. 2024. "Influence Factors of Pressure Swing Adsorption for Oxygen Production by the Orthogonal Method and the Response Surface Method" Processes 12, no. 7: 1306. https://doi.org/10.3390/pr12071306




