Impact of Drying Processes for Camellia Seeds on the Volatile Compounds of Camellia Seed Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Oil Samples
2.2. Determination of Oil Contents and Quality Parameters
2.3. Gas Chromatography-Ion Mobility Spectrometry Analysis
2.4. Qualitative and Quantitative Analysis of GC-IMS
2.5. Determination of Key Aroma Compounds
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition and Quality Parameters of CO Samples
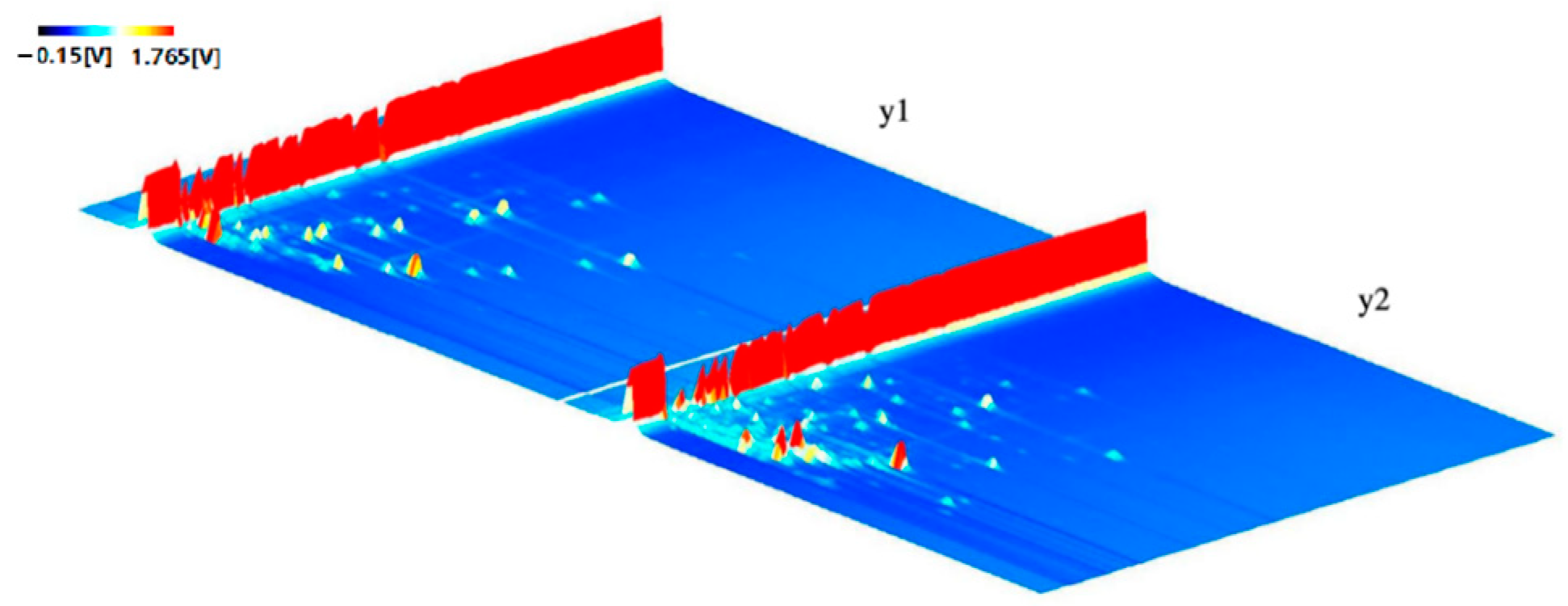
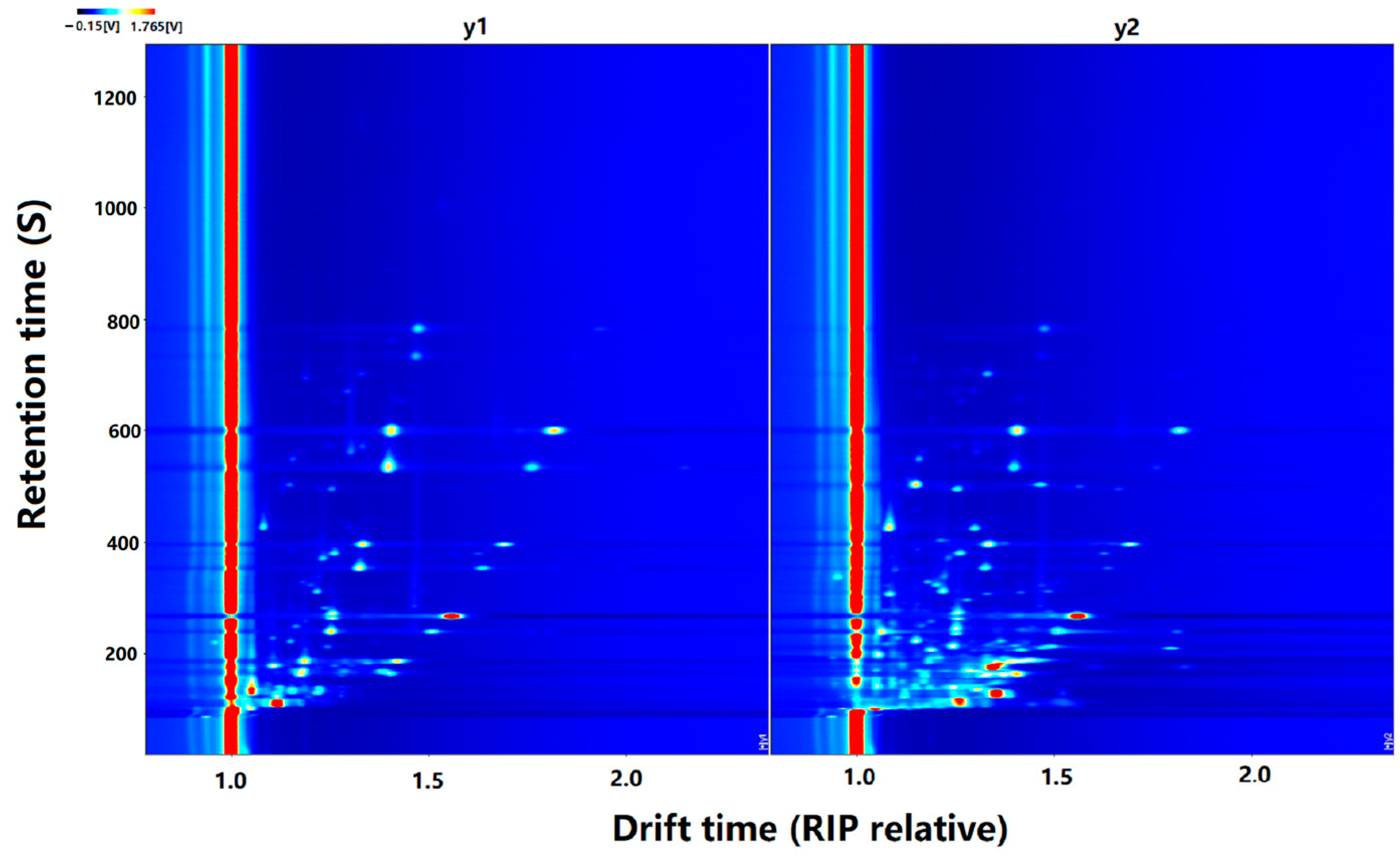
3.2. GC-IMS Topography of CO Samples
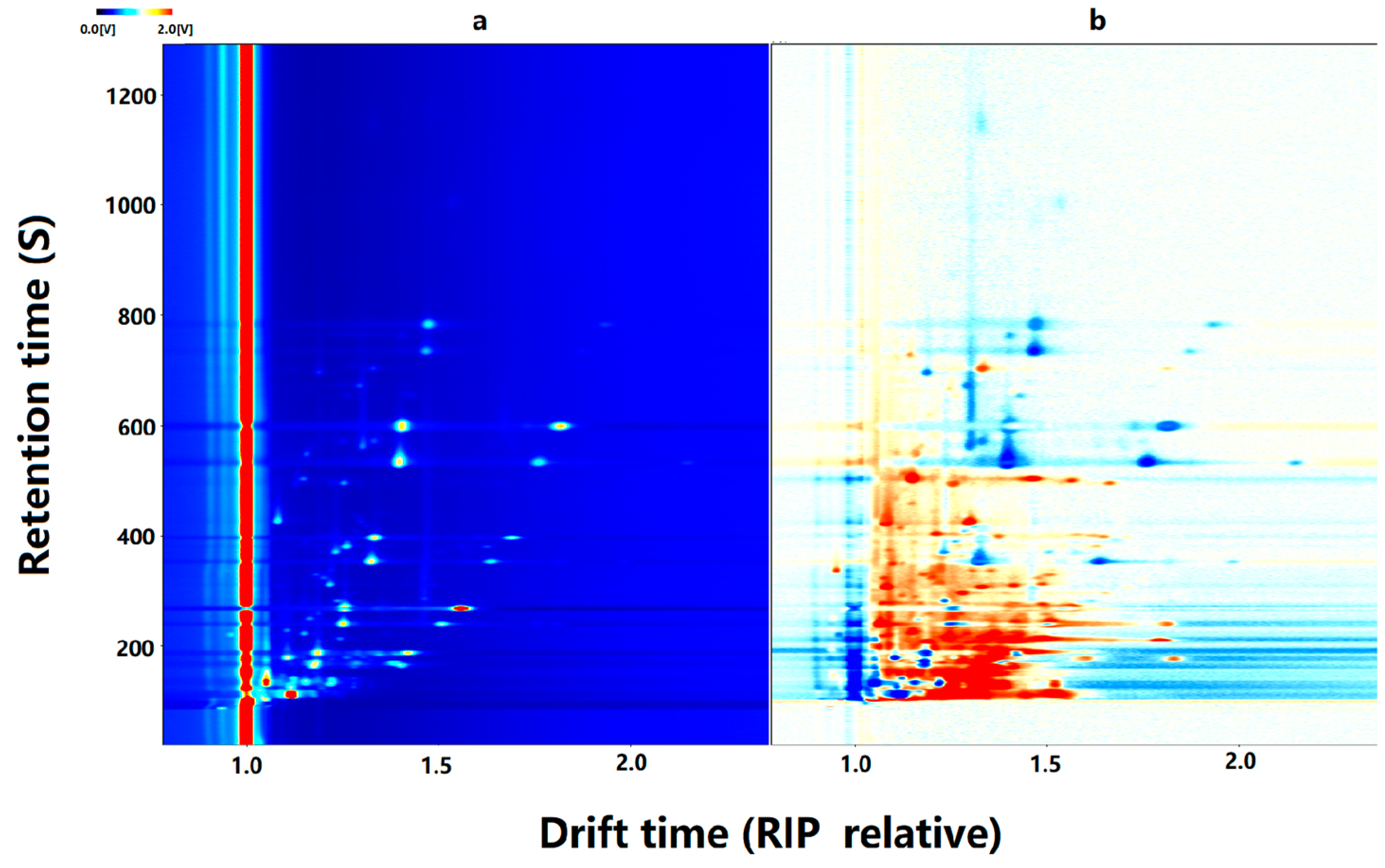
3.3. Fingerprint Study of VOCs in CO Samples
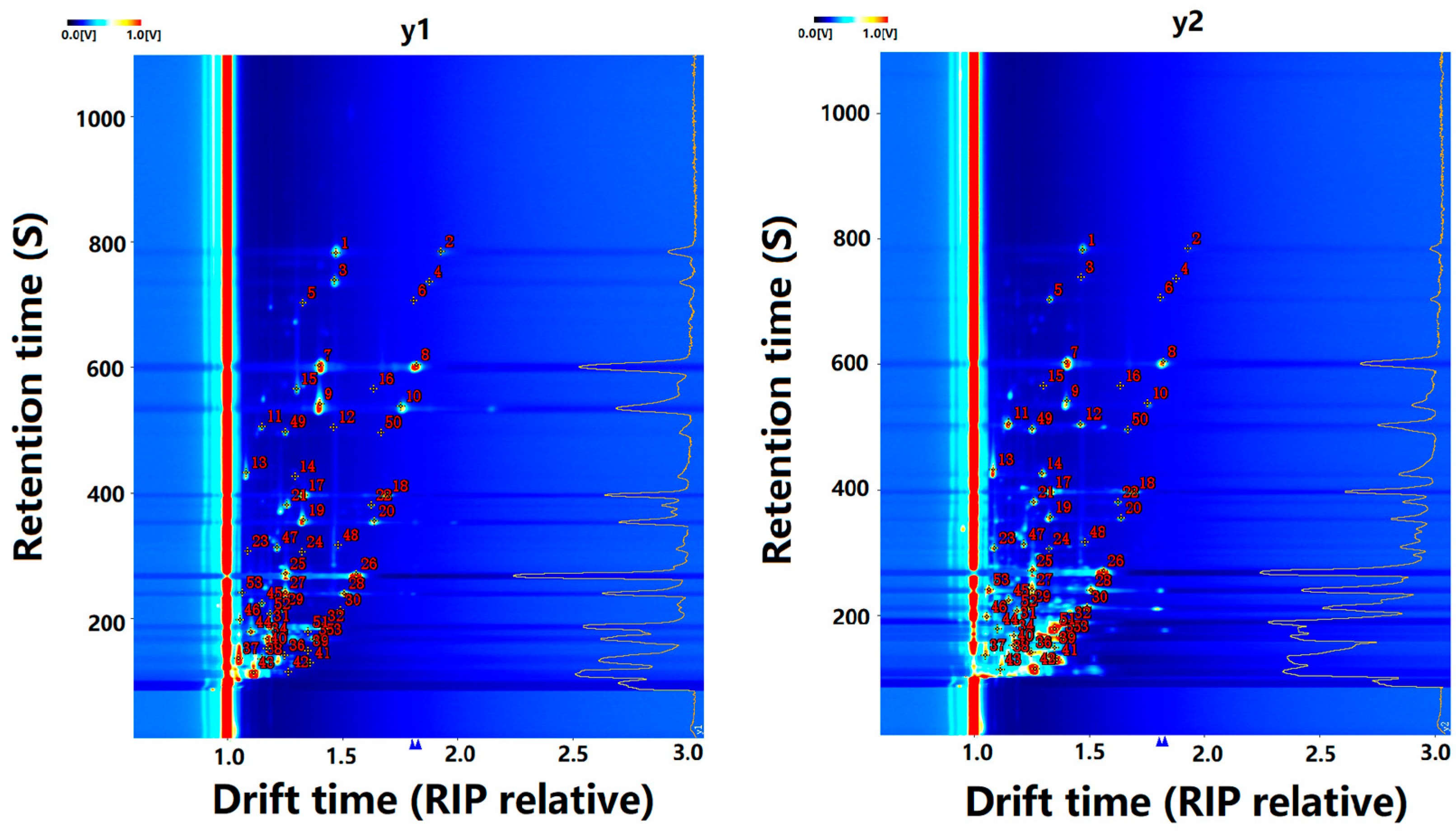
3.4. Identification of VOCs in CO Samples
3.5. Identification of Key Aroma Compounds
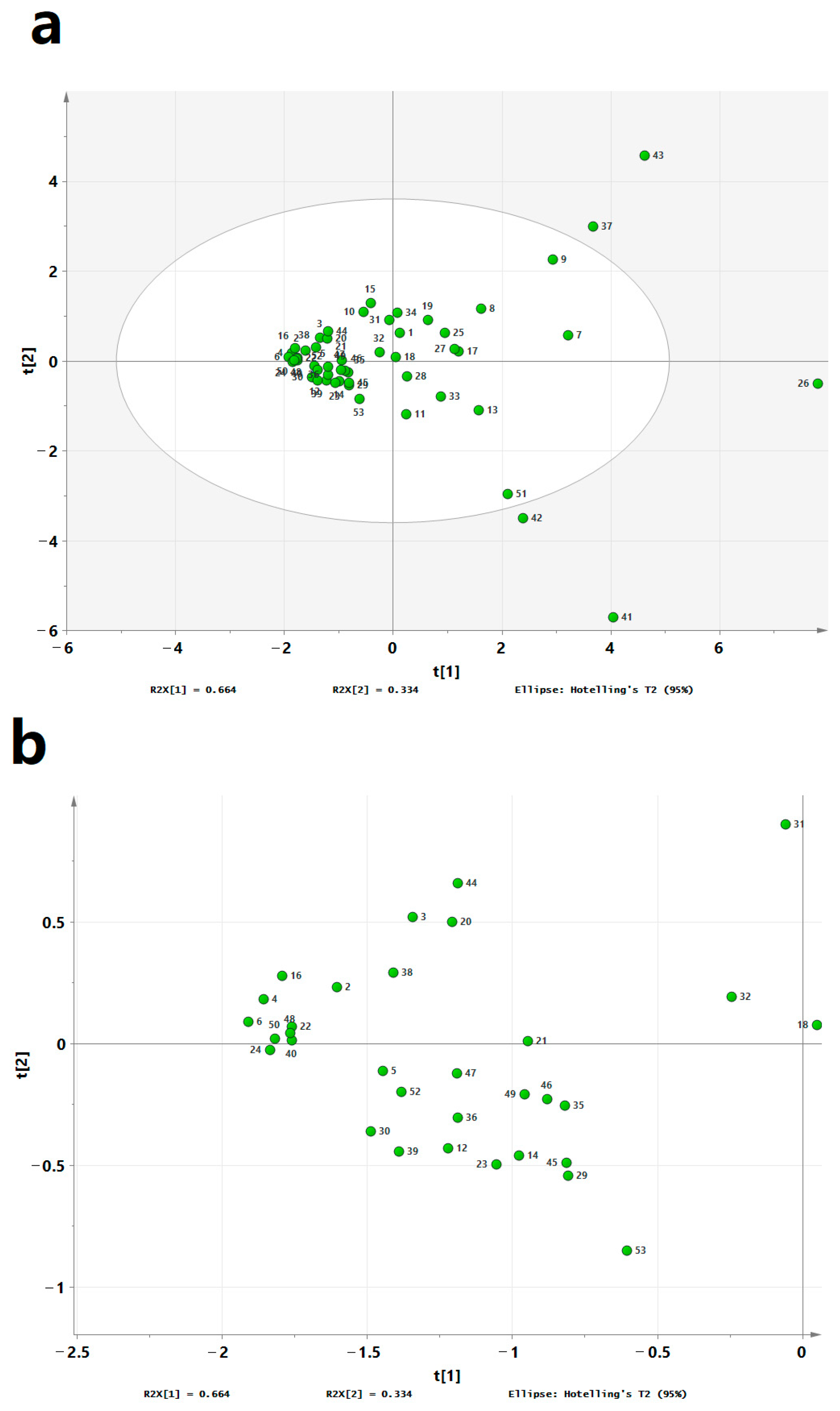
3.6. Principal Component Analysis of VOCs in CO Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Deng, S.; Ma, L.; Li, M.; Xie, B.; Gao, J.; Shao, M.; Chen, Y. Effects of soil properties and nutrients on the fruit economic parameters and oil nutrient contents of Camellia oleifera. Forests 2023, 14, 1786. [Google Scholar] [CrossRef]
- Li, G.; Ma, L.; Yan, Z.; Zhu, Q.; Cai, J.; Wang, S.; Yuan, Y.; Chen, Y.; Deng, S. Extraction of oils and phytochemicals from Camellia oleifera seeds: Trends, challenges, and innovations. Processes 2022, 10, 1489. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Q.; del Mar Contreras, M.; Wang, L. Profiling and quantification of phenolic compounds in camellia seed oils: Natural tea polyphenols in vegetable oil. Food Res. Int. 2017, 102, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Shen, J.; Wei, J.; Chen, Y. A critical review of the bioactive ingredients and biological functions of Camellia oleifera oil. Curr. Res. Food Sci. 2024, 8, 100753. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Li, M.; Zhang, B.; Huang, X.; Tao, L.; Liao, W.; Huang, K. Camellia oil inhibits oxidative stress and inflammatory response to ameliorate LPS-induced acute kidney injury via downregulation of TLR4-mediated activation of the NF-κB/AP-1/IRF3 and NLRP3 pathways. J. Funct. Foods 2020, 68, 103908. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, G.; Ma, L.; Chen, B.; Zhang, D.; Gao, J.; Deng, S.; Chen, Y. Virgin camellia seed oil improves glycolipid metabolism in the kidney of high fat-fed rats through AMPK-SREBP pathway. Nutrients 2023, 15, 4888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, D.; Zhang, X.; Zhao, H.; Gong, G.; Tang, X.; Li, L. Drying performance and energy consumption of Camellia oleifera seeds under microwave-vacuum drying. Food Sci. Biotechnol. 2023, 32, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fei, X.; Shi, X.; Ye, X.; Guo, S.; Luo, F. Effects of harvest time and treatment methods on nutrient accumulation of oil-tea camellia seed and its oil. China Oils Fats 2017, 42, 20–46. [Google Scholar]
- Ma, L.; Zhong, H.; Chen, Y.; Peng, S.; Zhu, N.; Chen, L.; Wang, R. The effect of post-harvest treatment of camellia fruit on quality of camellia seed. J. Chin. Cereals Oils Assoc. 2014, 29, 73–76. [Google Scholar]
- Luo, F.; Fei, X.; Guo, S.; Chen, J.; Li, K. Effects of harvest time and drying methods on quality of oil-tea camellia seed oil. China Oils Fats 2015, 40, 69–73. [Google Scholar]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using Gas Chromatography–Ion Mobility Spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Delgado, R.; Dobao-Prieto, M.M.; Arce, L.; Valcárcel, M. Determination of volatile compounds by GC–IMS to assign the quality of virgin olive oil. Food Chem. 2015, 187, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Zhao, P.; Zhang, T.; Luan, J.; Huang, L.; Dong, J.; Gao, H. Characteristic volatiles fingerprints and profiles determination in different grades of co-conut oil by HS-GC-IMS and HS-SPME-GC-MS. Int. J. Food Sci. Technol. 2020, 55, 3670–3679. [Google Scholar] [CrossRef]
- Liu, R.; Wei, B. Physical pressing process practice of oil-tea camellia seed. China Oils Fats 2017, 42, 155–157. [Google Scholar]
- Hao, Z.; Wu, S.; Jiang, M.; Guan, Y.; Feng, Z. Effect of pretreatment methods of fresh Camellia oleifera fruit on the quality of low-temperature pressed oil-tea camellia seed oil. China Oils Fats 2023, 48, 19–23. [Google Scholar]
- Zhang, L.; Zhou, C.; Wang, B.; Yagoub, A.E.G.A.; Ma, H.; Zhang, X.; Wu, M. Study of ultrasonic cavitation during extraction of peanut oil at varying frequencies. Ultrason. Sonochem. 2017, 37, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Y.; Liu, Y.; Wang, D. Analyzing volatile compounds of young and mature Docynia delavayi fruit by HS-SPME-GC-MS and rOAV. Foods 2023, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; He, J.J.; Zhou, Y.Z.; Li, Y.L.; Zhou, H.J. Aroma effects of key volatile compounds in Keemun black tea at different grades: HS-SPME-GC-MS, sensory evaluation, and chemometrics. Food Chem. 2022, 373, 131587. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Birch, J. Oxidative stability, thermal decomposition, and oxidation onset prediction of carrot, flax, hemp, and canola seed oils in relation to oil composition and positional distribution of fatty acids. Eur. J. Lipid Sci. Technol. 2016, 118, 1042–1052. [Google Scholar] [CrossRef]
- Schulte, L.R.; Ballard, T.; Samarakoon, T.; Yao, L.; Vadlani, P.; Staggenborg, S.; Rezac, M. Increased growing temperature reduces content of polyunsaturated fatty acids in four oilseed crops. Ind. Crops Prod. 2013, 51, 212–219. [Google Scholar] [CrossRef]
- GB 11765-2018; National Standard for Oil-Tea Camellia Seed Oil. State Administration for Market Regulation: Beijing, China, 2018.
- He, J.; Wu, X.; Zhou, Y.; Xu, X.; Huang, K. Effects of different preheat treatments on volatile compounds of camellia (Camellia oleifera Abel.) seed oil and formation mechanism of key aroma compounds. Food Biochem. 2021, 45, e13649. [Google Scholar] [CrossRef] [PubMed]
- Kraljic, K.; Stjepanovic, T.; Obranovic, M.; Skevin, D.; Medic, H. Influence of conditioning temperature on the quality, nutritional properties and volatile profile of virgin rapeseed oil. Food Technol. Biotechnol. 2018, 56, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Dierkes, G.; Bongartz, A.; Guth, H.; Buettner, A. Quality evaluation of olive oil by statistical analysis of multicomponent stable isotope dilution assay data of aroma active compounds. J. Agric. Food Chem. 2012, 60, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Deng, Q.; Yang, Y.; Jiang, L.; Xie, J.; Xie, M. Unraveling of the aroma-active compounds in virgin camellia oil (Camellia oleifera Abel.) using Gas Chromatography-Mass Spectrometry-Olfactometry, Aroma Recombination, and Omission Studies. J. Agric. Food Chem. 2021, 69, 9043–9055. [Google Scholar] [CrossRef]
- Kesen, S.; Kelebek, H.; Selli, S. Characterization of the key aroma compounds in Turkish olive oils from different geographic origins by application of Aroma Extract Dilution Analysis (AEDA). J. Agric. Food Chem. 2014, 62, 391–401. [Google Scholar] [CrossRef]
- Neugebauer, A.; Schieberle, P.; Granvogl, M. Characterization of the key odorants causing the musty and fusty/muddy sediment off-flavors in olive oils. J. Agric. Food Chem. 2021, 69, 14878–14892. [Google Scholar] [CrossRef]
- Reboredo-Rodriguez, P.; Gonzalez-Barreiro, C.; Cancho-Grande, B.; Simal-Gandara, J. Concentrations of aroma compounds and Odor Activity Values of odorant series in different Olive cultivars and their oils. J. Agric. Food Chem. 2013, 61, 5252–5259. [Google Scholar] [CrossRef]
- Rios-Reina, R.; Aparicio-Ruiz, R.; Morales, M.T.; García-González, D.L. Contribution of specific volatile markers to green and ripe fruity attributes in extra virgin olive oils studied with three analytical methods. Food Chem. 2023, 399, 133942. [Google Scholar] [CrossRef] [PubMed]
- Luo, X. Study on the Effect of Roasting Conditions on Antioxidant Capacity and Flavor Substances of Camellia Seed Oil. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2023. [Google Scholar]
- Kesen, S.; Kelebek, H.; Sen, K.; Selli, S. GC-MS-Olfactometric characterization of the key aroma compounds in Turkish olive oils by application of the Aroma Extract Dilution Analysis. Food Res. Int. 2013, 54, 1987–1994. [Google Scholar] [CrossRef]
- Xi, B.; Zhang, J.; Xu, X.; Gao, Q.; Cao, J.; Wang, Y. Characterization and metabolism pathway of volatile compounds in walnut oil obtained from various ripening stages via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2024, 435, 137547. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, X.; Chen, Q.; Shi, J.; Bao, J. Oxidative stabilities of olive and camellia oils: Possible mechanism of aldehydes formation in oleic acid triglyceride at high temperature. LWT-Food Sci. Technol. 2020, 118, 108858. [Google Scholar] [CrossRef]
- Xiao, L.; Lee, J.; Zhang, G.; Ebeler, S.E.; Wickramasinghe, N.; Seiber, J.; Mitchell, A.E. HS-SPME GC/MS characterization of volatiles in raw and dry-roasted almonds (Prunus dulcis). Food Chem. 2014, 151, 31–39. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, X.; Chao, Y.; Cai, J.; Zeng, W. The effect of extraction methods on the components and quality of Camellia oleifera oil: Focusing on the flavor and lipidomics. Food Chem. 2024, 447, 139046. [Google Scholar] [CrossRef]
- Jia, X.; Zhou, Q.; Wang, J.; Yin, Y.; Huang, D. Identification of key aroma-active compounds in sesame oil from microwaved seeds using E-Nose and HS-SPME-GCxGC-TOF/MS. J. Food Biochem. 2019, 43, e12786. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Petisca, C. Coffee: Consumption and Health Implications; Farah, A., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2019; pp. 520–540. [Google Scholar]
- Xu, Y.; Bi, S.; Niu, X.; Wang, H.; Xu, X. Comparison of aroma active compounds in cold- and hot-pressed walnut oil by Comprehensive Two-Dimensional Gas Chromatography-Olfactory-Mass Spectrometry and Headspace-Gas Chromatography-Ion Mobility Spectrometry. Food Res. Int. 2023, 163, 112208. [Google Scholar] [CrossRef]
- Jia, X.; Zhou, Q.; Huang, D.; Yin, Y. Insight into the comparison of key aroma-active compounds between camellia oils from different processing technology. Food Chem. 2024, 430, 137090. [Google Scholar] [CrossRef]
- Cerezo, S.; Hernandez, M.; Palomo-Rios, E.; Luisa, M.; Sanchez-Ortiz, A. Modification of 13-hydroperoxide lyase expression in olive affects plant growth and results in altered volatile profile. Plant Sci. 2021, 313, 111083. [Google Scholar] [CrossRef]
- Sanchez-Ortiz, A.; Romero-Segura, C.; Gazda, V.; Sanz, C.; Perez, A.G. Factors limiting the synthesis of virgin olive oil volatile esters. J. Agric. Food Chem. 2012, 60, 1300–1307. [Google Scholar] [CrossRef]
- Zhang, Y.; Stoppelmann, F.; Zhu, L.; Rohm, H.; Scherf, K.A. A Comparative study on flavor trapping techniques from the viewpoint of odorants of hot-pressed rapeseed oil. Food Chem. 2023, 426, 136617. [Google Scholar] [CrossRef]
- Neugebauer, A.; Granvogl, M.; Schieberle, P. Characterization of the key odorants in high-quality extra virgin olive oils and certified off-flavor oils to elucidate aroma compounds causing a rancid off-flavor. J. Agric. Food Chem. 2020, 68, 5927–5937. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Huang, Y.; Zeng, W.; Zhang, X.; Chao, Y. Aroma identification and classification in 18 kinds of teas (Camellia sinensis) by sensory evaluation, HS-SPME-GC-IMS/GC × GC-MS, and Chemometrics. Foods 2023, 12, 2433–2447. [Google Scholar] [CrossRef]
- He, J.; Wu, X.; Yu, Z. Microwave pretreatment of camellia (Camellia oleifera Abel.) seeds: Effect on oil flavor. Food Chem. 2021, 364, 130388. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Compilation of Odour Threshold Values in Air, Water and Other Media, 2nd ed.; Science Publisher: Beijing, China, 2015. [Google Scholar]
- Terry, A.; Heinrich, A. Flavornet. 2004. Available online: http://www.flavornet.org (accessed on 10 March 2024).
- Krist, S.; Stuebiger, G.; Bail, S.; Unterweger, H. Analysis of Volatile compounds and triacylglycerol composition of fatty seed oil gained from flax and false flax. Eur. J. Lipid Sci. Technol. 2006, 108, 48–60. [Google Scholar] [CrossRef]
- Sun, X.; Wan, Y.; Han, J.; Zhang, Y.; Xu, X. Analysis of volatile compounds and flavor fingerprint in hot-pressed flaxseed oil processed under different roasting conditions using Headspace-Gas Chromatography-Ion Mobility Spectrometry. Food Anal. Methods 2023, 16, 888–899. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T.; Alonso, V. Authentication of European extra-virgin olive oils by their chemical compounds, sensory attributes, and consumers’ attitudes. J. Agric. Food Chem. 1997, 45, 1076–1083. [Google Scholar] [CrossRef]
- Kiritsakis, A. Flavor components of olive oil-a review. J. Am. Oil Chem. Soc. 1998, 75, 673–681. [Google Scholar] [CrossRef]
- Xia, X. The Analysis of Volatile Compounds and Nutrition Composition in Camellia Oil. Master’s Thesis, Nanchang University, Nanchang, China, 2015. [Google Scholar]
- Luo, F.; Guo, S.; Fei, X.; Yao, X.; Wang, Y.; Ye, X. The effects of pressing conditions on volatile organic compounds and the quality of crude camellia oil. J. Chin. Cereals Oils Assoc. 2015, 30, 61–66. [Google Scholar]
- Siegmund, B.; Murkovic, M. Changes in chemical composition of pumpkin seeds during the roasting process for production of pumpkin seed oil (part 2: Volatile compounds). Food Chem. 2004, 84, 367–374. [Google Scholar] [CrossRef]


| Compounds and Parameters | y1 | y2 |
|---|---|---|
| Palmitic acid (C16:0) | 5.18% | 5.88% |
| Stearic acid (C18:0) | 1.75% | 1.90% |
| Oleic acid (C18:1n9c) | 83.60% | 82.80% |
| Linoleic acid (C18:2n6c) | 8.83% | 8.69% |
| Linolenic acid (C18:3n3) | 0.14% | 0.15% |
| Arachidonic acid (C20:1n9c) | 0.51% | 0.56% |
| Vitamin E (mg/kg) | 184.71 | 186.52 |
| Acid value (mg/g) | 0.54 | 0.88 |
| Peroxide value (g/100 g) | 0.03 | 0.07 |
| No. | Label | CAS | Formula | MW A | RI B | Rt C [s] | Dt D [a.u.] | Relative Content (%) E | |
|---|---|---|---|---|---|---|---|---|---|
| y1 | y2 | ||||||||
| 1 | Nonanal M | 124-19-6 | C9H18O | 142.2 | 1102.2 | 782.118 | 1.47435 | 2.64 ± 0.01 | 1.45 ± 0.01 |
| 2 | Nonanal D | 124-19-6 | C9H18O | 142.2 | 1103.3 | 784.609 | 1.93263 | 0.52 ± 0.03 | 0.27 ± 0.03 |
| 3 | 1-Octanol M | 111-87-5 | C8H18O | 130.2 | 1082.4 | 738.529 | 1.47038 | 1.07 ± 0.03 | 0.25 ± 0.01 |
| 4 | 1-Octanol D | 111-87-5 | C8H18O | 130.2 | 1081.3 | 736.212 | 1.87906 | 0.22 ± 0.01 | 0.09 ± 0.01 |
| 5 | (E)-2-Octenal M | 2548-87-0 | C8H14O | 126.2 | 1065.1 | 702.55 | 1.3329 | 0.33 ± 0.02 | 0.71 ± 0.01 |
| 6 | (E)-2-Octenal D | 2548-87-0 | C8H14O | 126.2 | 1066.9 | 706.29 | 1.81446 | 0.07 ± 0.00 | 0.13 ± 0.01 |
| 7 | Octanal M | 124-13-0 | C8H16O | 128.2 | 1012 | 602.494 | 1.40315 | 5.67 ± 0.17 | 4.24 ± 0.03 |
| 8 | Octanal D | 124-13-0 | C8H16O | 128.2 | 1012.8 | 603.916 | 1.82745 | 4.68 ± 0.15 | 2.30 ± 0.02 |
| 9 | 1-Heptanol M | C111706 | C7H16O | 116.2 | 981 | 541.358 | 1.4056 | 7.08 ± 0.05 | 2.50 ± 0.06 |
| 10 | 1-Heptanol D | C111706 | C7H16O | 116.2 | 979 | 537.092 | 1.75632 | 2.44 ± 0.01 | 0.47 ± 0.01 |
| 11 | Benzaldehyde M | 100-52-7 | C7H6O | 106.1 | 964.1 | 506.07 | 1.15348 | 0.94 ± 0.03 | 3.15 ± 0.00 |
| 12 | Benzaldehyde D | 100-52-7 | C7H6O | 106.1 | 963.7 | 505.231 | 1.46625 | 0.24 ± 0.01 | 1.19 ± 0.03 |
| 13 | Butyrolactone M | 96-48-0 | C4H6O2 | 86.1 | 924.7 | 432.297 | 1.08595 | 2.37 ± 0.08 | 4.24 ± 0.06 |
| 14 | Butyrolactone D | 96-48-0 | C4H6O2 | 86.1 | 921.3 | 426.428 | 1.29743 | 0.45 ± 0.02 | 1.43 ± 0.07 |
| 15 | Hexanoic acid M | 142-62-1 | C6H12O2 | 116.2 | 991.5 | 564.753 | 1.30276 | 2.77 ± 1.35 | 0.41 ± 0.09 |
| 16 | Hexanoic acid D | 142-62-1 | C6H12O2 | 116.2 | 991.5 | 564.753 | 1.63685 | 0.37 ± 0.22 | 0.06 ± 0.00 |
| 17 | Heptanal M | 111-71-7 | C7H14O | 114.2 | 902.7 | 395.754 | 1.33669 | 3.30 ± 0.02 | 2.78 ± 0.04 |
| 18 | Heptanal D | 111-71-7 | C7H14O | 114.2 | 902.3 | 395.205 | 1.69772 | 2.01 ± 0.01 | 1.86 ± 0.01 |
| 19 | 1-Hexanol M | 111-27-3 | C6H14O | 102.2 | 874 | 356.774 | 1.33519 | 3.45 ± 0.00 | 1.66 ± 0.03 |
| 20 | 1-Hexanol D | 111-27-3 | C6H14O | 102.2 | 872.7 | 355.126 | 1.6408 | 1.18 ± 0.01 | 0.38 ± 0.01 |
| 21 | 2-Heptanone M | 110-43-0 | C7H14O | 114.2 | 892.8 | 380.381 | 1.26329 | 0.95 ± 0.02 | 1.05 ± 0.02 |
| 22 | 2-Heptanone D | 110-43-0 | C7H14O | 114.2 | 893.1 | 380.93 | 1.62732 | 0.20 ± 0.00 | 0.28 ± 0.00 |
| 23 | Furan-2-carbaldehyde M | 98-01-1 | C5H4O2 | 96.1 | 829.8 | 306.813 | 1.09251 | 0.34 ± 0.01 | 1.39 ± 0.04 |
| 24 | Furan-2-carbaldehyde | 98-01-1 | C5H4O2 | 96.1 | 829.3 | 306.264 | 1.3292 | 0.03 ± 0.01 | 0.29 ± 0.01 |
| 25 | Hexanal M | 66-25-1 | C6H12O | 100.2 | 794.1 | 271.675 | 1.2558 | 3.47 ± 0.03 | 2.18 ± 0.03 |
| 26 | Hexanal D | 66-25-1 | C6H12O | 100.2 | 792.9 | 270.577 | 1.56589 | 9.17 ± 0.08 | 9.21 ± 0.06 |
| 27 | 1-Pentanol M | 71-41-0 | C5H12O | 88.1 | 761.3 | 240.863 | 1.25576 | 3.28 ± 0.01 | 2.67 ± 0.05 |
| 28 | 1-Pentanol D | 71-41-0 | C5H12O | 88.1 | 760.3 | 239.983 | 1.51156 | 1.79 ± 0.02 | 2.41 ± 0.04 |
| 29 | 1-Butanol, 3-methyl M | 123-51-3 | C5H12O | 88.1 | 731.3 | 215.058 | 1.24701 | 0.54 ± 0.01 | 1.65 ± 0.02 |
| 30 | 1-Butanol, 3-methyl D | 123-51-3 | C5H12O | 88.1 | 729.1 | 213.299 | 1.49516 | 0.04 ± 0.00 | 0.89 ± 0.01 |
| 31 | Pentanal M | 110-62-3 | C5H10O | 86.1 | 695.4 | 187.787 | 1.1847 | 2.73 ± 0.00 | 1.05 ± 0.02 |
| 32 | n-pentanal D | 110-62-3 | C5H10O | 86.1 | 695.4 | 187.787 | 1.42192 | 1.84 ± 0.01 | 1.50 ± 0.07 |
| 33 | 2-Methylbutanal | 96-17-3 | C5H10O | 86.1 | 661.5 | 166.381 | 1.41099 | 1.96 ± 0.05 | 3.36 ± 0.13 |
| 34 | 1-Butanol M | 71-36-3 | C4H10O | 74.1 | 664 | 167.848 | 1.17705 | 3.05 ± 0.03 | 1.02 ± 0.03 |
| 35 | 1-Butanol D | 71-36-3 | C4H10O | 74.1 | 661 | 166.088 | 1.38147 | 0.81 ± 0.04 | 1.39 ± 0.02 |
| 36 | 2-Butanone | 78-93-3 | C4H8O | 72.1 | 614.2 | 140.87 | 1.25357 | 0.40 ± 0.01 | 1.11 ± 0.01 |
| 37 | Acetic acid M | 64-19-7 | C2H4O2 | 60.1 | 605.7 | 136.765 | 1.05242 | 8.56 ± 0.44 | 2.51 ± 0.12 |
| 38 | Acetic acid D | 64-19-7 | C2H4O2 | 60.1 | 603.3 | 135.592 | 1.15409 | 0.77 ± 0.05 | 0.39 ± 0.01 |
| 39 | 2-Methylpropan-1-ol D | 78-83-1 | C4H10O | 74.1 | 629.2 | 148.494 | 1.35524 | 0.05 ± 0.00 | 1.05 ± 0.02 |
| 40 | 2-Methylpropan-1-ol M | 78-83-1 | C4H10O | 74.1 | 635.3 | 151.72 | 1.17267 | 0.15 ± 0.01 | 0.33 ± 0.02 |
| 41 | Ethyl Acetate | 141-78-6 | C4H8O2 | 88.1 | 589.4 | 129.141 | 1.36398 | 0.20 ± 0.02 | 10.46 ± 0.09 |
| 42 | 1-Propanol D | 71-23-8 | C3H8O | 60.1 | 556.6 | 115.066 | 1.26778 | 0.75 ± 0.02 | 7.06 ± 0.16 |
| 43 | 1-Propanol M | 71-23-8 | C3H8O | 60.1 | 551.5 | 113.013 | 1.1202 | 11.09 ± 0.09 | 1.97 ± 0.29 |
| 44 | Propanoic acid | 79-09-4 | C3H6O2 | 74.1 | 680.9 | 178.111 | 1.10599 | 1.36 ± 0.02 | 0.26 ± 0.00 |
| 45 | Methyl butanoate | 623-42-7 | C5H10O2 | 102.1 | 742.3 | 224.148 | 1.15409 | 0.59 ± 0.12 | 1.60 ± 0.06 |
| 46 | 2-Butanone, 3-hydroxy- | 513-86-0 | C4H8O2 | 88.1 | 709.5 | 198.051 | 1.05898 | 0.78 ± 0.06 | 1.31 ± 0.03 |
| 47 | 3-methylbutanoic acid M | 503-74-2 | C5H10O2 | 102.1 | 835.6 | 312.998 | 1.21968 | 0.58 ± 0.20 | 0.94 ± 0.23 |
| 48 | 3-methylbutanoic acid D | 503-74-2 | C5H10O2 | 102.1 | 838.9 | 316.514 | 1.48612 | 0.17 ± 0.03 | 0.29 ± 0.08 |
| 49 | (E)-2-Heptenal M | 18829-55-5 | C7H12O | 112.2 | 959.8 | 497.383 | 1.25623 | 0.72 ± 0.00 | 1.23 ± 0.02 |
| 50 | (E)-2-Heptenal D | 18829-55-5 | C7H12O | 112.2 | 959.2 | 496.234 | 1.67137 | 0.09 ± 0.01 | 0.27 ± 0.01 |
| 51 | 1-Penten-3-ol | 616-25-1 | C5H10O | 86.1 | 681.6 | 178.563 | 1.35608 | 1.02 ± 0.01 | 6.35 ± 0.04 |
| 52 | 1 | Unidentified | 0 | 722.1 | 207.666 | 1.19022 | 0.31 ± 0.01 | 0.85 ± 0.00 | |
| 53 | 2 | Unidentified | 0 | 761.1 | 240.675 | 1.06706 | 0.43 ± 0.03 | 2.10 ± 0.02 | |
| Total | Aldehydes | 40.75 | 38.56 | ||||||
| Alcohols | 38.011 | 32.14 | |||||||
| Acids | 14.58 | 4.86 | |||||||
| Esters | 3.61 | 17.73 | |||||||
| Ketones | 2.33 | 3.75 | |||||||
| No. | Label | Odor Detection Threshold (mg/kg) | ROAV Value | Aroma/Flavor Description | |
|---|---|---|---|---|---|
| y1 | y2 | ||||
| 1 | Nonanal M | 0.0035 | 1.33 | 0.98 | Oily, citrus, grass |
| 2 | Nonanal D | 0.0035 | 0.26 | 0.18 | Oily, citrus, grass |
| 3 | 1-Octanol M | 0.054 | 0.03 | 0.01 | |
| 4 | 1-Octanol D | 0.054 | 0.01 | 0 | |
| 5 | (E)-2-Octenal M | 0.003 | 0.19 | 0.56 | Grass, nutty, oily |
| 6 | (E)-2-Octenal D | 0.003 | 0.04 | 0.1 | Grass, nutty, oily |
| 7 | Octanal M | 0.0001 | 100 | 100 | Oily, soap, lemon, grass |
| 8 | Octanal D | 0.0001 | 82.49 | 54.37 | Oily, soap, lemon, grass |
| 9 | 1-Heptanol M | 0.2 | 0.06 | 0.03 | |
| 10 | 1-Heptanol D | 0.2 | 0.02 | 0.01 | |
| 11 | Benzaldehyde M | 0.3 | 0.01 | 0.02 | |
| 12 | Benzaldehyde D | 0.3 | 0 | 0.01 | |
| 13 | Butyrolactone M | 10 | 0 | 0 | |
| 14 | Butyrolactone D | 10 | 0 | 0 | |
| 15 | Hexanoic acid M | 0.7 | 0.01 | 0 | |
| 16 | Hexanoic acid D | 0.7 | 0 | 0 | |
| 17 | Heptanal M | 0.05 | 0.12 | 0.13 | Oily, citrus |
| 18 | Heptanal D | 0.05 | 0.07 | 0.09 | Oily, citrus |
| 19 | 1-Hexanol M | 0.2 | 0.03 | 0.02 | |
| 20 | 1-Hexanol D | 0.2 | 0.01 | 0 | |
| 21 | 2-Heptanone M | 1.5 | 0 | 0 | |
| 22 | 2-Heptanone D | 1.5 | 0 | 0 | |
| 23 | Furan-2-carbaldehyde M | 0.7 | 0 | 0 | |
| 24 | Furan-2-carbaldehyde | 0.7 | 0 | 0 | |
| 25 | Hexanal M | 0.0075 | 0.81 | 0.69 | Grass, fatty, oily |
| 26 | Hexanal D | 0.0075 | 2.15 | 2.9 | Grass, fatty, oily |
| 27 | 1-Pentanol M | 0.47 | 0.01 | 0.01 | |
| 28 | 1-Pentanol D | 0.47 | 0.01 | 0.01 | |
| 29 | 1-Butanol, 3-methyl M | 0.25 | 0 | 0.02 | |
| 30 | 1-Butanol, 3-methyl D | 0.25 | 0 | 0.01 | |
| 31 | n-Pentanal M | 0.012 | 0.4 | 0.21 | Almond, malt |
| 32 | n-Pentanal D | 0.012 | 0.27 | 0.3 | Almond, malt |
| 33 | 2-Methylbutanal | 0.0125 | 0.28 | 0.63 | Cocoa, almond |
| 34 | 1-Butanol M | 100 | 0 | 0 | |
| 35 | 1-Butanol D | 100 | 0 | 0 | |
| 36 | 2-Butanone | 15 | 0 | 0 | |
| 37 | Acetic acid M | 0.5 | 0.03 | 0.01 | |
| 38 | Acetic acid D | 0.5 | 0 | 0 | |
| 39 | 2-Methylpropan-1-ol D | 100 | 0 | 0 | |
| 40 | 2-Methylpropan-1-ol M | 100 | 0 | 0 | |
| 41 | Ethyl Acetate | 1.7 | 0 | 0.01 | |
| 42 | 1-Propanol D | 270 | 0 | 0 | |
| 43 | 1-Propanol M | 270 | 0 | 0 | |
| 44 | Propanoic acid | 0.72 | 0 | 0 | |
| 45 | Methyl butanoate | 0.5 | 0 | 0.01 | |
| 46 | 2-Butanone, 3-hydroxy- | 5 | 0 | 0 | |
| 47 | 3-methylbutanoic acid M | 0.026 | 0.04 | 0.09 | |
| 48 | 3-methylbutanoic acid D | 0.026 | 0.01 | 0.03 | |
| 49 | (E)-2-Heptenal M | 0.013 | 0.1 | 0.22 | Soap, fatty, almond |
| 50 | (E)-2-Heptenal D | 0.013 | 0.01 | 0.05 | Soap, fatty, almond |
| 51 | 1-Penten-3-ol | 0.35 | 0.01 | 0.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Gao, J.; Zhang, Z.; Zhu, X.; Chen, B.; Chen, Y.; Deng, S.; Li, Z.; Chen, X. Impact of Drying Processes for Camellia Seeds on the Volatile Compounds of Camellia Seed Oil. Processes 2024, 12, 1332. https://doi.org/10.3390/pr12071332
Ma L, Gao J, Zhang Z, Zhu X, Chen B, Chen Y, Deng S, Li Z, Chen X. Impact of Drying Processes for Camellia Seeds on the Volatile Compounds of Camellia Seed Oil. Processes. 2024; 12(7):1332. https://doi.org/10.3390/pr12071332
Chicago/Turabian StyleMa, Li, Jing Gao, Zhen Zhang, Xiaoyang Zhu, Bolin Chen, Yongzhong Chen, Senwen Deng, Zhigang Li, and Xinzhi Chen. 2024. "Impact of Drying Processes for Camellia Seeds on the Volatile Compounds of Camellia Seed Oil" Processes 12, no. 7: 1332. https://doi.org/10.3390/pr12071332
APA StyleMa, L., Gao, J., Zhang, Z., Zhu, X., Chen, B., Chen, Y., Deng, S., Li, Z., & Chen, X. (2024). Impact of Drying Processes for Camellia Seeds on the Volatile Compounds of Camellia Seed Oil. Processes, 12(7), 1332. https://doi.org/10.3390/pr12071332










