Paraquat Removal from Water by Magnetic Nanoparticles Coated with Waste-Sourced Biobased Substances
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Magnetic Nanoparticles
2.3. Characterization Techniques
2.4. Adsorption Experiments
3. Results and Discussion
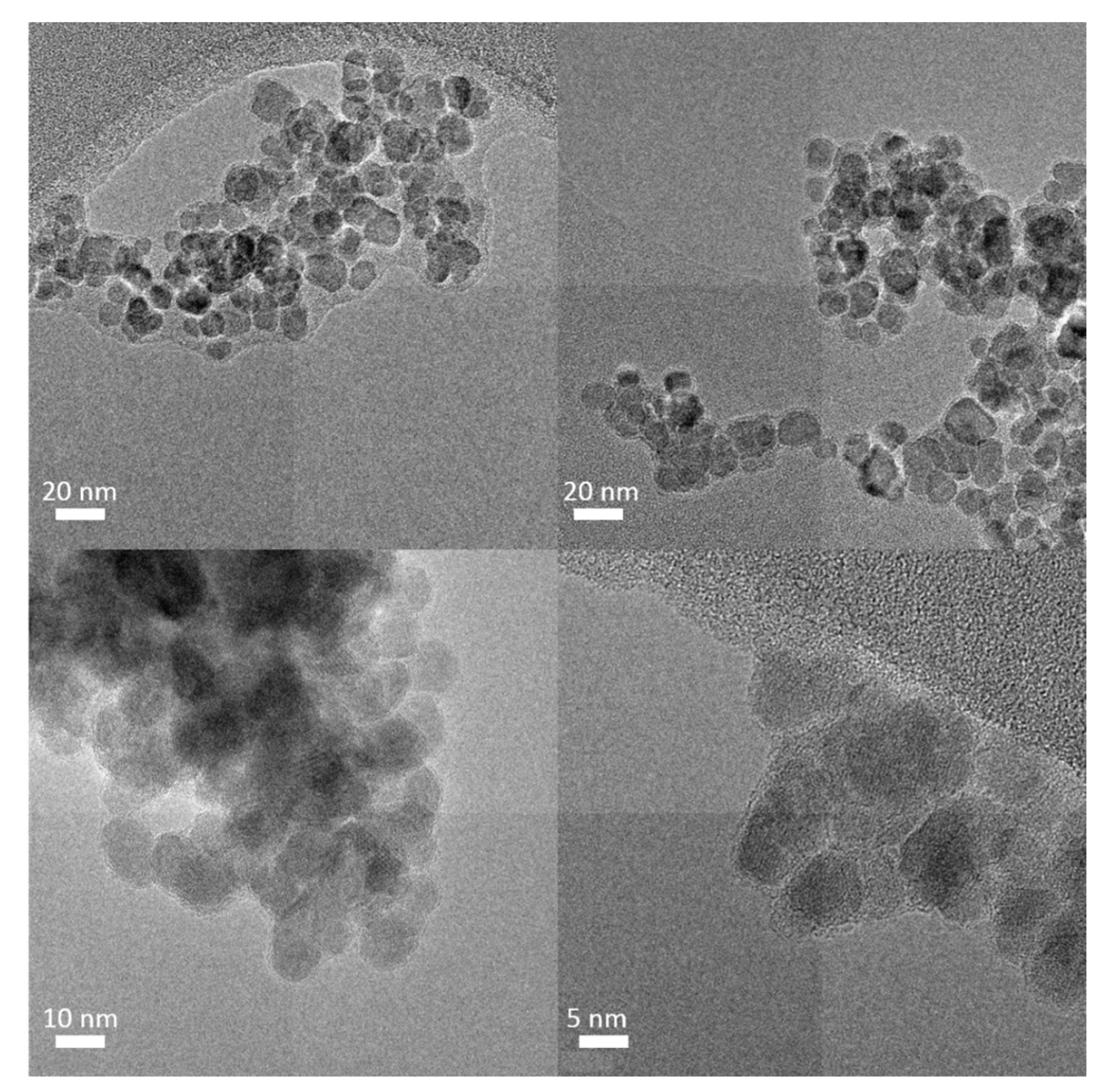
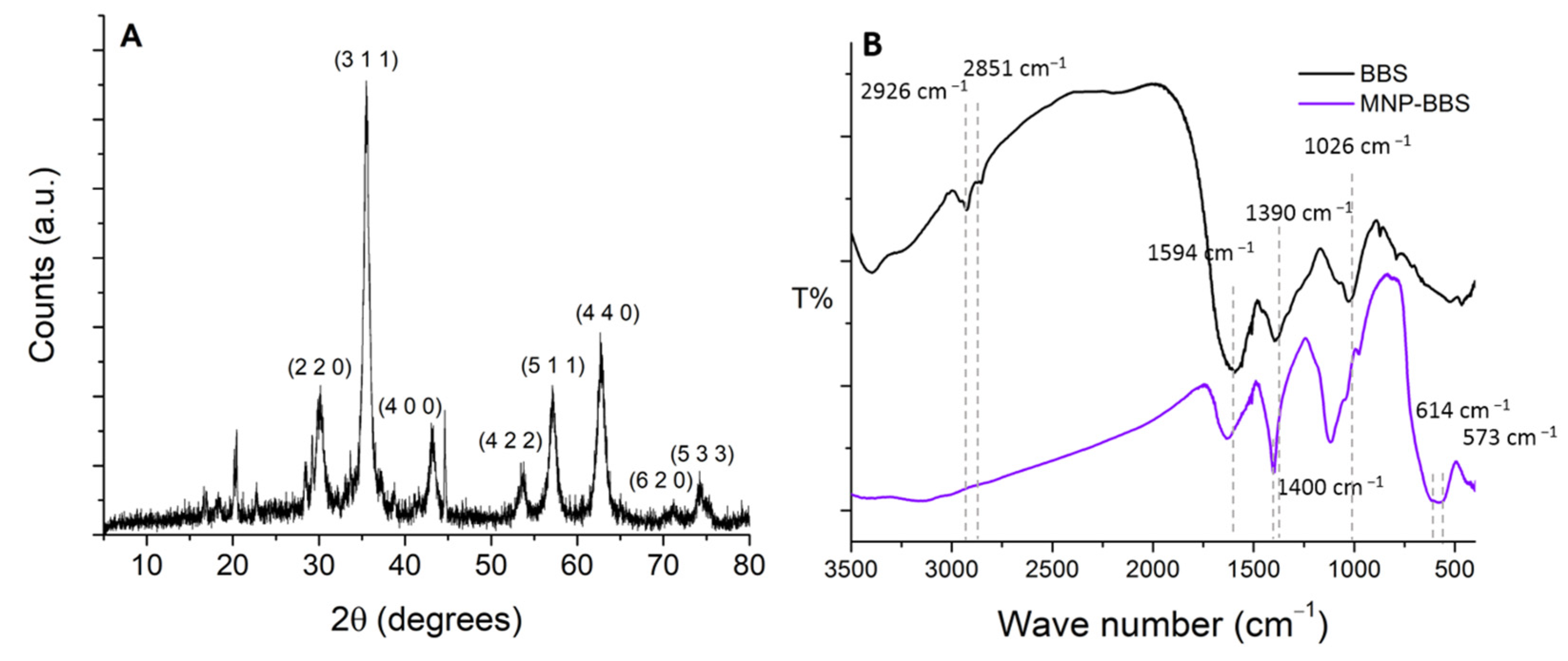
3.1. Characterization
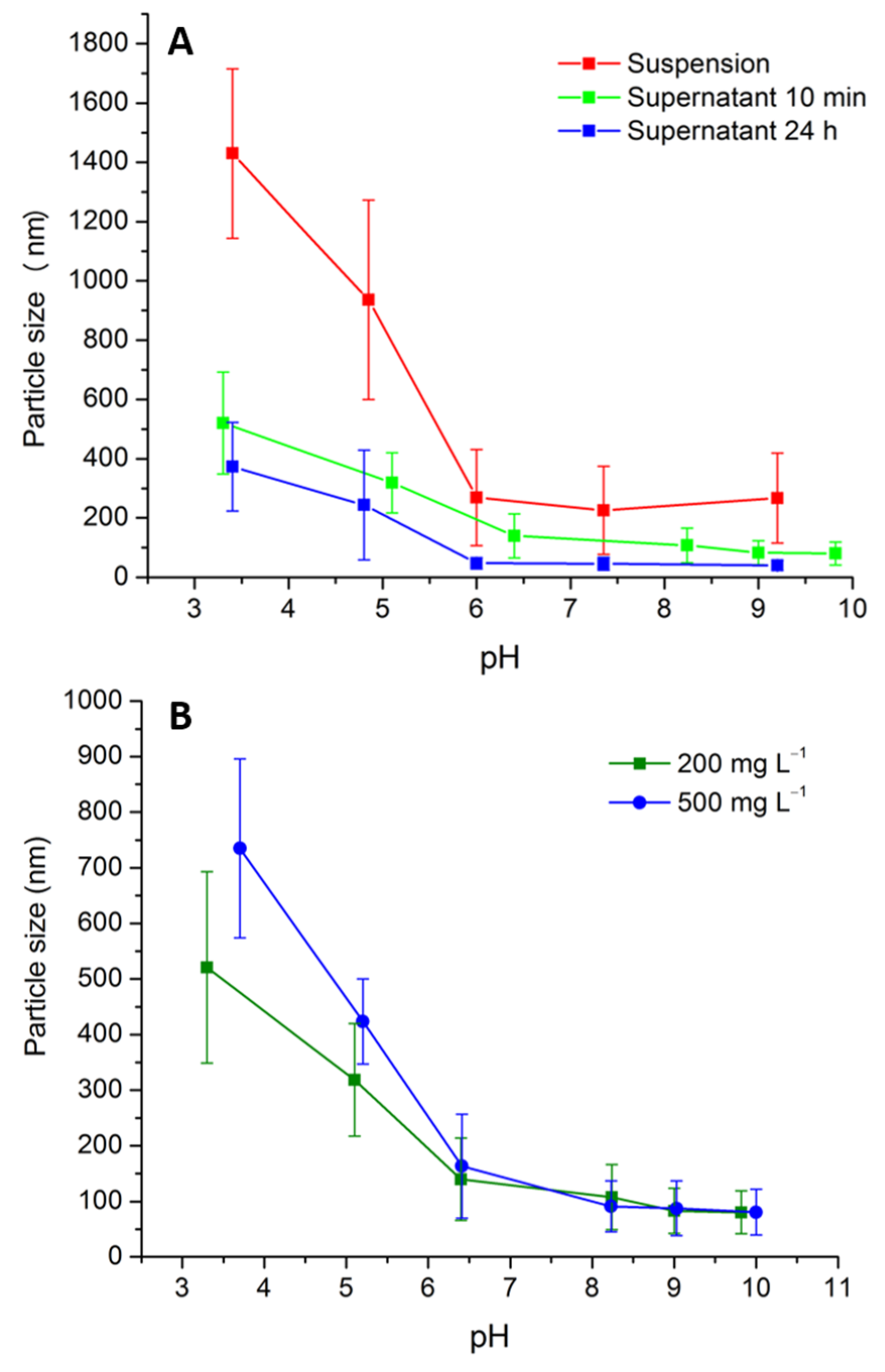
3.2. Aggregate Size and Magnetic Separation
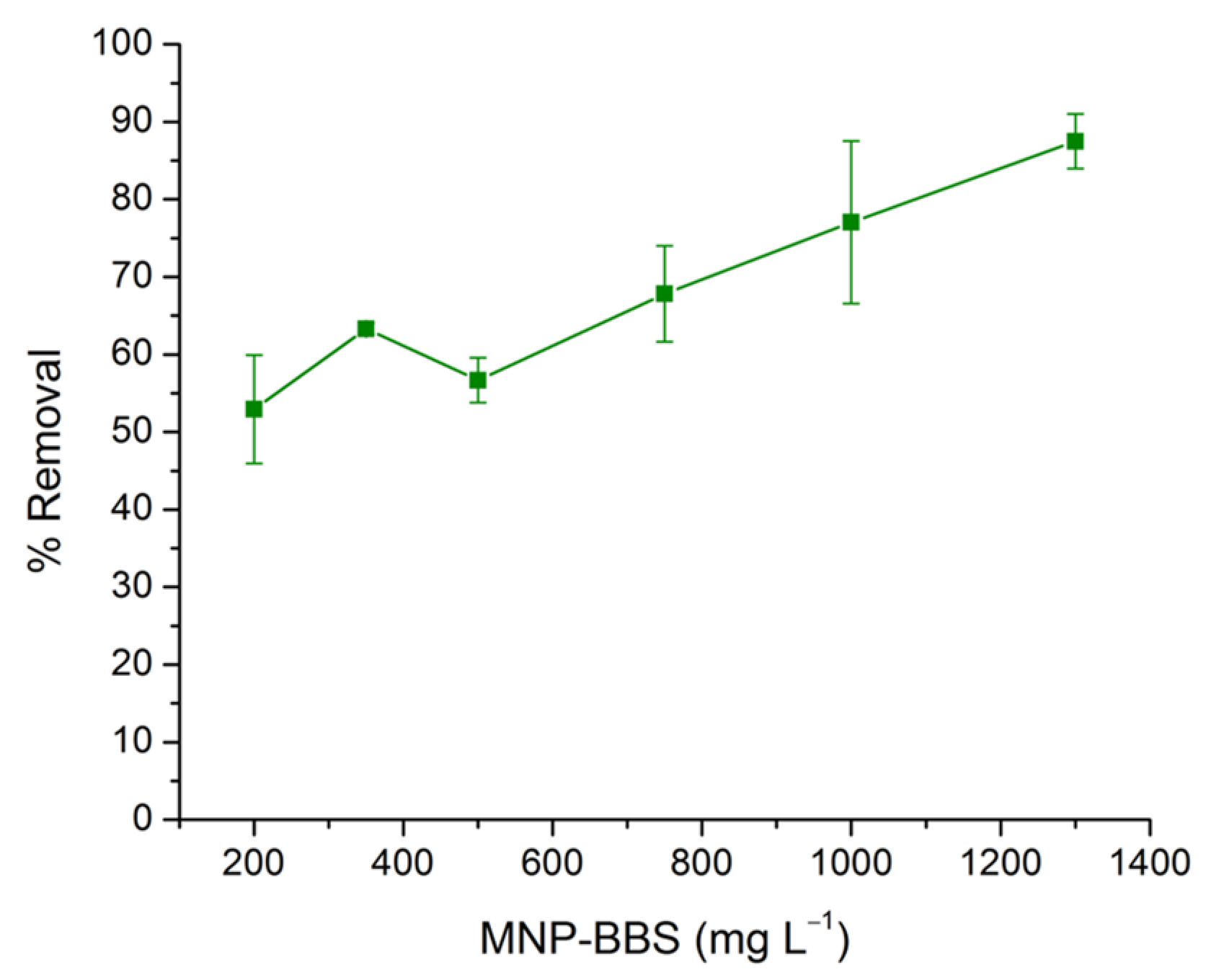
3.3. Adsorption Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loewy, R.M.; Monza, L.B.; Kirs, V.E.; Savini, M.C. Pesticide Distribution in an Agricultural Environment in Argentina. J. Environ. Sci. Health Part B 2011, 46, 37–41. [Google Scholar]
- Gensch, L.; Jantke, K.; Rasche, L.; Schneider, U.A. Pesticide Risk Assessment in European Agriculture: Distribution Patterns, Ban-Substitution Effects and Regulatory Implications. Environ. Pollut. 2024, 348, 123836. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Amin, S.M.N.; Rahman, M.A.; Juraimi, A.S.; Uddin, M.K.; Brown, C.L.; Arshad, A. Chronic Effects of Organic Pesticides on the Aquatic Environment and Human Health: A Review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100740. [Google Scholar] [CrossRef]
- Peña-Acevedo, L.M.; Ballesteros-Castro, D.A.; Sukumar, C.A. Paraquat. Encycl. Toxicol. Fourth Ed. 2023, 7, 269–281. [Google Scholar] [CrossRef]
- Donley, N. The USA Lags behind other Agricultural Nations in Banning Harmful Pesticides. Environ. Health 2019, 18, 44. [Google Scholar] [CrossRef]
- Franco, D.S.P.; Georgin, J.; Lima, E.C.; Silva, L.F.O. Advances Made in Removing Paraquat Herbicide by Adsorption Technology: A Review. J. Water Process. Eng. 2022, 49, 102988. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered Nanomaterials for Water Treatment and Remediation: Costs, Benefits, and Applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of Iron Oxide Nanomaterials in Wastewater Treatment: A Review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pastora, J.; Bringas, E.; Ortiz, I. Recent Progress and Future Challenges on the Use of High Performance Magnetic Nano-Adsorbents in Environmental Applications. Chem. Eng. J. 2014, 256, 187–204. [Google Scholar] [CrossRef]
- Peralta, M.E.; Ocampo, S.; Funes, I.G.; Medina, F.O.; Parolo, M.E.; Carlos, L. Nanomaterials with Tailored Magnetic Properties as Adsorbents of Organic Pollutants from Wastewaters. Inorganics 2020, 8, 24. [Google Scholar] [CrossRef]
- Ananpattarachai, J.; Aphaiphak, P.; Ard-Ong, R.; Kajitvichyanukul, P.; Hung, Y.T. Adsorption Isotherm and Kinetics of Paraquat Removal Using Activated Carbon/Iron Oxide Composite Material. Int. J. Environ. Waste Manag. 2016, 17, 189–202. [Google Scholar] [CrossRef]
- Fernandes, T.; Soares, S.F.; Trindade, T.; Daniel-Da-silva, A.L. Magnetic Hybrid Nanosorbents for the Uptake of Paraquat from Water. Nanomaterials 2017, 7, 68. [Google Scholar] [CrossRef]
- Ahmad, F.A. The Use of Agro-Waste-Based Adsorbents as Sustainable, Renewable, and Low-Cost Alternatives for the Removal of Ibuprofen and Carbamazepine from Water. Heliyon 2023, 9, e16449. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, L.; Luo, J.; Gong, H.; Zhu, N. A Sustainable Reuse Strategy of Converting Waste Activated Sludge into Biochar for Contaminants Removal from Water: Modifications, Applications and Perspectives. J. Hazard. Mater. 2022, 438, 129437. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Wang, J.; Liu, Y.; Zhang, M.; Zhao, L.; Gu, S.; Lin, R.; Chen, L. Research Progress on the Application of Natural Adsorbents in the Treatment of Livestock Wastewater. Desalination Water Treat 2024, 317, 100018. [Google Scholar] [CrossRef]
- Tsoutsa, E.K.; Tolkou, A.K.; Kyzas, G.Z.; Katsoyiannis, I.A. An Update on Agricultural Wastes Used as Natural Adsorbents or Coagulants in Single or Combined Systems for the Removal of Dyes from Wastewater. Water Air Soil Pollut. 2024, 235, 178. [Google Scholar] [CrossRef]
- Hamadi, N.K.; Swaminathan, S.; Chen, X.D. Adsorption of Paraquat dichloride from aqueous solution by activated carbon derived from used tires. J. Hazard. Mater. 2004, 112, 133–141. [Google Scholar] [CrossRef]
- Damdib, S.; Siyasukh, A.; Vanichsetakul, B.; Phamornpiboon, P.; Thanachayanont, C.; Punyapalakul, P.; Tonanon, N. Efficient Removal of Paraquat Pollutants Using Magnetic Biochar Derived from Corn Husk Waste: A Sustainable Approach for Water Remediation. Adsorpt Sci. Technol. 2023, 2023, 5512881. [Google Scholar]
- Nisticò, R.; Celi, L.R.; Bianco Prevot, A.; Carlos, L.; Magnacca, G.; Zanzo, E.; Martin, M. Sustainable Magnet-Responsive Nanomaterials for the Removal of Arsenic from Contaminated Water. J. Hazard. Mater. 2018, 342, 260–269. [Google Scholar] [CrossRef]
- Nisticò, R.; Cesano, F.; Franzoso, F.; Magnacca, G.; Scarano, D.; Funes, I.G.; Carlos, L.; Parolo, M.E. From Biowaste to Magnet-Responsive Materials for Water Remediation from Polycyclic Aromatic Hydrocarbons. Chemosphere 2018, 202, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Magnacca, G.; Allera, A.; Montoneri, E.; Celi, L.; Benito, D.E.; Gagliardi, L.G.; Gonzalez, M.C.; Mártire, D.O.; Carlos, L. Novel Magnetite Nanoparticles Coated with Waste-Sourced Biobased Substances as Sustainable and Renewable Adsorbing Materials. ACS Sustain. Chem. Eng. 2014, 2, 1518–1524. [Google Scholar] [CrossRef]
- Nisticò, R.; Barrasso, M.; Carrillo Le Roux, G.A.; Seckler, M.M.; Sousa, W.; Malandrino, M.; Magnacca, G. Biopolymers from Composted Biowaste as Stabilizers for the Synthesis of Spherical and Homogeneously Sized Silver Nanoparticles for Textile Applications on Natural Fibers. ChemPhysChem 2015, 16, 3902–3909. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.M.; Shukla, D.K.; Meneses, C.T.; Mendoza Zélis, P.; Singh, M.; Sharma, S.K. Synthesis, Phase Composition, Mössbauer and Magnetic Characterization of Iron Oxide Nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 9561–9568. [Google Scholar]
- Yamaura, M.; Camilo, R.L.; Sampaio, L.C.; Macêdo, M.A.; Nakamura, M.; Toma, H.E. Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- Ou, X.; Chen, S.; Quan, X.; Zhao, H. Photochemical Activity and Characterization of the Complex of Humic Acids with Iron(III). J. Geochem. Explor. 2009, 102, 49–55. [Google Scholar] [CrossRef]
- Gu, B.; Chen, Z.; Mccarthyt, J.F.; Divlslon, E.S.; Division, C.; Ridge, O. Adsorption and Desorption of Natural Organic Matter on Iron Oxide: Mechanisms and Models. Environ. Sci. Technol. 1994, 28, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Agmo Hernández, V. An overview of surface forces and the DLVO theory. ChemTexts 2023, 9, 10. [Google Scholar] [CrossRef]
- Stephens, J.R.; Beveridge, J.S.; Williams, M.E. Analytical Methods for Separating and Isolating Magnetic Nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 3280–3289. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, A.; López, R.; Gondar, D.; Antelo, J.; Fiol, S.; Arce, F. Effect of pH and Ionic Strength on the Binding of Paraquat and MCPA by Soil Fulvic and Humic Acids. Chemosphere 2009, 76, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gondar, D.; López, R.; Antelo, J.; Fiol, S.; Arce, F. Adsorption of Paraquat on Soil Organic Matter: Effect of Exchangeable Cations and Dissolved Organic Carbon. J. Hazard. Mater. 2012, 235–236, 218–223. [Google Scholar] [CrossRef]
- Seki, Y.; Yurdakoç, K. Paraquat Adsorption onto Clays and Organoclays from Aqueous Solution. J. Colloid Interface Sci. 2005, 287, 1–5. [Google Scholar] [CrossRef]
- Osakoo, N.; Pansakdanon, C.; Sosa, N.; Deekamwong, K.; Keawkumay, C.; Rongchapo, W.; Chanlek, N.; Jitcharoen, J.; Prayoonpokarach, S.; Wittayakun, J. Characterization and Comprehension of Zeolite NaY/Mesoporous SBA-15 Composite as Adsorbent for Paraquat. Mater. Chem. Phys. 2017, 193, 470–476. [Google Scholar] [CrossRef]
- Rongchapo, W.; Sophiphun, O.; Rintramee, K.; Prayoonpokarach, S.; Wittayakun, J. Paraquat Adsorption on Porous Materials Synthesized from Rice Husk Silica. Water Sci. Technol. 2013, 68, 863–869. [Google Scholar] [CrossRef] [PubMed]





| Kinetic Parameters | MNP-BBS (200 mg L−1) | MNP-BBS (500 mg L−1) | MNP-BBS (1000 mg L−1) |
|---|---|---|---|
| qe (mg g−1) | 0.41 | 0.37 | 0.28 |
| k (g mg−1 seg−1) | 0.13 | 0.24 | 1.59 |
| R2 | 0.993 | 0.999 | 0.998 |
| Adsorbents | Langmuir Model | Freundlich Model | Ref. | ||||
|---|---|---|---|---|---|---|---|
| qmax (mmol g−1) | KL (mmol−1) | R2 | KF (L−1 mmol(n−1/n) g−1) | n | R2 | ||
| MNP-BBS | 0.085 | 43 | 0.86 | 0.13 | 3.05 | 0.96 | this work |
| Sepiolite | 0.048 | 411 | 0.91 | 0.07 | 6.94 | - | [31] |
| Bentonite | 0.0165 | 732 | 0.99 | 0.19 | 15 | - | [31] |
| SBA-15 | 0.036 | - | 0.98 | [32] | |||
| MCM-41 | 0.083 | - | 0.98 | [33] | |||
| Activated carbon | 0.29 | 5.9 | 0.99 | [17] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocampo, S.; Parolo, M.E.; Carlos, L. Paraquat Removal from Water by Magnetic Nanoparticles Coated with Waste-Sourced Biobased Substances. Processes 2024, 12, 1339. https://doi.org/10.3390/pr12071339
Ocampo S, Parolo ME, Carlos L. Paraquat Removal from Water by Magnetic Nanoparticles Coated with Waste-Sourced Biobased Substances. Processes. 2024; 12(7):1339. https://doi.org/10.3390/pr12071339
Chicago/Turabian StyleOcampo, Santiago, María Eugenia Parolo, and Luciano Carlos. 2024. "Paraquat Removal from Water by Magnetic Nanoparticles Coated with Waste-Sourced Biobased Substances" Processes 12, no. 7: 1339. https://doi.org/10.3390/pr12071339
APA StyleOcampo, S., Parolo, M. E., & Carlos, L. (2024). Paraquat Removal from Water by Magnetic Nanoparticles Coated with Waste-Sourced Biobased Substances. Processes, 12(7), 1339. https://doi.org/10.3390/pr12071339







