Adsorption of Glyphosate in Water Using Iron-Based Water Treatment Residuals Derived from Drinking Water Treatment Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Fe-WTRs and Glyphosate Aqueous Solutions
2.2. Experimental Set-Up
2.3. Sample Analysis
2.4. Evaluation of Adsorption Isotherms, Kinetics, and Thermodynamics
2.4.1. Absorption Isotherms
2.4.2. Kinetics
2.4.3. Thermodynamics
3. Results and Discussion
3.1. Main Components and Microscopic Analysis of Fe-WTRs
3.2. Glyphosate Adsorption Capacity of Fe-WTRs
3.2.1. Effect of pH
3.2.2. Effect of Fe-WTR Particle Size
3.2.3. Adsorption Isotherms
3.2.4. Comparison of the Adsorption Capacity of Fe-WTRs with Other Adsorbents for Glyphosate
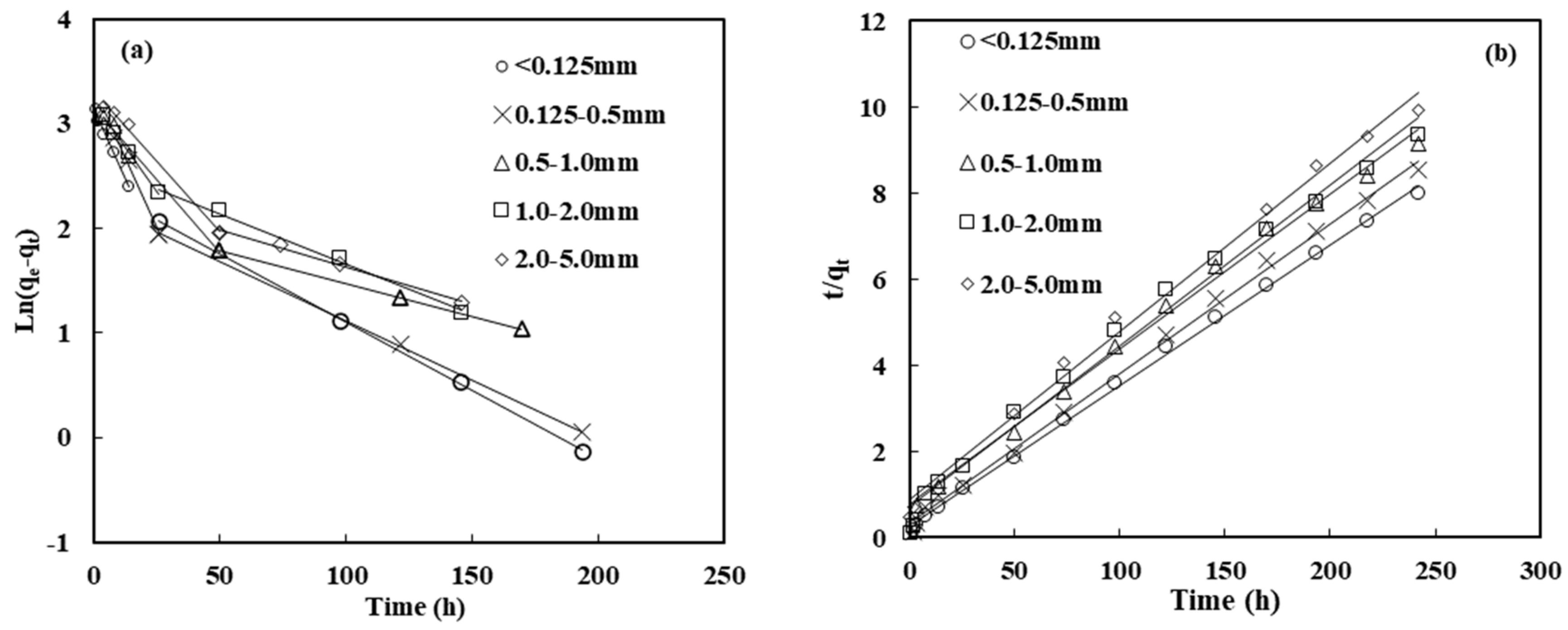
3.3. Adsorption Kinetics
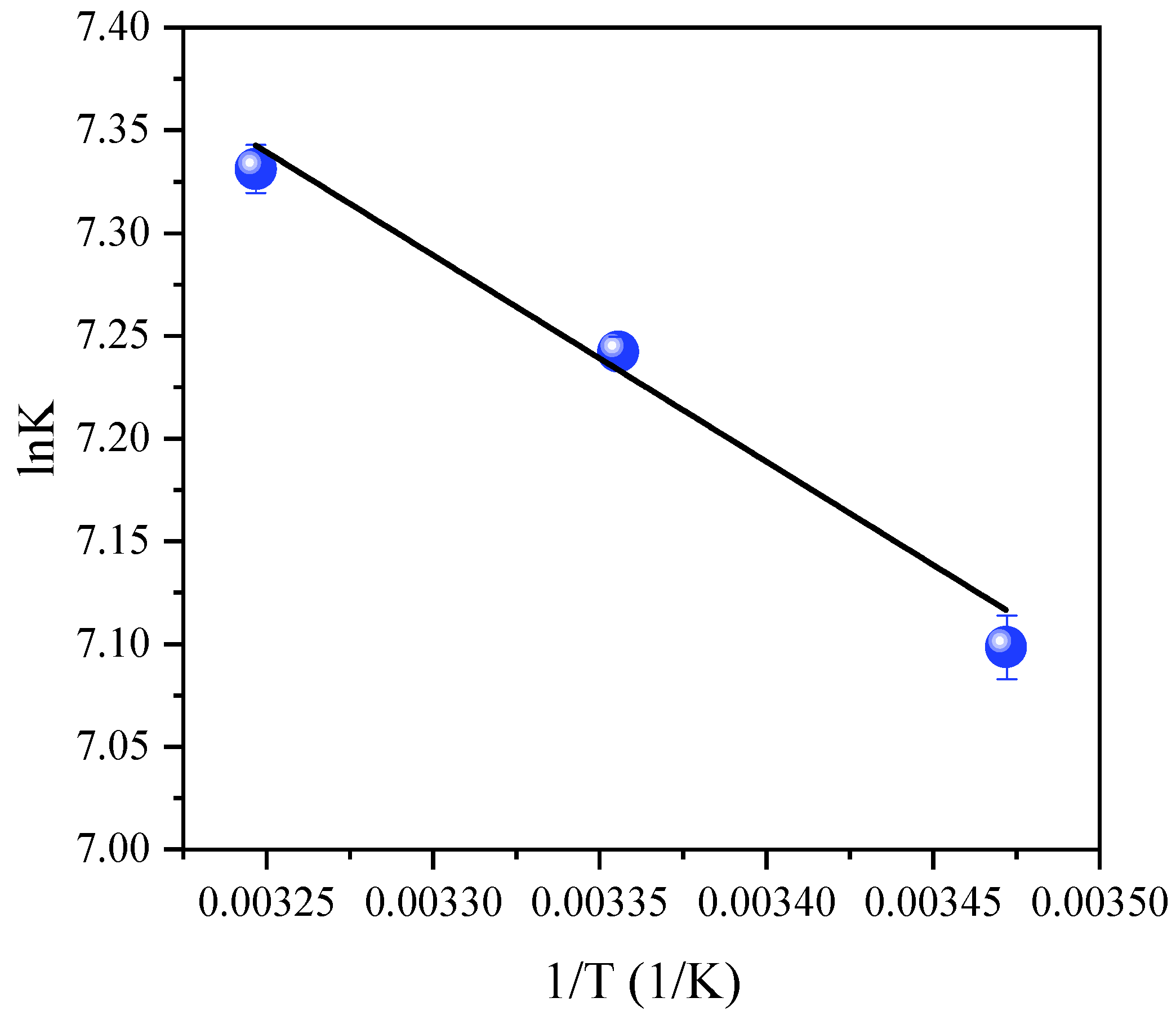
3.4. Thermodynamic Properties
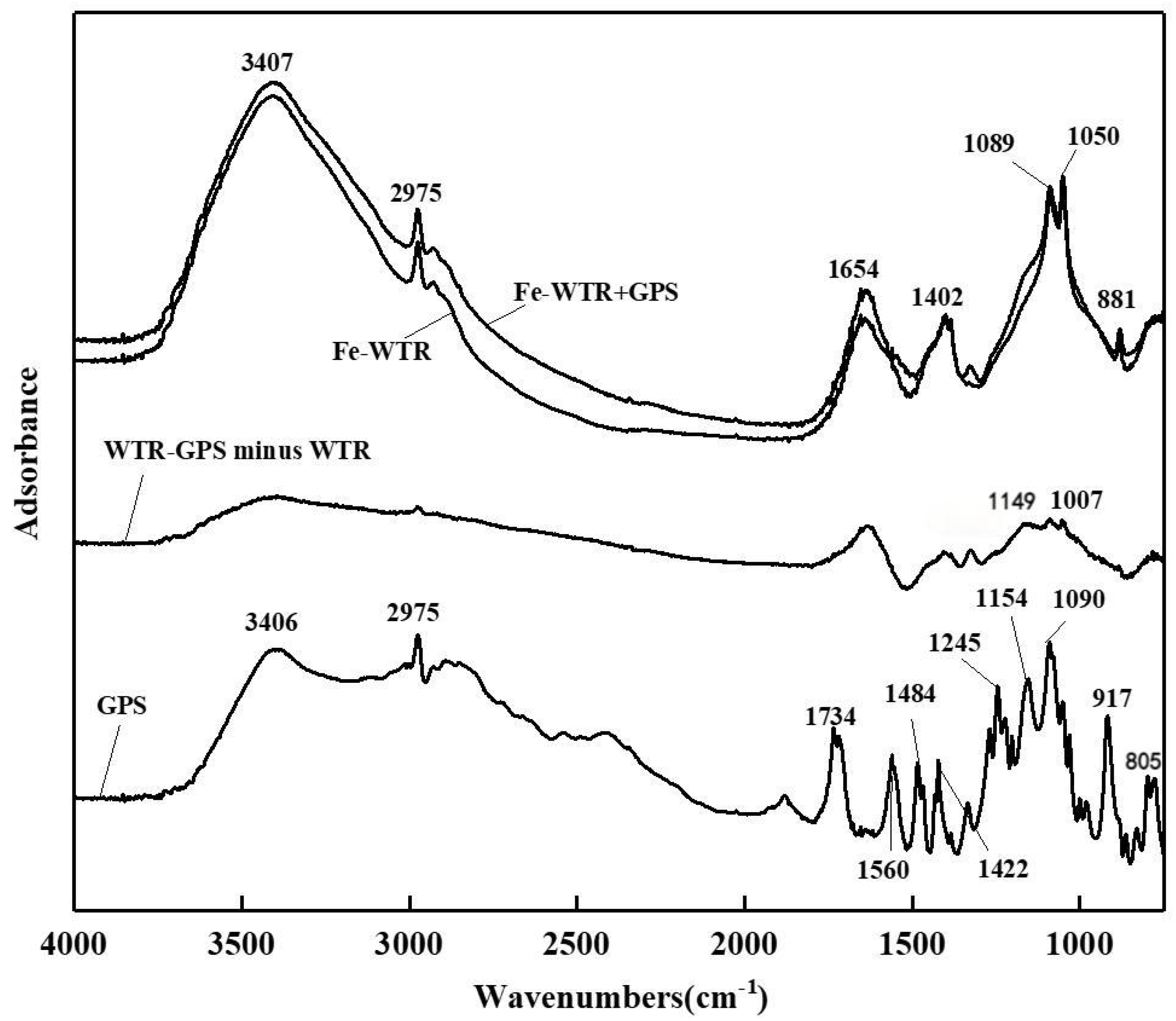
3.5. FTIR Spectral Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ighalo, J.O.; Adeniyi, A.G.; Eletta, O.A.A.; Arowoyele, L.T. Competitive Adsorption of Pb(II), Cu(II), Fe(II) and Zn(II) from Aqueous Media Using Biochar from Oil Palm (Elaeis guineensis) Fibers: A Kinetic and Equilibrium Study. Indian Chem. Eng. 2021, 63, 501–511. [Google Scholar] [CrossRef]
- Jeirani, Z.; Niu, C.H.; Soltan, J. Adsorption of Emerging Pollutants on Activated Carbon. Rev. Chem. Eng. 2017, 33, 491–522. [Google Scholar] [CrossRef]
- Hoppin, J.A.; Adgate, J.L.; Eberhart, M.; Nishioka, M.; Ryan, P.B. Environmental Exposure Assessment of Pesticides in Farmworker Homes. Environ. Health Perspect. 2006, 114, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.L.; Mello, F.C.C.; Alves-Balvedi, R.P.; Rodrigues, L.P.; Goulart, L.R. Glyphosate Detection: Methods, Needs and Challenges. Environ. Chem. Lett. 2019, 17, 291–317. [Google Scholar] [CrossRef]
- Sihtmäe, M.; Blinova, I.; Künnis-Beres, K.; Kanarbik, L.; Heinlaan, M.; Kahru, A. Ecotoxicological Effects of Different Glyphosate Formulations. Appl. Soil Ecol. 2013, 72, 215–224. [Google Scholar] [CrossRef]
- Wang, S.; Seiwert, B.; Kästner, M.; Miltner, A.; Schäffer, A.; Reemtsma, T.; Yang, Q.; Nowak, K.M. (Bio)Degradation of Glyphosate in Water-Sediment Microcosms—A Stable Isotope Co-Labeling Approach. Water Res. 2016, 99, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.A.; Schulz-Bull, D.E.; Kanwischer, M. The Challenge of Detecting the Herbicide Glyphosate and Its Metabolite AMPA in Seawater—Method Development and Application in the Baltic Sea. Chemosphere 2021, 262, 128327. [Google Scholar] [CrossRef] [PubMed]
- Villamar-Ayala, C.A.; Carrera-Cevallos, J.V.; Vasquez-Medrano, R.; Espinoza-Montero, P.J. Fate, Eco-Toxicological Characteristics, and Treatment Processes Applied to Water Polluted with Glyphosate: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1476–1514. [Google Scholar] [CrossRef]
- Mahler, B.J.; Van Metre, P.C.; Burley, T.E.; Loftin, K.A.; Meyer, M.T.; Nowell, L.H. Similarities and Differences in Occurrence and Temporal Fluctuations in Glyphosate and Atrazine in Small Midwestern Streams (USA) during the 2013 Growing Season. Sci. Total Environ. 2017, 579, 149–158. [Google Scholar] [CrossRef]
- Saunders, L.; Pezeshki, R. Glyphosate in Runoff Waters and in the Root-Zone: A Review. Toxics 2015, 3, 462–480. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Zhang, Y.; Wu, X.; Zhou, Z.; Huang, Y.; Zhao, Y.; Mishra, S.; Bhatt, P.; Chen, S. Characterization of a Novel Glyphosate-Degrading Bacterial Species, Chryseobacterium sp. Y16C, and Evaluation of Its Effects on Microbial Communities in Glyphosate-Contaminated Soil. J. Hazard. Mater. 2022, 432, 128689. [Google Scholar] [CrossRef]
- Yang, X.; Wang, F.; Bento, C.P.M.; Meng, L.; van Dam, R.; Mol, H.; Liu, G.; Ritsema, C.J.; Geissen, V. Decay Characteristics and Erosion-Related Transport of Glyphosate in Chinese Loess Soil under Field Conditions. Sci. Total Environ. 2015, 530–531, 87–95. [Google Scholar] [CrossRef]
- Gasnier, C.; Dumont, C.; Benachour, N.; Clair, E.; Chagnon, M.-C.; Séralini, G.-E. Glyphosate-Based Herbicides Are Toxic and Endocrine Disruptors in Human Cell Lines. Toxicology 2009, 262, 184–191. [Google Scholar] [CrossRef]
- Wunnapuk, K.; Gobe, G.; Endre, Z.; Peake, P.; Grice, J.E.; Roberts, M.S.; Buckley, N.A.; Liu, X. Use of a Glyphosate-Based Herbicide-Induced Nephrotoxicity Model to Investigate a Panel of Kidney Injury Biomarkers. Toxicol. Lett. 2014, 225, 192–200. [Google Scholar] [CrossRef]
- Maddalon, A.; Galbiati, V.; Colosio, C.; Mandić-Rajčević, S.; Corsini, E. Glyphosate-Based Herbicides: Evidence of Immune-Endocrine Alteration. Toxicology 2021, 459, 152851. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the Key Characteristics of Endocrine-Disrupting Chemicals as a Basis for Hazard Identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Tarone, R.E. On the International Agency for Research on Cancer Classification of Glyphosate as a Probable Human Carcinogen. Eur. J. Cancer Prev. 2018, 27, 82. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, M.; Yu, P.; Xu, Y.; Zhao, X. Pretreatment of Membrane Separation of Glyphosate Mother Liquor Using a Precipitation Method. Desalination 2013, 313, 140–144. [Google Scholar] [CrossRef]
- Rubí-Juárez, H.; Cotillas, S.; Sáez, C.; Cañizares, P.; Barrera-Díaz, C.; Rodrigo, M.A. Use of Conductive Diamond Photo-Electrochemical Oxidation for the Removal of Pesticide Glyphosate. Sep. Purif. Technol. 2016, 167, 127–135. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y. Study on the Photocatalytic Degradation of Glyphosate by TiO2 Photocatalyst. Chemosphere 2007, 67, 1010–1017. [Google Scholar] [CrossRef]
- Serra-Clusellas, A.; Angelis, L.D.; Beltramo, M.; Bava, M.; Frankenberg, J.D.; Vigliarolo, J.; Giovanni, N.D.; Stripeikis, J.D.; Rengifo-Herrera, J.A.; de Cortalezzi, M.M.F. Glyphosate and AMPA Removal from Water by Solar Induced Processes Using Low Fe(III) or Fe(II) Concentrations. Environ. Sci. Water Res. Technol. 2019, 5, 1932–1942. [Google Scholar] [CrossRef]
- Zheng, T.; Sun, Y.; Lin, Y.; Wang, N.; Wang, P. Study on Preparation of Microwave Absorbing MnOx/Al2O3 Adsorbent and Degradation of Adsorbed Glyphosate in MW–UV System. Chem. Eng. J. 2016, 298, 68–74. [Google Scholar] [CrossRef]
- Assalin, M.R.; De Moraes, S.G.; Queiroz, S.C.N.; Ferracini, V.L.; Duran, N. Studies on Degradation of Glyphosate by Several Oxidative Chemical Processes: Ozonation, Photolysis and Heterogeneous Photocatalysis. J. Environ. Sci. Health Part B 2009, 45, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Deng, Q.; Yan, W.; Jing, C.; Zhang, Y. Comparative Study of Glyphosate Removal on Goethite and Magnetite: Adsorption and Photo-Degradation. Chem. Eng. J. 2018, 352, 581–589. [Google Scholar] [CrossRef]
- Feng, D.; Soric, A.; Boutin, O. Treatment Technologies and Degradation Pathways of Glyphosate: A Critical Review. Sci. Total Environ. 2020, 742, 140559. [Google Scholar] [CrossRef]
- Pereira, H.A.; Hernandes, P.R.T.; Netto, M.S.; Reske, G.D.; Vieceli, V.; Oliveira, L.F.S.; Dotto, G.L. Adsorbents for Glyphosate Removal in Contaminated Waters: A Review. Environ. Chem. Lett. 2021, 19, 1525–1543. [Google Scholar] [CrossRef]
- Rallet, D.; Paltahe, A.; Tsamo, C.; Loura, B. Synthesis of Clay-Biochar Composite for Glyphosate Removal from Aqueous Solution. Heliyon 2022, 8, e09112. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.S.; Zhao, Y.Q.; Sorohan, B. Removal of Glyphosate from Aqueous Environment by Adsorption Using Water Industrial Residual. Desalination 2011, 271, 150–156. [Google Scholar] [CrossRef]
- Dotor-Robayo, M.Y.; Guerrero-Dallos, J.A.; Martínez-Cordón, M.J. Influence of Monoammonium Phosphate on Glyphosate Adsorption-Desorption in Tropical Soils: Effect of the Order of Sorbate Additions. Chemosphere 2022, 303, 135030. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Yang, Q.; Evans, D.G.; Forano, C.; Duan, X. Study on Adsorption of Glyphosate (N-Phosphonomethyl Glycine) Pesticide on MgAl-Layered Double Hydroxides in Aqueous Solution. J. Hazard. Mater. 2005, 125, 89–95. [Google Scholar] [CrossRef]
- Marin, P.; Bergamasco, R.; Módenes, A.N.; Paraiso, P.R.; Hamoudi, S. Synthesis and Characterization of Graphene Oxide Functionalized with MnFe2O4 and Supported on Activated Carbon for Glyphosate Adsorption in Fixed Bed Column. Process Saf. Environ. Prot. 2019, 123, 59–71. [Google Scholar] [CrossRef]
- Dissanayake Herath, G.A.; Poh, L.S.; Ng, W.J. Statistical Optimization of Glyphosate Adsorption by Biochar and Activated Carbon with Response Surface Methodology. Chemosphere 2019, 227, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Darabdhara, J.; Ahmaruzzaman, M. Recent Developments in MOF and MOF Based Composite as Potential Adsorbents for Removal of Aqueous Environmental Contaminants. Chemosphere 2022, 304, 135261. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, P.; Liang, Z.; Zhao, Z.; Cui, F. Selective Adsorption of Anions on Hydrotalcite-like Compounds Derived from Drinking Water Treatment Residuals. Chemosphere 2022, 300, 134508. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Wang, J.; Zhao, D.; Fu, K. Adsorption of Myo-Inositol Hexakisphosphate in Water Using Recycled Water Treatment Residual. Environ. Sci. Pollut. Res. 2018, 25, 29593–29604. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, M.K.; Gagnon, G.A. Understanding Removal of Phosphate or Arsenate onto Water Treatment Residual Solids. J. Hazard. Mater. 2011, 186, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, M.K.; Mortula, M.M.; Gagnon, G.A. Phosphorus Adsorption on Water Treatment Residual Solids. J. Water Supply Res. Technol.-AQUA 2009, 58, 1–10. [Google Scholar] [CrossRef]
- Chamberlain, K.; Evans, A.A.; Bromilow, R.H. 1-Octanol/Water Partition Coefficient (Kow) and pKa for Ionisable Pesticides Measured by apH-Metric Method. Pestic. Sci. 1996, 47, 265–271. [Google Scholar] [CrossRef]
- Pereira, R.C.; Anizelli, P.R.; Mauro, E.D.; Valezi, D.F.; da Costa, A.C.S.; Zaia, C.T.B.V.; Zaia, D.A.M. The Effect of pH and Ionic Strength on the Adsorption of Glyphosate onto Ferrihydrite. Geochem. Trans. 2019, 20, 1–14. [Google Scholar] [CrossRef]
- Guan, X.-H.; Shang, C.; Zhu, J.; Chen, G.-H. ATR-FTIR Investigation on the Complexation of Myo-Inositol Hexaphosphate with Aluminum Hydroxide. J. Colloid Interface Sci. 2006, 293, 296–302. [Google Scholar] [CrossRef]
- Bhaumik, R.; Mondal, N.K. Adsorption of Fluoride from Aqueous Solution by a New Low-Cost Adsorbent: Thermally and Chemically Activated Coconut Fibre Dust. Clean Technol. Environ. Policy 2015, 17, 2157–2172. [Google Scholar] [CrossRef]
- Flaten, T.P.; Alfrey, A.C.; Birchall, J.D.; Savory, J.; Yokel, R.A. Status and future concerns of clinical and environmental aluminum toxicology. J. Toxicol. Environ. Health 1996, 48, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Ueda Yamaguchi, N.; Bergamasco, R.; Hamoudi, S. Magnetic MnFe2O4–Graphene Hybrid Composite for Efficient Removal of Glyphosate from Water. Chem. Eng. J. 2016, 295, 391–402. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, C.; Li, G.; Peng, F. Thermodynamics and Kinetics of Glyphosate Adsorption on Resin D301. Arab. J. Chem. 2016, 9, S1665–S1669. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Zhang, S.; Wang, S. Kinetics and Thermodynamics of Efficient Phosphorus Removal by a Complex Material. Desalination Water Treat. 2015, 56, 1949–1954. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Ahad, A.; Alam, M. Characterization of Water Treatment Sludge and Its Reuse as Coagulant. J. Environ. Manag. 2016, 182, 606–611. [Google Scholar] [CrossRef]
- Riesgraf, D.A.; May, M.L. Infrared Spectra of Aluminum Hydroxide Chlorides. Appl. Spectrosc. 1978, 32, 362–366. [Google Scholar] [CrossRef]
- McBride, M.; Kung, K. Complexation of Glyphosate and Related Ligands with Iron (III). Soil Sci. Soc. Am. J. 1989, 53, 1668–1673. [Google Scholar] [CrossRef]
- Undabeytia, T.; Morillo, E.; Maqueda, C. FTIR Study of Glyphosate−Copper Complexes. J. Agric. Food Chem. 2002, 50, 1918–1921. [Google Scholar] [CrossRef]
- Sheals, J.; Sjöberg, S.; Persson, P. Adsorption of Glyphosate on Goethite: Molecular Characterization of Surface Complexes. Environ. Sci. Technol. 2002, 36, 3090–3095. [Google Scholar] [CrossRef]
- Barja, B.C.; Dos Santos Afonso, M. Aminomethylphosphonic Acid and Glyphosate Adsorption onto Goethite: A Comparative Study. Environ. Sci. Technol. 2005, 39, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xie, Y.; Cui, K.; Xie, J.; Zhang, Y.; Huang, Y. Adsorption Behavior and Adsorption Mechanism of Glyphosate in Water by Amino-MIL-101(Fe). J. Phys. Chem. Solids 2022, 161, 110403. [Google Scholar] [CrossRef]
- Li, X.; Zhang, R.; Dong, F.; Tang, Y.; Xu, L.; Sun, C.; Zhang, H. Exploring Adsorption Mechanism of Glyphosate on Pristine and Elemental Doped Graphene. Chem. Phys. Lett. 2021, 779, 138849. [Google Scholar] [CrossRef]
- Arai, Y.; Sparks, D.L. ATR–FTIR Spectroscopic Investigation on Phosphate Adsorption Mechanisms at the Ferrihydrite–Water Interface. J. Colloid Interface Sci. 2001, 241, 317–326. [Google Scholar] [CrossRef]
- Balasubramaniam, R.; Ramesh Kumar, A.V. Characterization of Delhi Iron Pillar Rust by X-Ray Diffraction, Fourier Transform Infrared Spectroscopy and Mössbauer Spectroscopy. Corros. Sci. 2000, 42, 2085–2101. [Google Scholar] [CrossRef]




| Chemical Element | SSA and Pore Volume (Particle Size < 0.125 mm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe (%) | Si (%) | Al (%) | Ca (%) | Mg (%) | C (%) | O (%) | N (%) | pH | BET-N2 (m2/g) | MPSA (m2/g) | TPV (cm3/g) | MPV (cm3/g) | TAAPW (nm) |
| 15.90 | 8.81 | 6.74 | 2.78 | 0.43 | 10.50 | 51.90 | 1.26 | 7.42 | 91.74 | 87.74 | 0.99 | 0.11 | 4.77 |
| Temperature (°C) | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qm | b | R2 | kF | n | R2 | B | kT | R2 | |
| 15 | 27.78 | 0.007 | 0.99 | 0.48 | 1.48 | 0.95 | 5.69 | 0.08 | 0.96 |
| 25 | 36.10 | 0.008 | 0.98 | 0.80 | 1.56 | 0.98 | 7.74 | 0.09 | 0.93 |
| 35 | 53.19 | 0.009 | 0.97 | 1.01 | 1.46 | 0.96 | 10.77 | 0.11 | 0.95 |
| Model | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | qe,exp (mg/g) | Fast Stage | Slow Stage | k2 (g/mg·h) | qe,cal (mg/g) | h (mg/g·h) | R2 | |||||
| k1f (1/h) | qe,cal (mg/g) | R2 | k1s (1/h) | qe,cal (mg/g) | R2 | |||||||
| Particle size (mm) | <0.125 | 30.25 | 0.054 | 22.87 | 0.99 | 0.012 | 9.61 | 0.98 | 0.004 | 30.58 | 3.70 | 0.99 |
| 0.125–0.5 | 28.33 | 0.052 | 27.23 | 0.99 | 0.010 | 8.08 | 0.95 | 0.004 | 28.65 | 3.06 | 0.99 | |
| 0.5–1 | 26.43 | 0.025 | 20.28 | 0.97 | 0.006 | 7.64 | 0.98 | 0.002 | 27.78 | 1.28 | 0.99 | |
| 1–2 | 25.86 | 0.029 | 22.22 | 0.95 | 0.011 | 14.04 | 0.96 | 0.002 | 26.67 | 1.42 | 0.99 | |
| 2–5 | 24.36 | 0.026 | 27.64 | 0.98 | 0.007 | 9.94 | 0.96 | 0.002 | 25.51 | 1.17 | 0.99 | |
| Temperature (K) | K or b (L/mol) | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (J/mol·K) | R2 |
|---|---|---|---|---|---|
| 288 | 1209.93 | −17.01 | 8.61 | 88.97 | 0.99 |
| 298 | 1397.07 | −17.90 | |||
| 308 | 1527.28 | −18.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, F.; Li, C.; Wang, S.; Li, S. Adsorption of Glyphosate in Water Using Iron-Based Water Treatment Residuals Derived from Drinking Water Treatment Plants. Processes 2024, 12, 1352. https://doi.org/10.3390/pr12071352
Qiu F, Li C, Wang S, Li S. Adsorption of Glyphosate in Water Using Iron-Based Water Treatment Residuals Derived from Drinking Water Treatment Plants. Processes. 2024; 12(7):1352. https://doi.org/10.3390/pr12071352
Chicago/Turabian StyleQiu, Fuguo, Chaoran Li, Shunxi Wang, and Shuang Li. 2024. "Adsorption of Glyphosate in Water Using Iron-Based Water Treatment Residuals Derived from Drinking Water Treatment Plants" Processes 12, no. 7: 1352. https://doi.org/10.3390/pr12071352
APA StyleQiu, F., Li, C., Wang, S., & Li, S. (2024). Adsorption of Glyphosate in Water Using Iron-Based Water Treatment Residuals Derived from Drinking Water Treatment Plants. Processes, 12(7), 1352. https://doi.org/10.3390/pr12071352





