Recent Progress in Poly (Lactic-co-Glycolic Acid)-Based Biodegradable Drug Delivery Carriers for Pain Management
Abstract
:1. Introduction
2. Materials and Methods
3. Pharmacological Interventions
3.1. Nonsteroidal Anti-Inflammatory Drug (NSAID) Pharmacological Interventions
3.2. Opioids
3.3. Local Anesthetics (LAs)
3.4. Capsaicin and Cannabinoids
3.5. Tricyclic Antidepressants and Serotonin and Norepinephrine Reuptake Inhibitors (SNRIs)
3.6. Muscle Relaxants
4. PLGA
5. Results
5.1. Neuropathic Pain
5.2. Inflammatory Pain
5.3. Traumatic Pain
5.4. Cancerous Pain
5.5. Angina
6. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Gottschalk, A.; Smith, D.S. New concepts in acute pain therapy: Preemptive analgesia. Am. Fam. Physician 2001, 63, 1979–1984. [Google Scholar] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H.; Dahl, J.B. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth. Analg. 1993, 77, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Todaro, B.; Moscardini, A.; Luin, S. Pioglitazone-Loaded PLGA Nanoparticles: Towards the Most Reliable Synthesis Method. Int. J. Mol. Sci. 2022, 23, 2522. [Google Scholar] [CrossRef] [PubMed]
- Kuldeep, N. Co-Delivery of Baclofen & Lamotrigine via Plga Nanoparticles, India. IN202011001014A, 9 January 2020. [Google Scholar]
- Yoo, J.; Won, Y.Y. Phenomenology of the Initial Burst Release of Drugs from PLGA Microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Peterson, K.; Helfand, M. Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: A systematic review. J. Pain Symptom Manag. 2004, 28, 140–175. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Antiinflamm Antiallergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Botting, R.M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998, 47 (Suppl. S2), S78–S87. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.J.; Fudin, J. Nonsteroidal Antiinflammatory Drugs for Acute and Chronic Pain. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 219–231. [Google Scholar] [CrossRef]
- James, D.S. The multisystem adverse effects of NSAID therapy. J. Am. Osteopath. Assoc. 1999, 99 (Suppl. S11), S1–S7. [Google Scholar] [CrossRef]
- Arfè, A.; Scotti, L.; Varas-Lorenzo, C.; Nicotra, F.; Zambon, A.; Kollhorst, B.; Schink, T.; Garbe, E.; Herings, R.; Straatman, H.; et al. Non-steroidal anti-inflammatory drugs and risk of heart failure in four European countries: Nested case-control study. BMJ 2016, 354, i4857. [Google Scholar] [CrossRef] [PubMed]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Dreischulte, T.; Morales, D.R.; Bell, S.; Guthrie, B. Combined use of nonsteroidal anti-inflammatory drugs with diuretics and/or renin-angiotensin system inhibitors in the community increases the risk of acute kidney injury. Kidney Int. 2015, 88, 396–403. [Google Scholar] [CrossRef]
- Bach-Rojecky, L.; Vađunec, D.; Žunić, K.; Kurija, J.; Šipicki, S.; Gregg, R.; Mikula, I.; Primorac, D. Continuing war on pain: A personalized approach to the therapy with nonsteroidal anti-inflammatory drugs and opioids. Per. Med. 2019, 16, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Yaksh, T.; Wallace, M. Opioids, Analgesia, and Pain Management. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13th ed.; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Crockett, S.D.; Wani, S.; Gardner, T.B.; Falck-Ytter, Y.; Barkun, A.N. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology 2018, 154, 1096–1101. [Google Scholar] [CrossRef]
- Wadlund, D.L. Local Anesthetic Systemic Toxicity. AORN J. 2017, 106, 367–377. [Google Scholar] [CrossRef]
- Macfarlane, A.J.R.; Gitman, M.; Bornstein, K.J.; El-Boghdadly, K.; Weinberg, G. Updates in our understanding of local anaesthetic systemic toxicity: A narrative review. Anaesthesia 2021, 76 (Suppl. S1), 27–39. [Google Scholar] [CrossRef]
- Pasierski, M.; Szulczyk, B. Beneficial Effects of Capsaicin in Disorders of the Central Nervous System. Molecules 2022, 27, 2484. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar]
- Brunetti, P.; Pichini, S.; Pacifici, R.; Busardò, F.P.; Del Rio, A. Herbal Preparations of Medical Cannabis: A Vademecum for Prescribing Doctors. Medicina 2020, 56, 237. [Google Scholar] [CrossRef]
- Poyatos, L.; Pérez-Acevedo, A.P.; Papaseit, E.; Pérez-Mañá, C.; Martin, S.; Hladun, O.; Siles, A.; Torrens, M.; Busardo, F.P.; Farré, M. Oral Administration of Cannabis and Δ-9-tetrahydrocannabinol (THC) Preparations: A Systematic Review. Medicina 2020, 56, 309. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Mathieson, S.; Lin, C.C.; Underwood, M.; Eldabe, S. Pregabalin and gabapentin for pain. BMJ. 2020, 369, m1315. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Sharma, M.S.; Brunoni, A.R.; Vieta, E.; Fava, G.A. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother. Psychosom. 2016, 85, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Kostev, K.; Rex, J.; Eith, T.; Heilmaier, C. Which adverse effects influence the dropout rate in selective serotonin reuptake inhibitor (SSRI) treatment? Results for 50,824 patients. Ger. Med. Sci. 2014, 12, Doc15. [Google Scholar]
- Browning, K.N. Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Front. Neurosci. 2015, 9, 413. [Google Scholar] [CrossRef]
- Qin, J.; Zhuang, L.; Yao, X.; Guo, D. Analysis of 233 cases of adverse drug reactions / events reported by SSRIs and SNRIs drugs. Drug Appl. Monit. China 2020, 17, 249–252. [Google Scholar]
- Gao, Y.J.; Ji, R.R. Targeting astrocyte signaling for chronic pain. Neurotherapeutics 2010, 7, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R.P. There’s plenty of room at the bottom [data storage]. J. Microelectromech. Syst. 1992, 1, 60–66. [Google Scholar] [CrossRef]
- Nielsen, R.V.; Fomsgaard, J.S.; Siegel, H.; Martusevicius, R.; Mathiesen, O.; Dahl, J.B. The effect of chlorzoxazone on acute pain after spine surgery. A randomized, blinded trial. Acta Anaesthesiol. Scand. 2016, 60, 1152–1160. [Google Scholar] [CrossRef]
- Barakat, A.; Hamdy, M.M.; Elbadr, M.M. Uses of fluoxetine in nociceptive pain management: A literature overview. Eur. J. Pharmacol. 2018, 829, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Ghanavatian, S.; Derian, A. Tizanidine. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Vázquez, N.; Sánchez-Arévalo, F.; Maciel-Cerda, A.; Garnica-Palafox, I.; Ontiveros-Tlachi, R.; Chaires-Rosas, C.; Piñón-Zarate, G.; Herrera-Enríquez, M.; Hautefeuille, M.; Vera-Graziano, R.; et al. Influence of the PLGA/gelatin ratio on the physical, chemical and biological properties of electrospun scaffolds for wound dressings. Biomed. Mater. 2019, 14, 045006. [Google Scholar] [CrossRef] [PubMed]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Parmar, A.; Kori, S.; Sandhir, R. PLGA-based nanoparticles: A new paradigm in biomedical applications. Trends Anal. Chem. 2016, 80, 30–40. [Google Scholar] [CrossRef]
- Dinarvand, R.; Sepehri, N.; Manoochehri, S.; Rouhani, H.; Atyabi, F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Park, T.G. Degradation of poly(lactic-co-glycolic acid) microspheres: Effect of copolymer composition. Biomaterials 1995, 16, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The Design of Poly(lactide-co-glycolide) Nanocarriers for Medical Applications. Front. Bioeng. Biotechnol. 2020, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhi, K.; Raji, B.; Nookala, A.R.; Khan, M.M.; Nguyen, X.H.; Sakshi, S.; Pourmotabbed, T.; Yallapu, M.M.; Kochat, H.; Tadrous, E.; et al. PLGANanoparticle-Based Formulations to Cross the Blood-Brain Barrier for Drug Delivery: From R&D to cGMP. Pharmaceutics 2021, 13, 500. [Google Scholar]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bee, S.-L.; Hamid, Z.A.A.; Mariatti, M.; Yahaya, B.H.; Lim, K.; Bee, S.-T.; Sin, L.T. Approaches to Improve Therapeutic Efficacy of Biodegradable PLA/PLGA Microspheres: A Review. Polym. Rev. 2018, 58, 495–536. [Google Scholar] [CrossRef]
- Xu, X.; Chang, S.; Zhang, X.; Hou, T.; Yao, H.; Zhang, S.; Zhu, Y.; Cui, X.; Wang, X. Fabrication of a controlled-release delivery system for relieving sciatica nerve pain using an ultrasound-responsive microcapsule. Front. Bioeng. Biotechnol. 2022, 10, 1072205. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Zhong, W.; Guo, L.; Ji, J.; Nie, H. Effect of Bufalin-PLGA Microspheres in the Alleviation of Neuropathic Pain via the CCI Model. Front. Pharmacol. 2022, 13, 910885. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.W.; Tseng, Y.Y.; Liu, K.S.; Liu, Y.W.; Chen, J.C.; He, H.L.; Kau, Y.C.; Liu, S.J. Anesthetics and human epidermal growth factor incorporated into anti-adhesive nanofibers provide sustained pain relief and promote healing of surgical wounds. Int. J. Nanomed. 2019, 14, 4007–4016. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.Y.; Lee, D.; Lee, T.C.; Chen, J.K.; Wu, R.C.; Liu, K.C.; Liu, S.J. Fabrication of Multi-Layered Lidocaine and Epinephrine-Eluting PLGA/Collagen Nanofibers: In Vitro and In Vivo Study. Polymers 2017, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Nigam, K.; Kaur, A.; Tyagi, A.; Nematullah, M.; Khan, F.; Gabrani, R.; Dang, S. Nose-to-brain delivery of lamotrigine-loaded PLGA nanoparticles. Drug Deliv. Transl. Res. 2019, 9, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Shen, S.J.; Hsu, Y.H.; Chou, Y.C.; Yu, P.C.; Liu, S.J. Tri-Layered Doxycycline-, Collagen- and Bupivacaine-Loaded Poly(lactic-co-glycolic acid) Nanofibrous Scaffolds for Tendon Rupture Repair. Polymers 2022, 14, 2659. [Google Scholar] [CrossRef] [PubMed]
- Berrocoso, E.; Rey-Brea, R.; Fernández-Arévalo, M.; Micó, J.A.; Martín-Banderas, L. Single oral dose of cannabinoid derivate loaded PLGA nanocarriers relieves neuropathic pain for eleven days. Nanomedicine 2017, 13, 2623–2632. [Google Scholar] [CrossRef]
- Rani, S.; Gothwal, A.; Pandey, P.K.; Chauhan, D.S.; Pachouri, P.K.; Gupta, U.D.; Gupta, U. HPMA-PLGA Based Nanoparticles for Effective In Vitro Delivery of Rifampicin. Pharm. Res. 2018, 36, 19. [Google Scholar] [CrossRef]
- Liu, K.S.; Chen, W.H.; Lee, C.H.; Su, Y.F.; Liu, S.J. Extended pain relief achieved by analgesic-eluting biodegradable nanofibers in the Nuss procedure: In vitro and in vivo studies. Int. J. Nanomed. 2018, 13, 8355–8364. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Cheng, Y.S.; Hsu, Y.H.; Yu, Y.H.; Liu, S.J. Biodegradable nanofiber-membrane for sustainable release of lidocaine at the femoral fracture site as a periosteal block: In vitro and in vivo studies in a rabbit model. Colloids Surf. B Biointerfaces 2016, 140, 332–341. [Google Scholar] [CrossRef]
- Nigam, K.; Kaur, A.; Tyagi, A.; Manda, K.; Gabrani, R.; Dang, S. Baclofen-Loaded Poly (D,L-Lactide-Co-Glycolic Acid) Nanoparticles for Neuropathic Pain Management: In Vitro and In Vivo Evaluation. Rejuvenation Res. 2019, 22, 235–245. [Google Scholar] [CrossRef]
- Kim, S.R.; Ho, M.J.; Kim, S.H.; Cho, H.R.; Kim, H.S.; Choi, Y.S.; Choi, Y.W.; Kang, M.J. Increased localized delivery of piroxicam by cationic nanoparticles after intra-articular injection. Drug Des. Dev. Ther. 2016, 10, 3779–3787. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, M.; Baskaran, P.; Arulsamy, N.; Thyagarajan, B. Preparation and Evaluation of PLGA-Coated Capsaicin Magnetic Nanoparticles. Pharm. Res. 2017, 34, 1255–1263. [Google Scholar] [CrossRef]
- Zhu, M.; Whittaker, A.K.; Smith, M.T.; Han, F.Y. Bioerodable Ketamine-Loaded Microparticles Fabricated Using Dissolvable Hydrogel Template Technology. J. Pharm. Sci. 2019, 108, 1220–1226. [Google Scholar] [CrossRef]
- Kandilli, B.; Ugur Kaplan, A.B.; Cetin, M.; Taspinar, N.; Ertugrul, M.S.; Aydin, I.C.; Hacimuftuoglu, A. Carbamazepine and levetiracetam-loaded PLGA nanoparticles prepared by nanoprecipitation method: In vitro and in vivo studies. Drug Dev. Ind. Pharm. 2020, 46, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ning, C.; Xu, W.; Hu, H.; Li, M.; Zhao, G.; Ding, J.; Chen, X. Precision-guided long-acting analgesia by Gel-immobilized bupivacaine-loaded microsphere. Theranostics 2018, 8, 3331–3347. [Google Scholar] [CrossRef]
- Wang, T.; Hurwitz, O.; Shimada, S.G.; Tian, D.; Dai, F.; Zhou, J.; Ma, C.; LaMotte, R.H. Anti-nociceptive effects of bupivacaine-encapsulated PLGA nanoparticles applied to the compressed dorsal root ganglion in mice. Neurosci. Lett. 2018, 668, 154–158. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, H.; Du, S.; Zhang, X.Q.; Lin, F.; Ji, F.; Tsou, Y.H.; Li, Z.; Feng, Y.; Ticehurst, K.; et al. Injectable PLGA-Coated Ropivacaine Produces A Long-Lasting Analgesic Effect on Incisional Pain and Neuropathic Pain. J. Pain. 2021, 22, 180–195. [Google Scholar] [CrossRef]
- Han, F.Y.; Whittaker, A.; Howdle, S.M.; Naylor, A.; Shabir-Ahmed, A.; Smith, M.T. Sustained-Release Hydromorphone Microparticles Produced by Supercritical Fluid Polymer Encapsulation. J. Pharm. Sci. 2019, 108, 811–814. [Google Scholar] [CrossRef]
- Rudnik-Jansen, I.; Schrijver, K.; Woike, N.; Tellegen, A.; Versteeg, S.; Emans, P.; Mihov, G.; Thies, J.; Eijkelkamp, N.; Tryfonidou, M.; et al. Intra-articular injection of triamcinolone acetonide releasing biomaterial microspheres inhibits pain and inflammation in an acute arthritis model. Drug Deliv. 2019, 26, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Dandamudi, M.; McLoughlin, P.; Behl, G.; Rani, S.; Coffey, L.; Chauhan, A.; Kent, D.; Fitzhenry, L. Chitosan-Coated PLGA Nanoparticles Encapsulating Triamcinolone Acetonide as a Potential Candidate for Sustained Ocular Drug Delivery. Pharmaceutics 2021, 13, 1590. [Google Scholar] [CrossRef]
- Shin, J.; Yin, Y.; Park, H.; Park, S.; Triantafillu, U.L.; Kim, Y.; Kim, S.R.; Lee, S.Y.; Kim, D.K.; Hong, J.; et al. p38 siRNA-encapsulated PLGA nanoparticles alleviate neuropathic pain behavior in rats by inhibiting microglia activation. Nanomedicine 2018, 13, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Malewicz, N.M.; Rattray, Z.; Oeck, S.; Jung, S.; Escamilla-Rivera, V.; Chen, Z.; Tang, X.; Zhou, J.; LaMotte, R.H. Topical Capsaicin in Poly(lactic-co-glycolic)acid (PLGA) Nanoparticles Decreases Acute Itch and Heat Pain. Int. J. Mol. Sci. 2022, 23, 5275. [Google Scholar] [CrossRef]
- Cavalier, M.; Benoit, J.P.; Thies, C. The formation and characterization of hydrocortisone-loaded poly((+/–)-lactide) microspheres. J. Pharm. Pharmacol. 1986, 38, 249–253. [Google Scholar] [CrossRef]
- Wu, X.S. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers: Part III. Drug delivery application. Artif. Cells Blood Substit. Immobil. Biotechnol. 2004, 32, 575–591. [Google Scholar] [CrossRef]
- Rudnik-Jansen, I.; Woike, N.; de Jong, S.; Versteeg, S.; Kik, M.; Emans, P.; Mihov, G.; Thies, J.; Eijkelkamp, N.; Tryfonidou, M.; et al. Applicability of a Modified Rat Model of Acute Arthritis for Long-Term Testing of Drug Delivery Systems. Pharmaceutics 2019, 11, 70. [Google Scholar] [CrossRef]
- Pramual, S.; Lirdprapamongkol, K.; Atjanasuppat, K.; Chaisuriya, P.; Niamsiri, N.; Svasti, J. PLGA-Lipid Hybrid Nanoparticles for Overcoming Paclitaxel Tolerance in Anoikis-Resistant Lung Cancer Cells. Molecules 2022, 27, 8295. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xie, L.Q.; Qin, J.; Jia, Y.; Cai, X.; Nan, W.; Yang, W.; Lv, F.; Zhang, Q.Q. Surface modification of PLGA nanoparticles with biotinylated chitosan for the sustained in vitro release and the enhanced cytotoxicity of epirubicin. Colloids Surf. B Biointerfaces 2016, 138, 1–9. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, J.S.; Park, M.; Ko, M.Y.; Yi, S.W.; Yoon, J.A.; Yang, S.; Shim, S.H.; Park, K.H.; Song, H. PLGA nanoparticles with multiple modes are a biologically safe nanocarrier for mammalian development and their offspring. Biomaterials 2018, 183, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.A.; Handa, M.; Garabadu, D.; Kumar, R.; Kushawaha, P.K.; Shukla, R. Transferrin decorated PLGA encumbered moxifloxacin nanoparticles and in vitro cellular studies. Drug Dev. Ind. Pharm. 2023, 49, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xie, Y.; Li, X.; Yao, X.; Wang, X.; Wang, M.; Li, Z.; Cao, F. Folic Acid/Peptides Modified PLGA-PEI-PEG Polymeric Vectors as Efficient Gene Delivery Vehicles: Synthesis, Characterization and Their Biological Performance. Mol. Biotechnol. 2021, 63, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Szok, D.; Tajti, J.; Nyári, A.; Vécsei, L. Therapeutic Approaches for Peripheral and Central Neuropathic Pain. Behav. Neurol. 2019, 2019, 8685954. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.C.; Blechschmidt, V.; Timmerman, H.; Wolff, A.; Treede, R.D. Challenges of neuropathic pain: Focus on diabetic neuropathy. J. Neural Transm. 2020, 127, 589–624. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.; Shin, H.J.; Yi, Y.; Beom, J.; Lee, W.; Lee, C.H.; Kim, D.W. p66shc siRNA-Encapsulated PLGA Nanoparticles Ameliorate Neuropathic Pain Following Spinal Nerve Ligation. Polymers 2020, 12, 1014. [Google Scholar] [CrossRef] [PubMed]
- Berliocchi, L.; Russo, R.; Maiarù, M.; Levato, A.; Bagetta, G.; Corasaniti, M.T. Autophagy impairment in a mouse model of neuropathic pain. Mol. Pain. 2011, 7, 83. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Z. Upregulated TLR3 promotes neuropathic pain by regulating autophagy in rat with l5 spinal nerve ligation model. Neurochem. Res. 2017, 42, 634–643. [Google Scholar] [CrossRef]
- Yi, M.H.; Shin, J.; Shin, N.; Yin, Y.; Lee, S.Y.; Kim, C.S.; Kim, S.R.; Zhang, E.; Kim, D.W. PINK1 mediates spinal cord mitophagy in neuropathic pain. J. Pain. Res. 2019, 12, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Shi, J.; Liu, K.; Liu, N.; Wang, Y.; Fu, Z.; Ding, J.; Jia, L.; Yuan, W. Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia 2013, 61, 504–512. [Google Scholar] [CrossRef]
- Bhat, S.S.; Anand, D.; Khanday, F.A. p66Shc as a switch in bringing about contrasting responses in cell growth: Implications on cell proliferation and apoptosis. Mol. Cancer 2015, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shin, H.J.; Noh, C.; Kim, S.I.; Ko, Y.K.; Lee, S.Y.; Lim, C.; Hong, B.; Yang, S.Y.; Kim, D.W.; et al. IKBKB siRNA-Encapsulated Poly (Lactic-co-Glycolic Acid) Nanoparticles Diminish Neuropathic Pain by Inhibiting Microglial Activation. Int. J. Mol. Sci. 2021, 22, 5657. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Shin, J.; Tran, Q.; Park, H.; Kwon, H.H.; Shin, N.; Hwang, J.A.; Shin, H.J.; Lee, J.; Lee, W.H.; et al. Application of PLGA nanoparticles to enhance the action of duloxetine on microglia in neuropathic pain. Biomater. Sci. 2021, 9, 6295–6307. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.; Duggan, S.T.; Keam, S.J. Triamcinolone Acetonide Extended-Release: A Review in Osteoarthritis Pain of the Knee. Drugs 2019, 79, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cao, Y.; Ihsan, A.U.; Khan, F.U.; Li, X.; Xie, D.; Cui, X.; Wang, W.; Liu, Z.; Li, C.; et al. Novel Treatment of Experimental Autoimmune Prostatitis by Nanoparticle-Conjugated Autoantigen Peptide T2. Inflammation 2019, 42, 1071–1081. [Google Scholar] [CrossRef]
- Jirkof, P. Side effects of pain and analgesia in animal experimentation. Lab Anim. 2017, 46, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Mattila, K.; Toivonen, J.; Janhunen, L.; Rosenberg, P.H.; Hynynen, M. Postdischarge symptoms after ambulatory surgery: First-week incidence, intensity, and risk factors. Anesth. Analg. 2005, 101, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; DeRoche, A.; Adams, L.; Slocum, K.V.; Clark, C.J.; Fino, N.F.; Shen, P. Effect of epidural compared to patient-controlled intravenous analgesia on outcomes for patients undergoing liver resection for neoplastic disease. J. Surg. Oncol. 2017, 115, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kosugi, K.; Suto, T.; Tobe, M.; Tabata, Y.; Yokoo, S.; Saito, S. Sustained-release lidocaine sheet for pain following tooth extraction: A randomized, single-blind, dose-response, controlled, clinical study of efficacy and safety. PLoS ONE 2018, 13, e0200059. [Google Scholar] [CrossRef]
- Fu, X.; Zeng, H.; Guo, J.; Liu, H.; Shi, Z.; Chen, H.; Li, D.; Xie, X.; Kuang, C. A PLGA-PEG-PLGA Thermosensitive Gel Enabling Sustained Delivery of Ropivacaine Hydrochloride for Postoperative Pain Relief. Chem. Pharm. Bull. 2017, 65, 229–235. [Google Scholar] [CrossRef]
- Pek, Y.S.; Pitukmanorom, P.; Ying, J.Y. Sustained release of bupivacaine for post-surgical pain relief using core-shell microspheres. J. Mater. Chem. B 2014, 2, 8194–8200. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Hsu, Y.H.; Chou, Y.C.; Fan, C.L.; Ueng, S.W.; Kau, Y.C.; Liu, S.J. Sustained relief of pain from osteosynthesis surgery of rib fracture by using biodegradable lidocaine-eluting nanofibrous membranes. Nanomedicine 2016, 12, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Grossberg, G.T. Phantom limb pain: Mechanisms and treatment approaches. Pain. Res. Treat. 2011, 2011, 864605. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.I.; Shin, D.M. Recent advances in head and neck cancer. N. Engl. J. Med. 2008, 359, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.B.; Krempl, G.A.; Canfield, V.; Bogardus, C.; Kojouri, K.; Kaneaster, S.K.; Medina, J.E. Treatment of base of tongue cancer with paclitaxel, ifosfamide, and cisplatinum induction chemotherapy followed by chemoradiotherapy. Laryngoscope 2008, 118, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Boese, S.E.; Luo, Z.; Nitin, N.; Gill, H.S. Drug coated microneedles for minimally-invasive treatment of oral carcinomas: Development and in vitro evaluation. Biomed. Microdevices 2015, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Dreher, M.R. Locoregional drug delivery using image-guided intra-arterial drug eluting bead therapy. J. Control Release 2012, 161, 338–350. [Google Scholar] [CrossRef]
- Cammà, C.; Schepis, F.; Orlando, A.; Albanese, M.; Shahied, L.; Trevisani, F.; Andreone, P.; Craxì, A.; Cottone, M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: Meta-analysis of randomized controlled trials. Radiology 2002, 224, 47–54. [Google Scholar] [CrossRef]
- Choi, J.W.; Park, J.H.; Baek, S.Y.; Kim, D.D.; Kim, H.C.; Cho, H.J. Doxorubicin-loaded poly(lactic-co-glycolic acid) microspheres prepared using the solid-in-oil-in-water method for the transarterial chemoembolization of a liver tumor. Colloids Surf. B Biointerfaces 2015, 132, 305–312. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Huang, Y.; Chen, Y.; Wang, J.; Xu, L.; Zhang, F.; Zhuge, Y.; Zou, X. Preparation of microspheres encapsulating sorafenib and catalase and their application in rabbit VX2 liver tumor. Biomed. Pharmacother. 2020, 129, 110512. [Google Scholar] [CrossRef] [PubMed]
- Qiu, E.; Liu, F. PLGA-based drug delivery systems in treating bone tumors. Front. Bioeng. Biotechnol. 2023, 11, 1199343. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, J.F. Management of vasospastic angina. Heart 2022, 109, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A.; Chaitman, B. Angina and Its Management. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, M.A.; Miaskowski, C. The symptom experience of angina in women. Pain. Manag. Nurs. 2000, 1, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, D.W.; Beatty, A.L.; Meyer, C.S.; Whooley, M.A. Longitudinal Association Between Angina Pectoris and Quality of Life. Am. J. Cardiol. 2022, 164, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shah, U.; Joshi, G.; Sawant, K. Improvement in antihypertensive and antianginal effects of felodipine by enhanced absorption from PLGA nanoparticles optimized by factorial design. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 35, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Misra, S.K.; Moitra, P.; Zhang, X.; Jeong, S.J.; Stitham, J.; Rodriguez-Velez, A.; Park, A.; Yeh, Y.S.; Gillanders, W.E.; et al. Use of acidic nanoparticles to rescue macrophage lysosomal dysfunction in atherosclerosis. Autophagy 2023, 19, 886–903. [Google Scholar] [CrossRef] [PubMed]
- Lalani, J.; Rathi, M.; Lalan, M.; Misra, A. Protein functionalized tramadol-loaded PLGA nanoparticles: Preparation, optimization, stability and pharmacodynamic studies. Drug Dev. Ind. Pharm. 2013, 39, 854–864. [Google Scholar] [CrossRef]
- Davda, J.; Labhasetwar, V. Characterization of nanoparticle uptake by endothelial cells. Int. J. Pharm. 2002, 233, 51–59. [Google Scholar] [CrossRef]
- Smith, D.J.; Trantolo, D.J.; King, W.F.; Gusek, E.J.; Fackler, P.H.; Gresser, J.D.; De Souza, V.L.; Wise, D.L. Induction of secretory immunity with bioadhesive poly (D,L-lactide-co-glycolide) microparticles containing Streptococcus sobrinus glucosyltransferase. Oral. Microbiol. Immunol. 2000, 15, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.K.; Bøgevig, S.; Balchen, T.; Springborg, A.H.; Royal, M.A.; Storgaard, I.K.; Lund, T.M.; Møller, K.; Werner, M.U. Dose safety and pharmacodynamics of subcutaneous bupivacaine in a novel extended-release microparticle formulation: A phase 1, dose-ascending study in male volunteers. Basic Clin. Pharmacol. Toxicol. 2024, 134, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Turk, D.C.; Fillingim, R.B.; Ohrbach, R.; Patel, K.V. Assessment of Psychosocial and Functional Impact of Chronic Pain. J. Pain. 2016, 17 (Suppl. S9), T21–T49. [Google Scholar] [CrossRef] [PubMed]

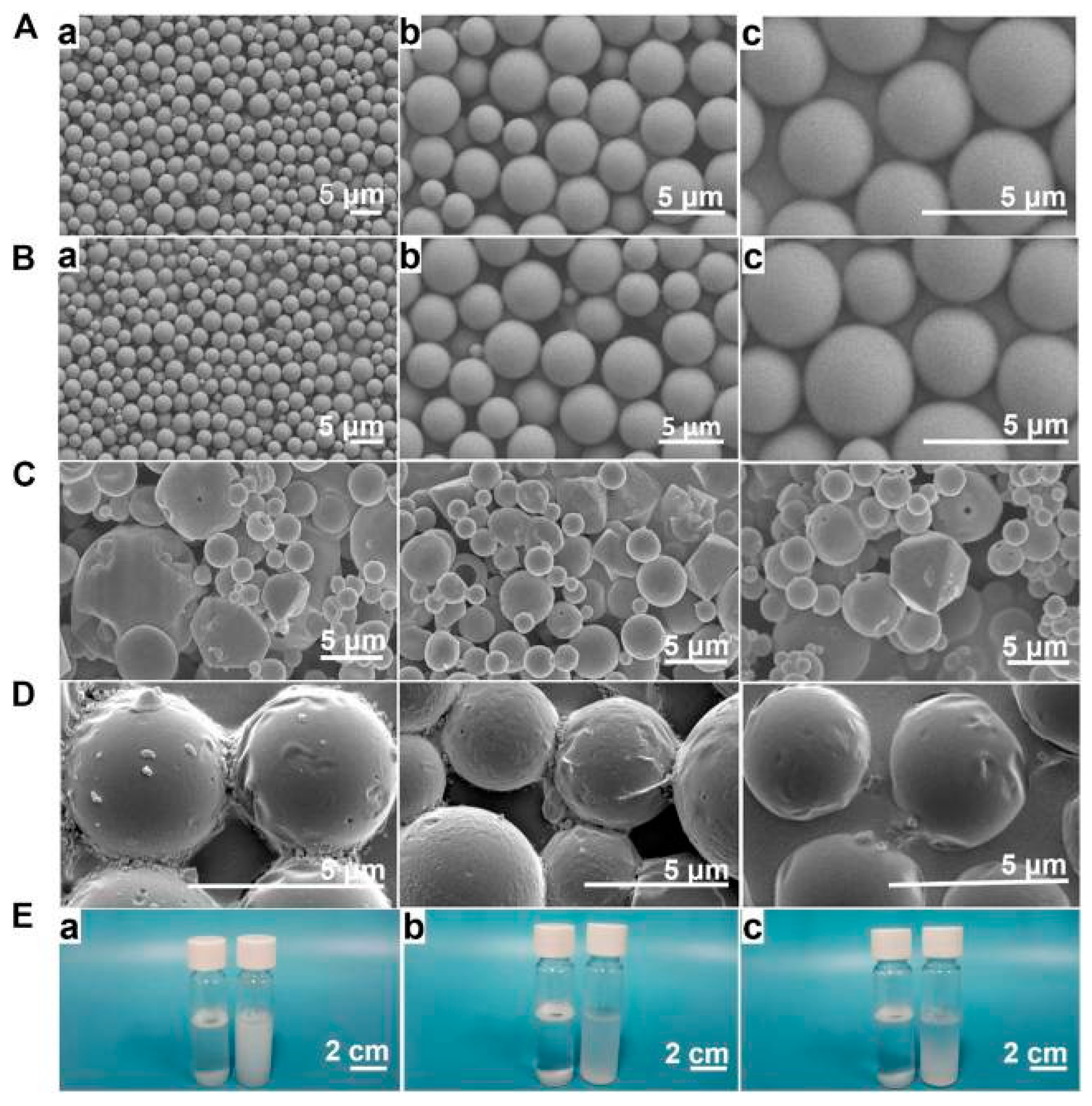



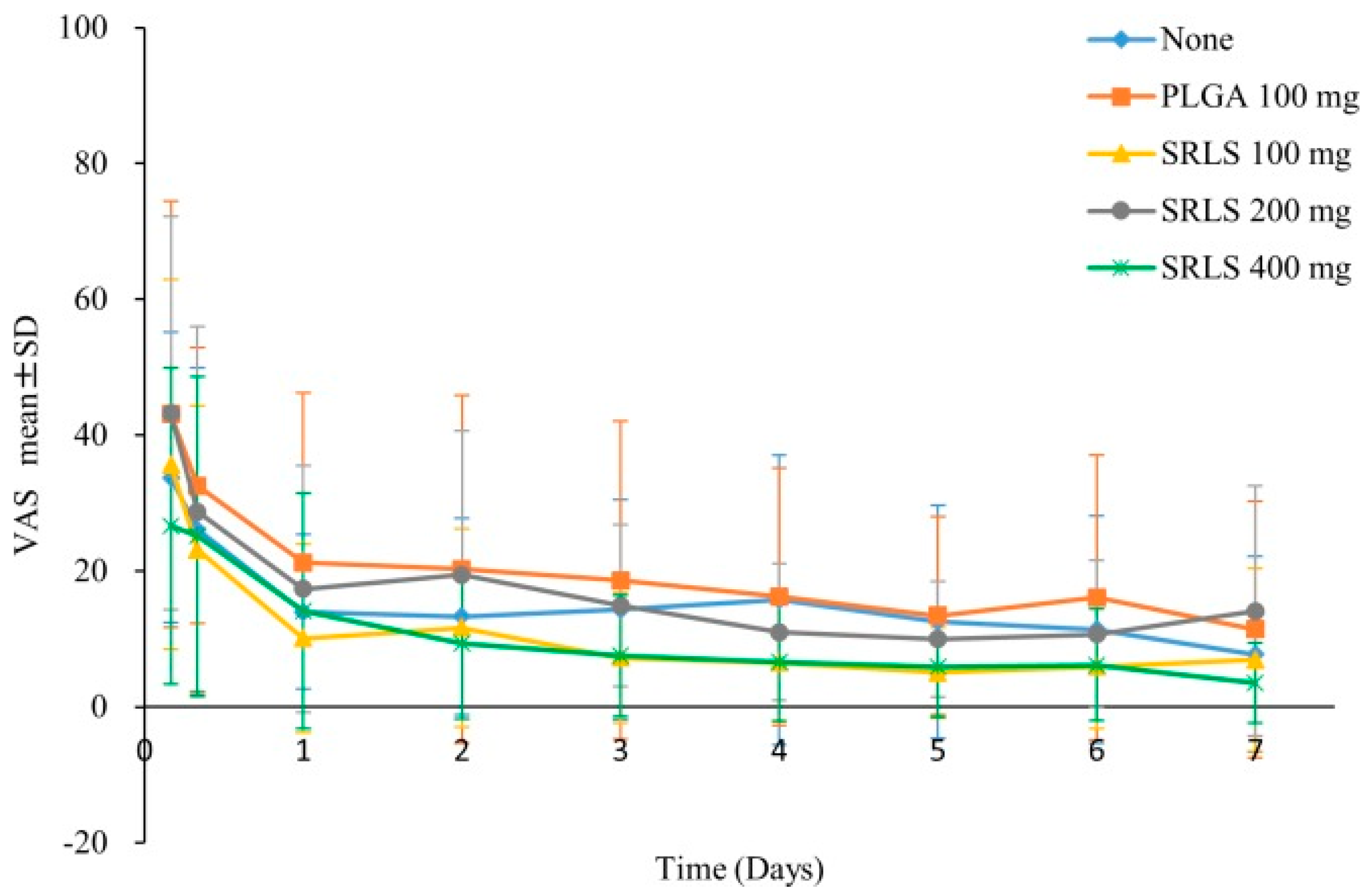

| Name of Drug | Character | Particle Size | Drug Efficacy |
|---|---|---|---|
| Doxycycline Collagen Bupivacaine | Microfiber | 227.7 ± 76.0 (doxycycline–PLGA), 215.0 ± 99.1 (collagen-PLGA), and 185.9 ± 75.2 nm (bupivacaine–PLGA) | The DCB-composite nanofibers provided the continuous release of doxycycline and bupivacaine for over 28 days in vivo. The experimental results illustrate that the strength of the repaired tendons is greater than that of normal tendons, compared with a simple administration mode, the sustained-release effect of the system is obviously improved [51]. |
| cannabinoid | Nanometer particles | <300 nm | Versus the free CB13, CB13-PLGA-PEG nanoparticles showed a very noticeable analgesic efficacy with the longest sustained pain-relieving effect, lasting up to 11 days after one oral dose [52]. |
| Rifampicin | Nanometer particles | 260.3 ± 2.21 nm | The pathogen-inhibition studies revealed that developed RMP-loaded HPMA-PLGA-NPs were approximately four times more effective with than pure drug against a sensitive Mycobacterium tuberculosis stain [53]. |
| Lidocaine and ketorolac | Nanofibers | 158~207 nm | Biodegradable nanofibers deliver higher doses of lidocaine and ketorolac over a 10-day period than when administered alone. Animals receiving the analgesic-eluting nanofiber-coated stainless steel rod implants showed significantly more food and water intake and physical activity than the blank control group, indicating effective pain relief [54]. |
| Lidocaine and hEGF | Nanofibers | 780.0 ± 552.1 nm | The nanofiber membrane effectively released lidocaine and hEGF in vivo for >2 weeks longer than the control group. In addition, rats implanted with lidocaine/hEGF nanofiber membranes showed greater activity than controls, demonstrating that lidocaine and hEGF sustained-release antiadhesive nanofiber membranes relieve postoperative pain and wound healing [48]. |
| Lidocaine | Nanofibers | 614 ± 213 nm, 623 ± 149 nm, 659 ± 204 nm | Compared with the blank control group, the rabbits in the experimental group had faster hemostasis and faster recovery of food and water intake after the operation. It is demonstrated that the multilayer biodegradable nanofiber membrane provides sufficient hemostatic effect and sustainable pain relief for the primary healing of palatal oral wounds [49]. |
| Lidocaine | Nanofiber | 221~496 nm | Rabbits implanted with lidocaine-loaded nanofibers showed faster recovery of activity after surgery compared to the group that simply fractured the femur and received fixation, confirming the analgesic efficacy of lidocaine-embedded nanofibers. Nanofibers with sustainable lidocaine release have sufficient efficacy and durability to provide pain relief in rabbits with segmental long bone fractures [55]. |
| Baclofen | Nanoparticles | 124.8 nm | In vivo γ-scintigraphy studies showed the prolonged retention of BCF-PLGA NPs in the brain, and unlike aqueous drugs, biodistribution studies confirmed PLGA as a suitable carrier for nanoparticles against neuropathic pain. Pharmacokinetics showed that the maximum cerebral concentration (Cmax) of 99Tc-BCF-PLGA nanoparticles after intranasal administration was 3.5%/g, which was higher than that after intravenous administration (2.65%/g), and the area under the curve (AUC) of rats after intranasal administration was 41%·h/g, which was significantly higher than the 33.52%·h/g of the rats administered intravenously [56]. |
| Piroxicam | Cationic nanoparticles | ~220 nm | The drug concentration in the joint tissue 24 h after the administration of cationic NPs was 3.2-fold and 1.8-fold higher than that in the drug solution and neutrally charged NPs, respectively [57]. |
| Lamotrigine | Polymer nanoparticles for intranasal pathway | ~185 nm | The oral, intranasal, and intravenous routes of LTG-PLGA NP showed better biodistribution, demonstrating that PLGA is a suitable carrier system for lamotrigine and a suitable route for the treatment of neuropathic pain [56]. |
| Sustained | Nanoparticles | 10–20 nm | PCMN provided the longer release of capsaicin in vitro, increased the solubility of capsaicin, and inhibited carrageenan-induced inflammatory pain in an in vivo mouse model [58]. |
| Ketamine | Microparticle | 20 µm | The in vitro microsomal release lasts for at least 14 days, which is higher than the usual mode of administration [59]. |
| Carbamazepie and levetiracetam | Nanoparticles | 180.62 ± 6.260 nm | Compared with carbamazepine (CBZ) and levetiracil (LEV), the CBZ + LEV combination and CBZ + LEV-PLGA NPs generally have higher antiepileptic activity. However, the anticonvulsant effect of CBZ + LEV-PLGA NPs was more pronounced [60]. |
| Bupivacaine | Microparticle | 15 μm | The injectable gel-MS system consisting of PLGA-PEG-PLGA gel and BUP-loaded PLGA-MS realizes a precisely guided drug release and retention system and shows long-term effective analgesia in vivo [61]. |
| Bupivacaine | Nanometer particles | 150 ± 10 nm | PLGA bupivacaine is an extended-release preparation of a local anesthetic that provides postoperative analgesia for up to 72 h [62]. |
| Ropivacaine | Microparticle | 1.7 ± 0.2 μm | The analgesic system may produce a more long-lasting analgesic effect lasting up to at least 6 days with a single administration via local sciatic nerve periinjection [63]. |
| Ropivacaine | Microparticle | 20–45 μm | The sustained-release effect of PLGA-supported hydromorphone was about 35 days in vitro, which prolonged the sustained release effect [64]. |
| Hydromorphinone | Microparticle | 38–50 um | Compared with triamcinolone acetonide alone, triamcinolone acetonide polylactic-co-glycolic acid (PLGA) prolongs the pain relief time of osteoarthritis to some extent [65]. |
| Triamcinolone acetonide | Nanoparticles | 334 ± 67.95 to 386 ± 15.14 nm | Poly (lactic-co-glycolic acid) (PLGA) nanoparticles support the controlled release of the encapsulated drug while prolonging the mucoadhesive capacity, thereby improving the bioavailability of the drug [66]. |
| SiRNA | Nanofiber | 153.1 ± 68.08 nm | P38 siRNA NPs significantly reduced mechanical allodynia and microgliosis in the dorsal horn of SNL rats, consistent with the down-regulation of p38-associated proinflammatory mediators [67]. |
| Capsaicin | Nanometer particles | 108.11 ± 17.36 and 110.45 ± 18.33 nm | Treat itching and maintain long-term desensitization and treat pain [68]. |
| Bufalin | Microsphere | Compared with free bufalin, bufalin-PLGA-MS can significantly prolong the duration of analgesia and reduce the number of administrations [47]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, T.; Gao, J.; Feng, R.; Zheng, Y.; Tian, K.; Chen, J.; Xu, X. Recent Progress in Poly (Lactic-co-Glycolic Acid)-Based Biodegradable Drug Delivery Carriers for Pain Management. Processes 2024, 12, 1372. https://doi.org/10.3390/pr12071372
Liang T, Gao J, Feng R, Zheng Y, Tian K, Chen J, Xu X. Recent Progress in Poly (Lactic-co-Glycolic Acid)-Based Biodegradable Drug Delivery Carriers for Pain Management. Processes. 2024; 12(7):1372. https://doi.org/10.3390/pr12071372
Chicago/Turabian StyleLiang, Tao, Jingjing Gao, Ruiquan Feng, Yu Zheng, Kewei Tian, Jianer Chen, and Xiaoling Xu. 2024. "Recent Progress in Poly (Lactic-co-Glycolic Acid)-Based Biodegradable Drug Delivery Carriers for Pain Management" Processes 12, no. 7: 1372. https://doi.org/10.3390/pr12071372





