Zinc Extraction from Primary Lead Smelting Slags by Oxidant Alkaline Leaching
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
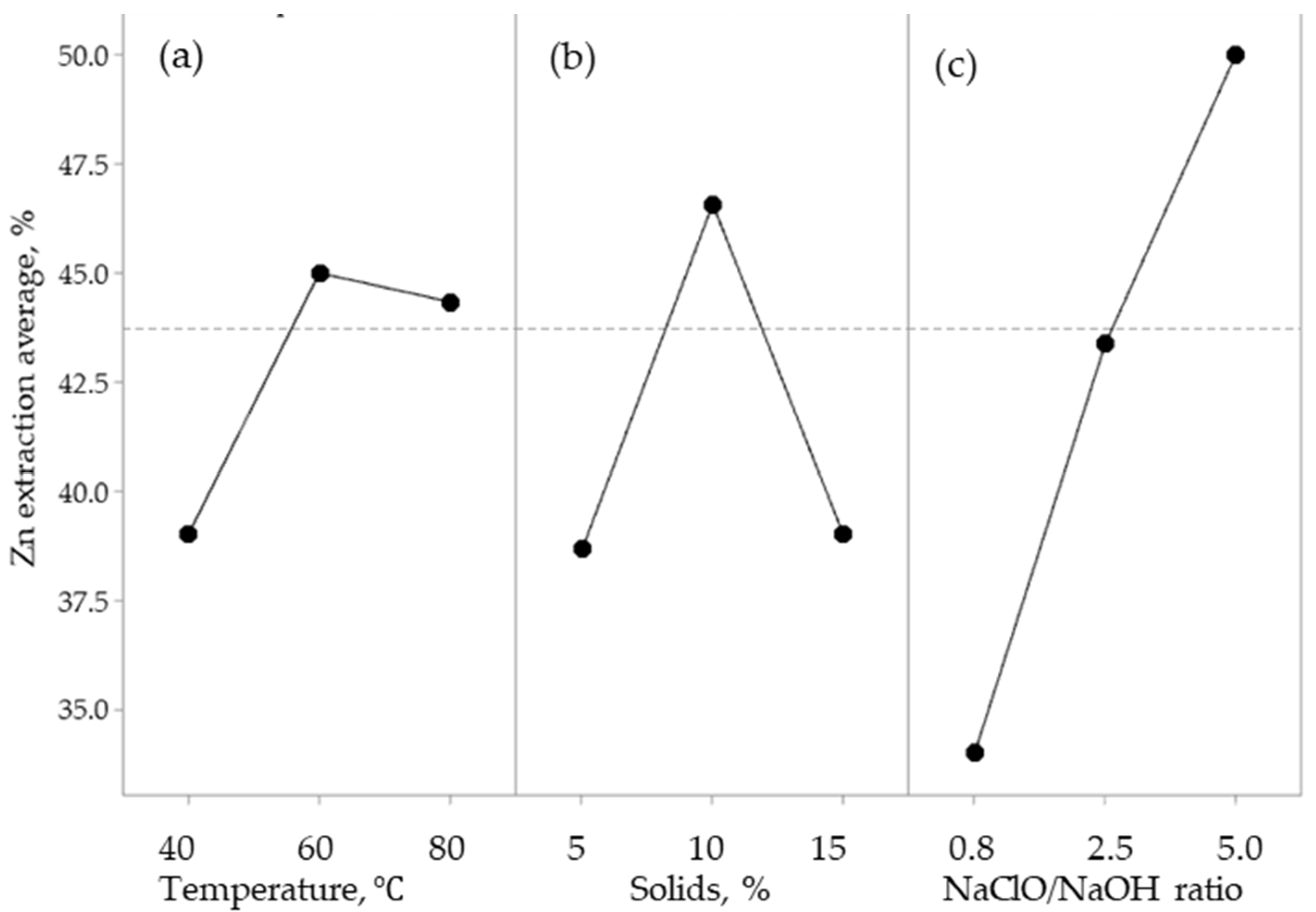
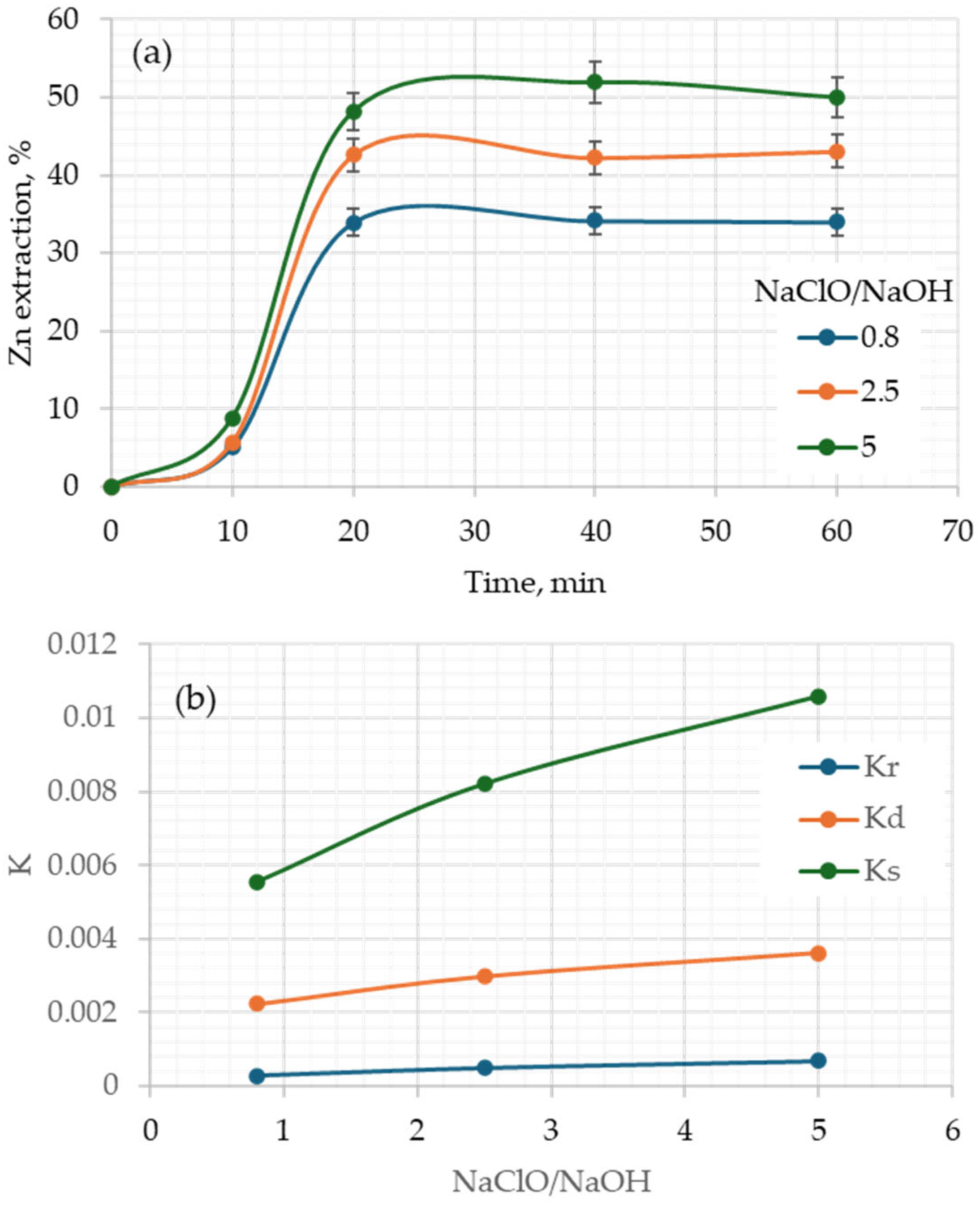
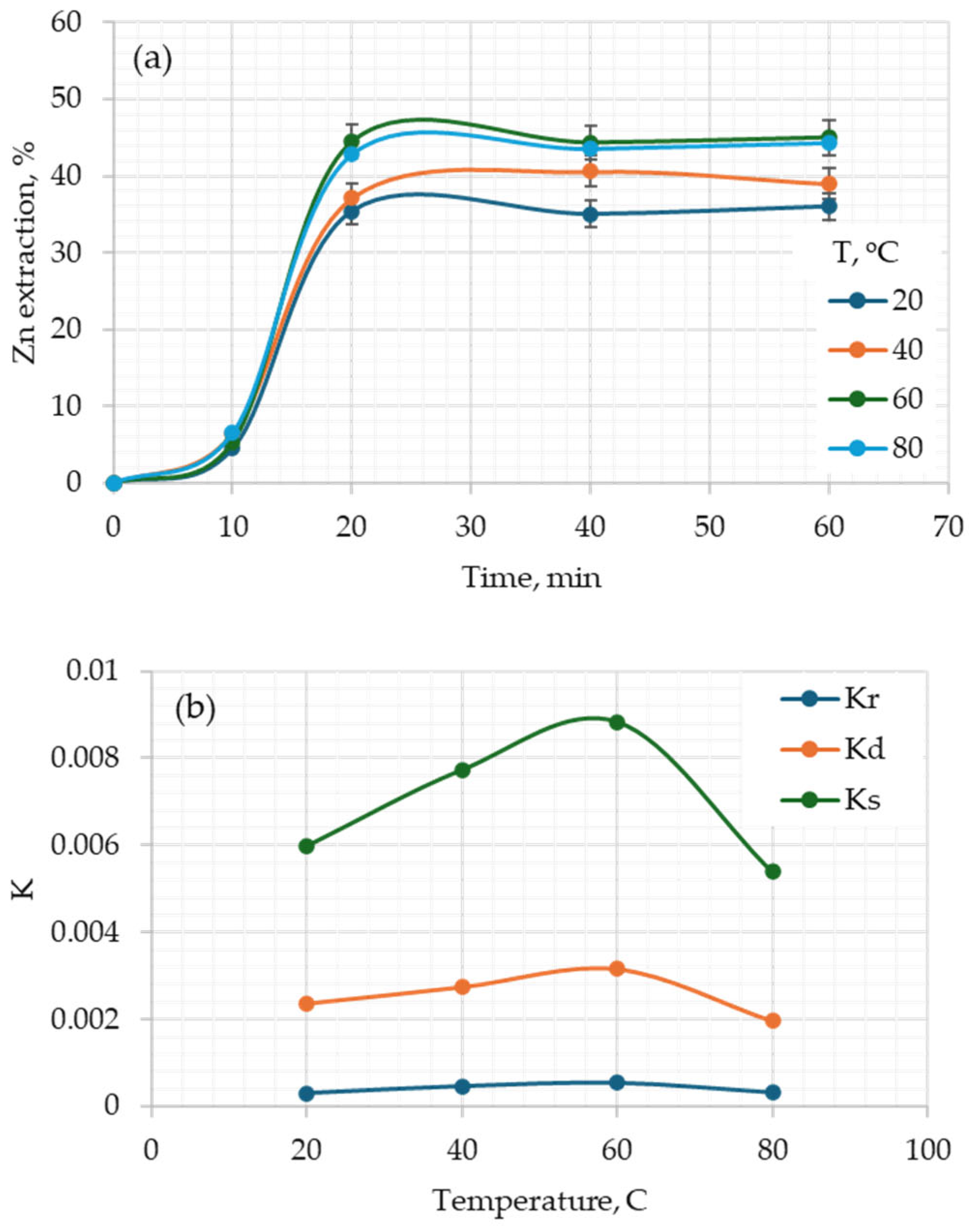
3.1. Effect of the Main Parameters
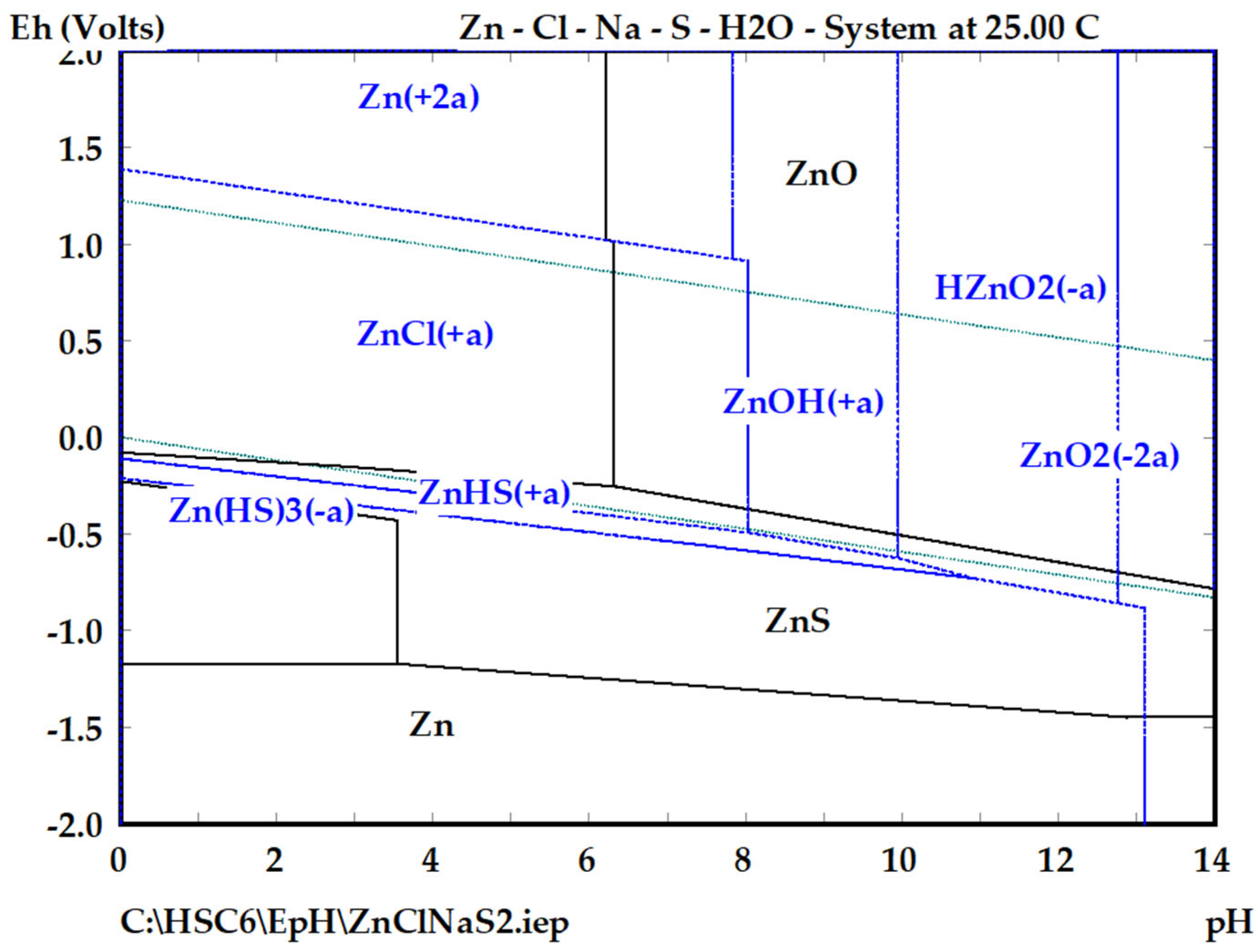
3.2. Thermodynamics
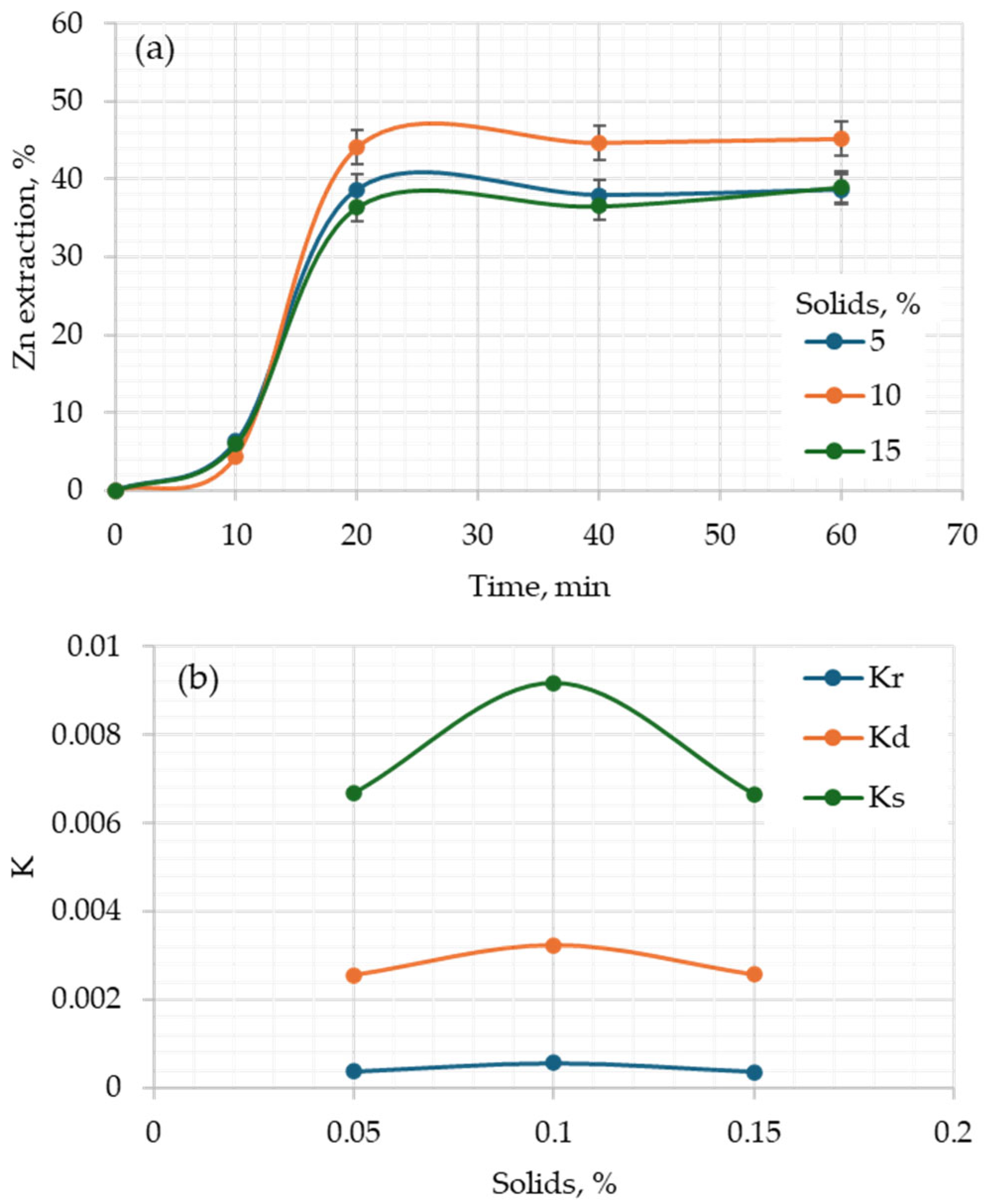
3.3. Kinetic Analysis
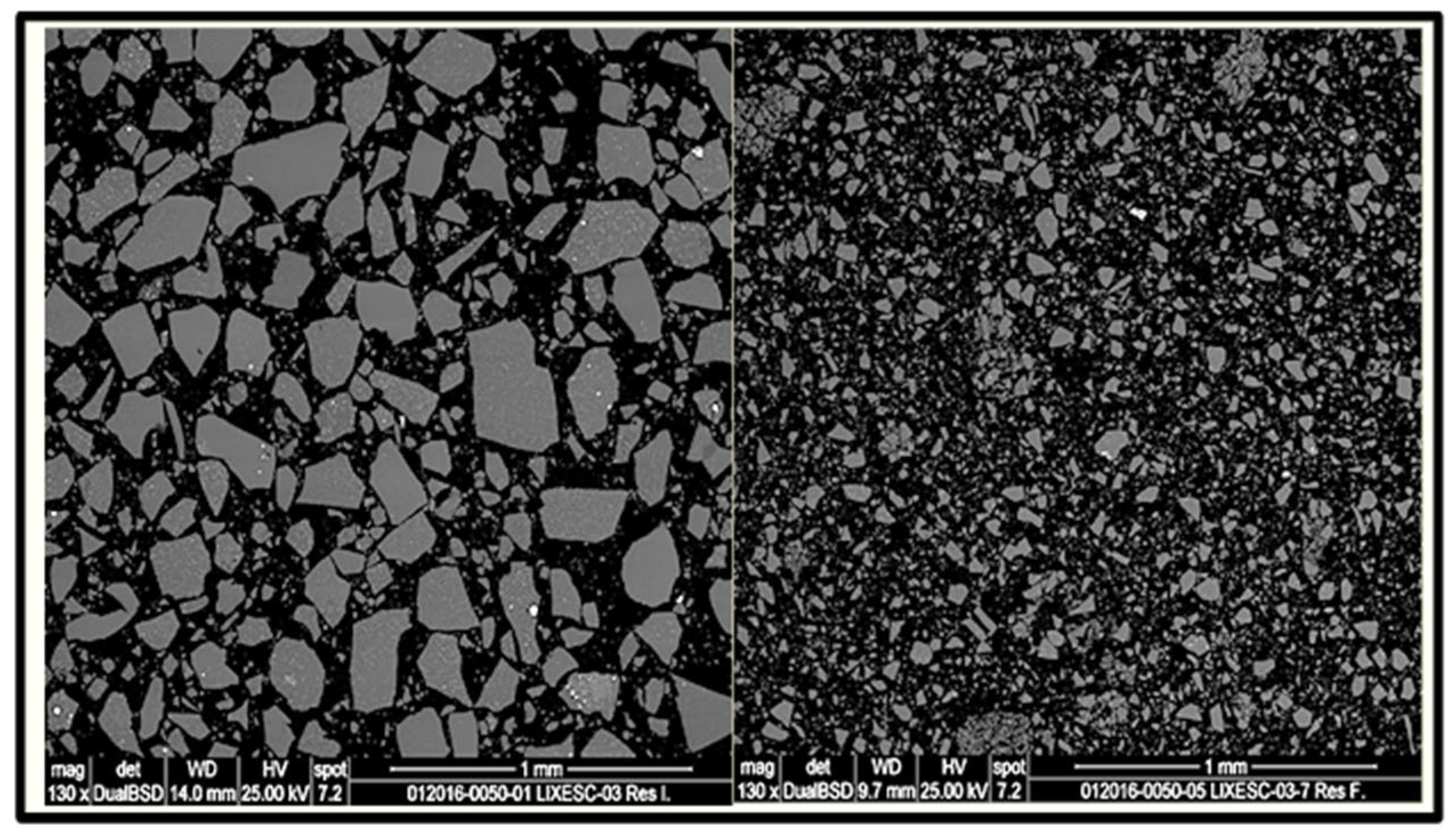
Identification of the Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, D.; Li, L.; Tian, X.; Wu, Y.; Cheng, N.; Yu, H. A review on lead slag generation, characteristics, and utilization. In Resources, Conservation and Recycling; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 146, pp. 140–155. [Google Scholar] [CrossRef]
- Yin, N.H.; Sivry, Y.; Guyot, F.; Lens, P.N.L.; van Hullebusch, E.D. Evaluation on chemical stability of lead blast furnace (LBF) and imperial smelting furnace (ISF) slags. J. Environ. Manag. 2016, 180, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, W.R.; da Silva, F.B.V.; Araújo, P.R.M.; do Nascimento, C.W.A. Assessing human health risks and strategies for phytoremediation in soils contaminated with As, Cd, Pb, and Zn by slag disposal. Ecotoxicol. Environ. Saf. 2017, 144, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Raj, K.; Prasad, A. Lead pollution: Impact on environment and human health and approach for a sustainable solution. Environ. Chem. Ecotoxicol. 2023, 5, 79–85. [Google Scholar] [CrossRef]
- Król, A.; Mizerna, K.; Bożym, M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef] [PubMed]
- Alwaeli, M. Application of granulated lead-zinc slag in concrete as an opportunity to save natural resources. Radiat. Phys. Chem. 2013, 83, 54–60. [Google Scholar] [CrossRef]
- Kanneboina, Y.Y.; Jothi Saravanan, T.; Kabeer, K.I.S.A.; Bisht, K. Valorization of lead and zinc slags for the production of construction materials—A review for future research direction. In Construction and Building Materials; Elsevier Ltd.: Amsterdam, The Netherlands, 2023; Volume 367. [Google Scholar] [CrossRef]
- Nath, S.K. Fly ash and zinc slag blended geopolymer: Immobilization of hazardous materials and development of paving blocks. J. Hazard. Mater. 2020, 387, 121673. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.; Misra, A.; Chaudhary, S. Strength and Abrasion Characteristics of ISF Slag Concrete. J. Mater. Civ. Eng. 2013, 25, 1611–1618. [Google Scholar] [CrossRef]
- Xia, M.; Muhammad, F.; Zeng, L.; Li, S.; Huang, X.; Jiao, B.; Shiau, Y.C.; Li, D. Solidification/stabilization of lead-zinc smelting slag in composite based geopolymer. J. Clean. Prod. 2019, 209, 1206–1215. [Google Scholar] [CrossRef]
- Zhang, P.; Muhammad, F.; Yu, L.; Xia, M.; Lin, H.; Huang, X.; Jiao, B.; Shiau, Y.C.; Li, D. Self-cementation solidification of heavy metals in lead-zinc smelting slag through alkali-activated materials. Constr. Build. Mater. 2020, 249, 118756. [Google Scholar] [CrossRef]
- Peng, P.; Xie, H.; Lu, L. Leaching of a sphalerite concentrate with H2SO4-HNO3 solutions in the presence of C2Cl4. Hydrometallurgy 2005, 80, 265–271. [Google Scholar] [CrossRef]
- Erdem, M.; Özverdi, A. Environmental risk assessment and stabilization/solidification of zinc extraction residue: II. Stabilization/solidification. Hydrometallurgy 2011, 105, 270–276. [Google Scholar] [CrossRef]
- Pang, L.; Wang, D.; Wang, H.; An, M.; Wang, Q. Occurrence and leaching behaviors of heavy-metal elements in metallurgical slags. Constr. Build. Mater. 2022, 330, 127268. [Google Scholar] [CrossRef]
- Kukurugya, F.; Vindt, T.; Havlík, T. Behavior of zinc, iron and calcium from electric arc furnace (EAF) dust in hydrometallurgical processing in sulfuric acid solutions: Thermodynamic and kinetic aspects. Hydrometallurgy 2015, 154, 20–32. [Google Scholar] [CrossRef]
- Lampinen, M.; Laari, A.; Turunen, I. Kinetic model for direct leaching of zinc sulfide concentrates at high slurry and solute concentration. Hydrometallurgy 2015, 153, 160–169. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Yan, X.; Wang, X.; Zhang, J.; Huang, J.; Zhao, J. Heavy metal chemical extraction from industrial and municipal mixed sludge by ultrasound-assisted citric acid. J. Ind. Eng. Chem. 2015, 27, 368–372. [Google Scholar] [CrossRef]
- Ashtari, P.; Pourghahramani, P. Selective mechanochemical alkaline leaching of zinc from zinc plant residue. Hydrometallurgy 2015, 156, 165–172. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Jiang, J.; Zhang, C. Optimized Hydrometallurgical Route to Produce Ultrafine Zinc Powder from Industrial Wastes in Alkaline Medium. Procedia Environ. Sci. 2012, 16, 674–682. [Google Scholar] [CrossRef]
- Stefanova, A.; Aromaa, J.; Forsen, O. Alkaline leaching of zinc from argon oxygen decolonization dust from stainless steel production. Physicochem. Probl. Miner. Process. 2013, 49, 37–46. [Google Scholar] [CrossRef]
- Song, S.; Sun, W.; Wang, L.; Liu, R.; Han, H.; Hu, Y.; Yang, Y. Recovery of cobalt and zinc from the leaching solution of zinc smelting slag. J. Environ. Chem. Eng. 2019, 7, 102777. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, H.; Zhang, C.; Liu, B.; Wang, L.; Peng, W.; Cao, Y.; Song, X.; Zhu, X. A novel method for the separation of zinc and cobalt from hazardous zinc–cobalt slag via an alkaline glycine solution. Sep. Purif. Technol. 2021, 273, 119009. [Google Scholar] [CrossRef]
- Xin, C.; Xia, H.; Jiang, G.; Zhang, Q.; Zhang, L.; Xu, Y. Studies on Recovery of Valuable Metals by Leaching Lead–Zinc Smelting Waste with Sulfuric Acid. Minerals 2022, 12, 1200. [Google Scholar] [CrossRef]
- Ma, A.; Zheng, X.; Li, S.; Wang, Y.; Zhu, S. Zinc recovery from metallurgical slag and dust by coordination leaching in NH3–CH3COONH4–H2O system. R. Soc. Open Sci. 2018, 5, 180660. [Google Scholar] [CrossRef] [PubMed]
- Alkan, M.S.; Rüşen, A.; Topçu, M.A. Recovery of Lead and Zinc from Complex Industrial Waste of Zinc Process with Ammonium Acetate. JOM 2023, 75, 1158–1168. [Google Scholar] [CrossRef]
- Wang, L.; Gao, H.; Song, S.; Xue, N.; Zhang, J.; Yang, S.; Liu, C. Experimental and kinetic study of zinc leaching from metallurgical slag by 5-sulfosalicylic acid. Physicochem. Probl. Miner. Process. 2021, 57, 8–20. [Google Scholar] [CrossRef]
- Varga, T.; Bokányi, L.; Török, T. On the Aqueous Recovery of Zinc from Dust and Slags of the Iron and Steel Production Technologies. Int. J. Metall. Mater. Eng. 2016, 212, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, A.; Aromaa, J.; Forsen, O. Alkaline leaching of zinc from stainless steel electric arc furnace dusts. Physicochem. Probl. Miner. Process. 2015, 51, 293–302. [Google Scholar]
- Dahal, M. Selective Leach Recovery of Zinc from a Composite Sulphide Ore Deposit, Tailings, Crushed Ore or Mine Sludge. U.S. Patent No. US 8,961,911 B2, 24 February 2015. [Google Scholar]
- Roine, A.; Lamberg, P.; Mansikk-Aho, J.; Björklund, P.; Kentala, J.; Talonet, T.; Vartiainen, A. HSC Chemistry 6. version 6.12. Outokumpu Research Oy: Helsinki, Finland, 2007. [Google Scholar]
- Levenspiel, O.; Conesa, J. Ingeniería de las Reacciones Químicas; Limusa: Ciudad de México, Mexico, 2004. [Google Scholar]
- Ciminelli, V.; Osseo, K. Kinetics of pyrite oxidation in sodium carbonate solutions. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 1995, 26, 677–685. [Google Scholar] [CrossRef]
- Habashi, F. Kinetics of Metallurgical Process; Métallurgie Extractive: Québec City, QC, Canada, 1999. [Google Scholar]

| Type of Sample (Zn Content and Main Zn Species) | Leaching Medium | Test Conditions | % Zn Extraction | Reference |
|---|---|---|---|---|
| Zinc smelting slag (Zn, 44%, as ZnSO4 and ZnSO3) | H2SO4 | 2 mol/L de H2SO4, T = 85 °C, t = 1 h, S/L = 1:4, agitation = 300 rpm | 98% | [21] |
| Zinc–cobalt slag (Zn, 4.9% as ZnO) | Glycine | pH = 10, T = 45 °C, Glycine = 100 g/L, S/L = 1:40, t = 1.5 h | 94% | [22] |
| Zinc–lead smelting slag (Zn, 42% as ZnO mainly) | H2SO4 | 160 g/L de H2SO4, T = 90 °C, t = 1 h, S/L = 1:5, agitation = 400 rpm | 83.22% | [23] |
| Slag and metallurgical dust from zinc–lead smelting (Zn 24.7% as ZnO, mainly) | CH3COONH4-NH3-H2O | 5 mol/L de NH3, T = 25 °C, t = 1 h, ammonia/ammonium ratio = 1:1, agitation = 300 rpm | 83.76% | [24] |
| Zinc and lead waste | NH4CH3COO | 3 mol/L de NH4CH3COO, T = 70 °C, t = 1 h, S/L = 1/10 g/mL, agitation = 300 rpm | 34% | [25] |
| Zinc metallurgical slag (Zn 22.6% as ZnO) | 5-sulfosalicylic acid | 0.3 mol/L de 5-sulfosalicylic acid, T = 50 °C, t = 1 h, agitation = 450 rpm, d90 = 65 µm of size fraction | 94.20% | [26] |
| Residues from the steel process. (Zn 35%, as [(Zn,Fe,Mn)(Fe,Mn)2O4] and ZnO | NaOH | NaOH 8 M, T = 250 °C, t = 0.5 h, S/L = 1:5, agitation = 400 rpm | 50% | [27] |
| SPECIES | % | ELEMENTS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Ca | Fe | K | Mg | Mo | O | Pb | S | Si | Zn | ||
| FeO | 24.57 | 19.1 | 5.47 | |||||||||
| Fe3O4 | 2.88 | 2.08 | 0.8 | |||||||||
| ZnO | 5.62 | 1.1 | 4.52 | |||||||||
| ZnS | 7.12 | 2.3 | 4.82 | |||||||||
| CaSiO3 | 32.16 | 11.1 | 13.29 | 7.78 | ||||||||
| Ca2Al2SiO7 | 7.12 | 1.4 | 2.08 | 2.91 | 0.73 | |||||||
| PbSiO3 | 1.9 | 0.32 | 1.39 | 0.19 | ||||||||
| MgO | 1.5 | 0.9 | 0.6 | |||||||||
| ZnFe2O4 | 5.98 | 2.77 | 1.59 | 1.62 | ||||||||
| Ca2ZnSi2O7 | 2.23 | 0.57 | 0.80 | 0.40 | 0.46 | |||||||
| K2O | 1.68 | 1.39 | 0.29 | |||||||||
| MoO3 | 1.00 | 0.67 | 0.33 | |||||||||
| Total * | 95.03 | 1.4 | 13.75 | 23.95 | 1.39 | 0.9 | 0.67 | 26.5 | 1.39 | 2.3 | 9.1 | 11.42 |
| Factor | DF | SS | MS | F |
|---|---|---|---|---|
| Temperature, °C | 2 | 55.5152 | 27.7576 | 0.45754261 |
| Solids, % | 2 | 283.3333 | 141.6667 | 2.3351641 |
| NaClO/NaOH | 3 | 372.6667 | 124.2222 | 2.04761756 |
| Error | 3 | 60.6667 | 20.2222 | |
| Total | 10 | 772.1818 |
| Model | Ea (kJ/mol) | R2 |
|---|---|---|
| Chemical reaction control | 13.27 | 0.989 |
| Diffusion control | 12.69 | 0.989 |
| Stochastic model | 15.26 | 0.9816 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najera Ibarra, J.M.; Soria-Aguilar, M.d.J.; Martínez-Luevanos, A.; Picazo-Rodriguez, N.G.; Almaguer-Guzman, I.; Chaidez-Felix, J.; Carrillo-Pedroza, F.R. Zinc Extraction from Primary Lead Smelting Slags by Oxidant Alkaline Leaching. Processes 2024, 12, 1409. https://doi.org/10.3390/pr12071409
Najera Ibarra JM, Soria-Aguilar MdJ, Martínez-Luevanos A, Picazo-Rodriguez NG, Almaguer-Guzman I, Chaidez-Felix J, Carrillo-Pedroza FR. Zinc Extraction from Primary Lead Smelting Slags by Oxidant Alkaline Leaching. Processes. 2024; 12(7):1409. https://doi.org/10.3390/pr12071409
Chicago/Turabian StyleNajera Ibarra, Juana María, Ma. de Jesus Soria-Aguilar, Antonia Martínez-Luevanos, Nallely Guadalupe Picazo-Rodriguez, Isaias Almaguer-Guzman, Josue Chaidez-Felix, and Francisco Raúl Carrillo-Pedroza. 2024. "Zinc Extraction from Primary Lead Smelting Slags by Oxidant Alkaline Leaching" Processes 12, no. 7: 1409. https://doi.org/10.3390/pr12071409







