Carboxymethyl-Cellulose-Based Hydrogels Incorporated with Cellulose Nanocrystals Loaded with Vitamin D for Controlled Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carboxymethyl Cellulose Hydrogel Conjugation with Cellulose Nanocrystals and Vitamin D3
2.3. Carboxymethyl Cellulose Hydrogel Conjugated with CNCs and Vitamin D3 Characterisation
2.4. Biological Analyses of Carboxymethyl Cellulose Hydrogels Conjugated with CNCs and Vitamin D3
2.4.1. Cell Culture
2.4.2. Cell Viability Assay with Resazurin
2.5. In Vitro Accumulative Kinetic Drug Delivery in Carboxymethyl Cellulose Hydrogels Conjugated with CNCs and Vitamin D3
3. Results and Discussion
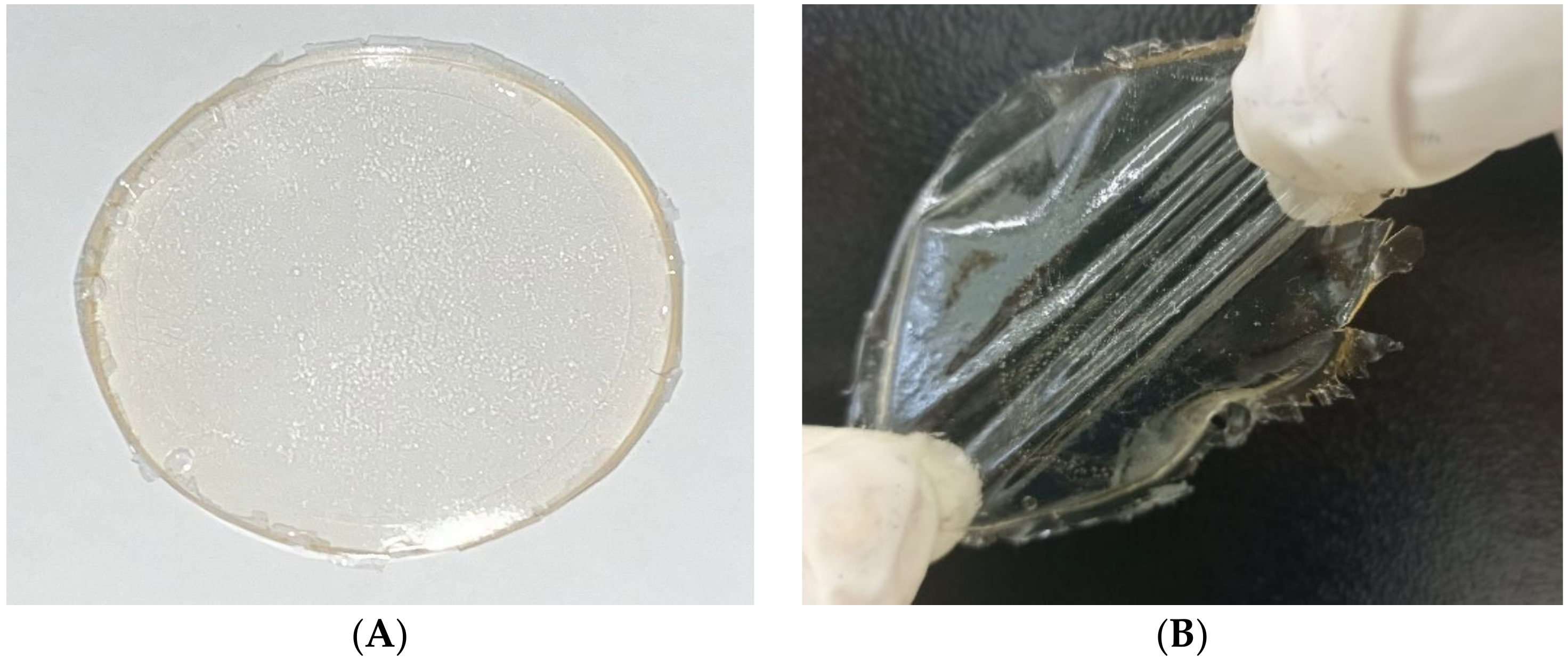
3.1. Visual Characterisation of Hydrogels
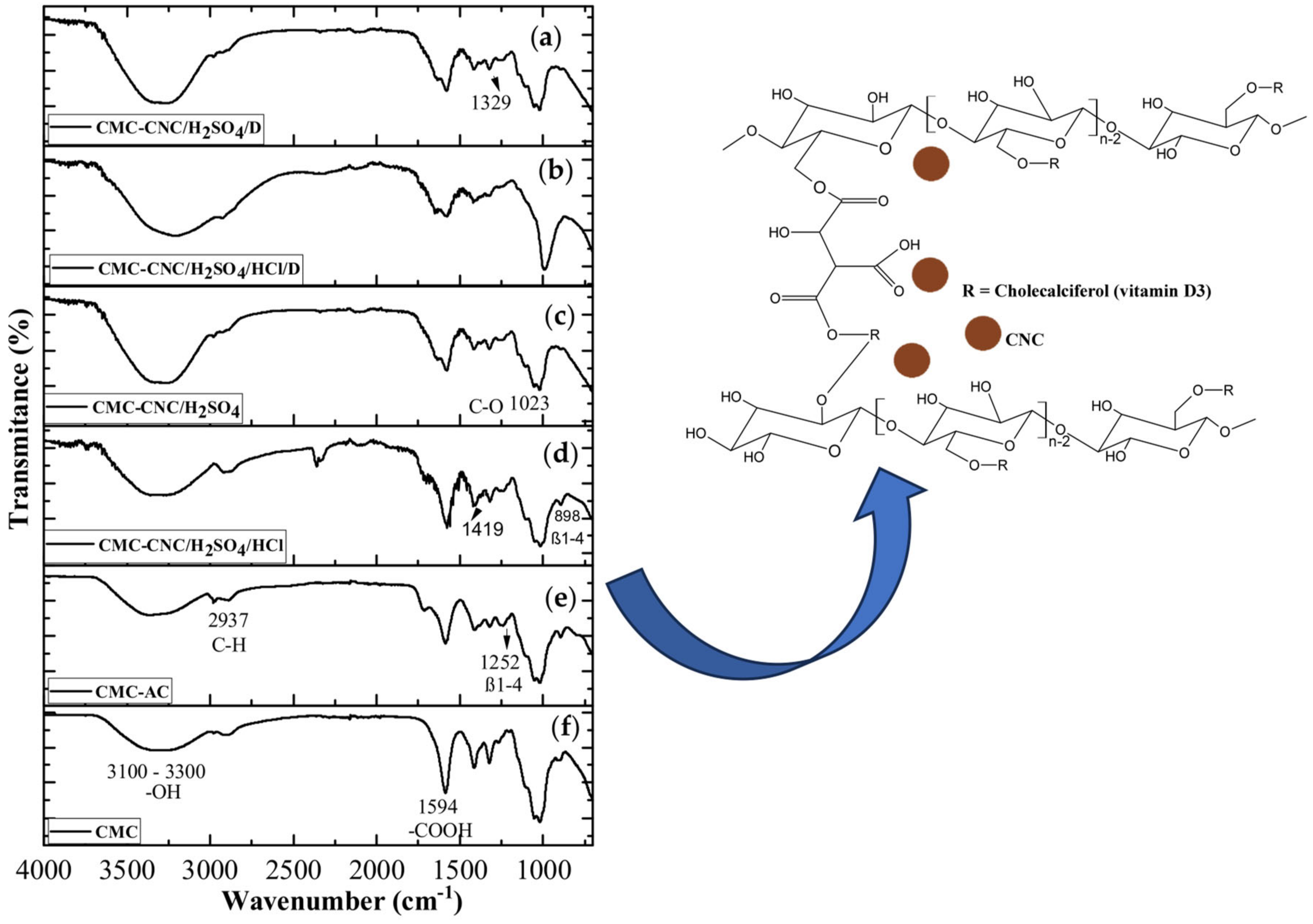
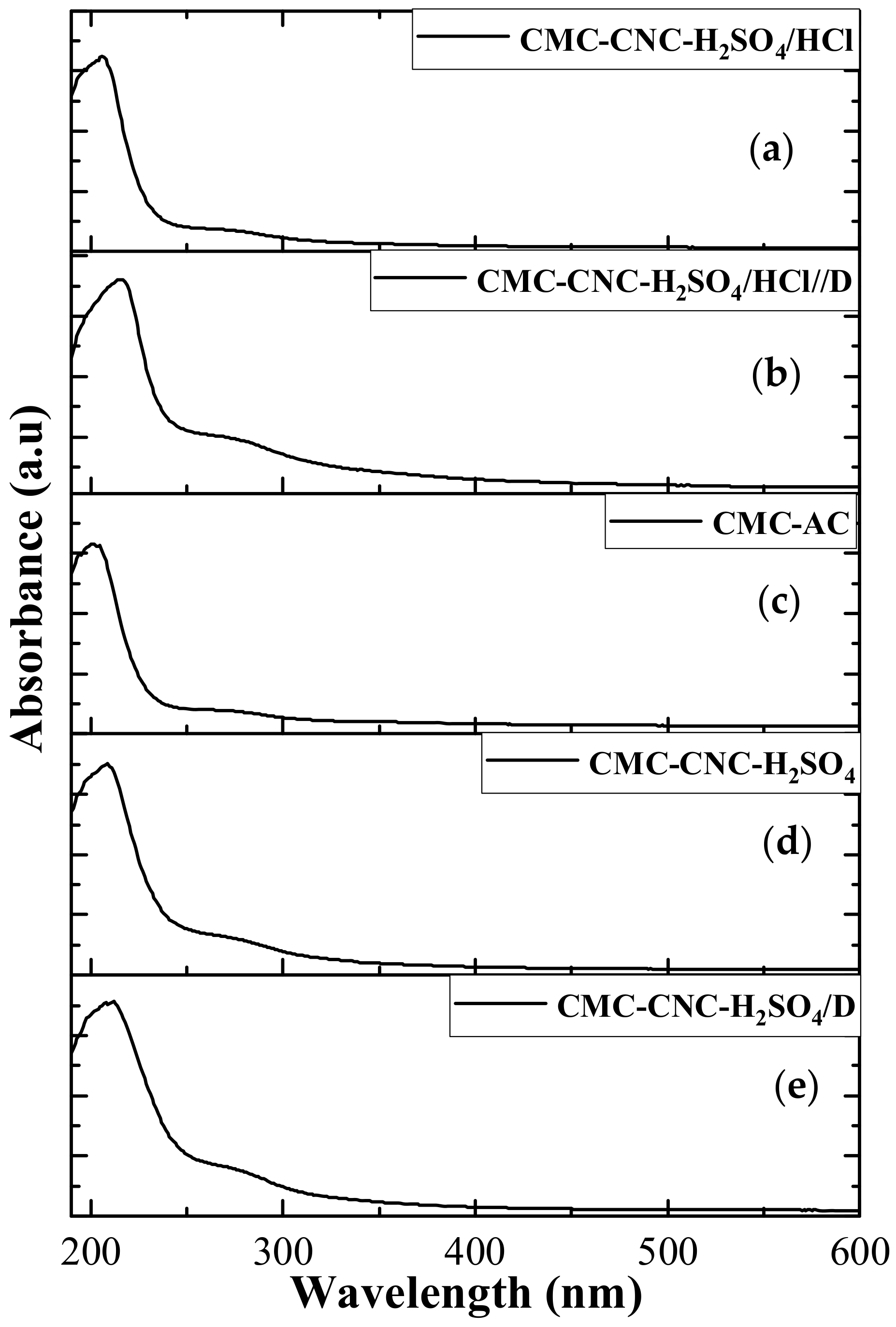
3.2. Spectroscopy Analyses
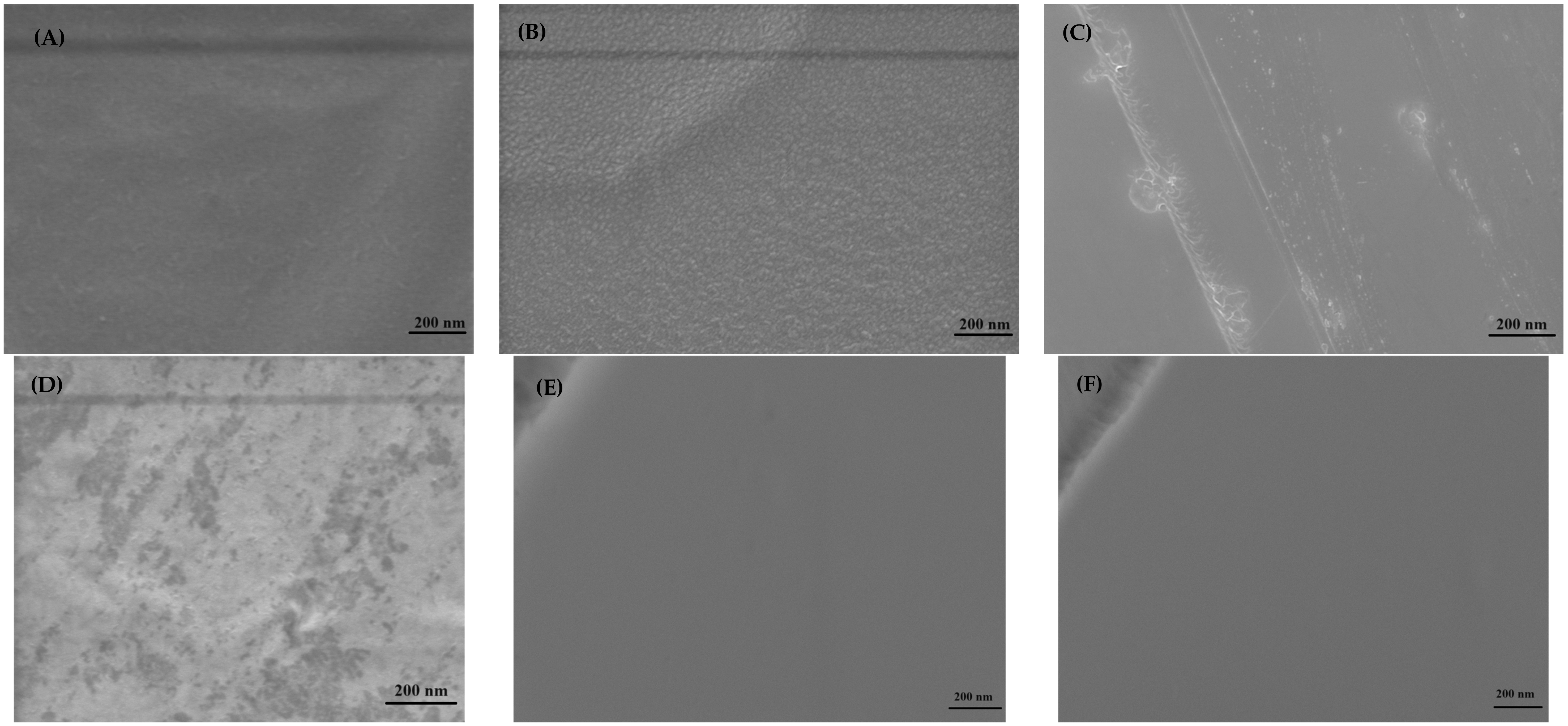
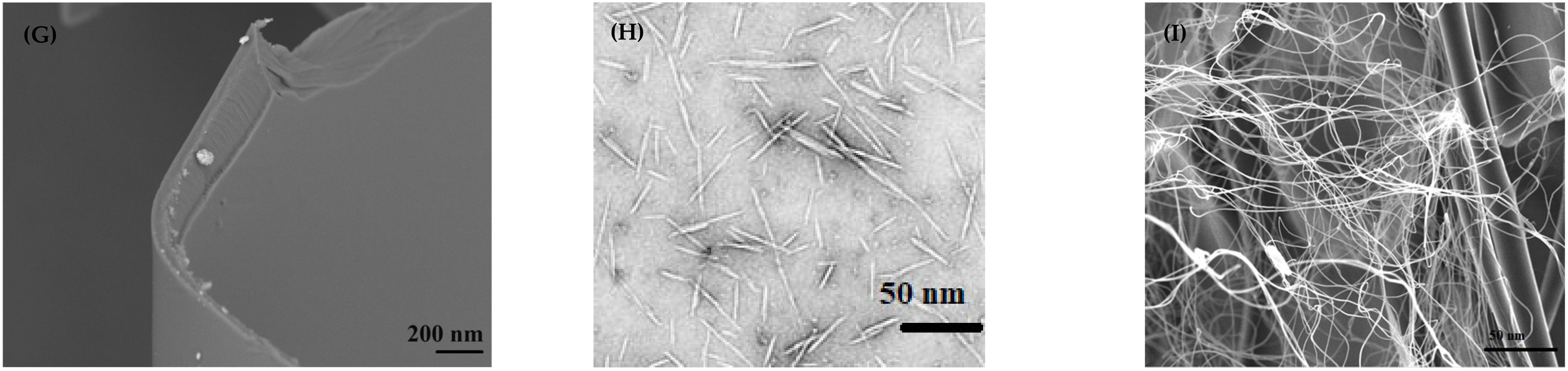
3.3. Microscopy Analysis
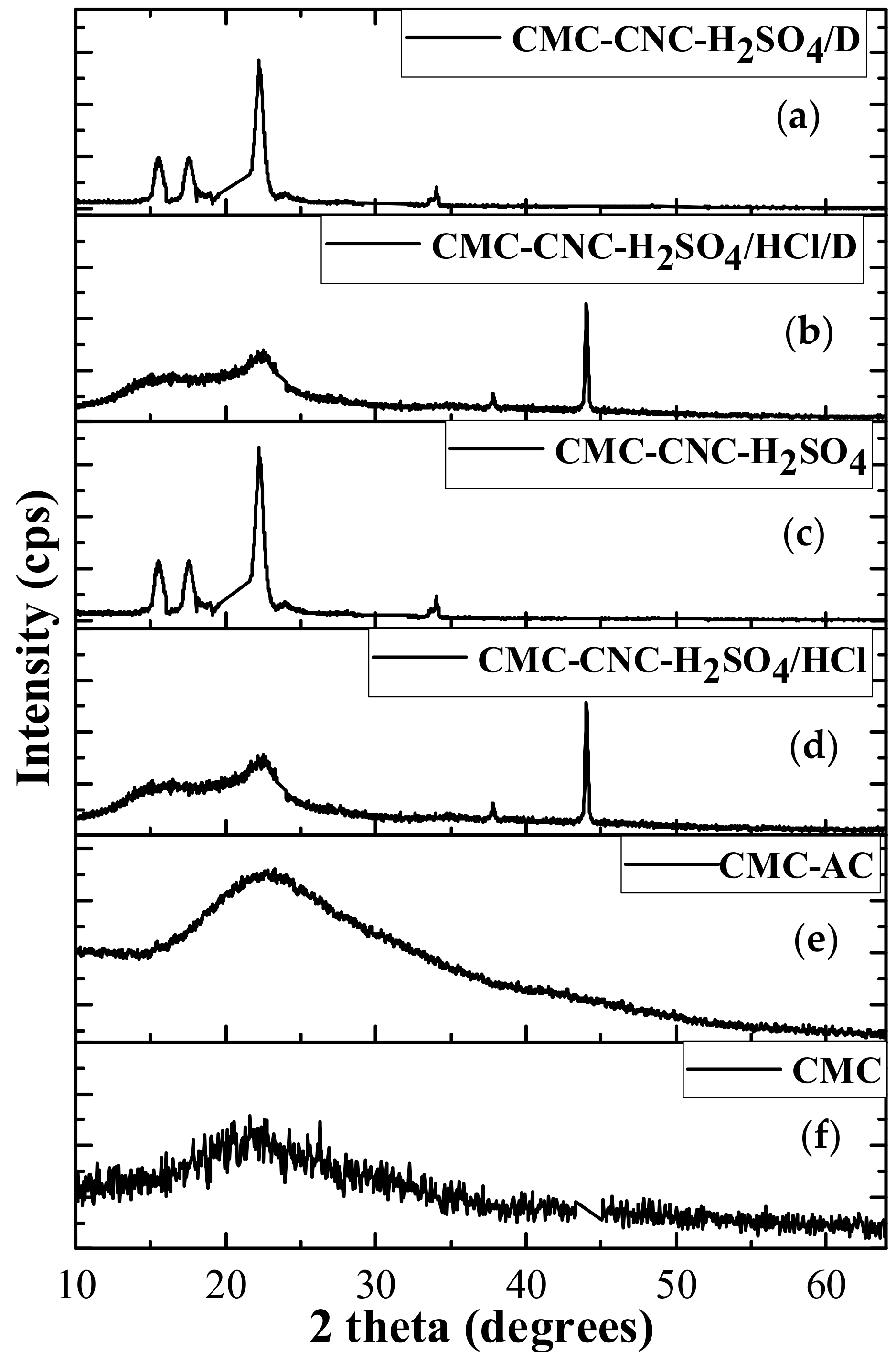
3.4. XRD Analysis of Hydrogels Loaded with Nanocrystals and Nanofibres Modified with Vitamin D
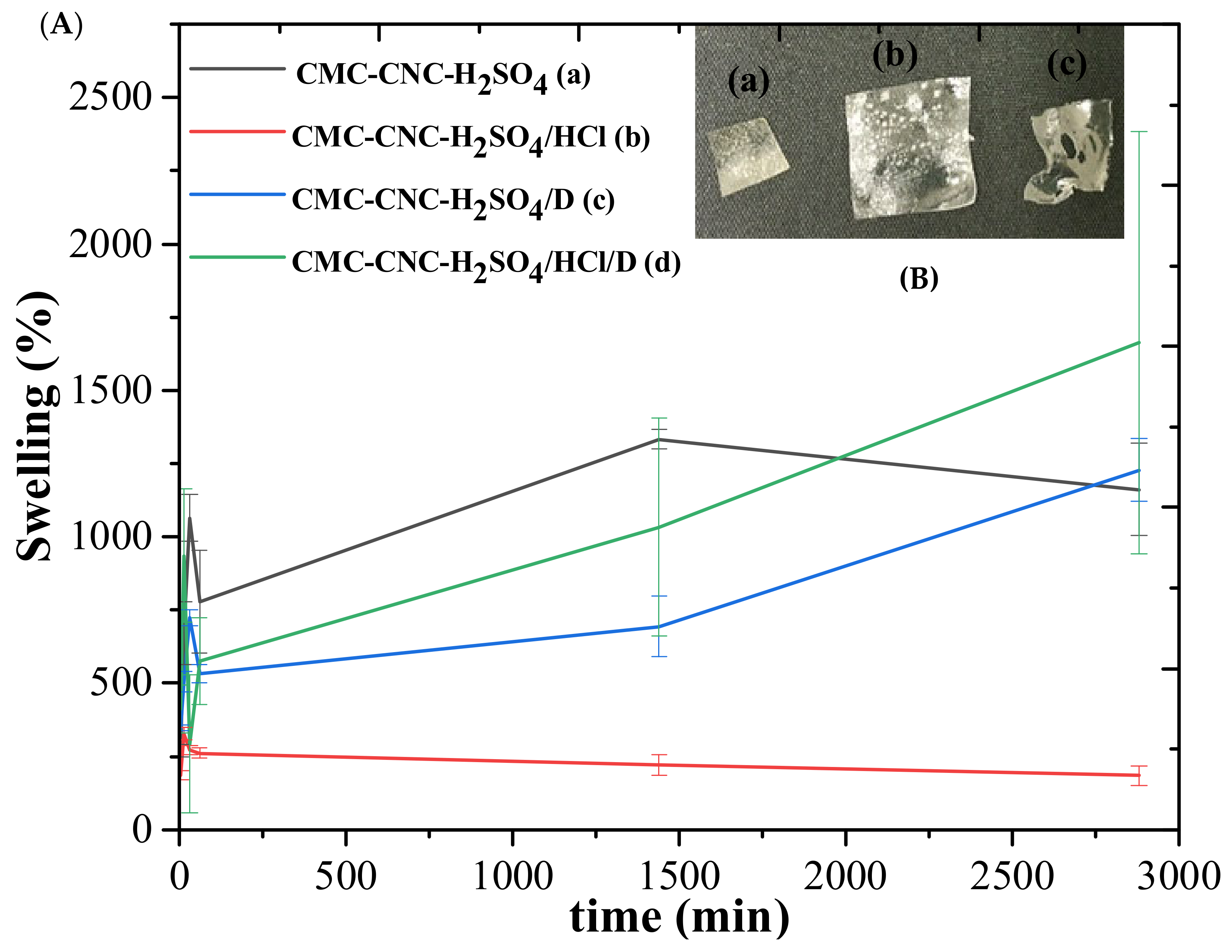
3.5. Swelling and Gel Fraction of Hydrogels Loaded with Nanocrystals and Nanofibres Modified with Vitamin D
3.6. In Vitro Kinetic Studies of Vitamin D Release
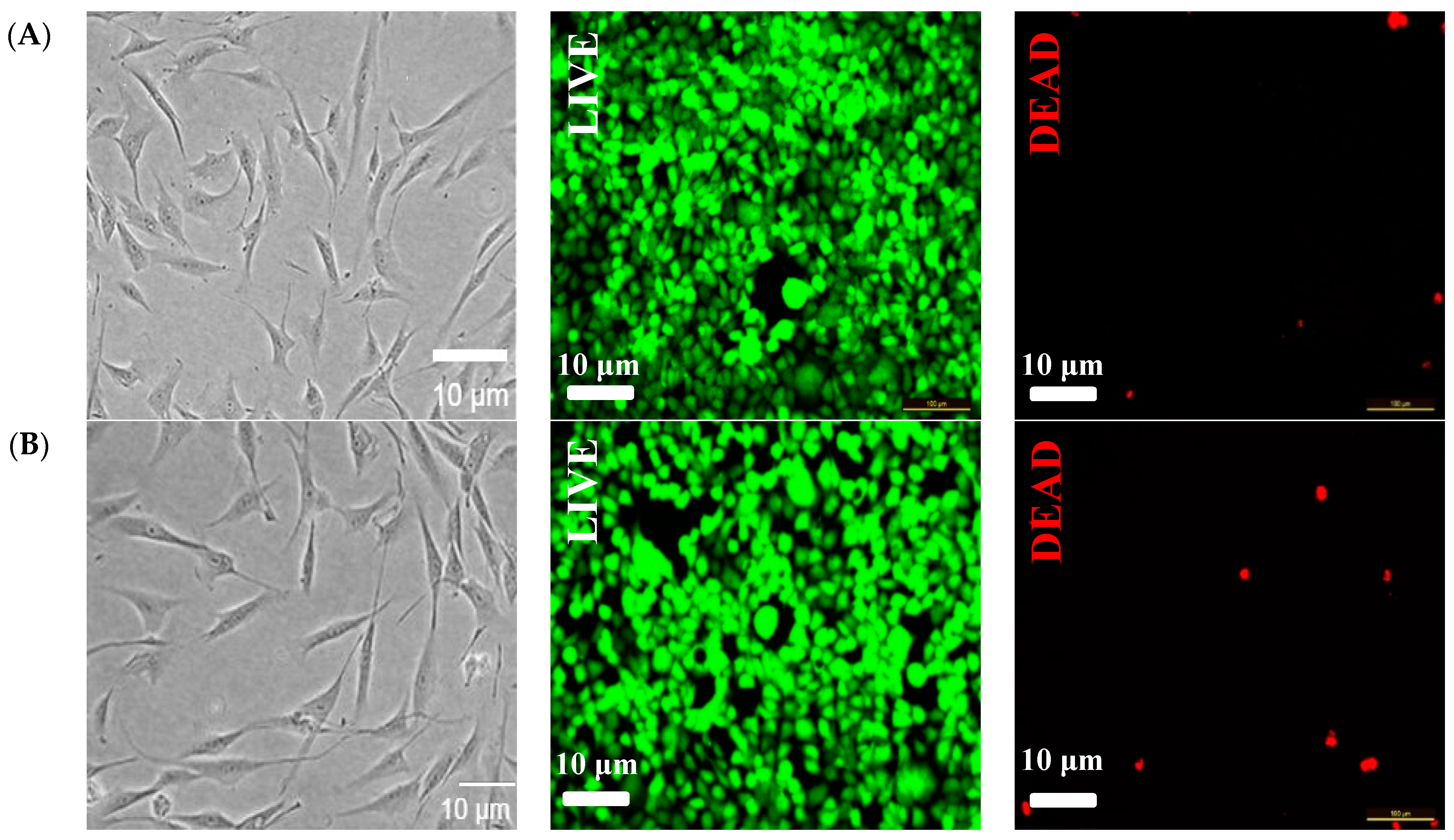
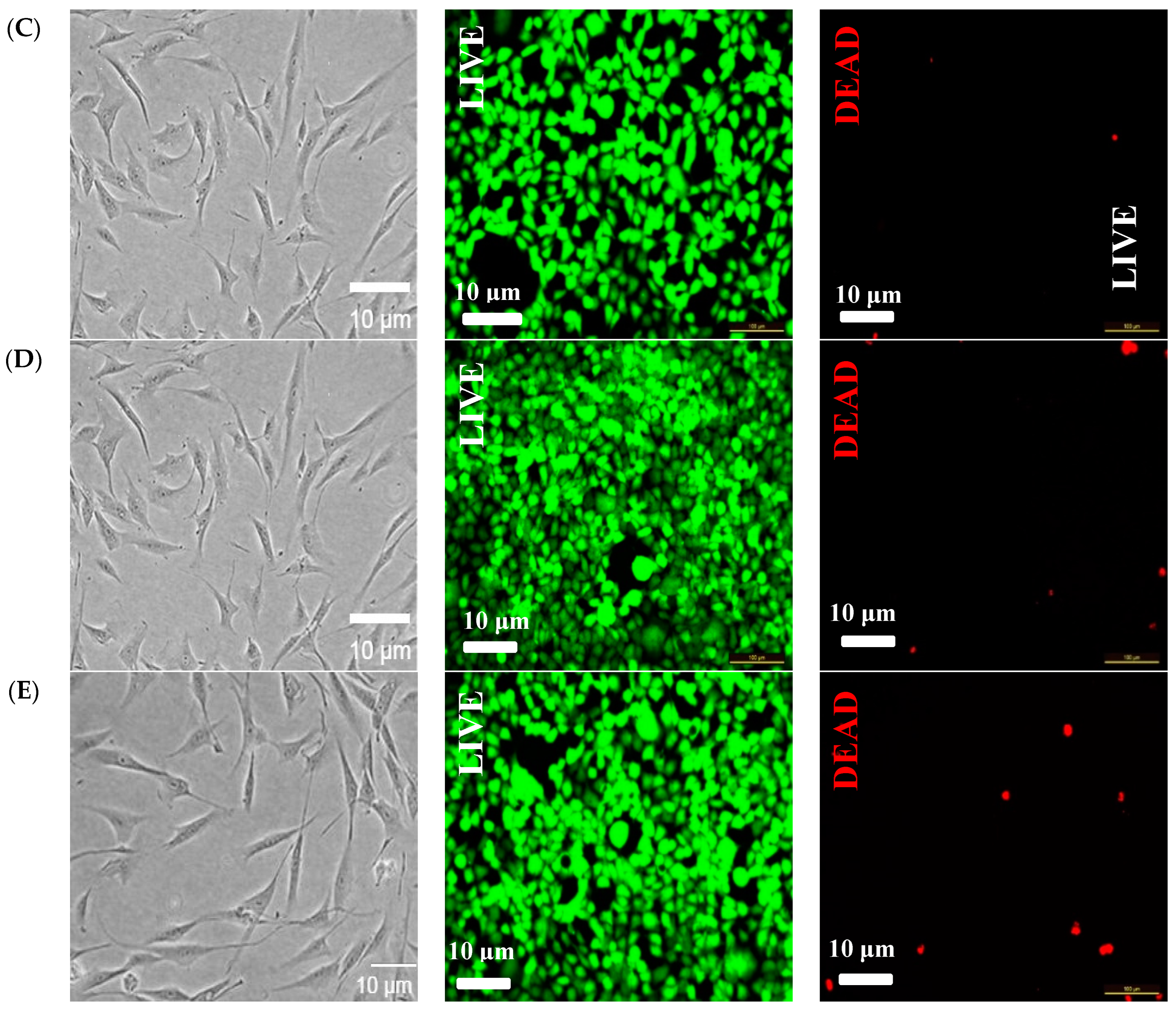
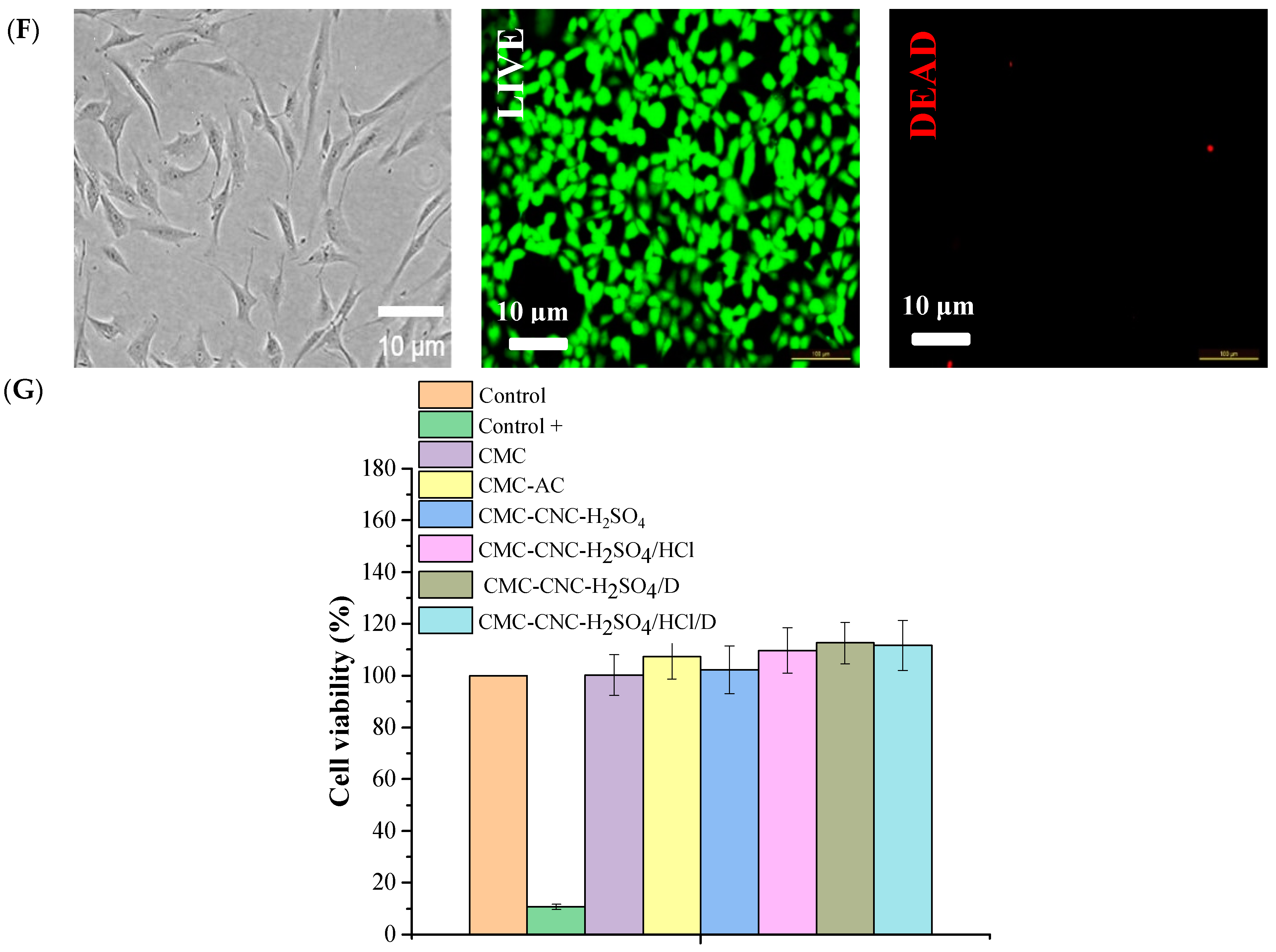
3.7. Cytotoxicity Evaluation of Hydrogels Loaded with Nanocrystals and Nanofibres Modified with Vitamin D
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- UN. The ODS in Brazil. 2024. Available online: https://brasil.un.org/pt-br/sdgs (accessed on 29 April 2024).
- Silva, N.C.; Rodrigues, J.S.; Bernardes, M.S.; Gonçalves, M.P.; Medeiros Borsagli, F.G.L. Cellulose nanocrystals and nano-fiber from sub-wear out Brazilian semiarid source for biological applications. J. Cluster Sci. 2024, 1, 1–10. [Google Scholar]
- Junges, A.; Denti, A.F.; Bernardi, J.L.; Polina, C.C.; Meregalli, M.M.; Vanz, J.B.; Mignoni, M.L. Application of nanotechnology in food engineering: A review. Res. Soc. Dev. 2022, 11, 1–10. [Google Scholar]
- Borsagli, F.G.L.M.; Borsagli, A. Luminescent carbon dots for environmental photocatalytic. In Inorganic-Organic Composites for Water and Wastewater Treatment; Lichtfouse, E., Muthu, S.S., Khadir, A., Eds.; Springer: Singapore, 2021; pp. 201–228. [Google Scholar]
- Paiva, A.E.; Guin, J.P.; Vasquez, J.F.B.; Thampi, K.R.; Sullivan, J.A.; Borsagli, F.G.L.M.; Morris, M.A. Degradation of an antibiotic via a photo-Fenton process on block copolymernanopatterned iron oxide coated substrates. Chem. Eng. J. 2023, 466, 142925. [Google Scholar] [CrossRef]
- Rodrigues, J.S.; Silva, C.J.T.; Silva, N.C.; Aguiar, R.A.; Bernardes, M.S.; Soares, C.M.; Madureira, J.A.; Borsagli, F.G.L.M. Carbohydrate polymer hydrogels: An environmental and eco-friendly choice for biomedical applications? J. Polym. Res. 2023, 30, 382. [Google Scholar] [CrossRef]
- Thomas, P.; Duolikun, T.; Rumjit, N.P.; Moosavi, S.; Lai, C.W.; Johan, M.R.B.; Fen, L.B. Comprehensive review on nanocellulose: Recent developments, challenges and future prospects. J. Mech. Behav. Biomed. Mater. 2020, 110, 103884. [Google Scholar] [CrossRef]
- Newman, L.; Jasim, D.A.; Prestat, E.; Lozano, N.; Lazaro, I.; Nam, Y.; Assas, B.M.; Pennock, J.; Haigh, S.J.; Bussy, C.; et al. Splenic capture and in vivo intracellular biodegradation of biological-grade graphene oxide sheets. ACS Nano 2020, 14, 10168–10186. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Choi, H.; Kang, K.; Shin, M.; Son, D. Soft stretchable conductive carboxymethylcellulose hydrogels for wearable sensors. Gels 2022, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Sadak, O.; Ramirez, I.A.; Iverson, N. Stimuli-bioresponsive hydrogels as new generation materials for implantable, wearable, and disposable biosensors for medical diagnostics: Principles, opportunities, and challenges. Adv. Colloid Interface Sci. 2023, 317, 102920. [Google Scholar] [CrossRef] [PubMed]
- Fadl, F.I.A.E.; Hegazy, D.E.; Maziad, N.A.; Ghobashy, M.M. Effect of nano-metal oxides (TiO2, MgO, CaO, and ZnO) on antibacterial property of (PEO/PEC-co-AAm) hydrogel synthesized by gamma irradiation. Int. J. Biol. Macromol. 2023, 250, 126248. [Google Scholar] [CrossRef] [PubMed]
- Borsagli, F.G.L.M.; Souza, A.J.M.; Paiva, A.E. Ecofriendly multifunctional thiolated carboxymethyl chitosan-based 3D scaffolds with luminescent properties for skin repair and theragnostic of tissue regeneration. Int. J. Biol. Macromol. 2020, 165, 3051–3064. [Google Scholar] [CrossRef] [PubMed]
- Parfenyuk, E.V.; Dolinina, E.S. Silica hydrogel composites as a platform for soft drug formulations and cosmetic compositions. Mater. Chem. Phys. 2022, 287, 126160. [Google Scholar] [CrossRef]
- Ijaz, F.; Tahir, H.M.; Ali, S.; Ali, A.; Khan, H.A.; Muzamil, A.; Manzoor, H.H.; Qayyum, K.A. Biomolecules based hydrogels and their potential biomedical applications: A comprehensive review. Int. J. Biol. Macromol. 2023, 253, 127362. [Google Scholar] [CrossRef]
- Ghorbanzadeh, M.; Golmohammadzadeh, S.; Karimi, M.; Farhadian, N. Evaluation of vitamin D3 serum level of microemulsion based hydrogel containing Calcipotriol drug. Mater. Todays Commun. 2022, 33, 104409. [Google Scholar] [CrossRef]
- Franco, P.; Marco, I. Contact Lenses as Ophthalmic Drug Delivery Systems: A review. Polymers 2021, 17, 1102. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Lu, K.; Lu, Y.; Zhu, S.; Yue, Y.; Xu, X.; Mei, C.; Xiao, H.; Wu, Q.; Han, J. A stretchable, self-healing conductive hydrogels based on nanocellulose supported graphene towards wearable monitoring of human motion. Carbohyd. Polym. 2020, 250, 116905. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xin, L.; Li, H.; Sun, P.; Liu, C.; Fang, L. Mussel-inspired controllable drug release hydrogel for transdermal drug delivery: Hydrogen bond and ion-dipole interactions. J. Control. Release 2024, 365, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Chng, A.L.B.; Tan, K.H.; Shub, A.; Tan, T.; Hian, T.K.; Lee, R.W.K.; Ling, L.S.; Kuma, K.; Siew, C.Y. Clinical practice of vitamin D screening and supplementation in pregnancy in Asia-pacific countries: A cross-sectional study. Heliyon 2023, 9, 21186. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Nie, X.-L.; Wu, S.; Cai, J. Vitamin D and hypertension: Prospective study and meta-analysis. PLoS ONE 2017, 12, e0174298. [Google Scholar] [CrossRef]
- Haitchi, S.; Moliterno, P.; Widhalm, K. Prevalence of vitamin D deficiency in seniors—A retrospective study. Clin. Nutr. ESPEN 2023, 57, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Holick, M. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.; Seidler, K.; Neil, J. Vitamin D deficiency and female infertility: A mechanism review examining the role of vitamin D in ovulatory dysfunction as a symptom of polycystic ovary syndrome. J. Reprod. Immunol. 2022, 151, 103633. [Google Scholar] [CrossRef] [PubMed]
- Seraphin, G.; Reiger, S.; Hewison, M.; Capobianco, E.; Lisse, T.S. The impact of vitamin D on cancer: A mini review. J. Steroid Biochem. Mol. Biol. 2023, 231, 106308. [Google Scholar] [CrossRef] [PubMed]
- Mousa, H.M.; Fahmy, H.S.; Abouzeid, R.; Abdel-Jaber, G.T.; Ali, W.Y. Polyvinylidene fluoride-cellulose nanocrystals hybrid nanofiber membrane for energy harvesting and oil-water separation applications. Mater. Lett. 2022, 306, 130965. [Google Scholar] [CrossRef]
- Sobrinho, M.S.; Tabatinga, G.M.; Machado, I.C.; Lopes, A.V. Pollination biology and mating system of Angelonia hirta (Scrophulariaceae), a neotropical shrub with uncommon floral traits. Acta Bot. Bras. 2013, 27, 456. [Google Scholar] [CrossRef]
- Costa, L.A.S.; Fonsêca, A.F.; Pereira, F.V.; Druzian, J.I. Biodegradability of nanocomposites based on cassava starch and clay. Cellul. Chem. Technol. 2015, 49, 127–134. [Google Scholar]
- Medeiros Borsagli, F.G.L.; Rodrigues, J.S.; Aguiar, R.A.; Paiva, A.E.; Vasquez, J.F.B.; Ramos, W.T.S.; Allibrandini, P.; Rocha, E.P.A.; Gonçalves, M.P.; Souza, F.E. Low-cost luminescent scaffolds-based on thiol chitosans by microwave radiation for vertebral disc repair/theragnostic. Int. J. Biol. Macromol. 2022, 209, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Durjava, M.K.; Kouba, M.; Lopez-Afonso, M.; Puente, S.L.; Marcon, F.; et al. Safety and efficacy of sodium carboxymethyl cellulose for all animal species. EFSA J. 2020, 18, 6211. [Google Scholar]
- Paiva, A.E.; Medeiros Borsagli, F.G.L. Ecofriendly multiphase aqueous colloidal based on carboxymethylcellulose nanoconjugates with luminescence properties for potential bioimaging cancer cells. J. Polym. Environ. 2020, 23, 3076–3096. [Google Scholar] [CrossRef]
- Molaveisi, M.; Shahidi-Noghabi, M.; Naji-Tabasi, S. Vitamin D3-loaded nanophytosomes for enrichment purposes: Formulation, structure optimization, and controlled release. Food Process Eng. 2020, 2020, 13560. [Google Scholar] [CrossRef]
- Semalty, A.; Semalty, M.; Rawat, M.S.M.; Franceschi, F. Supramolecular phospholipids–polyphenolics interactions: The PHYTOSOME® strategy to improve the bioavailability of phytochemicals. Fitoterapia 2010, 81, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, Z.; Zhang, W.; Wang, W.; Chang, J.; Kai, J. Fluorescent carbon dots as nanoprobe for determination of lidocaine hydrochloride. Sens. Actuators B Chem. 2018, 262, 928. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Cao, J.; Jiang, W. Improving the performance of edible food packaging films by using nanocellulose as an additive. Int. J. Biol. Macromol. 2020, 166, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Liu, C. Film transparency and opacity measurements. Food Anal. Methods 2022, 15, 2840–2846. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.Z.; Shah, A.A.; Noreen, Z.; Usman, S.; Zafar, S.; Yasin, N.A.; Sayed, S.R.M.; Al-Mana, R.A.; Elansary, H.O.; Ahmad, A.; et al. Calcium oxide nanoparticles mitigate lead stress in Abelmoschus esculentus through improving the key antioxidative enzymes, nutritional content and modulation of stress markers. Plant Physiol. Biochem. 2024, 206, 108171. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takacs, E.; Wojnarovitis, L. Synthesis and characterization of superabsorbent hydrogels based on hydroxyethylcellulose and acrylic acid. Carbohyd. Polym. 2017, 166, 300–308. [Google Scholar] [CrossRef] [PubMed]
- French, A.D. Correction to: Increment in evolution of cellulose crystallinity analysis. Cellulose 2020, 27, 5445–5448. [Google Scholar] [CrossRef]
- French, A.D.; Santiago Cintrón, M. Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 2020, 20, 583–588. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Yu, N.Y.; Fang, W.D.; Tan, Q.-G.; Ji, R.; Yang, L.-Y.; Wei, S.; Zhang, X.-W.; Miao, A.-J. Photodegradation of carbon dots cause cytotoxicity. Nat. Commun. 2021, 12, 812. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, T.; Grabovac, V.; Palmberger, T.F.; Hoffer, M.H.; Bernkop-Schnurch, A. Synthesis and characterization of a chitosan-N-acetyl cysteine conjugate. Int. J. Pharm. 2008, 347, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Mensaha, A.; Li, D.; Wang, Q.; Wei, Q. A plant-inspired long-lasting adhesive bilayer nanocomposite hydrogel based on redox-active Ag/Tannic acid-Cellulose nanofibers. Carbohydr. Polym. 2021, 255, 117508. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, R.; Zarrintaj, P.; Kamrava, S.K.; Bagher, Z.; Farhadi, M.; Heidari, F.; Komeili, A.; Gutierrez, T.J.; Saeb, M.R. Conductive hydrogels based on agarose/alginate/chitosan for neural disorder therapy. Carbohydr. Polym. 2019, 224, 115161. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Kim, J.H.; Baek, S.-W.; Lee, J.-K.; Park, S.-Y.; Choi, B.; Kim, T.-H.; Min, K.; Han, D.K. Controlled vitamin D delivery with injectable hyaluronic acid-based hydrogel for restoration of tendinopathy. J. Tissue Eng. 2022, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Tsuprykov, O.; Chen, X.; Elitok, S.; Krämer, B.K.; Hocher, B. Relationship between vitamin d and hormones important for human fertility in reproductive-aged women. Front. Endocrinol. 2021, 12, 666667. [Google Scholar] [CrossRef] [PubMed]
- Simioni, Y.R.; Ricatti, F.; Salvay, A.G.; Jerez, H.E.; Schilrreff, P.; Romero, E.L.; Morilla, M.J. Activity of hydrogel-vitamin D3/bacterioruberin nanoparticles on imiquimod-induced fibroblasts-keratinocytes spheroids. J. Drug Deliv. Sci. Technol. 2024, 97, 105738. [Google Scholar] [CrossRef]
- Eslami, M.; Shahedi, M.; Fath, M. Development of hydrogels for entrapment of vitamin D3: Physicochemical characterization and release study. Food Biophys. 2018, 13, 284–291. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). Vitamin D. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 30 April 2024).
- Desfrançois, C.; Auzély, R.; Texier, I. Lipid nanoparticles and their hydrogel composites for drug delivery: A review. Pharmaceuticals 2018, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, E.C.; Yang, E.; Lee, C.-J.; Lee, H.-W.; DiMaio, D.; Hwang, E.-S. Rapid induction of senescence in human cervical carcinoma cells. Proc. Natl. Acad. Sci. USA 2000, 97, 10978–10983. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Ruibo, Z.; Gao, H.; Li, W.; Yun, X.; Liu, J.; Zhao, X.; Zhao, G.; Zhang, F. One-step, green, and economic synthesis of water-soluble photoluminescent carbon dots by hydrothermal treatment of wheat straw and their bio-applications in labeling, imaging, and sensing. Appl. Surf. Sci. 2015, 355, 1136–1144. [Google Scholar] [CrossRef]
- Thankam, F.G.; Muthu, J.; Sankar, V.; Gopal, R.K. Growth and survival of cells in biosynthetic poly vinyl alcohol–alginate IPN hydrogels for cardiac applications. Colloids Surf. B Biointerfaces 2013, 107, 137–145. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, N.d.C.; Silva, C.J.T.; Gonçalves, M.P.; Borsagli, F.G.L.M. Carboxymethyl-Cellulose-Based Hydrogels Incorporated with Cellulose Nanocrystals Loaded with Vitamin D for Controlled Drug Delivery. Processes 2024, 12, 1437. https://doi.org/10.3390/pr12071437
Silva NdC, Silva CJT, Gonçalves MP, Borsagli FGLM. Carboxymethyl-Cellulose-Based Hydrogels Incorporated with Cellulose Nanocrystals Loaded with Vitamin D for Controlled Drug Delivery. Processes. 2024; 12(7):1437. https://doi.org/10.3390/pr12071437
Chicago/Turabian StyleSilva, Nathália da Cunha, Carla Jeany Teixeira Silva, Max Pereira Gonçalves, and Fernanda G. L. Medeiros Borsagli. 2024. "Carboxymethyl-Cellulose-Based Hydrogels Incorporated with Cellulose Nanocrystals Loaded with Vitamin D for Controlled Drug Delivery" Processes 12, no. 7: 1437. https://doi.org/10.3390/pr12071437
APA StyleSilva, N. d. C., Silva, C. J. T., Gonçalves, M. P., & Borsagli, F. G. L. M. (2024). Carboxymethyl-Cellulose-Based Hydrogels Incorporated with Cellulose Nanocrystals Loaded with Vitamin D for Controlled Drug Delivery. Processes, 12(7), 1437. https://doi.org/10.3390/pr12071437







