A Study on Effect Analysis and Process Parameter Optimization of Viscous Acid Acidification in a Porous Heterogeneous Carbonate Reservoir
Abstract
:1. Introduction
2. Experimental Scheme for Adaptability Evaluation of Viscous Acid in the Target Reservoir
2.1. Particle Dissolution Experiment
2.2. Steel Sheet Corrosion Experiment
2.3. Acid-Rock Reaction Kinetics Experiment
2.4. Acid Flow and Wormhole Formation Experiment
2.5. Residual Acid Damage Experiment
3. Viscous Acid Performance Test Results and Analysis
3.1. Basic Performance of Acid Liquid Test Results and Analysis
3.2. Important Indicators of the Performance Test Results and Analysis
3.2.1. Acid-Rock Reaction Kinetics Experimental Results
3.2.2. Acid-Rock Reaction Heterogeneous Acid Etching Degree Analysis
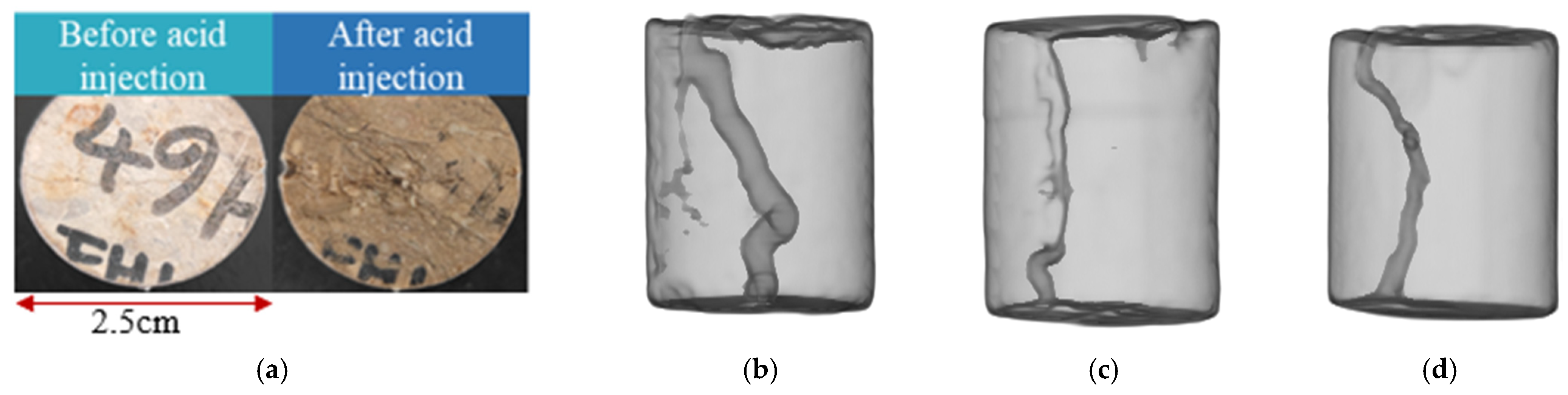
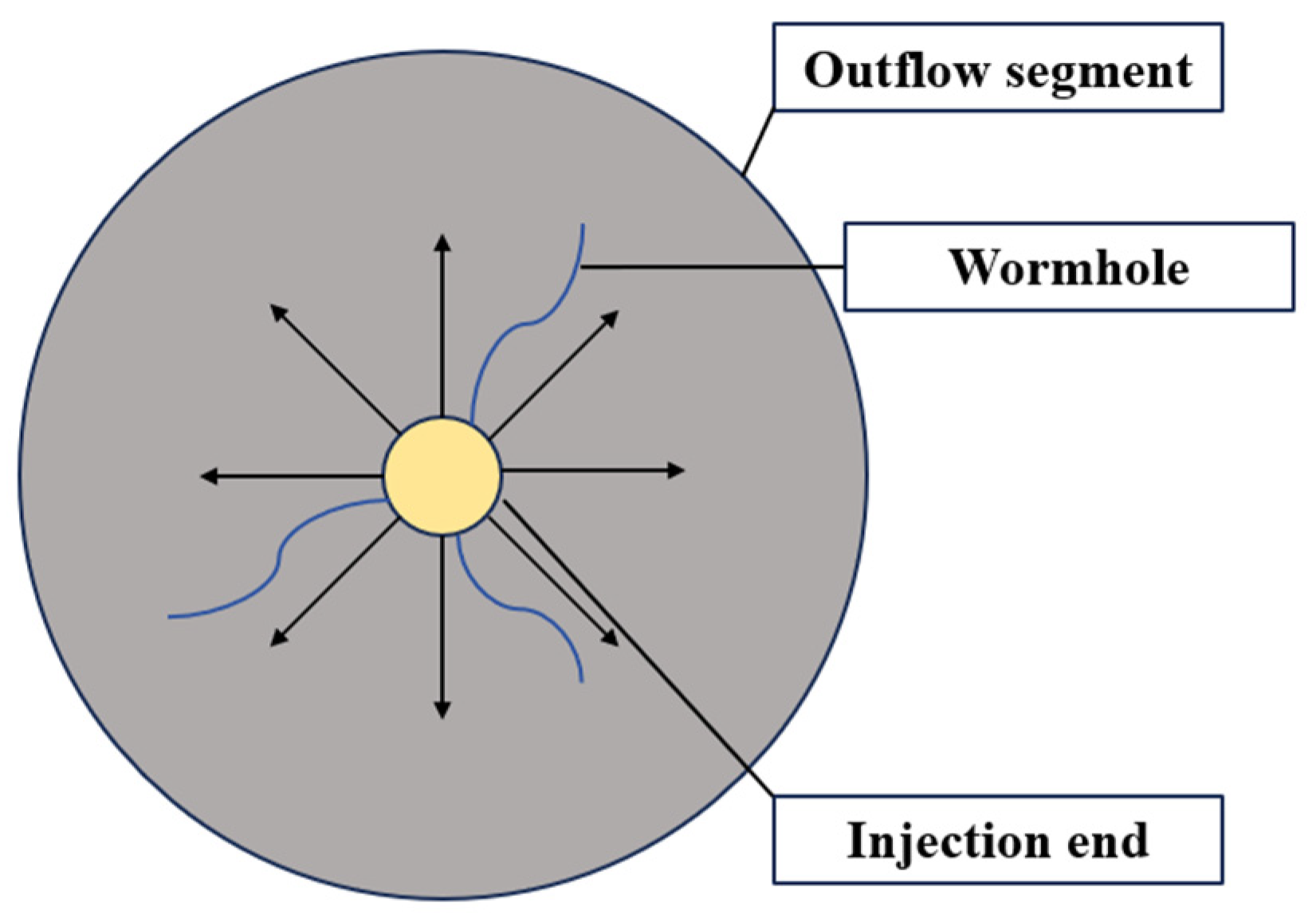
3.2.3. Acid Flow Experimental Results
3.2.4. Residual Acid damage Experimental Results
3.2.5. Acid Temperature Resistance and Shear Resistance Experimental Results
4. Acidification Construction Target Layer Selection
5. Acidizing Construction Parameter Optimization Simulation and Application Effect Analysis
5.1. Acid Pump Rate Optimization
5.2. Acidizing Fluid Volume Optimization
5.3. Acidizing Stimulation Effect Simulation Analysis
6. Conclusions and Suggestions
- The dissolution rate of the four layers is more than 95%. The dissolution rate is high, and the residue content is low. There is no problem that the reservoir cannot be stimulated by acidification. The experimental results of steel sheet corrosion and residual acid damage ensure the safety of acidizing construction, and there is no reservoir damage. The core damage rate of residual acid to the MB, MC, MD, and ME layers is low (<5%). It shows that the cores of MB, MC, MD, and ME almost do not cause damage after being invaded by residual acid.
- The experimental results of acid-rock reaction kinetics show that the reaction rates of the four reservoirs are 1.7434 × 10−5 mol/cm2·s, 3.6652 × 10−5 mol/cm2·s, 2.8245 × 10−5 mol/cm2·s, and 2.9781 × 10−5 mol/cm2·s, respectively. After acid etching, the corrosion concavity of the end face of each layer is different, and the uniformity degree of the four layers is MB, MD, MC, and ME in order from large to small. The activation energy of the reaction between the acid system and the natural core is about 4350 J/mol.
- The results of the acid flow experiments show that MB with low initial permeability cannot form effective acid wormholes. The cores of the MC, MD, and ME layers can form wormholes by acidification. The larger the initial permeability, the larger the wormholes and the higher the permeability after acidification. According to the initial permeability, the size of the wormholes formed in the four layers is MC > ME > MD >> MB.
- According to the distribution of reservoir porosity and permeability, the MD and ME layers are determined as the target layers of viscous acid acidification, and the acid pump rate and acidizing fluid volume are optimized by numerical simulation software. The simulation results of the acidizing stimulation effect show that viscous acid acidification can significantly increase the production of the MD and ME layers in the X-24 well with decreasing daily production and obtain a better stimulation effect. The viscous acid fully meets the needs of the acidizing field and has a good application prospect in the acidizing operation of porous heterogeneous carbonate reservoirs.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclatures
| CT | Computerized tomography |
| 3D | Three dimensions |
References
- Xu, Z.-X.; Li, S.-Y.; Li, B.-F.; Chen, D.-Q.; Liu, Z.-Y.; Li, Z.-M. A review of development methods and EOR technologies for carbonate reservoirs. Pet. Sci. 2020, 17, 990–1013. [Google Scholar] [CrossRef]
- Sun, H.; Li, S.; Liu, Z.; Chang, B.; Shen, C.; Cao, W. EOR technologies for fractured-vuggy carbonate condensate gas reservoirs. Nat. Gas Ind. B 2023, 10, 352–360. [Google Scholar] [CrossRef]
- Farhadi, H.; Ayatollahi, S.; Fatemi, M. Impact of rock morphology on the dominating enhanced oil recovery mechanisms by low salinity water flooding in carbonate rocks. Fuel 2022, 324, 124769. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Z.; Jiang, H.; Fu, X.; Xie, W.; Xu, A.; Shen, A.; Shi, S.; Huang, S.; Jiang, Q. Exploration status of the deep Sinian strata in the Sichuan Basin: Formation conditions of old giant carbonate oil/gas fields. Nat. Gas Ind. B 2020, 7, 462–472. [Google Scholar] [CrossRef]
- Somoza, A.; García-Mayoral, M.F.; Soto, A. A formulation based on a cationic surface-active ionic liquid and an anionic surfactant for enhanced oil recovery at a carbonate reservoir. Fuel 2023, 346, 128363. [Google Scholar] [CrossRef]
- Galkin, S.V.; Martyushev, D.A.; Osovetsky, B.M.; Kazymov, K.P.; Song, H. Evaluation of void space of complicated potentially oil-bearing carbonate formation using X-ray tomography and electron microscopy methods. Energy Rep. 2022, 8, 6245–6257. [Google Scholar] [CrossRef]
- Catinat, M.; Brigaud, B.; Fleury, M.; Thomas, H.; Antics, M.; Ungemach, P. Characterizing facies and porosity-permeability heterogeneity in a geothermal carbonate reservoir with the use of NMR-wireline logging data. Geothermics 2023, 115, 102821. [Google Scholar] [CrossRef]
- Jora, M.Z.; de Souza, R.N.; da Silva Barbosa, M.; Speglich, C.; Sabadini, E. pH-responsive wormlike micelles for acid stimulation in carbonate reservoirs. J. Pet. Sci. Eng. 2022, 218, 110975. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, L.; Chen, S.; He, Y.; Yang, X.; Duan, L.; Cai, J. Effects of L-glutamic acid, N, N-diacetic acid as chelating agent on acidification of carbonate reservoirs in acidic environments. J. Nat. Gas Sci. Eng. 2020, 82, 103494. [Google Scholar] [CrossRef]
- Akanni, O.; Nasr-El-Din, H. The accuracy of carbonate matrix-acidizing models in predicting optimum injection and wormhole propagation rates. In Proceedings of the SPE Middle East Oil & Gas Show and Conference, Manama, Bahrain, 8–11 March 2015. SPE-172575-MS. [Google Scholar]
- Alameedy, U.; A.Alrazzaq, A.A. Wormholes Models for the Optimum Matrix Acidizing in Mi4 Unit-Ahdeb Oil Field. J. Eng. 2022, 28, 34–55. [Google Scholar] [CrossRef]
- Prouvost, L.P.; Economides, M.J. Applications of real-time matrix-acidizing evaluation method. SPE Prod. Eng. 1989, 4, 401–407. [Google Scholar] [CrossRef]
- Zhao, K.; Xu, H.; Wang, J.; Jiang, H.; Zhang, L. Study on Optimization of Stimulation Technology of Heterogeneous Porous Carbonate Reservoir. Processes 2024, 12, 1191. [Google Scholar] [CrossRef]
- Du, M.; Yang, Z.; Jiang, E.; Lv, J.; Yang, T.; Wang, W.; Wang, J.; Zhang, Y.; Li, H.; Xu, Y. Using digital cores and nuclear magnetic resonance to study pore-fracture structure and fluid mobility in tight volcanic rock reservoirs. J. Asian Earth Sci. 2024, 259, 105890. [Google Scholar] [CrossRef]
- Egiebor, N.O.; Oni, B. Acid rock drainage formation and treatment: A review. Asia-Pac. J. Chem. Eng. 2007, 2, 47–62. [Google Scholar] [CrossRef]
- Zhou, F. Theory and Application of Diverting Acidizing and Acid Fracturing in Carbonate Reservoirs; Science Press: Beijing, China, 2021. [Google Scholar]
- Shen, X.; Wang, S.; Guo, J.; Chen, F.; Xu, B.; Wang, Z.; Liu, Y. Effect of carbon chain lengths of cationic surfactant on inhibition rate of acid-rock reaction. J. Pet. Sci. Eng. 2021, 196, 107793. [Google Scholar] [CrossRef]
- Gomaa, A. Proper design of polymer-based in-situ gelled acids to improve carbonate reservoir productivity. In Proceedings of the SPE Annual Technical Conference and Exhibition, Florence, Italy, 19–22 September 2010. SPE-141123-STU. [Google Scholar]
- Yeager, V.; Shuchart, C. In situ gels improve formation acidizing. Oil Gas J. 1997, 95. [Google Scholar]
- AlOtaibi, F.; Dahlan, M.; Khaldi, M.; AlGhamdi, A.; Al-Hulail, I. Evaluation of inorganic-crosslinked-based gelled acid system for high-temperature applications. In Proceedings of the SPE International Conference and Exhibition on Formation Damage Control, Lafayette, LA, USA, 19–21 February 2020. D021S009R004. [Google Scholar]
- Kalgaonkar, R.; Baqader, N. Surface modified nanoparticles based novel gelled acid system—A unique formation damage free well stimulation technology. In Proceedings of the International Petroleum Technology Conference, Riyadh, Saudi Arabia, 21–23 February 2022. D021S053R002. [Google Scholar]
- Wolf, K.; Easton, A.; Warne, S. Techniques of examining and analyzing carbonate skeletons, minerals, and rocks. In Developments in Sedimentology; Elsevier: Amsterdam, The Netherlands, 1967; Volume 9, pp. 253–341. [Google Scholar]
- Fan, Y.; Peng, H.; Chen, G.; Peng, J.; Han, H.; Qin, Y.; Wang, L.; Liu, D. Experimental study of the influences of different factors on the acid-rock reaction rate of carbonate rocks. J. Energy Storage 2023, 63, 107064. [Google Scholar] [CrossRef]
- Alameedy, U.; Fatah, A.; Abbas, A.K.; Al-Yaseri, A. Matrix acidizing in carbonate rocks and the impact on geomechanical properties: A review. Fuel 2023, 349, 128586. [Google Scholar] [CrossRef]
- Lucas, C.R.d.S.; Neyra, J.R.; Araújo, E.A.; da Silva, D.N.N.; Lima, M.A.; Ribeiro, D.A.M.; Aum, P.T.P. Carbonate acidizing—A review on influencing parameters of wormholes formation. J. Pet. Sci. Eng. 2023, 220, 111168. [Google Scholar] [CrossRef]
- Karimi, M.; Ayatollahi, S. A new insight into wormhole propagation prediction in carbonate matrix acidizing based on experimental approaches. Gas Sci. Eng. 2023, 116, 205039. [Google Scholar] [CrossRef]
- Maheshwari, P.; Ratnakar, R.; Kalia, N.; Balakotaiah, V. 3-D simulation and analysis of reactive dissolution and wormhole formation in carbonate rocks. Chem. Eng. Sci. 2013, 90, 258–274. [Google Scholar] [CrossRef]
- Ghommem, M.; Zhao, W.; Dyer, S.; Qiu, X.; Brady, D. Carbonate acidizing: Modeling, analysis, and characterization of wormhole formation and propagation. J. Pet. Sci. Eng. 2015, 131, 18–33. [Google Scholar] [CrossRef]
- Panga, M.K.R.; Ziauddin, M.; Balakotaiah, V. Two-scale continuum model for simulation of wormholes in carbonate acidization. AIChE J. 2005, 51, 3231–3248. [Google Scholar] [CrossRef]

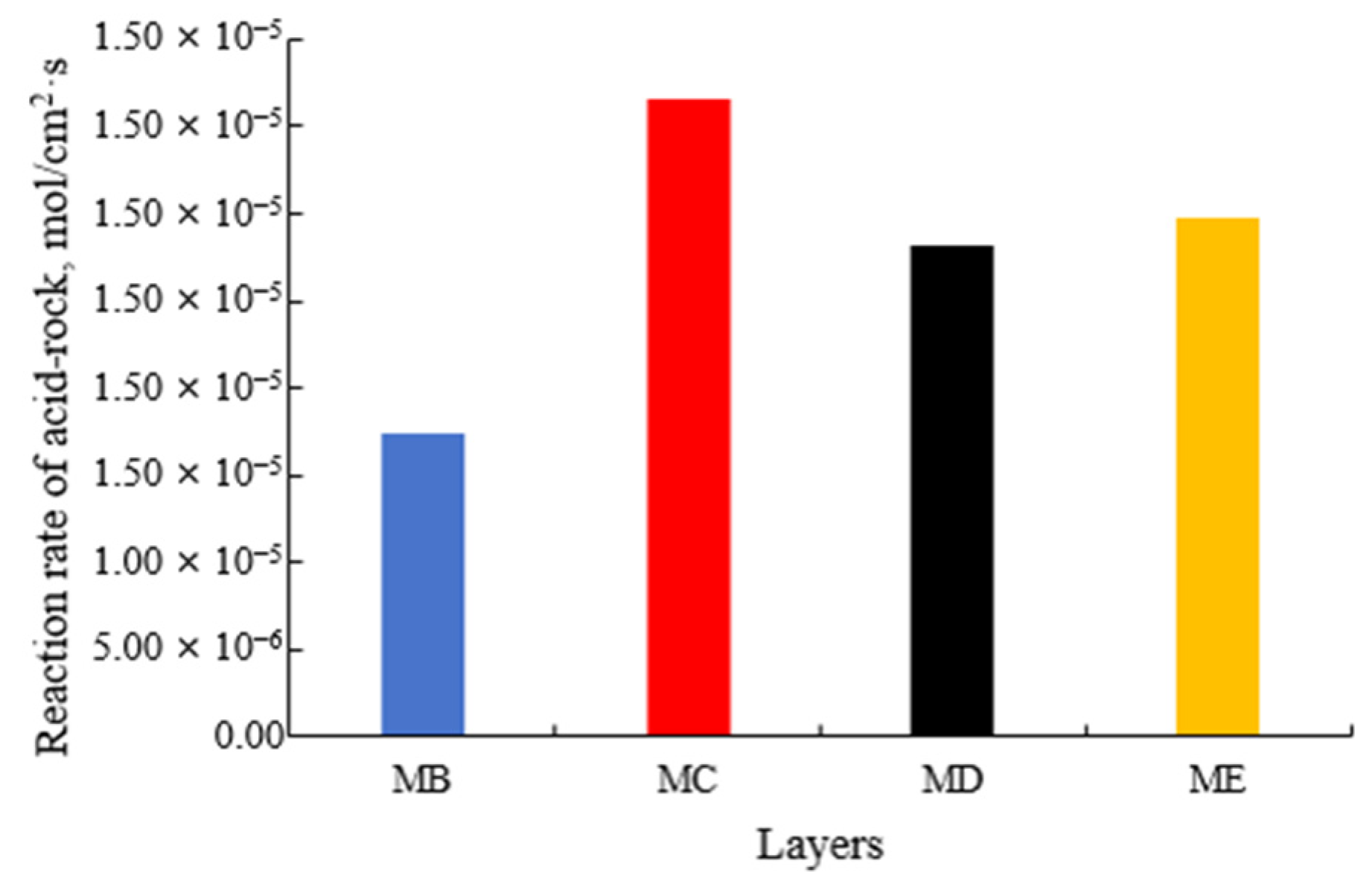
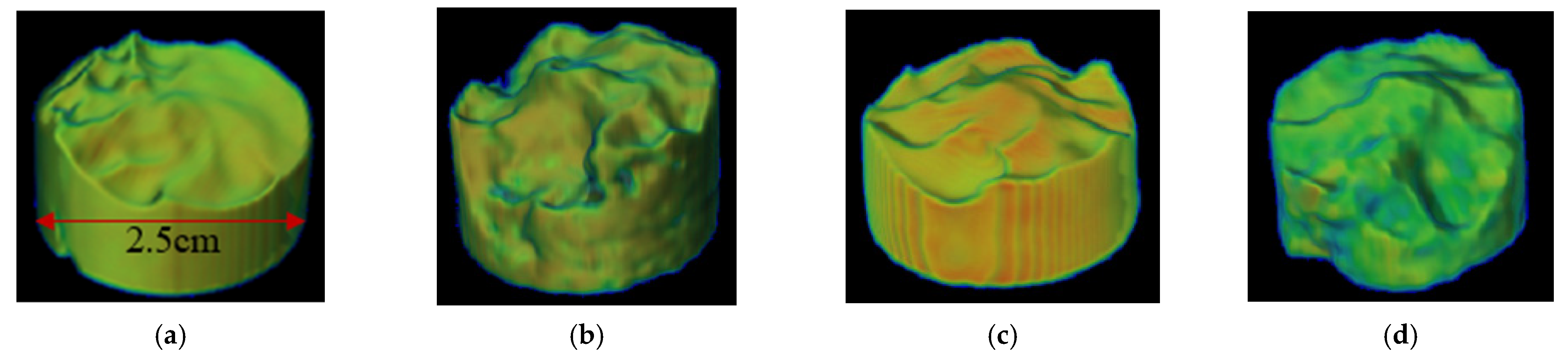
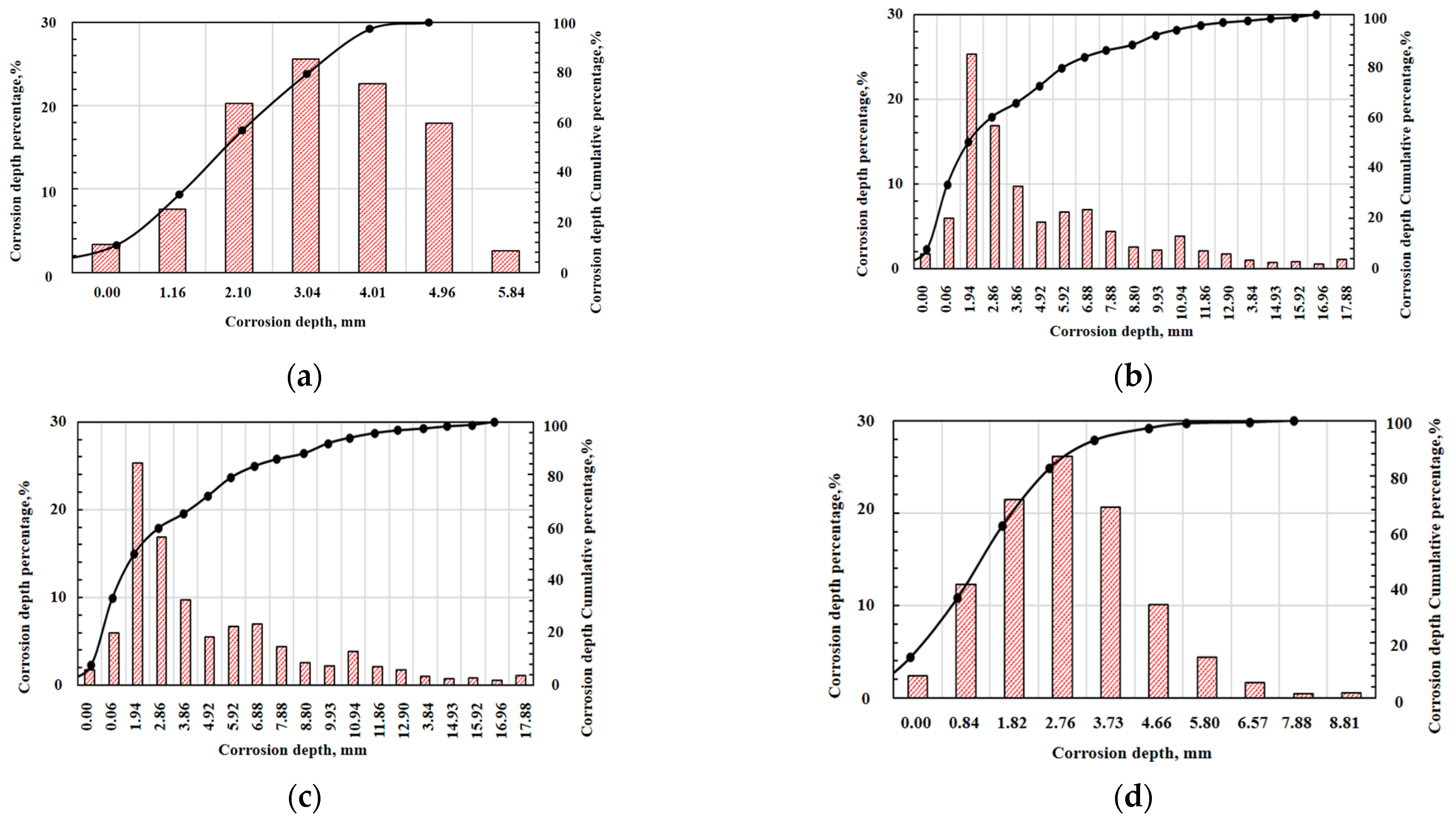
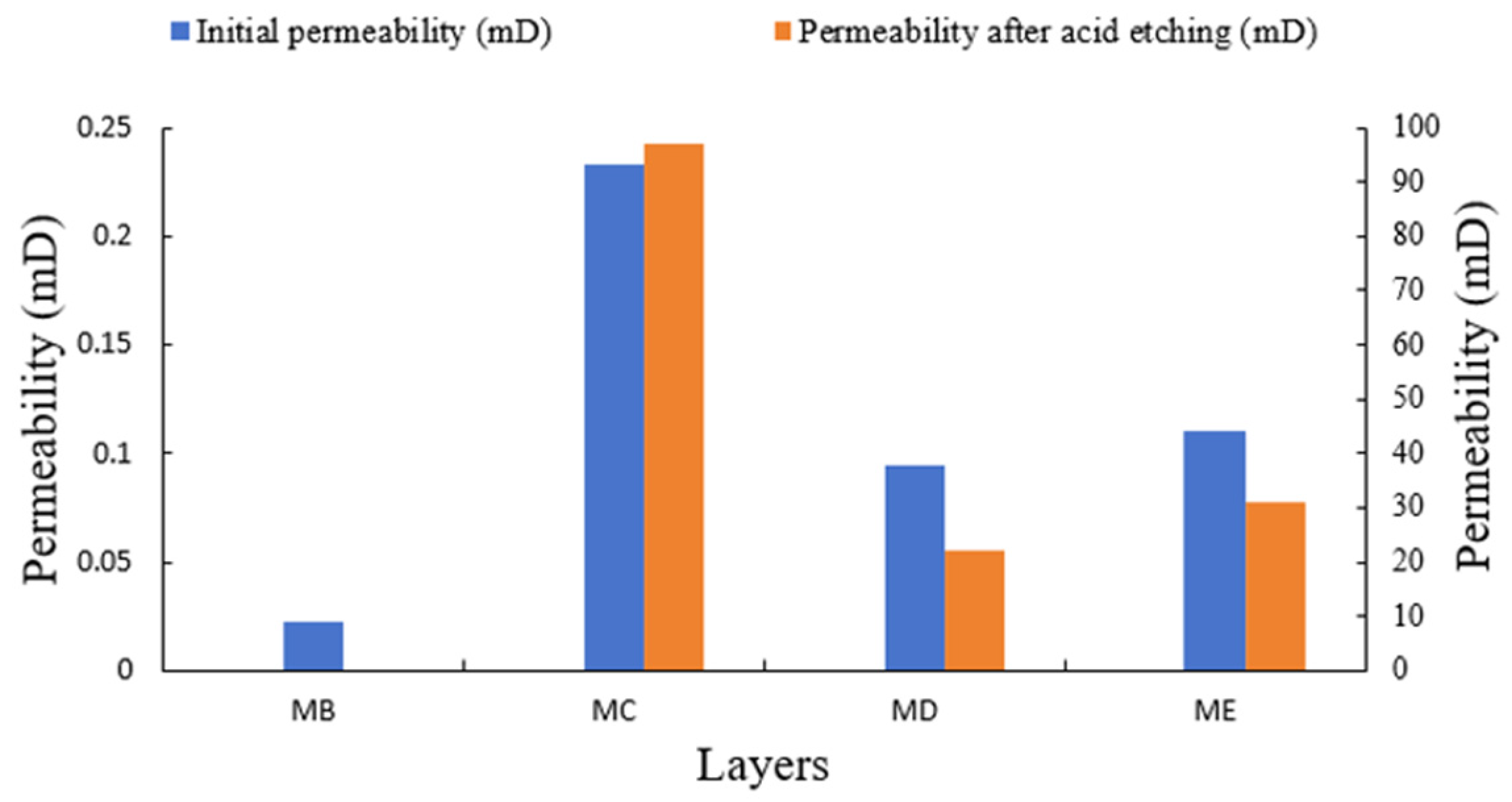




| Layer | Reaction Rate Constant K | Reaction Order m | Reaction Kinetics Equations | Reaction Activation Energy Ea (J/mol) |
|---|---|---|---|---|
| MB | 6.78 × 10−8 | 3.98 | J = 6.78 × 10−8 C3.98 | 4350.7 |
| MC | 6.72 × 10−8 | 3.96 | J = 6.72 × 10−8 C3.96 | |
| MD | 6.68 × 10−8 | 3.95 | J = 6.68 × 10−8 C3.95 | |
| ME | 6.66 × 10−8 | 3.94 | J = 6.66 × 10−8 C3.94 |
| Layer | Diameter (cm) | Length (cm) | Porosity (%) | Initial Permeability (mD) | Permeability After Acid Etching (mD) | Breakthrough PV |
|---|---|---|---|---|---|---|
| MB | 2.522 | 2.402 | 3.17 | 0.023 | / | Difficult to inject |
| MC | 2.518 | 2.321 | 18.78 | 0.233 | 97.02 | 7.23 |
| MD | 2.512 | 2.204 | 13.10 | 0.095 | 22.16 | 7.97 |
| ME | 2.519 | 2.451 | 10.32 | 0.110 | 30.93 | 7.54 |
| Layer | Diameter (cm) | Length (cm) | Permeability before Damage (mD) | Permeability after Damage (mD) | Damage Rate (%) |
|---|---|---|---|---|---|
| MB | 2.496 | 2.502 | 0.033 | 0.032 | 3.03 |
| MC | 2.487 | 2.595 | 7.410 | 7.130 | 3.78 |
| MD | 2.501 | 2.522 | 2.405 | 2.354 | 2.12 |
| ME | 2.486 | 2.546 | 1.076 | 1.043 | 3.01 |
| Layer | Depth (m) | Thickness (m) | Description of Porosity and Permeability |
|---|---|---|---|
| MB | 2807.2 | 99.7 | (1) Permeability is generally poor (78%: 0.01~6.3 mD, 12%: 6.3~54.28 mD). (2) The proportion of low to moderate porosity is high, followed by medium preference (56%: 2.17~10.48%, 44%: 11.38~26.53%). |
| MC | 2906.9 | 33.9 | |
| MD | 2940.8 | 55.4 | (1) Permeability is generally poor (84%: 0.08~7.81 mD, 16%: 10.57~25.79 mD). (2) The proportion of moderately good porosity is in the majority (28%: 2.5~10.4%, 72%: 11.43~30.78%). |
| ME | 2996.2 | 82.1 | (1) Permeability was generally poor (41%: 0.01~7.55 mD, 59%: 17.71~127.61 mD). (2) Porosity preference was predominant (18%: 3~9.8%, 82%: 16.16~30.78%). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Xu, H.; Zhao, K.; Wang, J.; Jiang, H. A Study on Effect Analysis and Process Parameter Optimization of Viscous Acid Acidification in a Porous Heterogeneous Carbonate Reservoir. Processes 2024, 12, 1443. https://doi.org/10.3390/pr12071443
Zhang L, Xu H, Zhao K, Wang J, Jiang H. A Study on Effect Analysis and Process Parameter Optimization of Viscous Acid Acidification in a Porous Heterogeneous Carbonate Reservoir. Processes. 2024; 12(7):1443. https://doi.org/10.3390/pr12071443
Chicago/Turabian StyleZhang, Liangjun, Hualei Xu, Kangjia Zhao, Jie Wang, and Houshun Jiang. 2024. "A Study on Effect Analysis and Process Parameter Optimization of Viscous Acid Acidification in a Porous Heterogeneous Carbonate Reservoir" Processes 12, no. 7: 1443. https://doi.org/10.3390/pr12071443





