Photocatalytic Degradation of Azo Dyes in Aqueous Solution Using TiO2 Doped with rGO/CdS under UV Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Photocatalyst
2.2. Photocatalytic Degradation Experiment
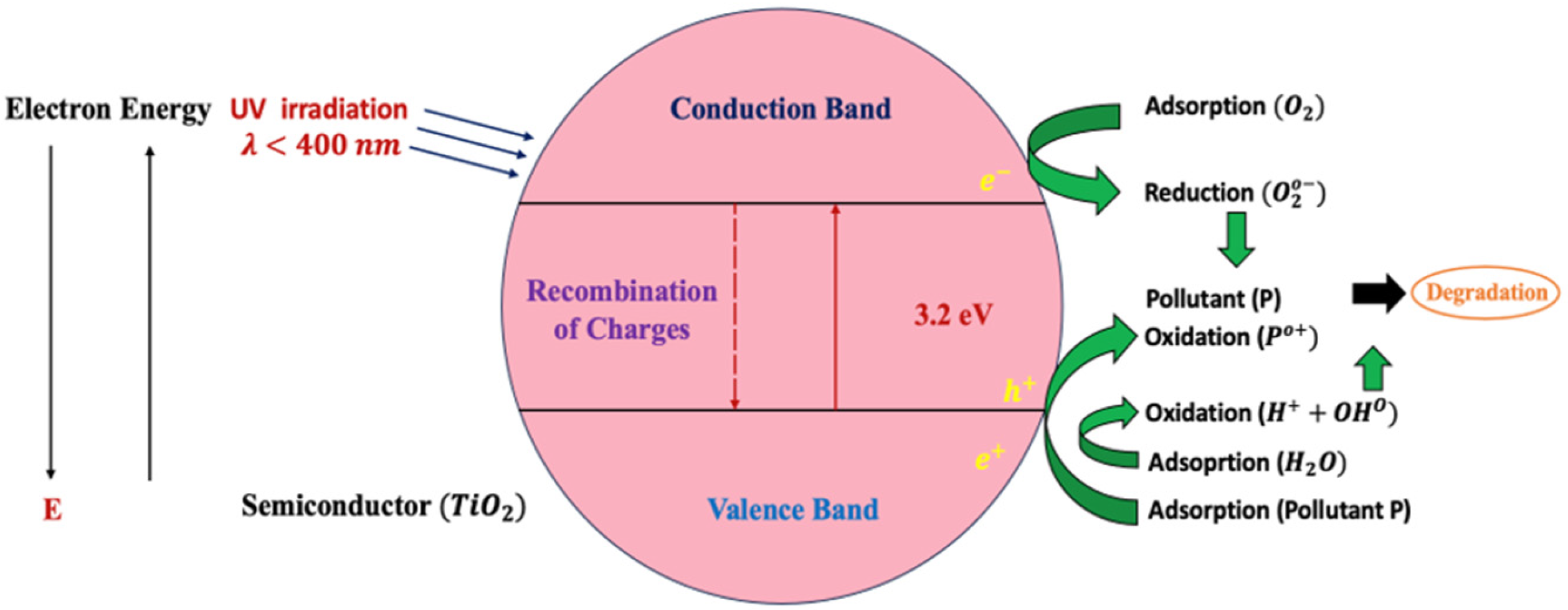
2.3. Photocatalytic Mechanism
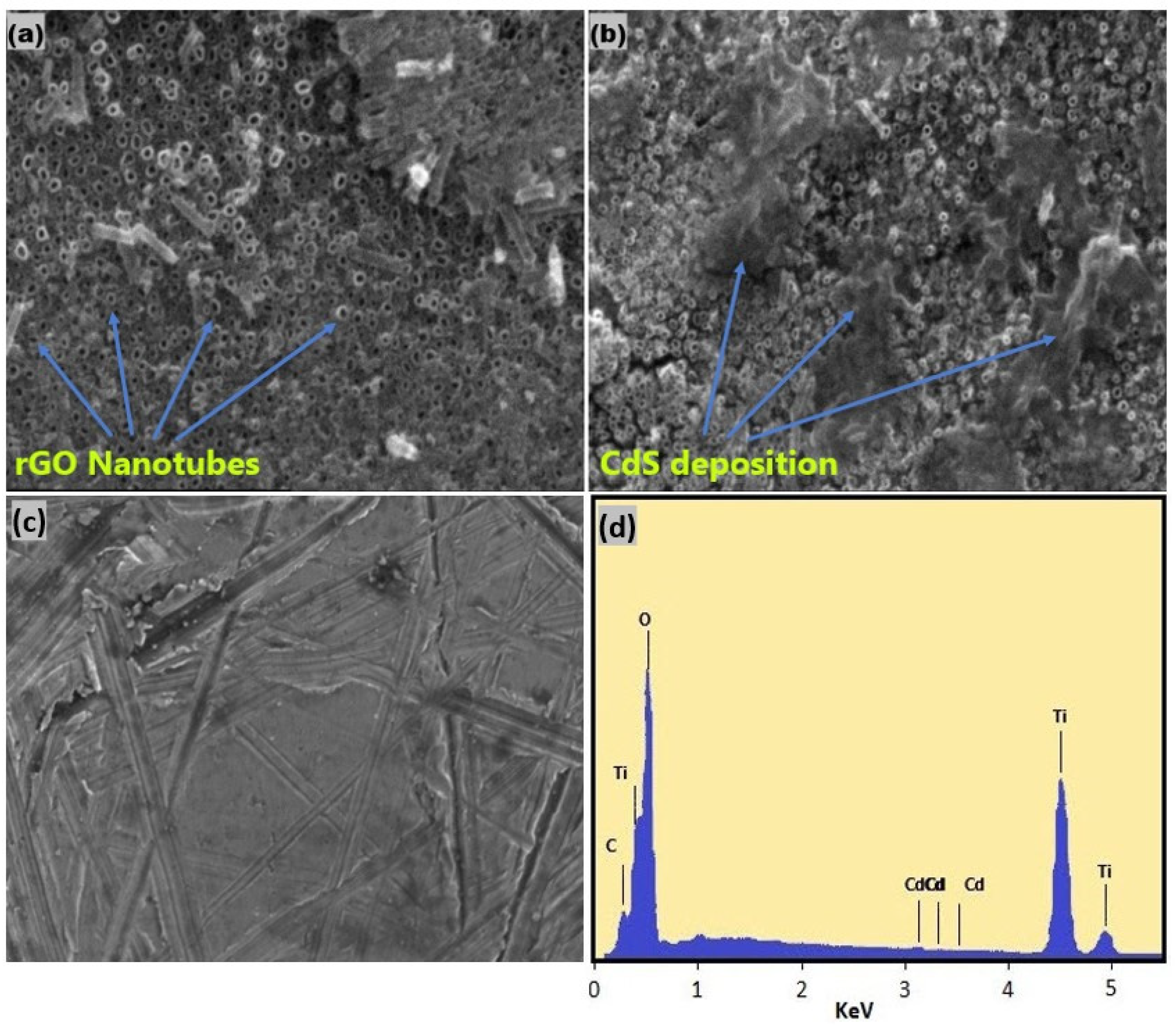
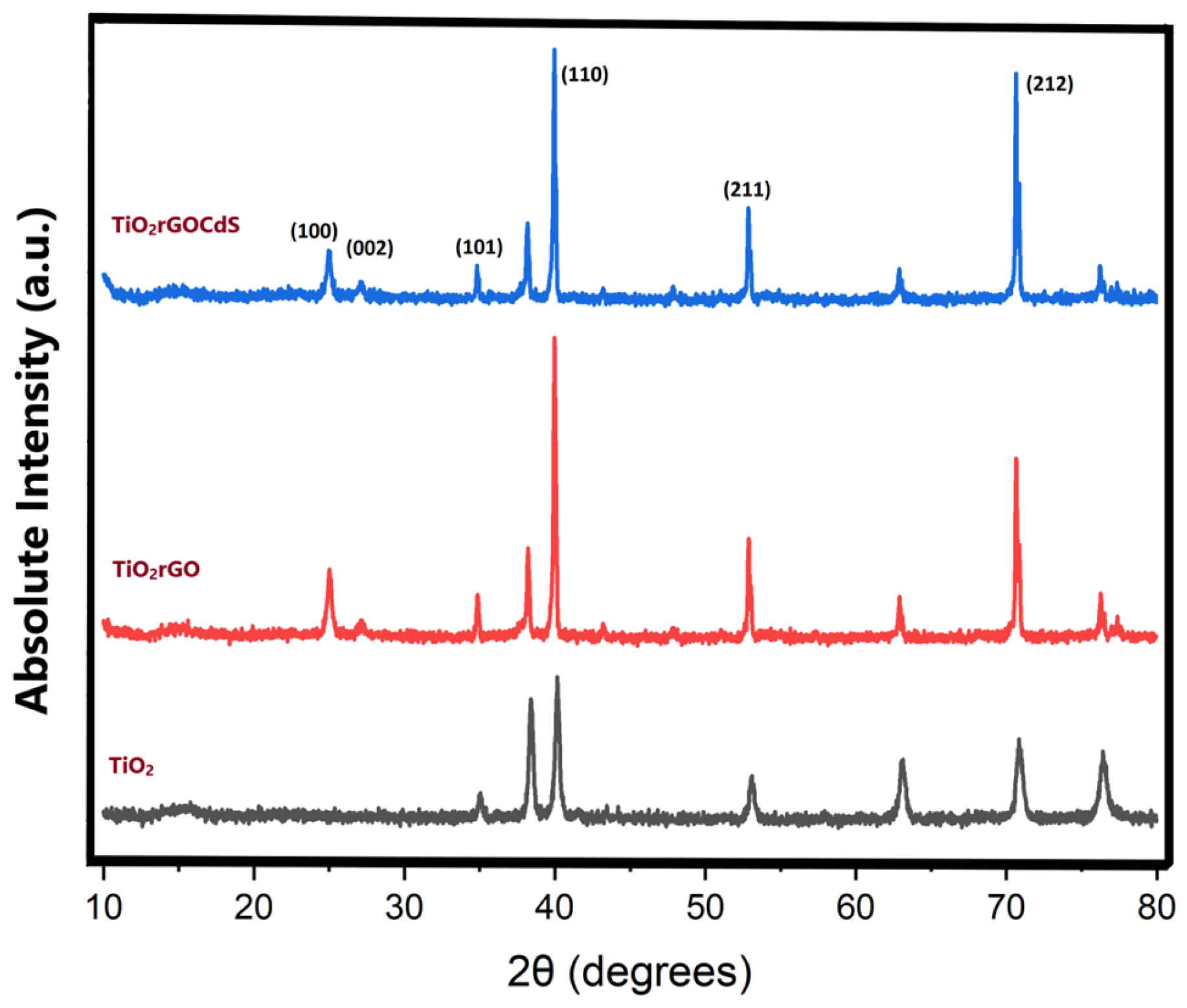
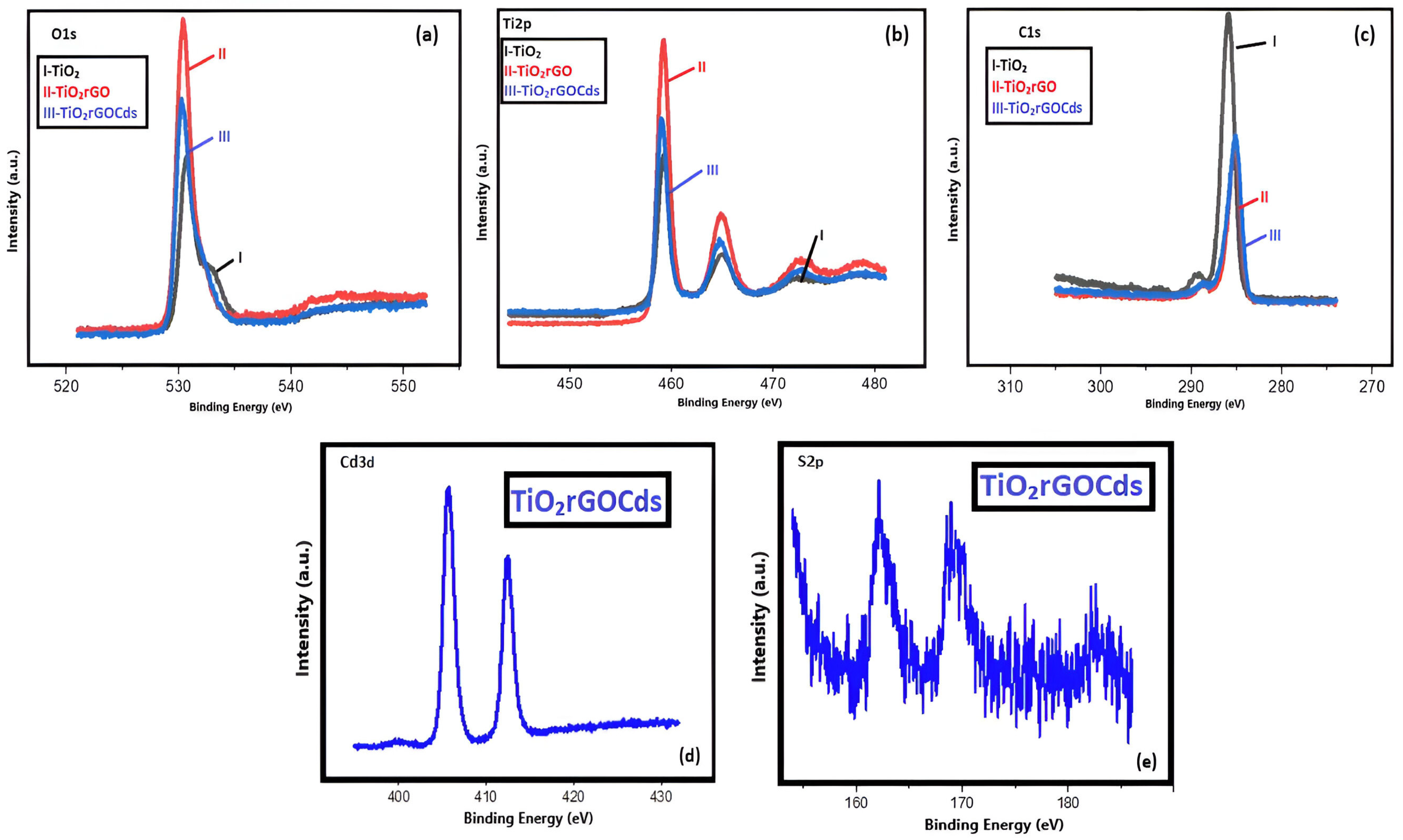
2.4. Characterization
2.5. First-Order Kinetic Model of the Photocatalytic Degradation
3. Results and Discussion
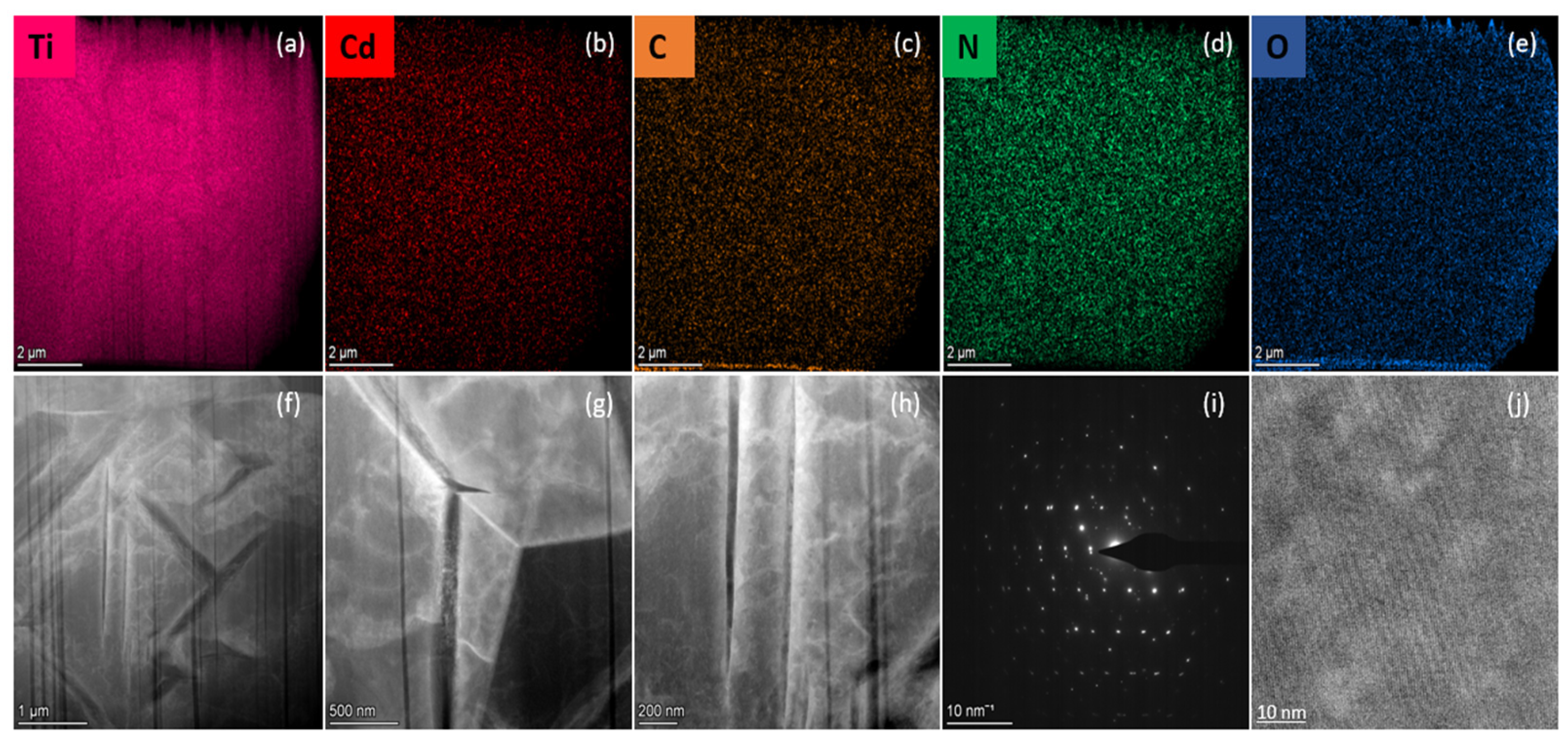
3.1. Characterization of TRCP
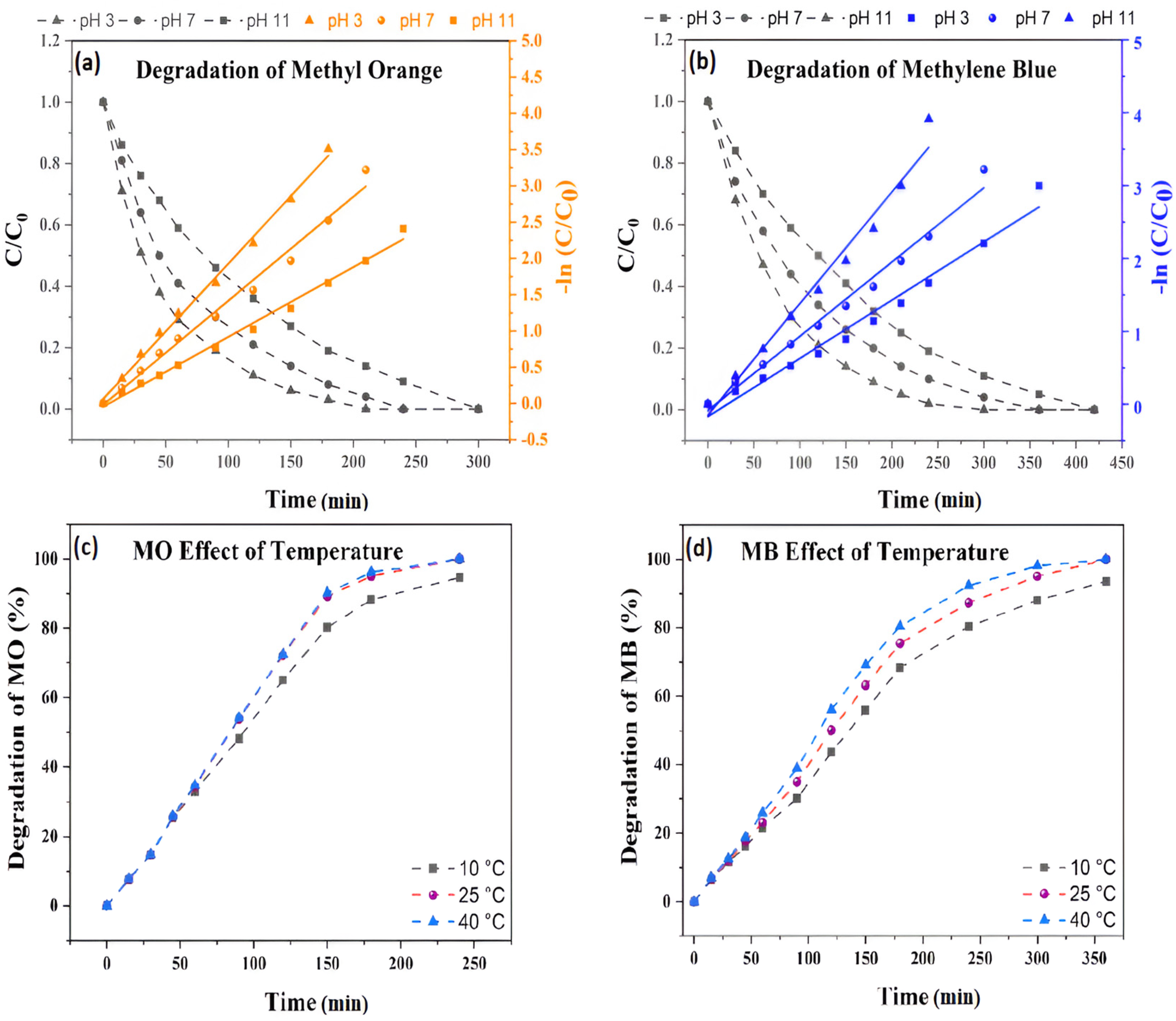
3.2. Photocatalytic Degradation Study of TRCP
3.3. Photocatalytic Mechanism of TRCP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, W.; Chen, X.; Ismail, M.; Wei, L.; Hu, B. Simulating the synergy of electron donors and different redox mediators on the anaerobic decolorization of azo dyes: Can AQDS-chitosan globules replace the traditional redox mediators? Chemosphere 2021, 275, 130025. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, G.; Wang, H. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015, 78, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Mezgebe, K.; Mulugeta, E. Synthesis and pharmacological activities of azo dye derivatives incorporating heterocyclic scaffolds: A review. RSC Adv. 2022, 12, 25932–25946. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Cui, M.-H.; Liang, B.; Liu, W.-Z.; Tang, Z.-E.; Wang, A.-J. Mutual effect between electrochemically active bacteria (EAB) and azo dye in bio-electrochemical system (BES). Chemosphere 2020, 239, 124787. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Pozhidaev, E.; Chigrinov, V.; Cheung, H.; Ho, Y.; Kwok, H. Photo-aligned ferroelectric liquid crystal displays based on azo-dye layers. Displays 2004, 25, 21–29. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, N.; Zhang, H.; Tian, W.; Zhang, T.; Wu, P.; Jiang, W. Visible-light-driven photocatalysis-assisted adsorption of azo dyes using Ag2O. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124105. [Google Scholar] [CrossRef]
- Zhao, B.; Zhu, Z.; Qin, X.D.; Li, Z.; Zhang, H. Highly efficient and stable CuZr-based metallic glassy catalysts for azo dye degradation. J. Mater. Sci. Technol. 2020, 46, 88–97. [Google Scholar] [CrossRef]
- Navarro, A.; Sanz, F. Dye aggregation in solution: Study of C.I. direct red I. Dye. Pigment. 1999, 40, 131–139. [Google Scholar] [CrossRef]
- Ravi, B.N.; Keshavayya, J.; Kumar, V.; Kandgal, S. Synthesis, characterization and pharmacological evaluation of 2-aminothiazole incorporated azo dyes. J. Mol. Struct. 2020, 1204, 127493. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; Osibote, O.A.; Darmokoesoemo, H.; Kusuma, H.S. Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: A review. J. Mater. Res. Technol. 2021, 14, 2751–2774. [Google Scholar] [CrossRef]
- Cruz, D.R.; de Jesus, G.K.; Santos, C.A.; Silva, W.R.; Wisniewski, A.; Cunha, G.C.; Romão, L.P. Magnetic nanostructured material as heterogeneous catalyst for degradation of AB210 dye in tannery wastewater by electro-Fenton process. Chemosphere 2021, 280, 130675. [Google Scholar] [CrossRef]
- Duan, Y.; Zhao, J.; Qiu, X.; Deng, X.; Ren, X.; Ge, W.; Yuan, H. Coagulation performance and floc properties for synchronous removal of reactive dye and polyethylene terephthalate microplastics. Process Saf. Environ. Prot. 2022, 165, 66–76. [Google Scholar] [CrossRef]
- Li, N.; Lou, T.J.; Wang, W.; Li, M.; Jing, L.C.; Yang, Z.X.; Chang, R.Y.; Li, J.; Geng, H.Z. MXene-PANI/PES composite ultrafiltration membranes with conductive properties for anti-fouling and dye removal. J. Membr. Sci. 2023, 668, 121271. [Google Scholar] [CrossRef]
- Kaur, K.; Jindal, R. Comparative study on the behaviour of Chitosan-Gelatin based Hydrogel and nanocomposite ion exchanger synthesized under microwave conditions towards photocatalytic removal of cationic dyes. Carbohydr. Polym. 2019, 207, 398–410. [Google Scholar] [CrossRef]
- An, M.; Gutierrez, L.; D’Haese, A.; Tan, L.; Verliefde, A.; Cornelissen, E. In-situ modification of nanofiltration and reverse osmosis membranes for organic micropollutants and salts removal: A review. Desalination 2023, 565, 116861. [Google Scholar] [CrossRef]
- Navarathna, C.M.; Dewage, N.B.; Karunanayake, A.G.; Farmer, E.L.; Perez, F.; Hassan, E.B.; Mlsna, T.E.; Pittman, C.U., Jr. Rhodamine B Adsorptive Removal and Photocatalytic Degradation on MIL-53-Fe MOF/Magnetic Magnetite/Biochar Composites. J. Inorg. Organomet. Polym. Mater. 2020, 30, 214–229. [Google Scholar] [CrossRef]
- Madduri, S.; Elsayed, I.; Hassan, E.B. Novel oxone treated hydrochar for the removal of Pb(II) and methylene blue (MB) dye from aqueous solutions. Chemosphere 2020, 260, 127683. [Google Scholar] [CrossRef]
- Chandrabose, G.; Dey, A.; Gaur, S.S.; Pitchaimuthu, S.; Jagadeesan, H.; Braithwaite, N.S.J.; Selvaraj, V.; Kumar, V.; Krishnamurthy, S. Removal and degradation of mixed dye pollutants by integrated adsorption-photocatalysis technique using 2-D MoS2/TiO2 nanocomposite. Chemosphere 2021, 279, 130467. [Google Scholar] [CrossRef]
- Ahmad, A.; Ali, M.; Al-Sehemi, A.G.; Al-Ghamdi, A.A.; Park, J.-W.; Algarni, H.; Anwer, H. Carbon-integrated semiconductor photocatalysts for removal of volatile organic compounds in indoor environments. Chem. Eng. J. 2023, 452, 139436. [Google Scholar] [CrossRef]
- Aswini, R.; Padmanaban, A.; Vigneshwaran, S.; Valdes, H.; Arunachalam, S. A review on versatile nano-photocatalysts for environmental remediation: Carbon-decorated bismuth-based nanomaterials. Nano-Struct. Nano-Objects 2023, 35, 100991. [Google Scholar] [CrossRef]
- Bibi, S.; Shah, S.S.; Muhammad, F.; Siddiq, M.; Kiran, L.; Aldossari, S.A.; Mushab, M.S.S.; Sarwar, S. Cu-doped mesoporous TiO2 photocatalyst for efficient degradation of organic dye via visible light photocatalysis. Chemosphere 2023, 339, 139583. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Yan, X.; Xu, J.; Wang, C.; Wang, L. Fabrication of hollow type-II and Z-scheme In2O3/TiO2/Cu2O photocatalyst based on In-MIL-68 for efficient catalytic degradation of tetracycline. Sep. Purif. Technol. 2021, 265, 118487. [Google Scholar] [CrossRef]
- Sun, P.; Xing, Z.; Li, Z.; Zhou, W. Recent advances in quantum dots photocatalysts. Chem. Eng. J. 2023, 458, 141399. [Google Scholar] [CrossRef]
- Wang, X.; He, J.; Chen, X.; Ma, B.; Zhu, M. Metal halide perovskites for photocatalytic CO2 reduction: An overview and prospects. Coord. Chem. Rev. 2023, 482, 215076. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Wu, J.; Zhou, W. Recent progress in defective TiO2 photocatalysts for energy and environmental applications. Renew. Sustain. Energy Rev. 2022, 156, 111980. [Google Scholar] [CrossRef]
- Xu, H.; Yang, J.; Li, Y.; Fu, F.; Da, K.; Cao, S.; Chen, W.; Fan, X. Fabrication of Bi2O3 QDs decorated TiO2/BiOBr dual Z-scheme photocatalysts for efficient degradation of gaseous toluene under visible-light. J. Alloys Compd. 2023, 950, 169959. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Zhao, Y.; Linghu, X.; Shu, Y.; Zhang, J.; Chen, Z.; Wu, Y.; Shan, D.; Wang, B. Classification and catalytic mechanisms of heterojunction photocatalysts and the application of titanium dioxide (TiO2)-based heterojunctions in environmental remediation. J. Environ. Chem. Eng. 2022, 10, 108077. [Google Scholar] [CrossRef]
- Azam, M.U.; Tahir, M.; Umer, M.; Tahir, B.; Shehzad, N.; Siraj, M. In-situ synthesis of TiO2/La2O2CO3/rGO composite under acidic/basic treatment with La3+/Ti3+ as mediators for boosting photocatalytic H2 evolution. Int. J. Hydrogen Energy 2019, 44, 23669–23688. [Google Scholar] [CrossRef]
- Yin, H.; Cao, Y.; Fan, T.; Qiu, B.; Zhang, M.; Yao, J.; Li, P.; Liu, X.; Chen, S. Construction of carbon bridged TiO2/CdS tandem Z-scheme heterojunctions toward efficient photocatalytic antibiotic degradation and Cr (VI) reduction. J. Alloys Compd. 2020, 824, 153915. [Google Scholar] [CrossRef]
- Hossen, M.A.; Solayman, H.M.; Leong, K.H.; Sim, L.C.; Yaacof, N.; Abd Aziz, A.; Wu, L.; Monir, M.U. Recent progress in TiO2-Based photocatalysts for conversion of CO2 to hydrocarbon fuels: A systematic review. Results Eng. 2022, 16, 100795. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.W.; Lee, G.M.; Lee, B.T.; Yun, S.T.; Kim, S.O. Monitoring of TiO2-catalytic UV-LED photo-oxidation of cyanide contained in mine wastewater and leachate. Chemosphere 2016, 143, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Fang, S.; Si, L.; Xia, Y.; Ho, W.; Li, M. Fabrication of TiO2 nanorod assembly grafted rGO (rGO@TiO2-NR) hybridized flake-like photocatalyst. Appl. Surf. Sci. 2017, 391, 218–227. [Google Scholar] [CrossRef]
- Ge, M.; Li, Q.; Cao, C.; Huang, J.; Li, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. One-dimensional TiO2 Nanotube Photocatalysts for Solar Water Splitting. Adv. Sci. 2017, 4, 1600152. [Google Scholar] [CrossRef]
- Soares, T.A.; Gonzalez-Moya, J.R.; Byun, Y.; Thesing, A.; Dares, C.; Coskun, A.; Machado, G. One-step anodization-electrophoretic deposition of titanium nanotubes-graphene nanoribbon framework for water oxidation. J. Electroanal. Chem. 2021, 902, 115802. [Google Scholar] [CrossRef]
- Rajaitha, P.; Hajra, S.; Sahu, M.; Mistewicz, K.; Toroń, B.; Abolhassani, R.; Panda, S.; Mishra, Y.; Kim, H. Unraveling highly efficient nanomaterial photocatalyst for pollutant removal: A comprehensive review and future progress. Mater. Today Chem. 2022, 23, 100692. [Google Scholar] [CrossRef]
- Rambabu, Y.; Jaiswal, M.; Roy, S.C. Enhanced photoelectrochemical performance of multi-leg TiO2 nanotubes through efficient light harvesting. J. Phys. D Appl. Phys. 2015, 48, 295302. [Google Scholar] [CrossRef]
- Rodríguez-Lazcano, Y.; Barrios-Salgado, E.; Flores-Castañeda, M.; Camacho-López, S.; Pérez-Alvarez, J.; Chávez-Chávez, A.; Quiñones-Galván, J.G. Physical properties of Sb2S3–Cu nanocomposite thin films synthesized by chemical bath deposition and laser ablation of solids in liquids. J. Mater. Res. Technol. 2023, 24, 6604–6613. [Google Scholar] [CrossRef]
- Sahnesarayi, M.K.; Rastegari, S.; Sarpoolaky, H. Enhanced photoelectrochemical water splitting performance of vertically aligned Bi2O3 nanosheet arrays derived from chemical bath deposition method by controlling chemical bath temperature and complexing agent concentration. Surf. Interfaces 2022, 30, 101819. [Google Scholar] [CrossRef]
- Qian, T.; Yin, X.; Li, J.; Xu, H.; Deng, Y.; Wang, X. Nano-TiO2 Decorated Radial-Like Mesoporous Silica: Preparation, Characterization, and Adsorption-Photodegradation Behavior. J. Mater. Sci. Technol. 2017, 33, 1314–1322. [Google Scholar] [CrossRef]
- Tichapondwa, S.M.; Newman, J.P.; Kubheka, O. Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys. Chem. Earth Parts A/B/C 2020, 118–119, 102900. [Google Scholar] [CrossRef]
- Sun, T.; Zhao, Z.; Liang, Z.; Liu, J.; Shi, W.; Cui, F. Efficient degradation of p-arsanilic acid with arsenic adsorption by magnetic CuO-Fe3O4 nanoparticles under visible light irradiation. Chem. Eng. J. 2018, 334, 1527–1536. [Google Scholar] [CrossRef]
- Xu, J.; Shen, X.; Wang, D.; Zhao, C.; Liu, Z.; Pozdnyakov, I.P.; Wu, F.; Xia, J. Kinetics and mechanisms of pH-dependent direct photolysis of p-arsanilic acid under UV-C light. Chem. Eng. J. 2018, 336, 334–341. [Google Scholar] [CrossRef]
- Eldoma, M.A.; Alaswad, S.O.; Mahmoud, M.A.; Qudsieh, I.Y.; Hassan, M.; Bakather, O.Y.; Elawadi, G.A.; Abouatiaa, A.F.; Alomar, M.S.; Elhassan, M.S.; et al. Enhancing photocatalytic performance of Co-TiO2 and Mo-TiO2-based catalysts through defect engineering and doping: A study on the degradation of organic pollutants under UV light. J. Photochem. Photobiol. A Chem. 2024, 446, 115164. [Google Scholar] [CrossRef]
- Wang, Y.; He, D.; Chen, H.; Wang, D. Catalysts in electro-, photo- and photoelectrocatalytic CO2 reduction reactions. J. Photochem. Photobiol. C Photochem. Rev. 2019, 40, 117–149. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Vengust, D.; Radošević, T.; Shvalya, V.; Gonçalves, G.; Podlogar, M. Synergistic Remediation of Organic Dye by Titanium Dioxide/Reduced Graphene Oxide Nanocomposite. Molecules 2023, 28, 7326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Zhang, C.; Wu, J.; Cui, K.; Li, Q. Degradation of AO7 through RGO/TiO2 nanotube arrays photoanode in UV/electrical coupling system: Performance and mechanism analysis. Opt. Mater. 2024, 149, 115030. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, L.; Yue, X.; Li, B.; Qin, X.; Li, Q.; Zhang, J. One-pot synthesis of CdS nanoparticles with surface-phase junctions to catalyze the photo-reduction of CO2 into CO and CH4 selectively. Mater. Today Commun. 2023, 37, 107205. [Google Scholar] [CrossRef]
- Kalita, L.; Soni, A.; Kalita, S.J.; Basyach, P.; Guha, A.K.; Jain, S.L.; Saikia, L. Synergistic CdS@CeO2 nanocomposites: Harnessing Z-scheme electron transfer for enhanced photocatalytic CO2 conversion and aqueous Cr(VI) reduction. J. Environ. Chem. Eng. 2024, 12, 112930. [Google Scholar] [CrossRef]
- Guo, Y.; Sui, M.; Liu, S.; Li, T.; Lv, X.; Yu, M.; Mo, Y. Insight into cobalt substitution in LaFeO3-based catalyst for enhanced activation of peracetic acid: Reactive species and catalytic mechanism. J. Hazard. Mater. 2024, 461, 132662. [Google Scholar] [CrossRef]
- Yadav, I.; Dutta, S.; Pandey, A.; Yadav, N.; Goyal, A.; Chatterjee, R. Evolution of TiOx-SiOx nano-composite during annealing of ultrathin titanium oxide films on Si substrate. Ceram. Int. 2020, 46, 19935–19941. [Google Scholar] [CrossRef]
- Kozakov, A.; Kochur, A.; Kumar, N.; Panda, K.; Nikolskii, A.; Sidashov, A. Determination of sp2 and sp3 phase fractions on the surface of diamond films from C1s, valence band X-ray photoelectron spectra and CKVV X-ray-excited Auger spectra. Appl. Surf. Sci. 2021, 536, 147807. [Google Scholar] [CrossRef]
- Caloudova, H.; Blahova, J.; Mares, J.; Richtera, L.; Franc, A.; Garajova, M.; Tichy, F.; Lenz, J.; Caloudova, J.; Enevova, V.; et al. The effects of dietary exposure to Magneli phase titanium suboxide and titanium dioxide on rainbow trout (Oncorhynchus mykiss). Chemosphere 2022, 293, 133689. [Google Scholar] [CrossRef]
- Ahluwalia, S.; Prakash, N.T.; Prakash, R.; Pal, B. Improved degradation of methyl orange dye using bio-co-catalyst Se nanoparticles impregnated ZnS photocatalyst under UV irradiation. Chem. Eng. J. 2016, 306, 1041–1048. [Google Scholar] [CrossRef]
- Bergamonti, L.; Predieri, G.; Paz, Y.; Fornasini, L.; Lottici, P.; Bondioli, F. Enhanced self-cleaning properties of N-doped TiO2 coating for Cultural Heritage. Microchem. J. 2017, 133, 1–12. [Google Scholar] [CrossRef]
- Balarabe, B.Y.; Paria, S.; Keita, D.S.; Baraze, A.R.I.; Kalugendo, E.; Tetteh, G.N.T.; Meringo, M.M.; Oumarou, M.N.I. Enhanced UV-light active α-Bi2O3 nanoparticles for the removal of methyl orange and ciprofloxacin. Inorg. Chem. Commun. 2022, 146, 110204. [Google Scholar] [CrossRef]
- Bakar, S.A.; Byzynski, G.; Ribeiro, C. Synergistic effect on the photocatalytic activity of N-doped TiO2 nanorods synthesised by novel route with exposed (110) facet. J. Alloys Compd. 2016, 666, 38–49. [Google Scholar] [CrossRef]
- Shah, R.K. Efficient photocatalytic degradation of methyl orange dye using facilely synthesized α-Fe2O3 nanoparticles. Arab. J. Chem. 2023, 16, 104444. [Google Scholar] [CrossRef]
- Khan, R.; Hassan, M.S.; Jang, L.-W.; Yun, J.H.; Ahn, H.-K.; Khil, M.-S.; Lee, I.-H. Low-temperature synthesis of ZnO quantum dots for photocatalytic degradation of methyl orange dye under UV irradiation. Ceram. Int. 2014, 40, 14827–14831. [Google Scholar] [CrossRef]
- Khan, R.; Hassan, M.S.; Cho, H.-S.; Polyakov, A.Y.; Khil, M.-S.; Lee, I.-H. Facile low-temperature synthesis of ZnO nanopyramid and its application to photocatalytic degradation of methyl orange dye under UV irradiation. Mater. Lett. 2014, 133, 224–227. [Google Scholar] [CrossRef]
- Isai, K.A.; Shrivastava, V.S. Photocatalytic degradation of methylene blue using ZnO and 2%Fe–ZnO semiconductor nanomaterials synthesized by sol–gel method: A comparative study. SN Appl. Sci. 2019, 1, 1247. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Mengting, Z.; Fu, D.; Yeap, S.K.; Othman, M.H.D.; Avtar, R.; Ouyang, T. Functionalizing TiO2 with graphene oxide for enhancing photocatalytic degradation of methylene blue (MB) in contaminated wastewater. J. Environ. Manag. 2020, 270, 110871. [Google Scholar] [CrossRef] [PubMed]
- Qamar, M.A.; Shahid, S.; Javed, M.; Iqbal, S.; Sher, M.; Akbar, M.B. Highly efficient g-C3N4/Cr-ZnO nanocomposites with superior photocatalytic and antibacterial activity. J. Photochem. Photobiol. A Chem. 2020, 401, 112776. [Google Scholar] [CrossRef]
- Goyal, P.; Chakraborty, S.; Misra, S.K. Multifunctional Fe3O4-ZnO nanocomposites for environmental remediation applications. Environ. Nanotechnol. Monit. Manag. 2018, 10, 28–35. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Dong, S.; Zhou, X.; Dong, S. N-doped carbon quantum dots/TiO2 hybrid composites with enhanced visible light driven photocatalytic activity toward dye wastewater degradation and mechanism insight. J. Photochem. Photobiol. A Chem. 2016, 325, 104–110. [Google Scholar] [CrossRef]
- Abu Bakar, S.; Ribeiro, C. An insight toward the photocatalytic activity of S doped 1-D TiO2 nanorods prepared via novel route: As promising platform for environmental leap. J. Mol. Catal. A Chem. 2016, 412, 78–92. [Google Scholar] [CrossRef]
- Sabouri, Z.; Akbari, A.; Hosseini, H.A.; Khatami, M.; Darroudi, M. Egg white-mediated green synthesis of NiO nanoparticles and study of their cytotoxicity and photocatalytic activity. Polyhedron 2020, 178, 114351. [Google Scholar] [CrossRef]

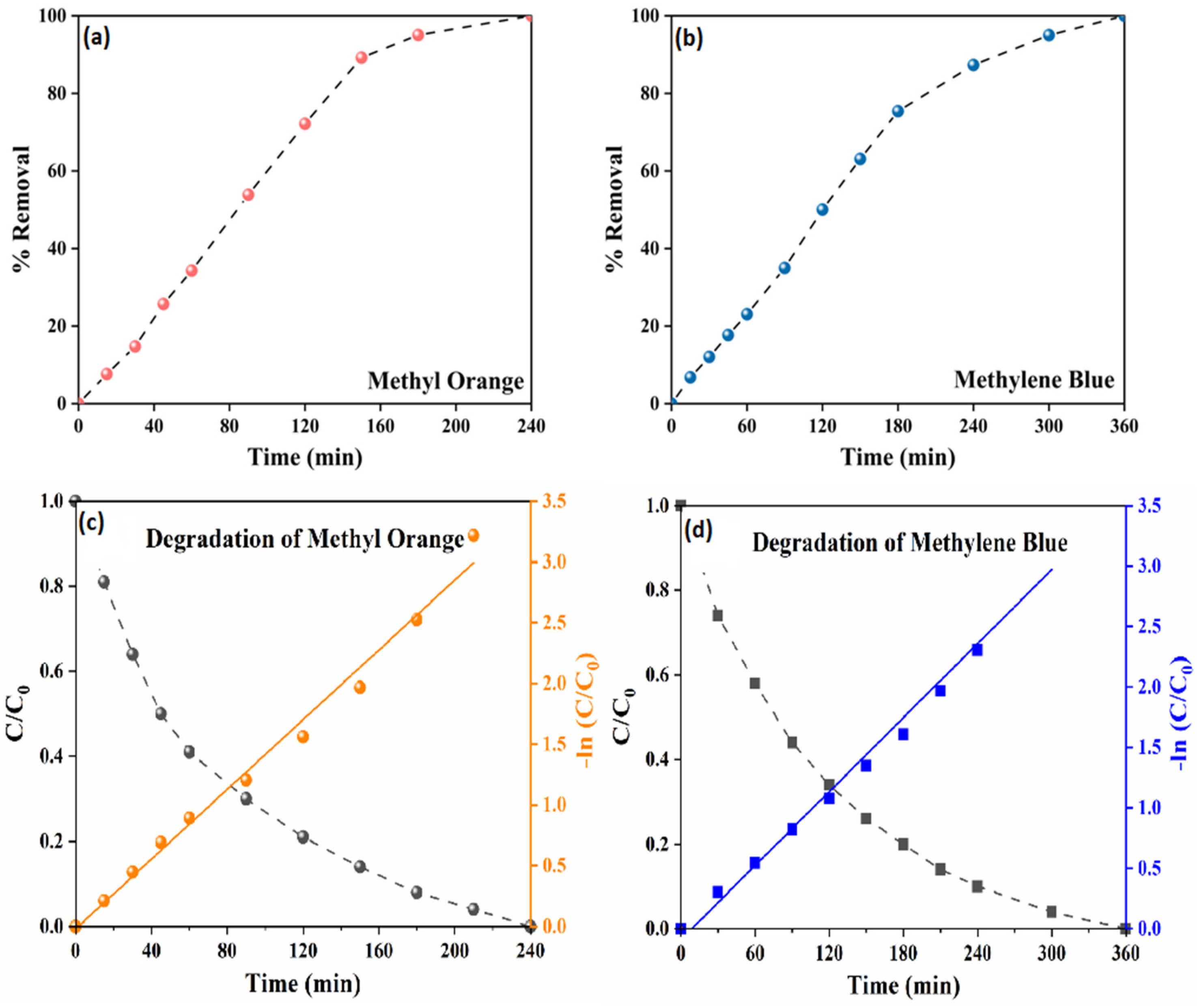

| Experiments | Reaction Rate Constant (min−1) | R-Square (R2) |
|---|---|---|
| MO | 0.0143 | 0.988 |
| MB | 0.0101 | 0.986 |
| Photocatalyst | Dye | Irradiation Time (min) | Efficiency (%) | Reference |
|---|---|---|---|---|
| Se-ZnSNCS | MO | 160 | 95.00 | [56] |
| N-TiO2 | MO | 200 | 90.00 | [57] |
| α-Bi2O3 | MO | 150 | 95.00 | [58] |
| N-TiO2 nanorods | MO | 250 | 80.00 | [59] |
| α-Fe2O3 nanoparticles | MO | 100 | 95.31 | [60] |
| ZnO quantum dots | MO | 160 | 97.00 | [61] |
| ZnO nanopyramid | MO | 150 | 95.00 | [62] |
| Fe–ZnO | MB | 180 | 92 | [63] |
| GO/TiO2 | MB | 240 | 100 | [64] |
| Cd–ZnO | MB | 240 | 89 | [65] |
| Fe3O4–ZnO NCS | MB | 180 | 89.2 | [66] |
| N-Carbon quantum dots/TiO2 | MB | 420 | 82.00 | [67] |
| S-TiO2 nanorods | MB | 240 | 92.00 | [68] |
| Egg-NiO | MB | 240 | 79.00 | [69] |
| TiO2rGOCdS | MO | 240 | 100 | This work |
| TiO2rGOCdS | MB | 360 | 100 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madduri, S.B.; Kommalapati, R.R. Photocatalytic Degradation of Azo Dyes in Aqueous Solution Using TiO2 Doped with rGO/CdS under UV Irradiation. Processes 2024, 12, 1455. https://doi.org/10.3390/pr12071455
Madduri SB, Kommalapati RR. Photocatalytic Degradation of Azo Dyes in Aqueous Solution Using TiO2 Doped with rGO/CdS under UV Irradiation. Processes. 2024; 12(7):1455. https://doi.org/10.3390/pr12071455
Chicago/Turabian StyleMadduri, Sunith B., and Raghava R. Kommalapati. 2024. "Photocatalytic Degradation of Azo Dyes in Aqueous Solution Using TiO2 Doped with rGO/CdS under UV Irradiation" Processes 12, no. 7: 1455. https://doi.org/10.3390/pr12071455






