Investigation of Partial Oxidation of Methane at Different Reaction Parameters by Adding Ni to CeO2 and ZrO2 Supported Cordierite Monolith Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Catalysts
2.2. Characterizations
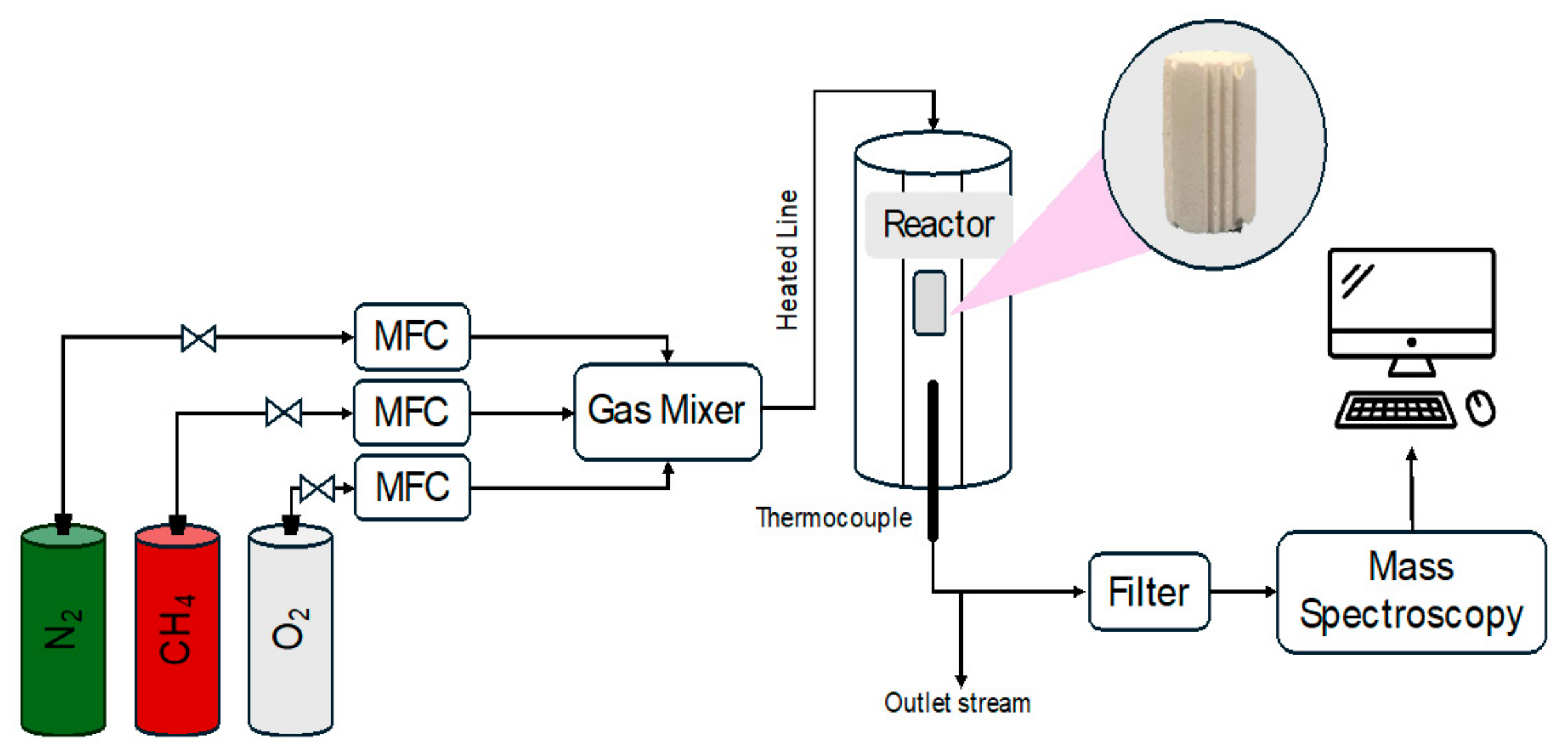
2.3. Catalytic Tests
3. Results and Discussion
3.1. Characterization of Catalyst
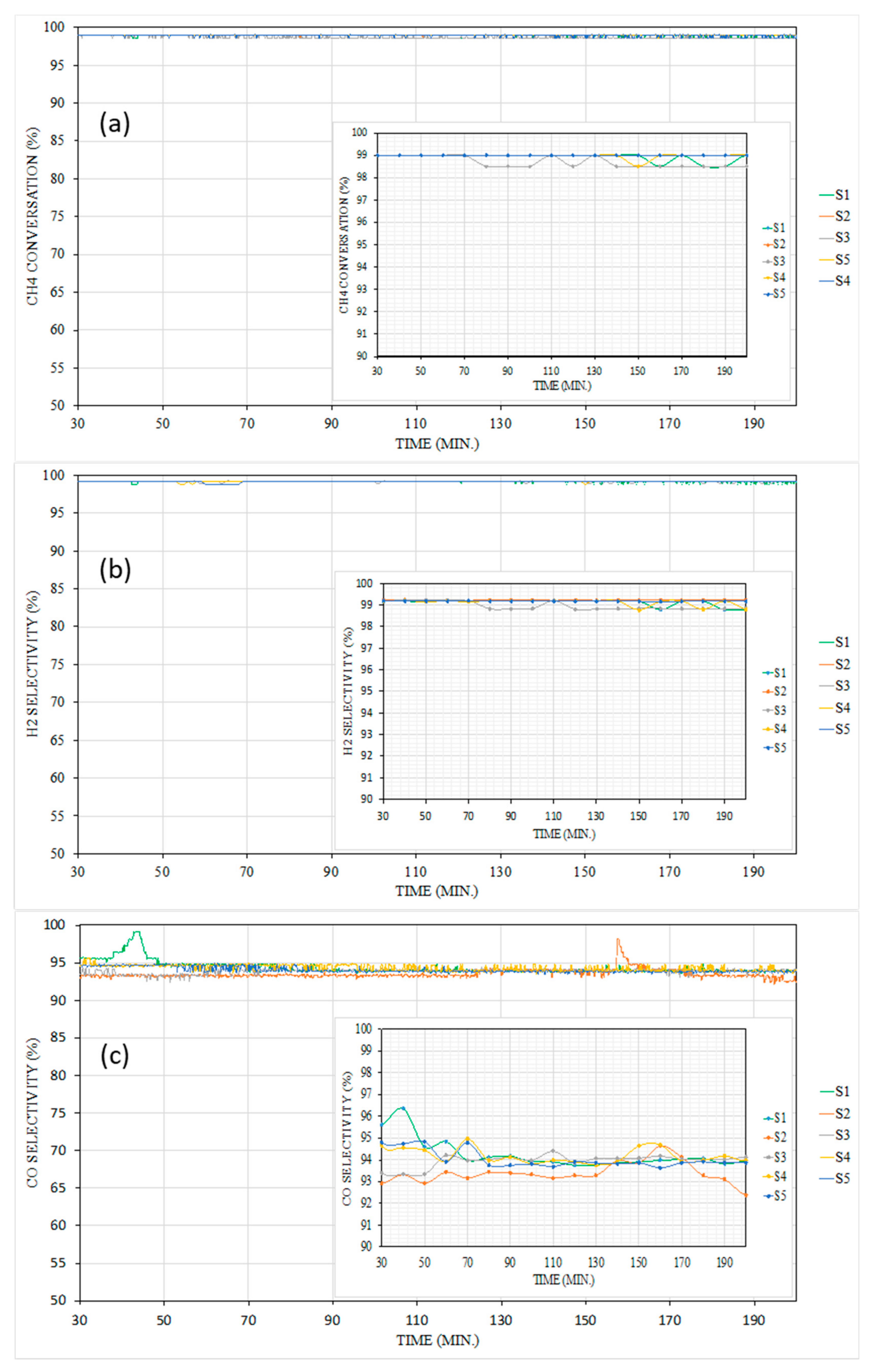
3.2. Catalytic Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, J.; Su, S.; Yu, X.X.; Weng, Y. Thermodynamic characteristics of a low concentration methane catalytic combustion gas turbine. Appl. Energy 2010, 87, 2102–2108. [Google Scholar] [CrossRef]
- International Energy Agency. Net Zero Roadmap: A Global Pathway to Keep the 1.5 °C Goal in Reach. Available online: https://www.iea.org/reports/net-zero-roadmap-a-global-pathway-to-keep-the-15-0c-goal-in-reach (accessed on 1 September 2023).
- Borowski, P.F.; Karlikowska, B. Clean Hydrogen Is a Challenge for Enterprises in the Era of Low-Emission and Zero-Emission Economy. Energies 2023, 16, 1171. [Google Scholar] [CrossRef]
- International Energy Agency. Global Hydrogen Review. Available online: https://www.iea.org/reports/global-hydrogen-review-2023 (accessed on 1 December 2023).
- U.S. Department of Energy. Department of Energy Hydrogen Program Plan. Available online: https://www.hydrogen.energy.gov/docs/hydrogenprogramlibraries/pdfs/hydrogen-program-plan-2020.pdf (accessed on 1 November 2020).
- Ferreira-Aparicio, P.; Benito, M.J.; Sanz, J.L. New trends in reforming technologies: From hydrogen industrial plants to multifuel microreformers. Catal. Rev. 2005, 47, 491–588. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Enger, B.C.; Lødeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A Gen. 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Karaismailoğlu, M.; Figen, H.E.; Baykara, S.Z. Methane decomposition over Fe-based catalysts. Int. J. Hydrogen Energy 2020, 45, 34773–34782. [Google Scholar] [CrossRef]
- York, A.P.; Xiao, T.; Green, M.L. Brief overview of the partial oxidation of methane to synthesis gas. Top. Catal. 2003, 22, 345–358. [Google Scholar] [CrossRef]
- Hickman, D.A.; Schmidt, L.D. Production of syngas by direct catalytic oxidation of methane. Science 1993, 259, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Siang, T.J.; Jalil, A.A.; Abdulrasheed, A.A.; Hambali, H.U.; Nabgan, W. Thermodynamic equilibrium study of altering methane partial oxidation for Fischer–Tropsch synfuel production. Energy 2020, 198, 117394. [Google Scholar] [CrossRef]
- Pantu, P.; Gavalas, G.R. Methane partial oxidation on Pt/CeO2 and Pt/Al2O3 catalysts. Appl. Catal. A Gen. 2002, 223, 253–260. [Google Scholar] [CrossRef]
- Fazlikeshteli, S.; Vendrell, X.; Llorca, J. Bimetallic Ru–Pd supported on CeO2 for the catalytic partial oxidation of methane into syngas. Fuel 2023, 334, 126799. [Google Scholar] [CrossRef]
- Kondratenko, V.A.; Karimova, U.; Kasimov, A.A.; Kondratenko, E.V. Methane conversion into synthesis gas over supported well-defined Pt, Rh or Ru nanoparticles: Effects of metal and support. Appl. Catal. A Gen. 2021, 619, 118143. [Google Scholar] [CrossRef]
- Li, L.; MD Dostagir, N.H.; Shrotri, A.; Fukuoka, A.; Kobayashi, H. Partial oxidation of methane to syngas via formate intermediate found for a ruthenium–rhenium bimetallic catalyst. ACS Catal. 2021, 11, 3782–3789. [Google Scholar] [CrossRef]
- Balint, I.; Miyazaki, A.; Aika, K.I. The relevance of Ru nanoparticles morphology and oxidation state to the partial oxidation of methane. J. Catal. 2003, 220, 74–83. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Orooji, Y. Nickel-based nanocatalysts promoted over MgO-modified SBA-16 for dry reforming of methane for syngas production: Impact of support and promoters. J. Energy Inst. 2021, 97, 100–108. [Google Scholar] [CrossRef]
- Xu, J.; Yeung, C.M.; Ni, J.; Meunier, F.; Acerbi, N.; Fowles, M.; Tsang, S.C. Methane steam reforming for hydrogen production using low water-ratios without carbon formation over ceria coated Ni catalysts. Appl. Catal. A Gen. 2008, 345, 119–127. [Google Scholar] [CrossRef]
- Figen, H.E.; Baykara, S.Z. Hydrogen production by partial oxidation of methane over Co based, Ni and Ru monolithic catalysts. Int. J. Hydrogen Energy 2015, 40, 7439–7451. [Google Scholar] [CrossRef]
- Babakouhi, R.; Alavi, S.M.; Rezaei, M.; Jokar, F.; Varbar, M.; Akbari, E. Hydrogen production through combined dry reforming and partial oxidation of methane over the Ni/Al2O3–CeO2 catalysts. Int. J. Hydrogen Energy 2024, 60, 503–514. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries. Available online: https://pubs.usgs.gov/periodicals/mcs2024/mcs2024.pdf (accessed on 31 January 2024).
- Arku, P.; Regmi, B.; Dutta, A. A review of catalytic partial oxidation of fossil fuels and biofuels: Recent advances in catalyst development and kinetic modelling. Chem. Eng. Res. Des. 2018, 136, 385–402. [Google Scholar] [CrossRef]
- Ahmed, S.; Bibi, S.S.; Irshad, M.; Asif, M.; Khan, M.K.; Kim, J. Synthesis of long-chain paraffins over bimetallic Na–Fe0. 9Mg0. 1Ox by direct CO2 hydrogenation. Top. Catal. 2024, 67, 363–376. [Google Scholar] [CrossRef]
- Ahmed, S.; Irshad, M.; Yoon, W.; Karanwal, N.; Sugiarto, J.R.; Khan, M.K.; Kim, J. Evaluation of MgO as a promoter for the hydrogenation of CO2 to long-chain hydrocarbons over Fe-based catalysts. Appl. Catal. B Environ. 2023, 338, 123052. [Google Scholar] [CrossRef]
- Shah, M.; Mondal, P. Optimization of CO2 reforming of methane process for the syngas production over Ni–Ce/TiO2–ZrO2 catalyst using the Taguchi method. Int. J. Hydrogen Energy 2021, 46, 22799–22812. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic dry reforming of methane over high surface area ceria. Appl. Catal. B Environ. 2005, 60, 107–116. [Google Scholar] [CrossRef]
- Roh, H.S.; Potdar, H.S.; Jun, K.W. Carbon dioxide reforming of methane over co-precipitated Ni–CeO2, Ni–ZrO2 and Ni–Ce–ZrO2 catalysts. Catal. Today 2004, 93, 39–44. [Google Scholar] [CrossRef]
- Osorio–Zabala, M.A.; Baquero, E.A.; Daza, C. Dry reforming of methane using cordierite monoliths with immobilized Ni–Ce catalysts. Int. J. Hydrogen Energy 2024, 60, 1157–1169. [Google Scholar] [CrossRef]
- Rodrigues, C.P.; Schmal, M. Nickel–alumina washcoating on monoliths for the partial oxidation of ethanol to hydrogen production. Int. J. Hydrogen Energy 2011, 36, 10709–10718. [Google Scholar] [CrossRef]
- Pereira, V.G.F.; Rodrigues, C.P.; Toniolo, F.S. Ni/Al2O3 supported on cordierite monoliths for methane steam reforming: Influence of catalyst coating methodology. Catal. Commun. 2023, 183, 106759. [Google Scholar] [CrossRef]
- Tsai, S.B.; Ma, H. A research on preparation and application of the monolithic catalyst with interconnecting pore structure. Sci. Rep. 2018, 8, 16605. [Google Scholar] [CrossRef]
- Govender, S.; Friedrich, H.B. Monoliths: A review of the basics, preparation methods and their relevance to oxidation. Catalysts 2017, 7, 62. [Google Scholar] [CrossRef]
- Hickman, D.A.; Schmidt, L.D. The role of boundary layer mass transfer in partial oxidation selectivity. J. Catal. 1992, 136, 300–308. [Google Scholar] [CrossRef]
- Landi, G.; Barbato, P.S.; Di Benedetto, A.; Lisi, L. Optimization of the preparation method of CuO/CeO2 structured catalytic monolith for CO preferential oxidation in H2-rich streams. Appl. Catal. B Environ. 2016, 181, 727–737. [Google Scholar] [CrossRef]
- Peng, X.; Jin, Q. Molecular simulation of methane steam reforming reaction for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 7569–7585. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
- Nijhuis, T.A.; Beers, A.E.; Vergunst, T.; Hoek, I.; Kapteijn, F.; Moulijn, J.A. Preparation of monolithic catalysts. Catal. Rev. 2001, 43, 345–380. [Google Scholar] [CrossRef]
- Khani, Y.; Bahadoran, F.; Soltanali, S.; Ahari, J.S. Hydrogen production by methanol steam reforming on a cordierite monolith reactor coated with Cu–Ni/LaZnAlO4 and Cu–Ni/γ-Al2O3 catalysts. Res. Chem. Intermed. 2018, 44, 925–942. [Google Scholar] [CrossRef]
- Zhao, A.; Ying, W.; Zhang, H.; Hongfang, M.; Fang, D. Ni/Al2O3 catalysts for syngas methanation: Effect of Mn promoter. J. Nat. Gas Chem. 2012, 21, 170–177. [Google Scholar] [CrossRef]
- Cai, X.; Cai, Y.; Lin, W. Autothermal reforming of methane over Ni catalysts supported over ZrO2-CeO2-Al2O3. J. Nat. Gas Chem. 2008, 17, 201–207. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M.; Garbarino, G.; Busca, G. Preparation and characterization of mesoporous nanocrystalline La-, Ce-, Zr-, Sr-containing NiAl2O3 methane autothermal reforming catalysts. Int. J. Hydrogen Energy 2016, 41, 8855–8862. [Google Scholar] [CrossRef]
- Zhang, R.J.; Xia, G.F.; Li, M.F.; Yu, W.U.; Hong, N.I.E.; Li, D.D. Effect of support on the performance of Ni-based catalyst in methane dry reforming. J. Fuel Chem. Technol. 2015, 43, 1359–1365. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M. Ce promoting effect on the activity and coke formation of Ni catalysts supported on mesoporous nanocrystalline γ-Al2O3 in autothermal reforming of methane. Int. J. Hydrogen Energy 2017, 42, 11130–11138. [Google Scholar] [CrossRef]
- Tezel, E.; Figen, H.E.; Baykara, S.Z. Hydrogen production by methane decomposition using bimetallic Ni–Fe catalysts. Int. J. Hydrogen Energy 2019, 44, 9930–9940. [Google Scholar] [CrossRef]
- Zheng, X.; Qi, J.; Zheng, Y.; Liu, C. Synthesis and characterization of CeAlO3 via solid state method. J. Solid State Chem. 2022, 312, 123220. [Google Scholar] [CrossRef]
- Pantu, P.; Kim, K.; Gavalas, G.R. Methane partial oxidation on Pt/CeO2–ZrO2 in the absence of gaseous oxygen. Appl. Catal. A Gen. 2000, 193, 203–214. [Google Scholar] [CrossRef]
- Dajiang, M.; Yaoqiang, C.; Junbo, Z.; Zhenling, W.; Di, M.; Maochu, G. Catalytic partial oxidation of methane over Ni/CeO2-ZrO2-Al2O3. J. Rare Earths 2007, 25, 311–315. [Google Scholar] [CrossRef]
- Ding, C.; Ai, G.; Zhang, K.; Yuan, Q.; Han, Y.; Ma, X.; Liu, S. Coking resistant Ni/ZrO2@SiO2 catalyst for the partial oxidation of methane to synthesis gas. Int. J. Hydrogen Energy 2015, 40, 6835–6843. [Google Scholar] [CrossRef]
- Liu, T.; Snyder, C.; Veser, G. Catalytic partial oxidation of methane: Is a distinction between direct and indirect pathways meaningful? Ind. Eng. Chem. Res. 2007, 46, 9045–9052. [Google Scholar] [CrossRef]
- Jin, R.; Chen, Y.; Li, W.; Cui, W.; Ji, Y.; Yu, C.; Jiang, Y. Mechanism for catalytic partial oxidation of methane to syngas over a Ni/Al2O3 catalyst. Appl. Catal. A Gen. 2000, 201, 71–80. [Google Scholar] [CrossRef]
- Larimi, A.S.; Alavi, S.M. Ceria-Zirconia supported Ni catalysts for partial oxidation of methane to synthesis gas. Fuel 2012, 102, 366–371. [Google Scholar] [CrossRef]
- Figen, H.E.; Baykara, S.Z. Effect of ruthenium addition on molybdenum catalysts for syngas production via catalytic partial oxidation of methane in a monolithic reactor. Int. J. Hydrogen Energy 2018, 43, 1129–1138. [Google Scholar] [CrossRef]
- Meng, F.; Chen, G.; Wang, Y.; Liu, Y. Metallic Ni monolith–Ni/MgAl2O4 dual bed catalysts for the autothermal partial oxidation of methane to synthesis gas. Int. J. Hydrogen Energy 2010, 35, 8182–8190. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Hong, X.; Zhang, Z.; Fang, Z.; Pan, Y.; Han, Z. Partial oxidation of methane to syngas over nickel monolithic catalysts. AIChE J. 2006, 52, 4276–4279. [Google Scholar] [CrossRef]
- Pengpanich, S.; Meeyoo, V.; Rirksomboon, T. Methane partial oxidation over Ni/CeO2–ZrO2 mixed oxide solid solution catalysts. Catal. Today 2004, 93, 95–105. [Google Scholar] [CrossRef]
- Fazlikeshteli, S.; Vendrell, X.; Llorca, J. Low-temperature partial oxidation of methane over Pd–Ni bimetallic catalysts supported on CeO2. Int. J. Hydrogen Energy 2023, 48, 12024–12035. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Hong, X.; Li, B. Zirconia promoted metallic nickel catalysts for the partial oxidation of methane to synthesis gas. Catal. Commun. 2009, 10, 940–944. [Google Scholar] [CrossRef]
- Özdemir, H.; Öksüzömer, M.F.; Gürkaynak, M.A. Preparation and characterization of Ni based catalysts for the catalytic partial oxidation of methane: Effect of support basicity on H2/CO ratio and carbon deposition. Int. J. Hydrogen Energy 2010, 35, 12147–12160. [Google Scholar] [CrossRef]
- Eriksson, S.; Rojas, S.; Boutonnet, M.; Fierro, J.L.G. Effect of Ce-doping on Rh/ZrO2 catalysts for partial oxidation of methane. Appl. Catal. A Gen. 2007, 326, 8–16. [Google Scholar] [CrossRef]
- Fazlikeshteli, S.; Vendrell, X.; Llorca, J. Catalytic partial oxidation of methane over bimetallic Ru–Ni supported on CeO2 for syngas production. Int. J. Hydrogen Energy 2024, 51, 1494–1507. [Google Scholar] [CrossRef]


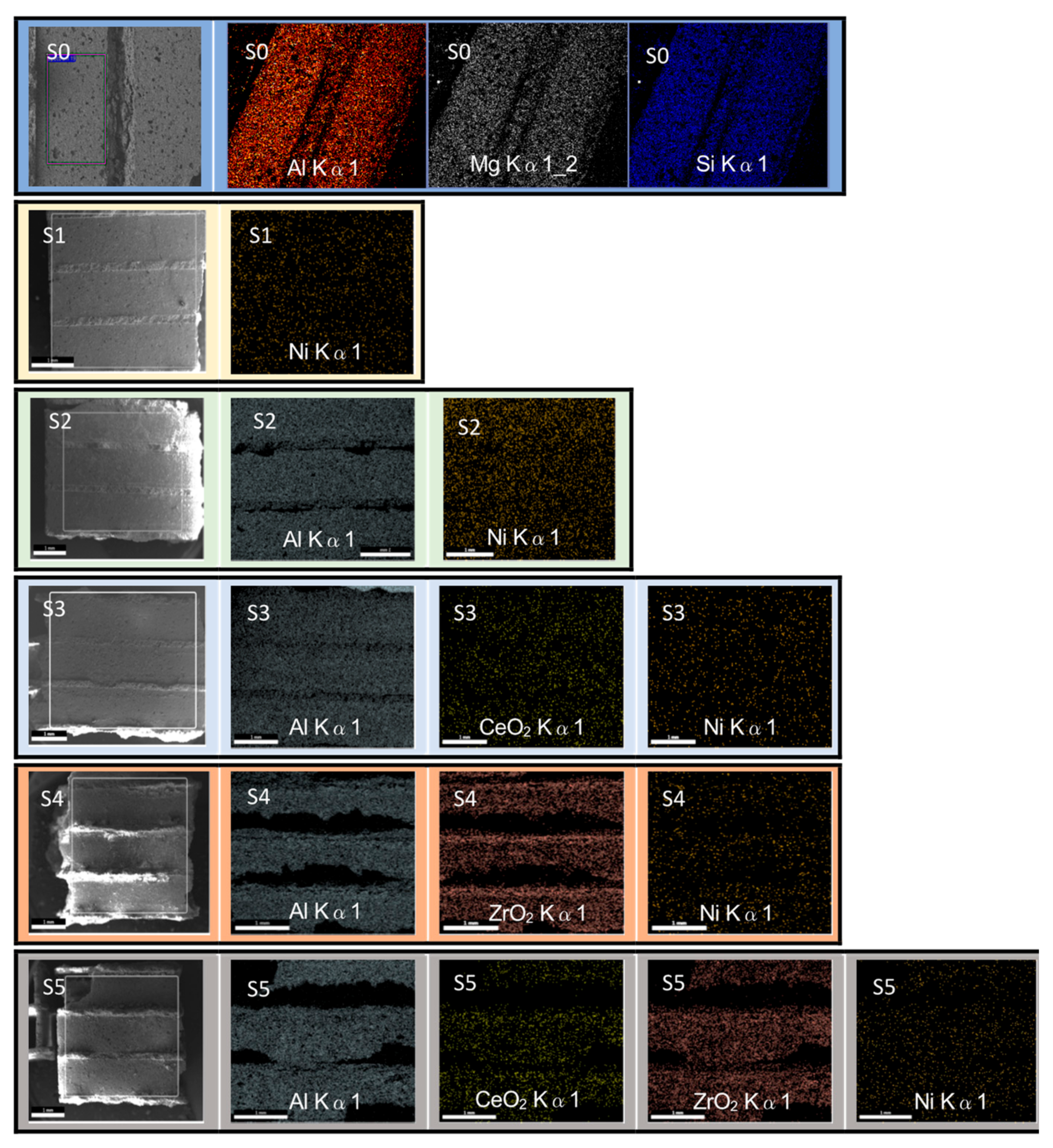
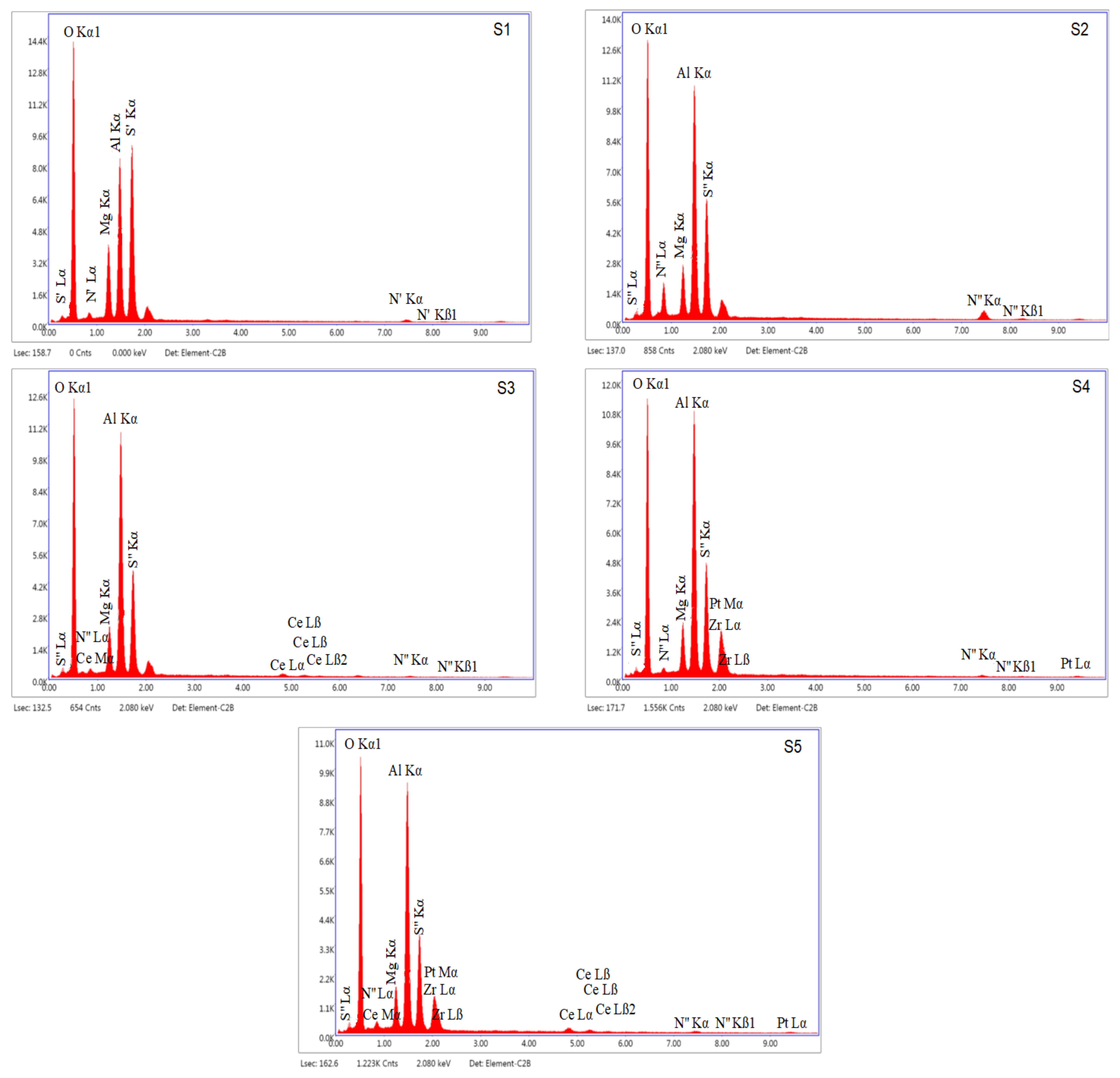
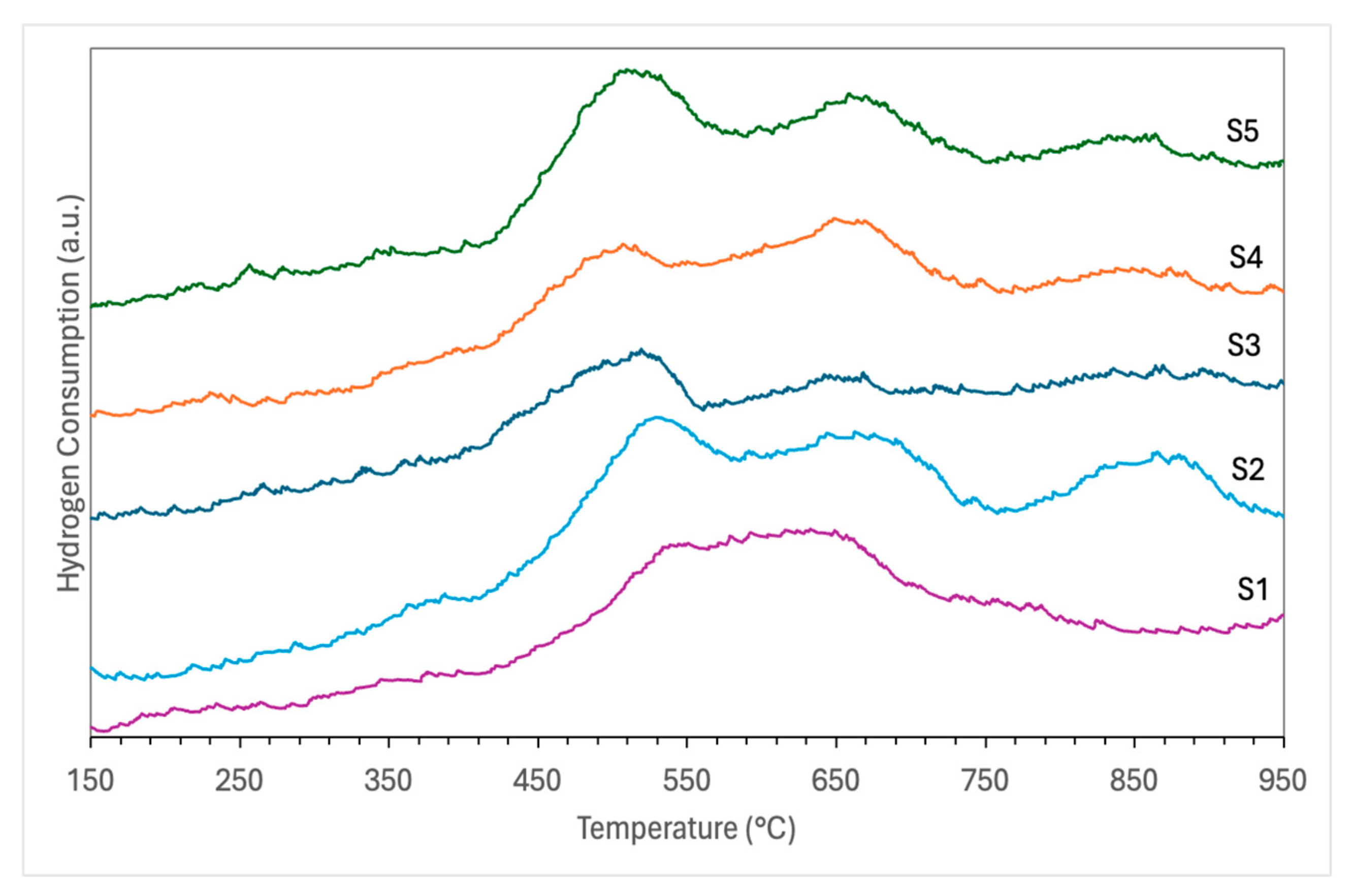
| Code | Catalyst | Crystal Phases | Crystal Size (nm) |
|---|---|---|---|
| S0 | Monolith Support | Mg2Al4Si5O18 (01-076-6037) | 38.33 |
| S1 | Ni | NiO (04-002-5335) | 38.86 |
| S2 | γ-Al2O3/Ni | NiO (04-002-5335) | 43.71 |
| S3 | γ-Al2O3/CeO2/Ni | NiO (04-002-5335) CeO2 (04-005-4553) | 49.00 35.53 |
| S4 | γ-Al2O3/ZrO2/Ni | NiO (04-002-5335) ZrO2 (04-007-0953) | 29.11 25.56 |
| S5 | γ-Al2O3/CeO2-ZrO2/Ni | NiO (04-002-5335) CeO2 (04-005-4553) ZrO2 (04-007-0953) | 29.11 29.61 14.07 |
| Catalyst | Surface Element Content (Mass Fraction %) | ||||||
|---|---|---|---|---|---|---|---|
| O | Mg | Al | Si | Ni | CeO2 | ZrO2 | |
| S1 | 40.42 | 9.27 | 21.38 | 25.49 | 3.44 | - | - |
| S2 | 35.61 | 6.17 | 29.35 | 16.02 | 12.86 | - | - |
| S3 | 39.74 | 6.11 | 32.51 | 16.46 | 1.30 | 3.87 | - |
| S4 | 40.23 | 5.67 | 30.18 | 13.98 | 3.22 | - | 6.72 |
| S5 | 37.55 | 5.32 | 29.84 | 12.08 | 3.56 | 6.59 | 5.06 |
| Catalyst | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| S1 | 0.9409 | 0.004721 | 20.068 |
| S2 | 2.5726 | 0.008115 | 12.618 |
| S3 | 2.4832 | 0.007582 | 12.231 |
| S4 | 2.9475 | 0.007748 | 10.515 |
| S5 | 1.0673 | 0.003538 | 13.259 |
| S1 | S2 | S3 | S4 | S5 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | CH4 feed (%) | XCH4 (%) | SH2 (%) | SCO (%) | XCH4 (%) | SH2 (%) | SCO (%) | XCH4 (%) | SH2 (%) | SCO (%) | XCH4 (%) | SH2 (%) | SCO (%) | XCH4 (%) | SH2 (%) | SCO (%) |
| 750 | 2 | 98.5 | 99.0 | 94.2 | 98.5 | 99.0 | 93.4 | 96.5 | 97.4 | 92.2 | 97.0 | 97.0 | 76.5 | 94.0 | 95.8 | 89.4 |
| 5 | 97.4 | 98.2 | 97.4 | 97.4 | 98.2 | 97.1 | 95.6 | 96.7 | 95.2 | 96.4 | 96.5 | 79.0 | 92.6 | 94.7 | 92.6 | |
| 10 | 95.3 | 96.4 | 96.0 | 95.2 | 96.4 | 95.7 | 92.8 | 94.2 | 93.4 | 95.5 | 95.4 | 79.1 | 90.8 | 92.9 | 90.5 | |
| 800 | 2 | 98.0 | 98.6 | 94.1 | 98.5 | 98.9 | 92.1 | 98.5 | 98.9 | 94.9 | 95.5 | 96.8 | 91.5 | 97.0 | 97.8 | 91.8 |
| 5 | 97.2 | 98.0 | 97.4 | 98.4 | 98.9 | 97.4 | 98.0 | 98.5 | 97.4 | 93.4 | 95.3 | 94.5 | 96.4 | 97.4 | 95.4 | |
| 10 | 95.3 | 96.4 | 96.7 | 97.2 | 97.9 | 97.2 | 96.1 | 97.0 | 96.7 | 90.7 | 92.8 | 92.7 | 95.0 | 96.2 | 95.2 | |
| 850 | 2 | 97.0 | 97.9 | 93.5 | 98.5 | 99.0 | 94.1 | 98.5 | 98.9 | 96.9 | 90.5 | 93.1 | 89.0 | 96.0 | 97.2 | 91.0 |
| 5 | 96.6 | 97.6 | 97.1 | 98.8 | 99.2 | 98.0 | 98.4 | 98.9 | 98.0 | 90.8 | 93.4 | 92.4 | 96.8 | 97.8 | 96.3 | |
| 10 | 95.2 | 96.3 | 96.7 | 98.1 | 98.6 | 98.2 | 97.3 | 97.9 | 97.8 | 89.1 | 91.6 | 91.5 | 96.1 | 97.1 | 96.8 | |
| S1 | S2 | S3 | S4 | S5 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | CH4 feed (%) | XCH4 (%) | SH2 (%) | SCO (%) | XCH4 (%) | SH2 (%) | SCO (%) | XCH4 (%) | SH2 (%) | SCO (%) | XCH4 (%) | SH2 (%) | SCO (%) | XCH4 (%) | SH2 (%) | SCO (%) |
| 750 | 2 | 99.0 | 99.2 | 95.0 | 99.0 | 99.2 | 94.2 | 99.0 | 99.2 | 93.5 | 98.5 | 98.3 | 76.8 | 98.0 | 98.4 | 91.0 |
| 5 | 95.2 | 96.5 | 98.4 | 95.2 | 96.6 | 98.7 | 95.6 | 96.8 | 97.2 | 99.0 | 99.1 | 84.1 | 95.2 | 96.6 | 96.1 | |
| 10 | 96.0 | 96.9 | 95.7 | 96.4 | 97.3 | 95.6 | 96.0 | 96.8 | 94.1 | 98.9 | 98.9 | 80.0 | 95.9 | 96.9 | 93.3 | |
| 800 | 2 | 99.0 | 99.2 | 94.1 | 99.0 | 99.2 | 91.9 | 99.0 | 99.2 | 96.0 | 97.5 | 98.0 | 92.3 | 99.0 | 99.2 | 91.8 |
| 5 | 95.2 | 96.6 | 99.0 | 96.6 | 97.6 | 98.0 | 96.4 | 97.4 | 98.3 | 94.0 | 95.8 | 98.2 | 96.6 | 97.6 | 98.2 | |
| 10 | 97.4 | 98.0 | 97.3 | 98.1 | 98.6 | 96.6 | 98.0 | 98.4 | 96.7 | 96.1 | 97.1 | 95.5 | 98.0 | 98.5 | 96.3 | |
| 850 | 2 | 98.5 | 98.8 | 93.4 | 99.0 | 99.2 | 92.7 | 99.0 | 99.2 | 97.5 | 97.5 | 98.0 | 92.3 | 99.0 | 99.2 | 93.3 |
| 5 | 95.8 | 97.0 | 99.3 | 97.4 | 98.2 | 99.0 | 97.0 | 97.9 | 98.9 | 95.0 | 96.5 | 99.3 | 97.2 | 98.1 | 99.3 | |
| 10 | 98.7 | 99.0 | 98.1 | 99.0 | 99.3 | 97.9 | 98.9 | 99.2 | 97.9 | 96.5 | 97.4 | 96.0 | 99.0 | 99.3 | 97.9 | |
| Catalyst | XCH4 (%) | SH2 (%) | SCO (%) | Temperature, °C | GHSV, h−1 | Ref. |
|---|---|---|---|---|---|---|
| Ni monolith catalyst | 81.49 | 82.72 | 78.75 | 800 | 1 × 104 | [20] |
| Mo-Ni monolith catalyst | 95.67 | 91.57 | 89.53 | 800 | 1 × 104 | [53] |
| Ru-Mo-Ni monolith catalyst | 95.31 | 92.72 | 91.21 | 800 | 1 × 104 | [53] |
| Ni-Ni/MgAl2O4 monolith catalyst | 85.30 | 91.50 | 93.00 | 700 | 1 × 105 | [54] |
| Ce-Zr/Ni monolith catalyst | 94.00 | 98.00 | 96.00 | 850 | 1 × 105 | [55] |
| Ni/CeO2 | 98.00 | 93.00 | 98.00 | 800 | 53 × 103 | [56] |
| Ni/ZrO2 | 96.00 | 96.00 | 98.00 | 800 | 53 × 103 | [56] |
| Ni/CeZrO2 | 98.00 | 95.00 | 98.00 | 800 | 53 × 103 | [56] |
| Pd–Ni/CeO2 | 75.80 | 66.10 | 22.80 | 550 | 12 × 103 | [57] |
| Zr/Ni sponge catalyst | 84.00 | 98.00 | 95.00 | 850 | 1 × 105 | [58] |
| Ni/Al2O3 | 95.30 | 97.40 | 98.00 | 800 | - | [59] |
| Rh/CeO2–ZrO2 | 97.00 | 87.00 | 96.00 | 750 | 99 × 103 | [60] |
| Ru/CeO2 | 74.10 | 65.10 | 21.70 | 600 | 12 × 103 | [61] |
| Ru–Ni/CeO2 | 76.40 | 63.70 | 20.60 | 600 | 12 × 103 | [61] |
| S1 | 99.00 | 99.20 | 94.10 | 800 | 1 × 104 | In this study |
| S2 | 99.00 | 99.20 | 91.90 | 800 | 1 × 104 | In this study |
| S3 | 99.00 | 99.20 | 96.00 | 800 | 1 × 104 | In this study |
| S4 | 97.50 | 98.00 | 92.30 | 800 | 1 × 104 | In this study |
| S5 | 99.00 | 99.20 | 91.80 | 800 | 1 × 104 | In this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilicak Bayraktar, I.; Figen, H.E. Investigation of Partial Oxidation of Methane at Different Reaction Parameters by Adding Ni to CeO2 and ZrO2 Supported Cordierite Monolith Catalyst. Processes 2024, 12, 1463. https://doi.org/10.3390/pr12071463
Ilicak Bayraktar I, Figen HE. Investigation of Partial Oxidation of Methane at Different Reaction Parameters by Adding Ni to CeO2 and ZrO2 Supported Cordierite Monolith Catalyst. Processes. 2024; 12(7):1463. https://doi.org/10.3390/pr12071463
Chicago/Turabian StyleIlicak Bayraktar, Ilke, and Halit Eren Figen. 2024. "Investigation of Partial Oxidation of Methane at Different Reaction Parameters by Adding Ni to CeO2 and ZrO2 Supported Cordierite Monolith Catalyst" Processes 12, no. 7: 1463. https://doi.org/10.3390/pr12071463
APA StyleIlicak Bayraktar, I., & Figen, H. E. (2024). Investigation of Partial Oxidation of Methane at Different Reaction Parameters by Adding Ni to CeO2 and ZrO2 Supported Cordierite Monolith Catalyst. Processes, 12(7), 1463. https://doi.org/10.3390/pr12071463






