Towards Sustainable Lithium-Ion Battery Recycling: Advancements in Circular Hydrometallurgy
Abstract
:1. Introduction
2. Methodology and Structure of the Article
3. Environmental and Resource Challenges in the Manufacture of LIBs
4. Overview of Recycling Processes for Lithium-Ion Batteries
| Process | Advantages | Disadvantages | Challenges |
|---|---|---|---|
| Pyrometallurgy |
|
|
|
| Hydrometa-llurgy |
|
|
|
| Direct recycling |
|
|
|
4.1. The Hydrometallurgical Process of LIB Recycling
4.2. Circular Hydrometallurgy
5. Electrodialysis
5.1. Basic Principles of Electrodialysis
5.2. Comparison with Other Separation Techniques
| Method | Advantages | Disadvantages |
|---|---|---|
| Precipitation |
|
|
| Solvent extraction |
|
|
| Ion exchange |
|
|
| Electrodialysis |
|
|
6. Role of Electrodialysis in Circular Hydrometallurgy
6.1. Separation of Metals
6.2. Combination of the Leaching and Separation Stages
6.3. Regeneration of Reactants
7. Challenges and Limitations of Electrodialysis
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2016, 164, A5019. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The Lithium-Ion Battery: State of the Art and Future Perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- International Energy Agency IEA. The Role of Critical Minerals in Clean Energy Transitions; IEA: Paris, France, 2021. [Google Scholar]
- International Energy Agency IEA. Global EV Outlook 2020; IEA: Paris, France, 2020. [Google Scholar]
- Kitchenham, B. Procedures for Performing Systematic Reviews; Keele University: Kelee, UK, 2004; Volume 33. [Google Scholar]
- Mrozik, W.; Rajaeifar, M.A.; Heidrich, O.; Christensen, P. Environmental Impacts, Pollution Sources and Pathways of Spent Lithium-Ion Batteries. Energy Environ. Sci. 2021, 14, 6099–6121. [Google Scholar] [CrossRef]
- European Comission. Study on the Critical Raw Materials for the EU 2023—Final Report; European Comission: Brussels, Belgium, 2023. [Google Scholar]
- Nalule, V.R. Social and Environmental Impacts of Mining. In Mining and the Law in Africa; Palgrave Pivot: Cham, Switzerland, 2020; pp. 51–81. [Google Scholar] [CrossRef]
- Joint Research Centre. The JRC Battery Raw Materials and Value Chain Tool (2021) and Ongoing RMIS Modelling Work; Joint Research Centre: Seville, Spain, 2021. [Google Scholar]
- European Union. Directive 2006/66/EC of the European Parliament and of the Council of 6 September 2006 on Batteries and Accumulators and Waste Batteries and Accumulators and Repealing Directive 91/157/EEC; European Union: Brussels, Belgium, 2006. [Google Scholar]
- European Union. Regulation (EU) 2023/1542 of the European Parliament and of the Council of 12 July 2023 Concerning Batteries and Waste Batteries, Amending Directive 2008/98/EC and Regulation (EU) 2019/1020 and Repealing Directive 2006/66/EC; European Union: Brussels, Belgium, 2023. [Google Scholar]
- Ma, X.; Azhari, L.; Wang, Y. Li-Ion Battery Recycling Challenges. Chem 2021, 7, 2843–2847. [Google Scholar] [CrossRef]
- Chen, M.; Ma, X.; Chen, B.; Arsenault, R.; Karlson, P.; Simon, N.; Wang, Y. Recycling End-of-Life Electric Vehicle Lithium-Ion Batteries. Joule 2019, 3, 2622–2646. [Google Scholar] [CrossRef]
- Khodadadmahmoudi, G.; Javdan Tabar, K.; Homayouni, A.H.; Chehreh Chelgani, S. Recycling Spent Lithium Batteries—An Overview of Pretreatment Flowsheet Development Based on Metallurgical Factors. Environ. Technol. Rev. 2023, 12, 2248559. [Google Scholar] [CrossRef]
- Xu, Z.; Zhiyuan, L.; Wenjun, M.; Qinxin, Z. Pretreatment Options for the Recycling of Spent Lithium-Ion Batteries: A Comprehensive Review. J. Energy Storage 2023, 72, 108691. [Google Scholar] [CrossRef]
- Dobó, Z.; Dinh, T.; Kulcsár, T. A Review on Recycling of Spent Lithium-Ion Batteries. Energy Rep. 2023, 9, 6362–6395. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical Options for Recycling Spent Lithium-Ion Batteries: A Comprehensive Review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Zhou, M.; Li, B.; Li, J.; Xu, Z. Pyrometallurgical Technology in the Recycling of a Spent Lithium Ion Battery: Evolution and the Challenge. ACS ES&T Eng. 2021, 1, 1369–1382. [Google Scholar] [CrossRef]
- Saleem, U.; Joshi, B.; Bandyopadhyay, S. Hydrometallurgical Routes to Close the Loop of Electric Vehicle (EV) Lithium-Ion Batteries (LIBs) Value Chain: A Review. J. Sustain. Metall. 2023, 9, 950–971. [Google Scholar] [CrossRef]
- Bai, Y.; Muralidharan, N.; Sun, Y.K.; Passerini, S.; Stanley Whittingham, M.; Belharouak, I. Energy and Environmental Aspects in Recycling Lithium-Ion Batteries: Concept of Battery Identity Global Passport. Mater. Today 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Montoya, A.T.; Yang, Z.; Dahl, E.U.; Pupek, K.Z.; Polzin, B.; Dunlop, A.; Vaughey, J.T. Direct Recycling of Lithium-Ion Battery Cathodes: A Multi-Stage Annealing Process to Recover the Pristine Structure and Performance. ACS Sustain. Chem. Eng. 2022, 10, 13319–13324. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling—Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Zhou, L.F.; Yang, D.; Du, T.; Gong, H.; Luo, W. Bin The Current Process for the Recycling of Spent Lithium Ion Batteries. Front. Chem. 2020, 8, 578044. [Google Scholar] [CrossRef] [PubMed]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Sharma, P.; Pandey, A.; Jang, M.; Jeon, B.-H.; Varjani, S.; Kim, S.-H. Recycling of Cathode Material from Spent Lithium-Ion Batteries: Challenges and Future Perspectives. J. Hazard. Mater. 2022, 429, 128312. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. The Twelve Principles of Circular Hydrometallurgy. J. Sustain. Metall. 2022, 9, 1–25. [Google Scholar] [CrossRef]
- Meshram, P.; Mishra, A.; Abhilash; Sahu, R. Environmental Impact of Spent Lithium Ion Batteries and Green Recycling Perspectives by Organic Acids—A Review. Chemosphere 2020, 242, 125291. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.X.; Chen, R.J.; Wu, F.; Amine, K. Ascorbic-Acid-Assisted Recovery of Cobalt and Lithium from Spent Li-Ion Batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
- Joulié, M.; Billy, E.; Laucournet, R.; Meyer, D. Current Collectors as Reducing Agent to Dissolve Active Materials of Positive Electrodes from Li-Ion Battery Wastes. Hydrometallurgy 2017, 169, 426–432. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Meng, Q.; Dong, P.; Ning, P.; Li, Q. Recovery of Valuable Metals from Mixed Spent Lithium-Ion Batteries by Multi-Step Directional Precipitation. RSC Adv. 2020, 11, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhao, Z. Recovery of Lithium from Spent Lithium-Ion Batteries Using Precipitation and Electrodialysis Techniques. Sep. Purif. Technol. 2018, 206, 335–342. [Google Scholar] [CrossRef]
- Chen, X.; Ma, H.; Luo, C.; Zhou, T. Recovery of Valuable Metals from Waste Cathode Materials of Spent Lithium-Ion Batteries Using Mild Phosphoric Acid. J. Hazard. Mater. 2017, 326, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Refly, S.; Floweri, O.; Mayangsari, T.R.; Aimon, A.H.; Iskandar, F. Green Recycle Processing of Cathode Active Material from LiNi1/3Co1/3Mn1/3O2 (NCM 111) Battery Waste through Citric Acid Leaching and Oxalate Co-Precipitation Process. Mater. Today Proc. 2021, 44, 3378–3380. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, S.; He, Y. Lithium Recycling and Cathode Material Regeneration from Acid Leach Liquor of Spent Lithium-Ion Battery via Facile Co-Extraction and Co-Precipitation Processes. Waste Manag. 2017, 64, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, N.; Legeai, S.; Balva, M.; Hazotte, C.; Comel, J.; Lapicque, F.; Billy, E.; Meux, E. Metals Recovery of Metals from Secondary Raw Materials by Coupled Electroleaching and Electrodeposition in Aqueous or Ionic Liquid Media. Metals 2018, 8, 556. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, S.; Song, X. Electrodeposition of Cobalt in Double-Membrane Three-Compartment Electrolytic Reactor. Trans. Nonferrous Met. Soc. China 2016, 26, 1706–1713. [Google Scholar] [CrossRef]
- Villen-Guzman, M.; Arhoun, B.; Vereda-Alonso, C.; Gomez-Lahoz, C.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Electrodialytic Processes in Solid Matrices. New Insights into Batteries Recycling. A Review. J. Chem. Technol. Biotechnol. 2019, 4, 1727–1738. [Google Scholar] [CrossRef]
- Vasconcelos, D.d.S.; Tenório, J.A.S.; Botelho Junior, A.B.; Espinosa, D.C.R. Circular Recycling Strategies for LFP Batteries: A Review Focusing on Hydrometallurgy Sustainable Processing. Metals 2023, 13, 543. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a Mature Technology with a Multitude of New Applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Arana Juve, J.M.; Christensen, F.M.S.; Wang, Y.; Wei, Z. Electrodialysis for Metal Removal and Recovery: A Review. Chem. Eng. J. 2022, 435, 134857. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.M.; Villen-Guzman, M.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Metal Recovery from Wastewater Using Electrodialysis Separation. Metals 2023, 14, 38. [Google Scholar] [CrossRef]
- Villen-Guzman, M.; Guedes, P.; Couto, N.; Ottosen, L.M.; Ribeiro, A.B.; Rodriguez-Maroto, J.M. Electrodialytic Phosphorus Recovery from Sewage Sludge Ash under Kinetic Control. Electrochim. Acta 2018, 287, 49–59. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Ion-Exchange Membrane Systems—Electrodialysis and Other Electromembrane Processes. Fundam. Model. Membr. Syst. Membr. Process Perform. 2018, 251–300. [Google Scholar] [CrossRef]
- McGovern, R.K.; Weiner, A.M.; Sun, L.; Chambers, C.G.; Zubair, S.M.; Lienhard V, J.H. On the Cost of Electrodialysis for the Desalination of High Salinity Feeds. Appl. Energy 2014, 136, 649–661. [Google Scholar] [CrossRef]
- Hopsort, G.; Cacciuttolo, Q.; Pasquier, D. Electrodialysis as a Key Operating Unit in Chemical Processes: From Lab to Pilot Scale of Latest Breakthroughs. Chem. Eng. J. 2024, 494, 153111. [Google Scholar] [CrossRef]
- Lin, X.; Pan, J.; Zhou, M.; Xu, Y.; Lin, J.; Shen, J.; Gao, C.; Van Der Bruggen, B. Extraction of Amphoteric Amino Acid by Bipolar Membrane Electrodialysis: Methionine Acid as a Case Study. Ind. Eng. Chem. Res. 2016, 55, 2813–2820. [Google Scholar] [CrossRef]
- Kırmızı, S.; Karabacakoğlu, B. Performance of Electrodialysis for Ni(II) and Cr(VI) Removal from Effluents: Effect of Process Parameters on Removal Efficiency, Energy Consumption and Current Efficiency. J. Appl. Electrochem. 2023, 53, 2039–2055. [Google Scholar] [CrossRef]
- Ozkul, S.; van Daal, J.J.; Kuipers, N.J.M.; Bisselink, R.J.M.; Bruning, H.; Dykstra, J.E.; Rijnaarts, H.H.M. Transport Mechanisms in Electrodialysis: The Effect on Selective Ion Transport in Multi-Ionic Solutions. J. Membr. Sci. 2023, 665, 121114. [Google Scholar] [CrossRef]
- Voutetaki, A.; Plakas, K.V.; Papadopoulos, A.I.; Bollas, D.; Parcharidis, S.; Seferlis, P. Pilot-Scale Separation of Lead and Sulfate Ions from Aqueous Solutions Using Electrodialysis: Application and Parameter Optimization for the Battery Industry. J. Clean. Prod. 2023, 410, 137200. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, H.; Wang, X.; Wang, Y.; Xu, T. A Closed Loop Production of Water Insoluble Organic Acid Using Bipolar Membranes Electrodialysis (BMED). J. Membr. Sci. 2016, 520, 345–353. [Google Scholar] [CrossRef]
- Gao, W.; Fang, Q.; Yan, H.; Wei, X.; Wu, K. Recovery of Acid and Base from Sodium Sulfate Containing Lithium Carbonate Using Bipolar Membrane Electrodialysis. Membranes 2021, 11, 152. [Google Scholar] [CrossRef]
- Karpiński, P.H.; Bałdyga, J. Precipitation Processes. In Handbook of Industrial Crystallization; Myerson, A.S., Erdemir, D., Lee, A.Y., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 216–265. ISBN 9780521196185. [Google Scholar]
- Lei, S.; Sun, W.; Yang, Y. Solvent Extraction for Recycling of Spent Lithium-Ion Batteries. J. Hazard. Mater. 2022, 424, 127654. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Z.; Si, X.; Lu, Y.; Liu, Q. Efficient Recovery of Lithium from Spent Lithium Ion Batteries Effluent by Solvent Extraction Using 2-Ethylhexyl Hydrogen [Bis(2-Ethylhexyl) Amino]Methyl Phosphonate Acid. Metals 2024, 14, 345. [Google Scholar] [CrossRef]
- Kanagasundaram, T.; Murphy, O.; Haji, M.N.; Wilson, J.J. The Recovery and Separation of Lithium by Using Solvent Extraction Methods. Coord. Chem. Rev. 2024, 509, 215727. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Dreisinger, D.B.; Espinosa, D.C.R. A Review of Nickel, Copper, and Cobalt Recovery by Chelating Ion Exchange Resins from Mining Processes and Mining Tailings. Min. Metall. Explor. 2019, 36, 199–213. [Google Scholar] [CrossRef]
- Jasim, A.Q.; Ajjam, S.K. Removal of Heavy Metal Ions from Wastewater Using Ion Exchange Resin in a Batch Process with Kinetic Isotherm. S. Afr. J. Chem. Eng. 2024, 49, 43–54. [Google Scholar] [CrossRef]
- Chan, K.H.; Malik, M.; Azimi, G. Recovery of Valuable Metals from End-of-Life Lithium-Ion Battery Using Electrodialysis. Miner. Met. Mater. Ser. 2021, 11–17. [Google Scholar] [CrossRef]
- Gmar, S.; Chagnes, A. Recent Advances on Electrodialysis for the Recovery of Lithium from Primary and Secondary Resources. Hydrometallurgy 2019, 189, 105124. [Google Scholar] [CrossRef]
- Gmar, S.; Chagnes, A.; Lutin, F.; Muhr, L. Application of Electrodialysis for the Selective Lithium Extraction Towards Cobalt, Nickel and Manganese from Leach Solutions Containing High Divalent Cations/Li Ratio. Recycling 2022, 7, 14. [Google Scholar] [CrossRef]
- Xing, Z.; Srinivasan, M. Lithium Recovery from Spent Lithium-Ion Batteries Leachate by Chelating Agents Facilitated Electrodialysis. Chem. Eng. J. 2023, 474, 145306. [Google Scholar] [CrossRef]
- Iizuka, A.; Yamashita, Y.; Nagasawa, H.; Yamasaki, A.; Yanagisawa, Y. Separation of Lithium and Cobalt from Waste Lithium-Ion Batteries via Bipolar Membrane Electrodialysis Coupled with Chelation. Sep. Purif. Technol. 2013, 113, 33–41. [Google Scholar] [CrossRef]
- Chan, K.H.; Malik, M.; Azimi, G. Separation of Lithium, Nickel, Manganese, and Cobalt from Waste Lithium-Ion Batteries Using Electrodialysis. Resour. Conserv. Recycl. 2022, 178, 106076. [Google Scholar] [CrossRef]
- Chen, L.; Liang, W.; Zhang, Y.; Wang, B.; Song, X.; Abdel-Ghafar, H.M.; Zhang, L.; Jiang, H. Metal Coordination Combining with Electrodialysis for One-Step Separation of Valuable Metals from Spent Lithium-Ion Batteries. Chem. Eng. J. 2024, 488, 150882. [Google Scholar] [CrossRef]
- Hansen, H.K.; Rojo, A.; Ottosen, L.M. Electrodialytic Remediation of Copper Mine Tailings. J. Hazard. Mater. 2005, 117, 179–183. [Google Scholar] [CrossRef]
- Pedersen, A.J.; Kristensen, I.V.; Ottosen, L.M.; Ribeiro, A.B.; Villumsen, A. Electrodialytic Remediation of CCA-Treated Waste Wood in Pilot Scale. Eng. Geol. 2005, 77, 331–338. [Google Scholar] [CrossRef]
- Ribeiro, A.B.; Mateus, E.P.; Ottosen, L.M.; Bech-Nielsen, G. Electrodialytic Removal of Cu, Cr, and As from Chromated Copper Arsenate-Treated Timber Waste. Environ. Sci. Technol. 2000, 34, 784–788. [Google Scholar] [CrossRef]
- Jensen, J.B.; Kueeš, V.; Kubal, M. Electrokinetic Remediation of Soils Polluted with Heavy Metals. Removal of Zinc and Copper Using a New Concept. Environ. Technol. 1994, 15, 1077–1082. [Google Scholar] [CrossRef]
- Hansen, H.K.; Ottosen, L.M.; Hansen, L.; Kliem, B.K.; Villumsen, A.; Bech-Nielsen, G. Electrodialytic Remediation of Soil Polluted With Heavy Metals: Key Parameters for Optimization of the Process. Chem. Eng. Res. Des. 1999, 77, 218–222. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Hansen, H.K.; Laursen, S.; Villumsen, A. Electrodialytic Remediation of Soil Polluted with Copper from Wood Preservation Industry. Environ. Sci. Technol. 1997, 31, 1711–1715. [Google Scholar] [CrossRef]
- Guedes, P.; Mateus, E.P.; Alshawabkeh, A.N.; Ribeiro, A.B. Ultrasound-Assisted Electrodialytic Separation of Cobalt from Tungsten Carbide Scrap Powder. Sustain. Chem. Pharm. 2024, 38, 101471. [Google Scholar] [CrossRef]
- Segall, B.A.; Matthias, J.; O’bannon, C.E. Electro-Osmosis Chemistry and Water Quality. J. Geotech. Eng. Div. 1980, 106, 1148–1152. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.M.; Villen-Guzman, M.; Vereda-Alonso, C.; Gomez-Lahoz, C.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Recovery of Li and Co from LiCoO2 via Hydrometallurgical–Electrodialytic Treatment. Appl. Sci. 2020, 10, 2367. [Google Scholar] [CrossRef]
- Diaz, L.A.; Strauss, M.L.; Adhikari, B.; Klaehn, J.R.; McNally, J.S.; Lister, T.E. Electrochemical-Assisted Leaching of Active Materials from Lithium Ion Batteries. Resour. Conserv. Recycl. 2020, 161, 104900. [Google Scholar] [CrossRef]
- Herrero-Gonzalez, M.; Diaz-Guridi, P.; Dominguez-Ramos, A.; Irabien, A.; Ibañez, R. Highly Concentrated HCl and NaOH from Brines Using Electrodialysis with Bipolar Membranes. Sep. Purif. Technol. 2020, 242, 116785. [Google Scholar] [CrossRef]
- Zouhri, N.; Habzize, S.A.; Amrani, M.E.; Taky, M.; Elmidaoui, A. Generation of Sulfuric Acid and Sodium Hydroxide from the Sodium Sulphate Salt by Electro-Electrodialysis (EED). Am. J. Appl. Chem. 2013, 1, 75–78. [Google Scholar] [CrossRef]
- Rongqiang, F.; Hoe, N. Electrodialysis Systems and Methods for Energy Generation and Waste Treatment. US Patent US20130288142A1, 28 October 2011. [Google Scholar]
- Blackburn, J.W. Electrodialysis Applications for Pollution Prevention in the Chemical Processing Industry. J. Air Waste Manag. Assoc. 1999, 49, 934–942. [Google Scholar] [CrossRef]
- Kang, B.; Kang, D.; Jung, J.C.-Y.J.; Asadi, A.; Sui, P.-C. Electrodialysis of a Lithium Sulphate Solution: An Experimental Investigation. J. Electrochem. Soc. 2022, 169, 063515. [Google Scholar] [CrossRef]
- Asadi, A.; Kang, D.; Bazargan Harandi, H.; Chung-Yen Jung, J.; Sui, P.C. Utilization of Lithium Sulphate Electrodialysis for Closed-Loop LIB Recycling: Experimental Study and Process Simulation. Sep. Purif. Technol. 2024, 343, 126989. [Google Scholar] [CrossRef]
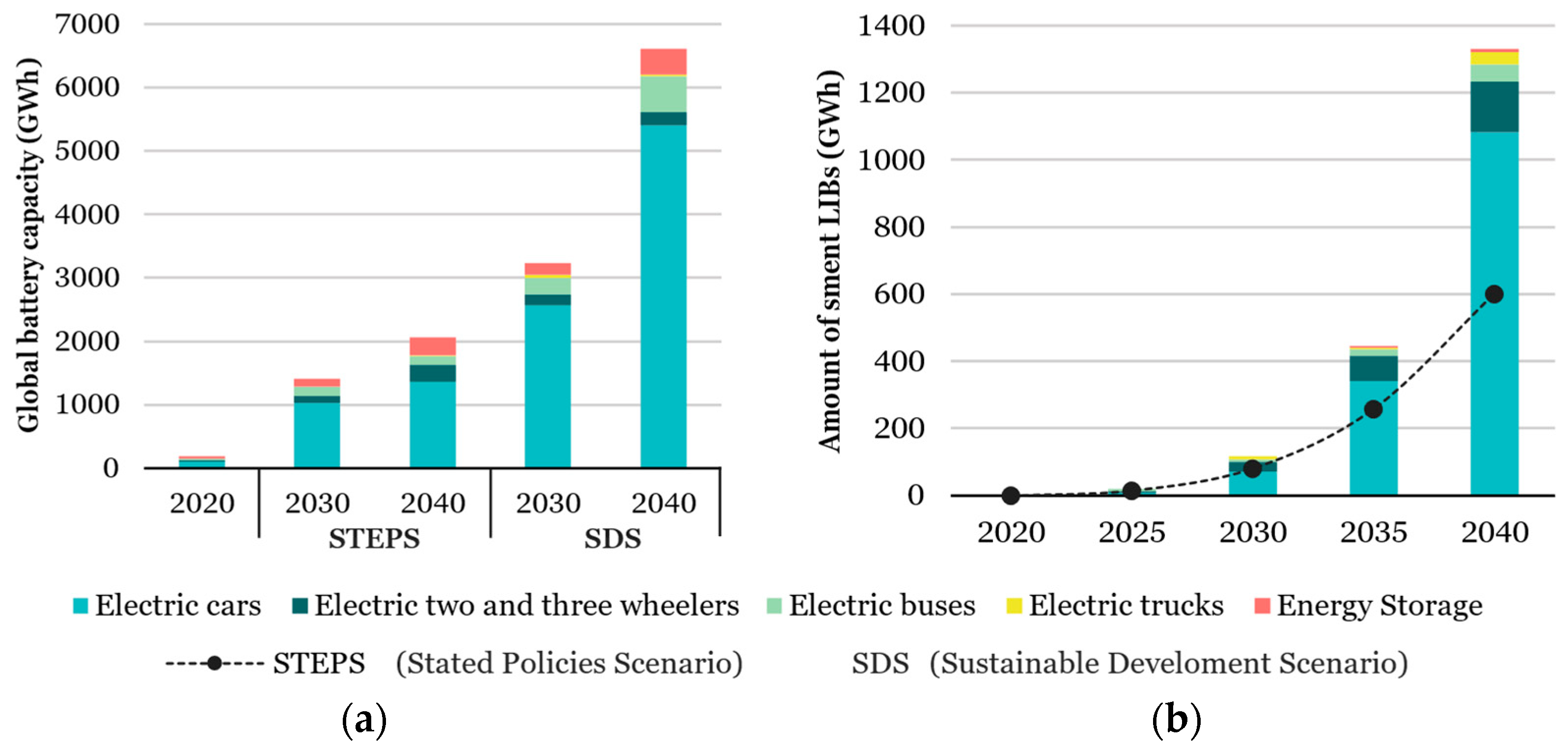




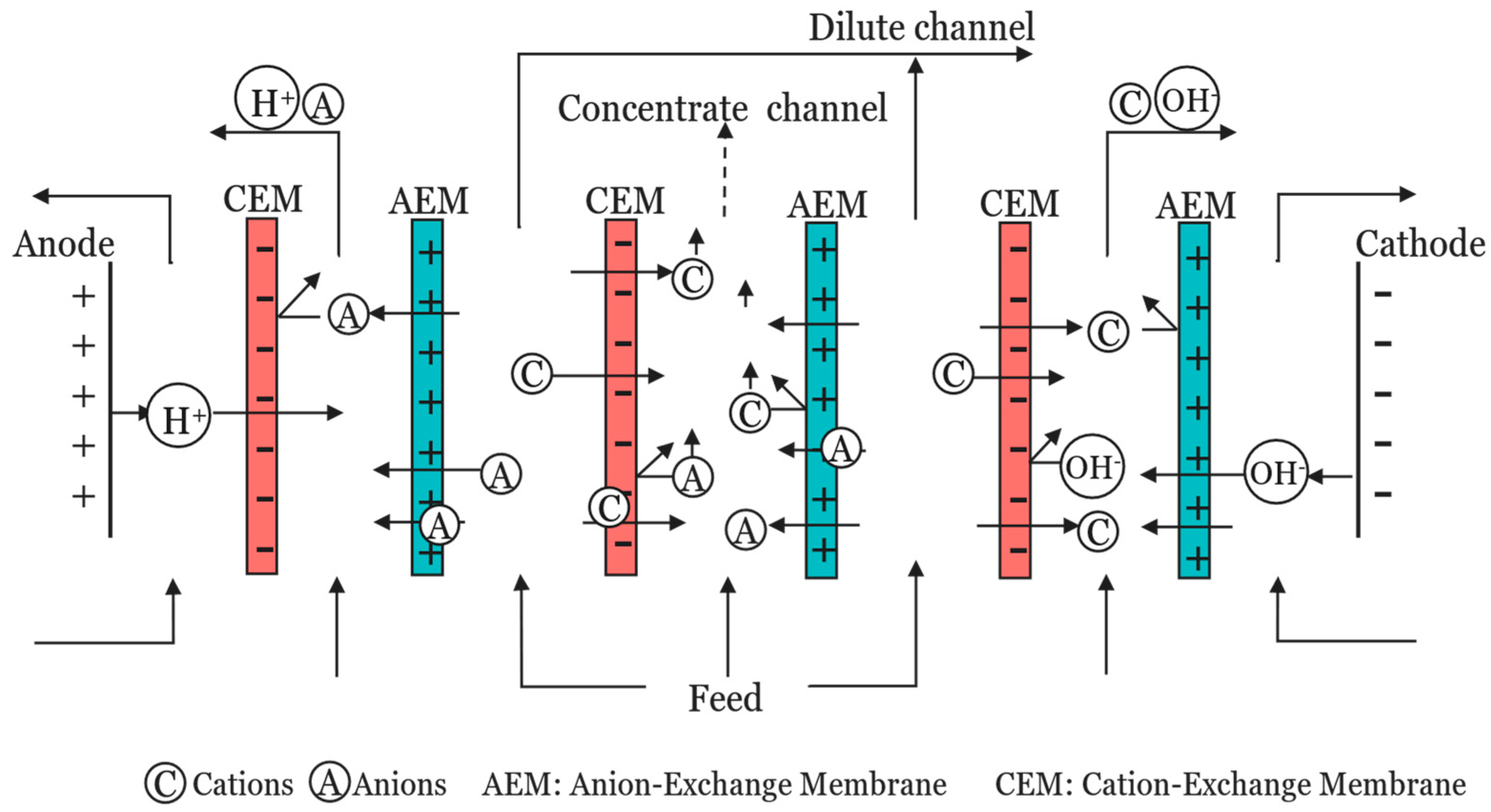
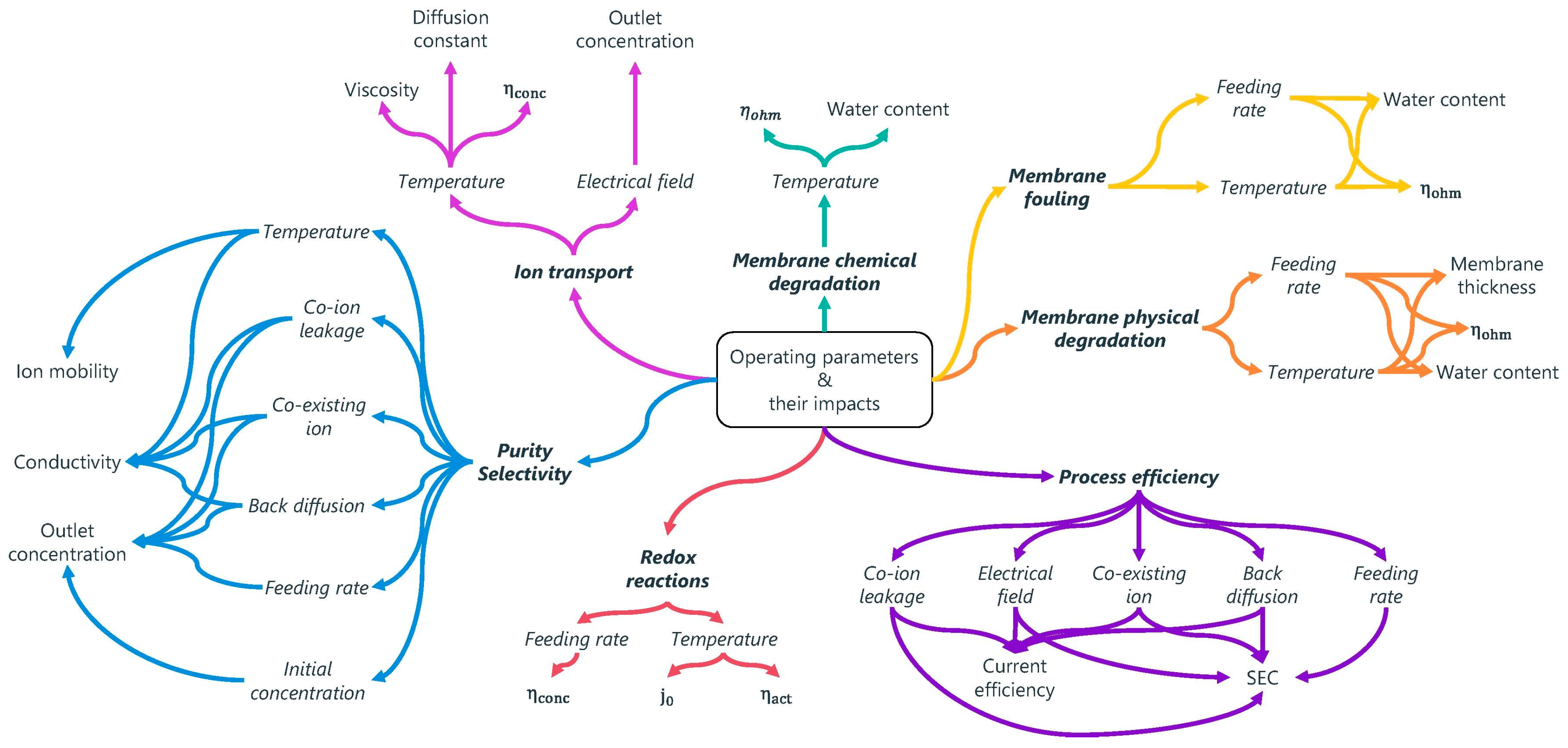
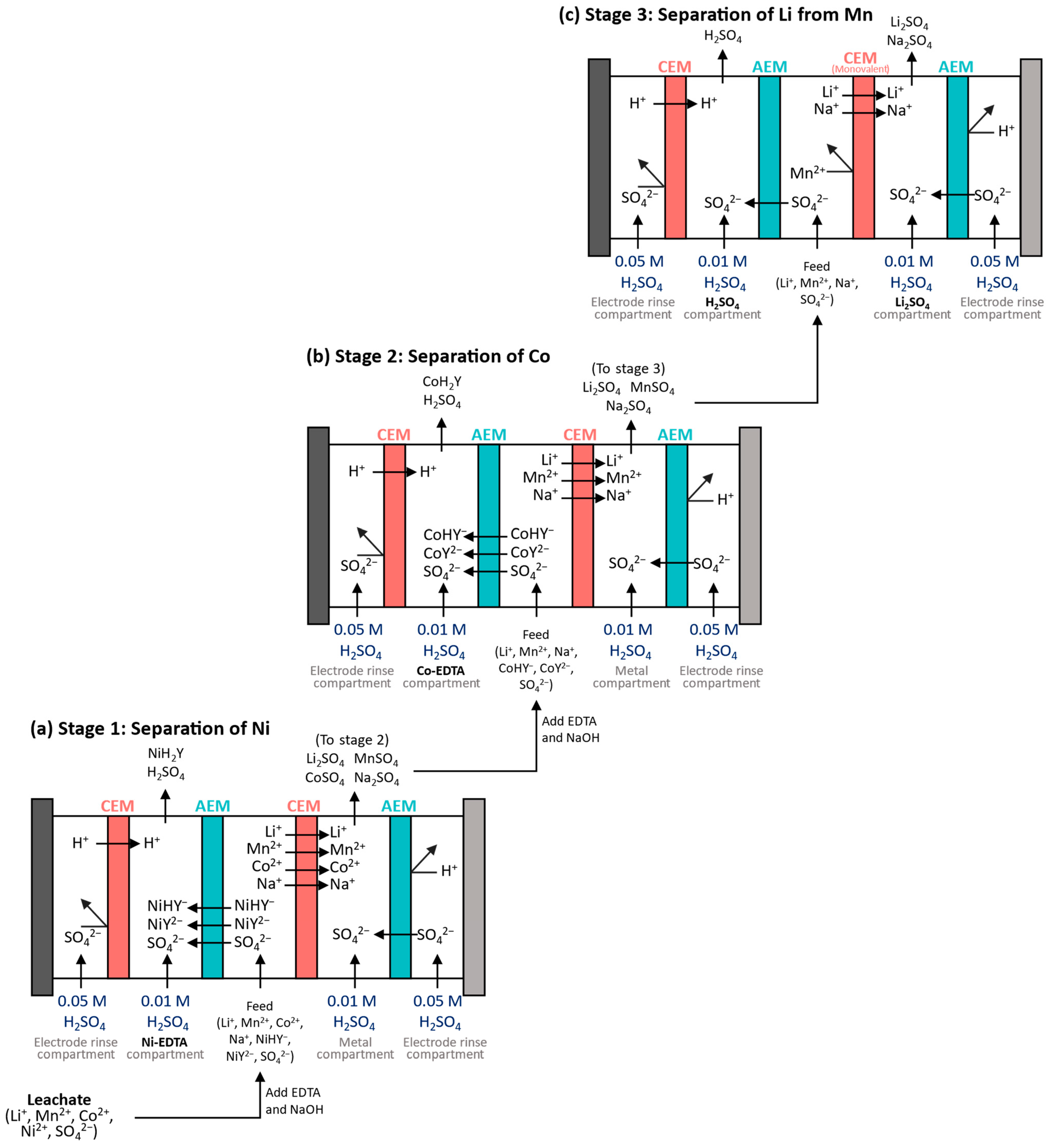


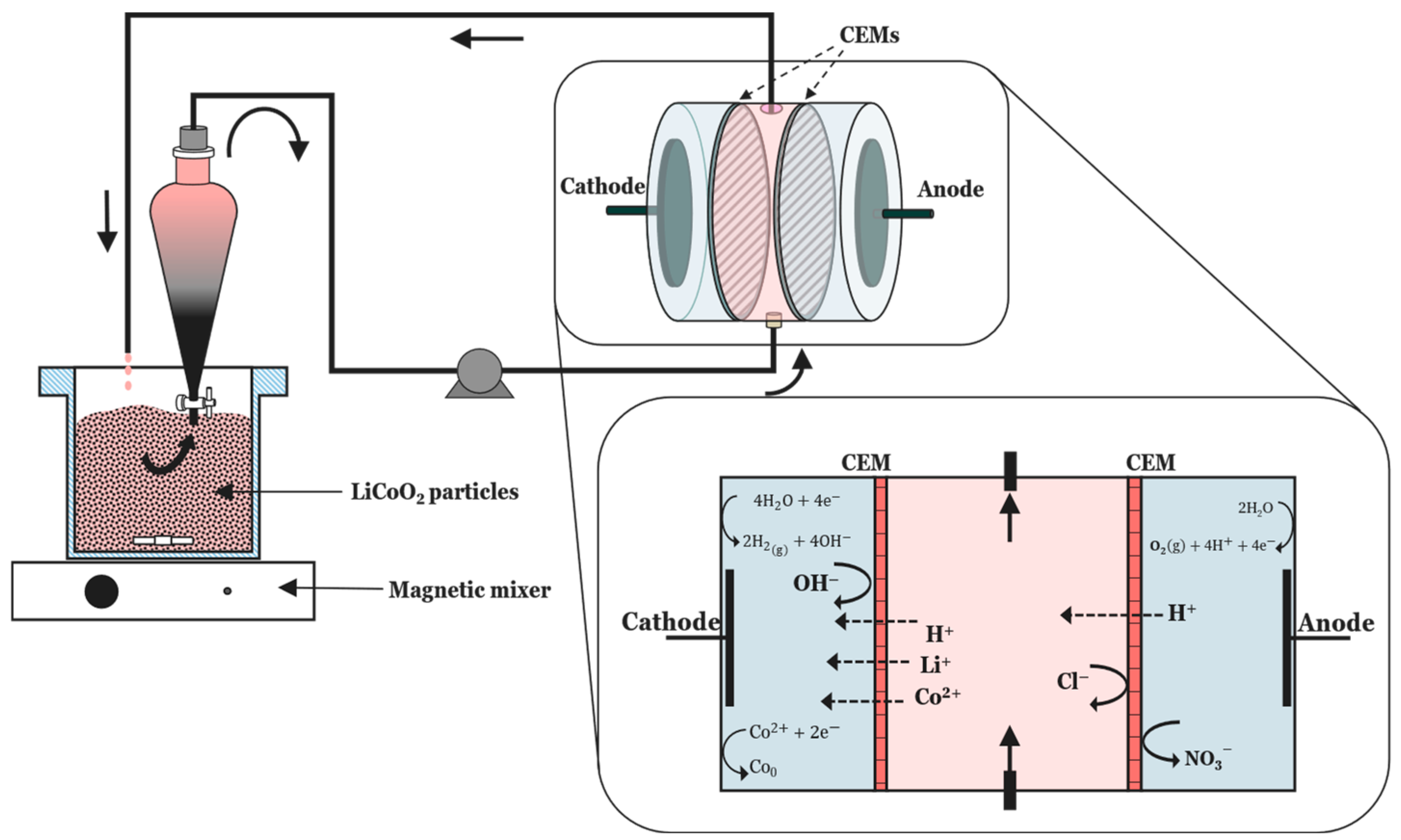
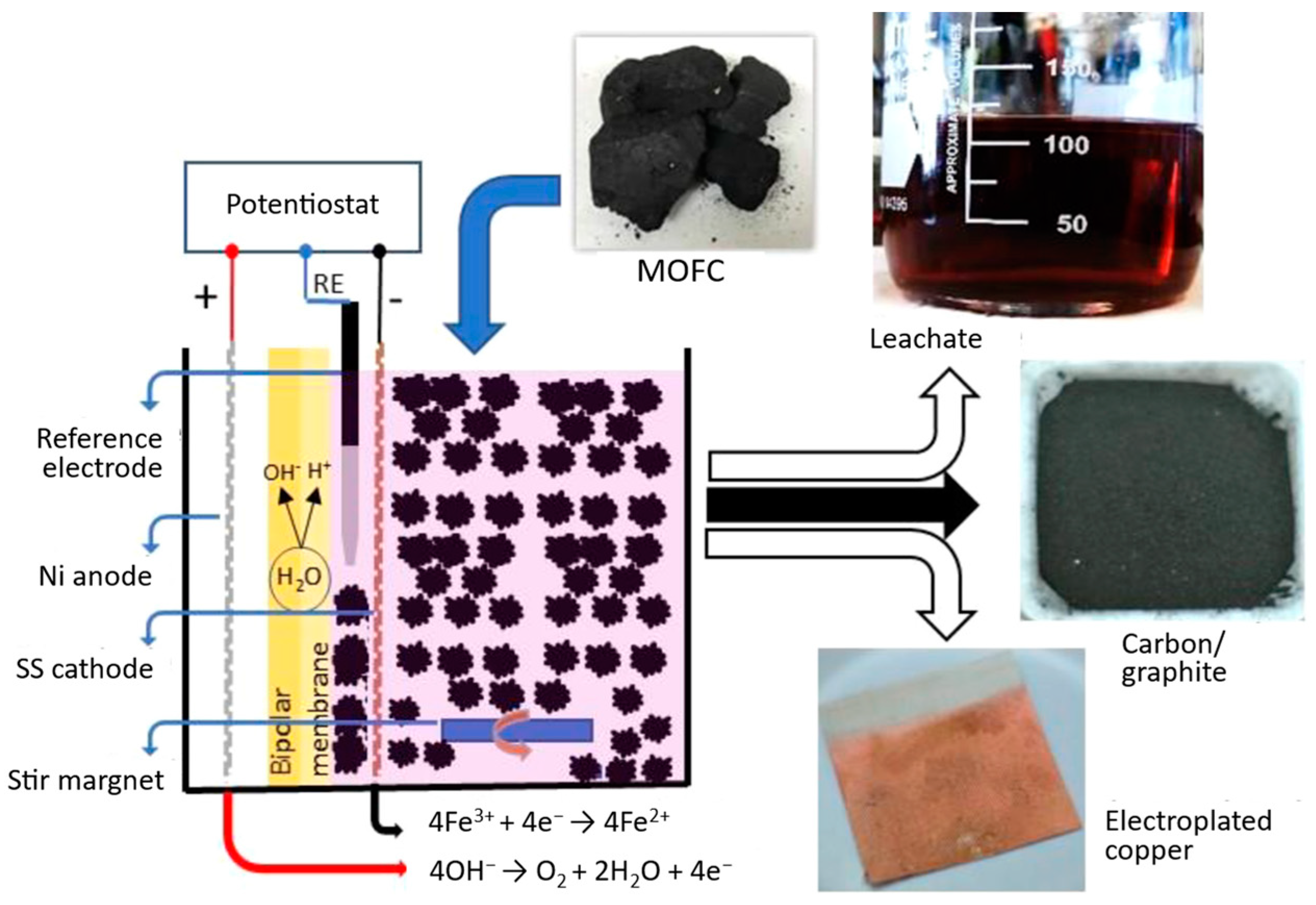
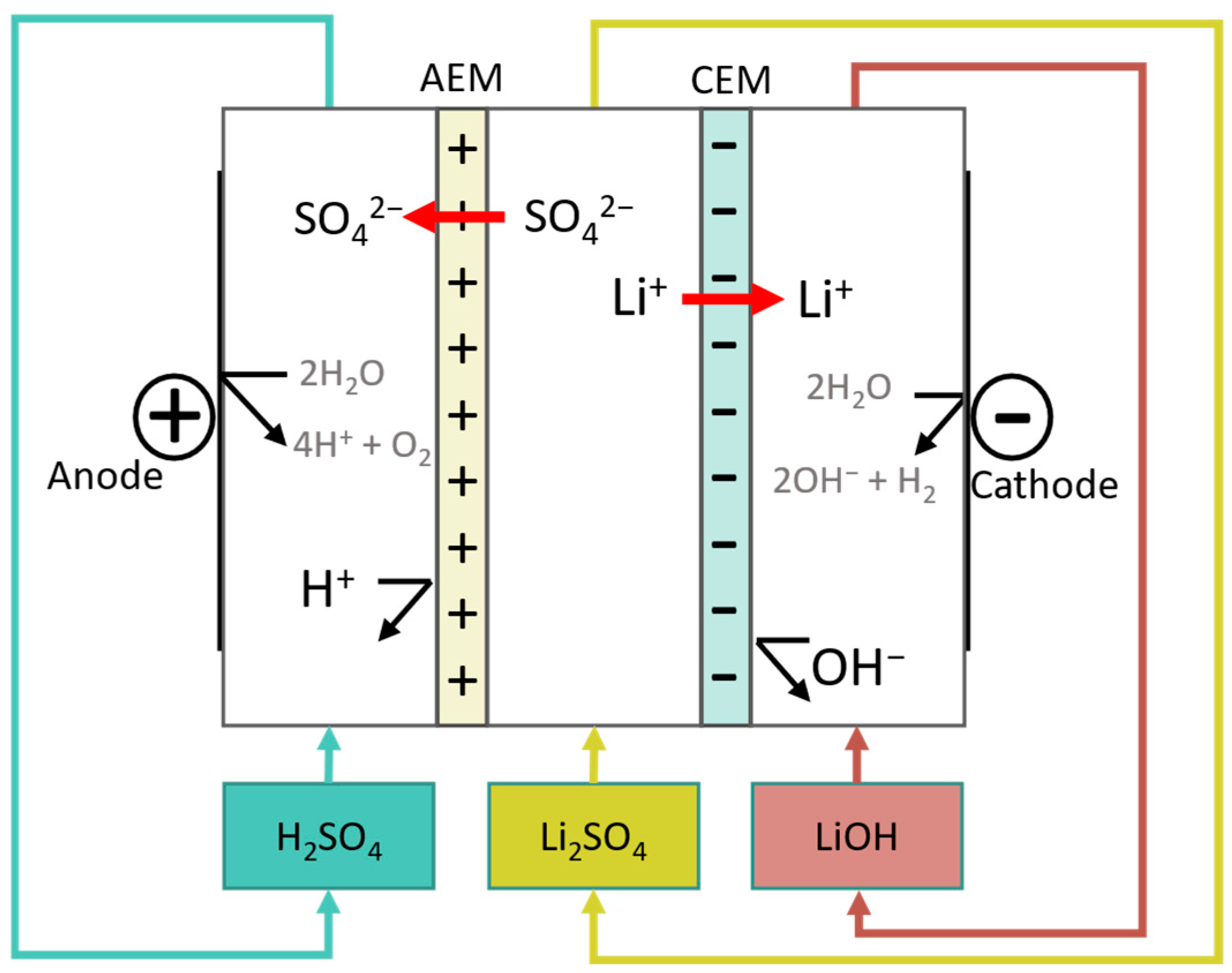

| Research Questions | |
|---|---|
| Q1 | What are the challenges of the manufacture and recycling process of LIBs? |
| Q2 | What are the current processes and technologies for recycling lithium-ion batteries? |
| Q3 | Can electrodialysis contribute to the concept of circular hydrometallurgy recycling? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerrillo-Gonzalez, M.d.M.; Villen-Guzman, M.; Vereda-Alonso, C.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Towards Sustainable Lithium-Ion Battery Recycling: Advancements in Circular Hydrometallurgy. Processes 2024, 12, 1485. https://doi.org/10.3390/pr12071485
Cerrillo-Gonzalez MdM, Villen-Guzman M, Vereda-Alonso C, Rodriguez-Maroto JM, Paz-Garcia JM. Towards Sustainable Lithium-Ion Battery Recycling: Advancements in Circular Hydrometallurgy. Processes. 2024; 12(7):1485. https://doi.org/10.3390/pr12071485
Chicago/Turabian StyleCerrillo-Gonzalez, Maria del Mar, Maria Villen-Guzman, Carlos Vereda-Alonso, Jose Miguel Rodriguez-Maroto, and Juan Manuel Paz-Garcia. 2024. "Towards Sustainable Lithium-Ion Battery Recycling: Advancements in Circular Hydrometallurgy" Processes 12, no. 7: 1485. https://doi.org/10.3390/pr12071485








