Decoding the Effect of Synthesis Factors on Morphology of Nanomaterials: A Case Study to Identify and Optimize Experimental Conditions for Silver Nanowires
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Overview of the Modified AgNW Synthesis
2.3. Quantitative Indicators for AgNW Synthesis
2.4. Materials Characterizations
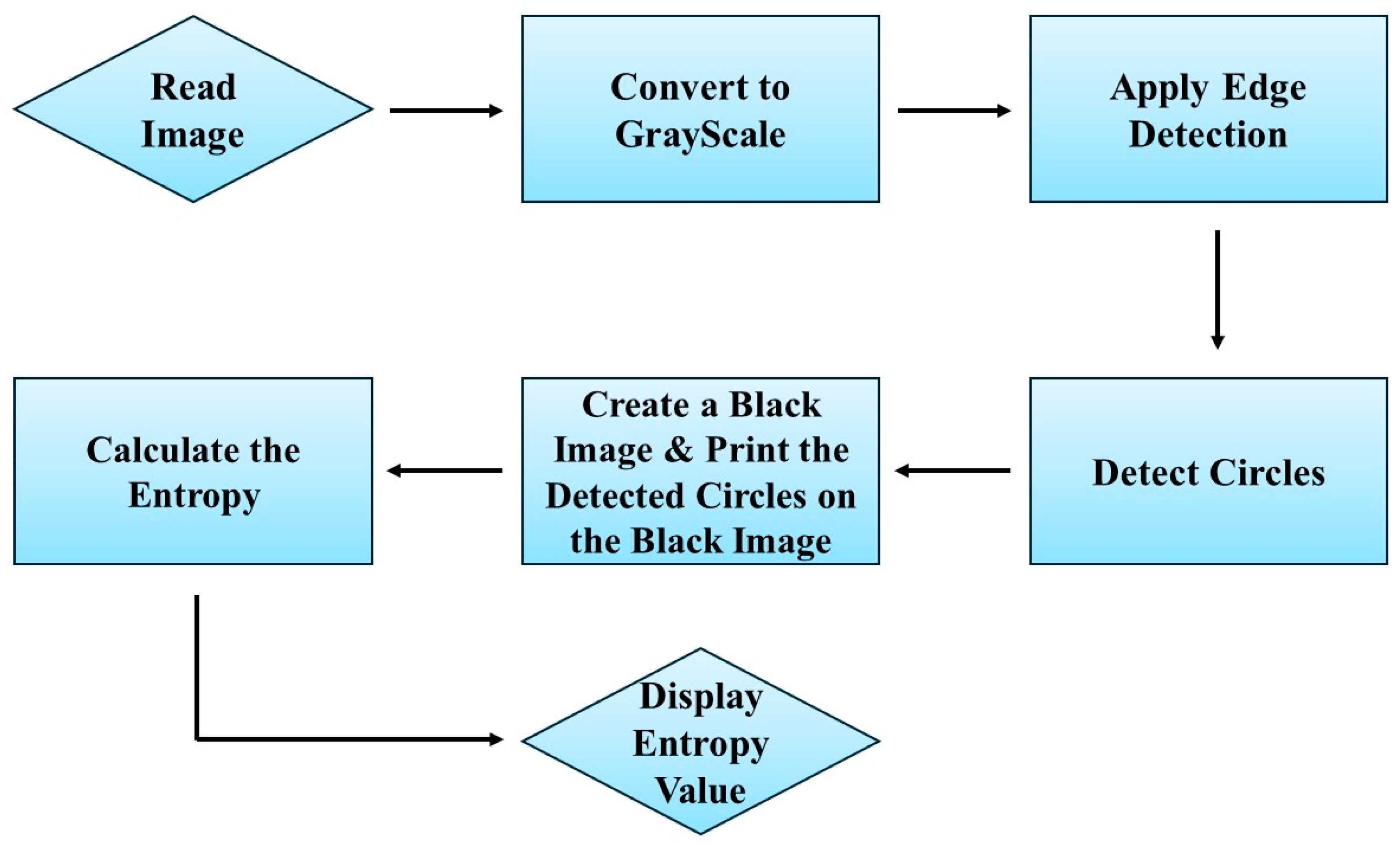
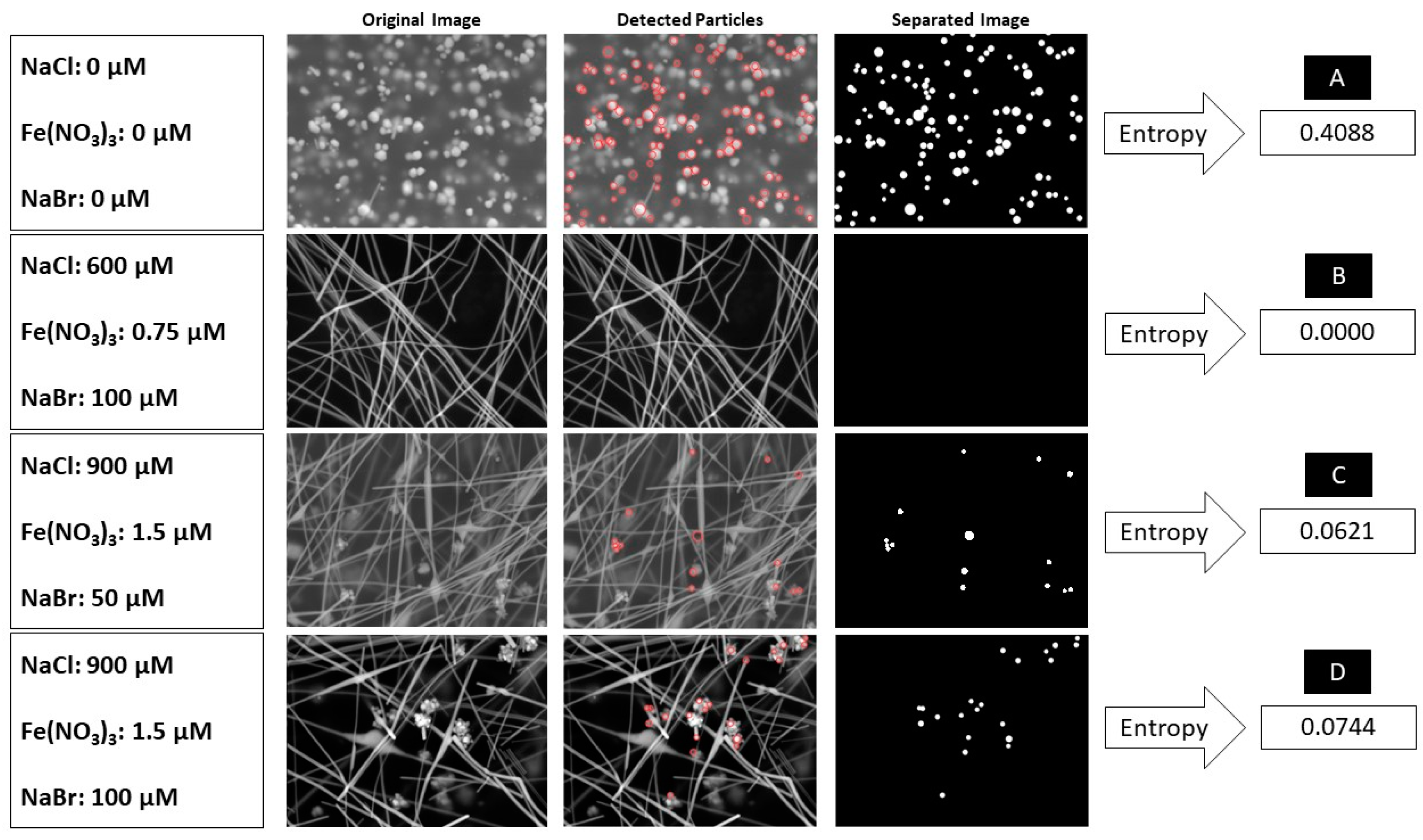
2.5. Entropy as an Indicator to Quantify Nanostructures
3. Results and Discussion
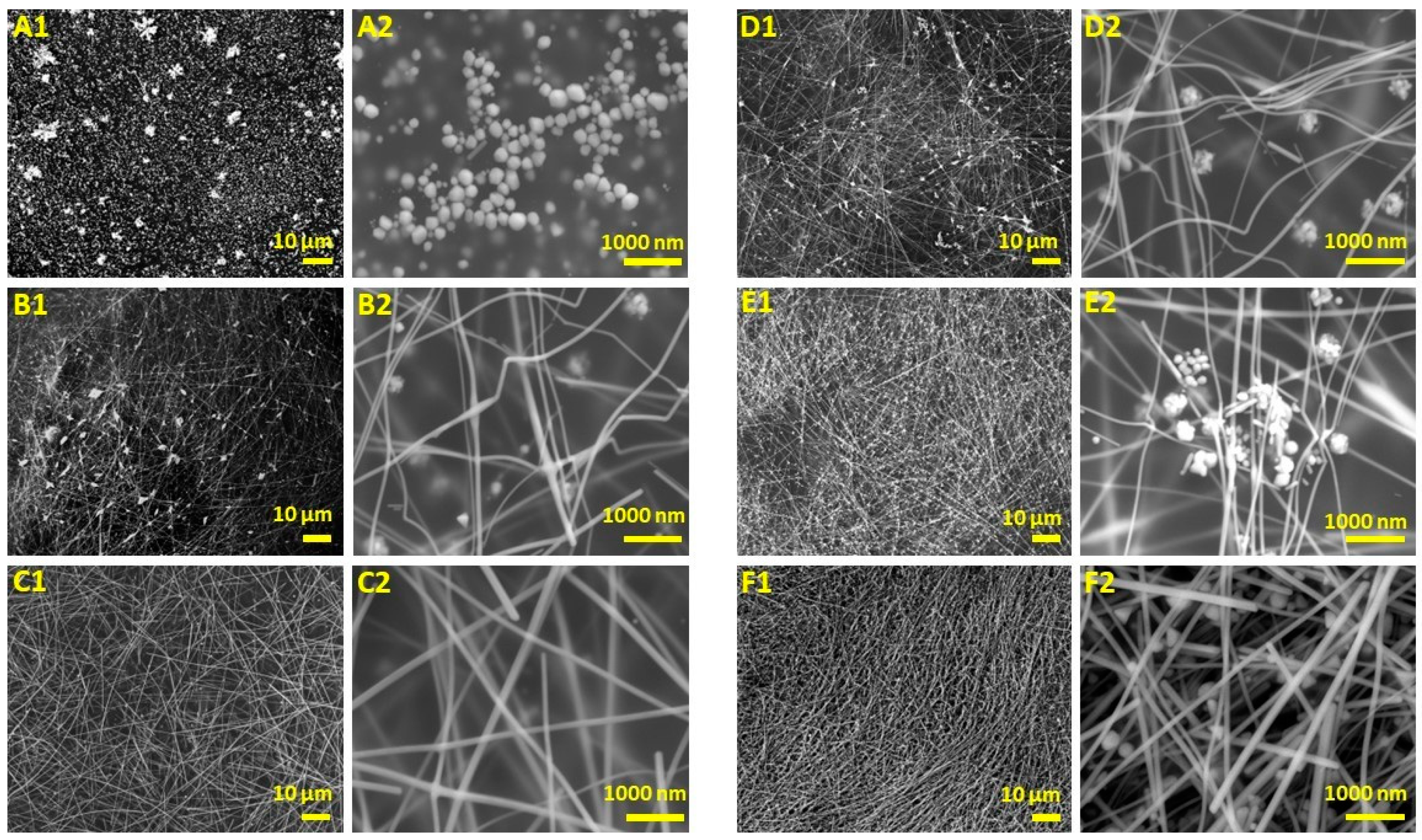
3.1. Effect of Nucleants on AgNW Synthesis
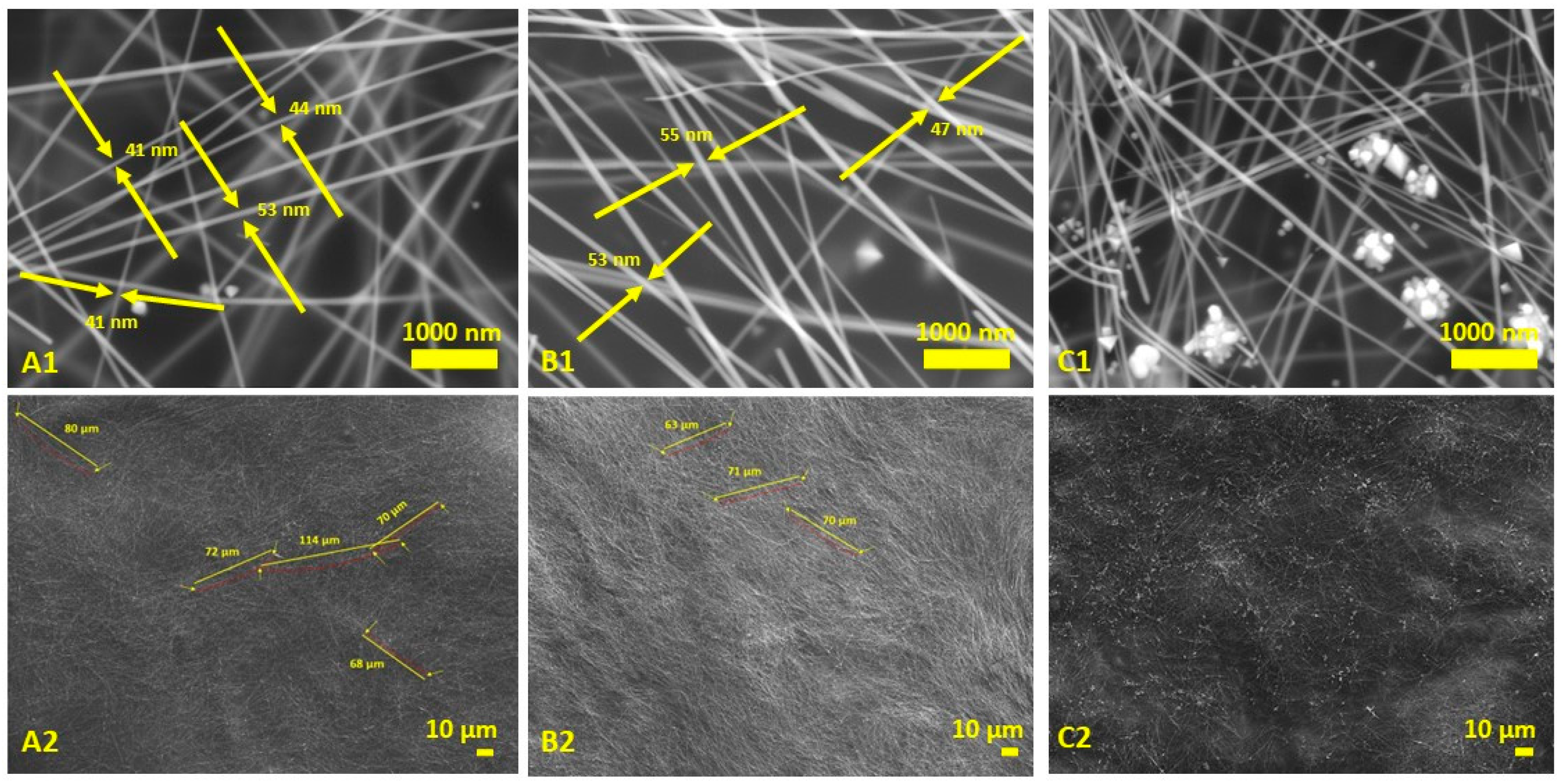
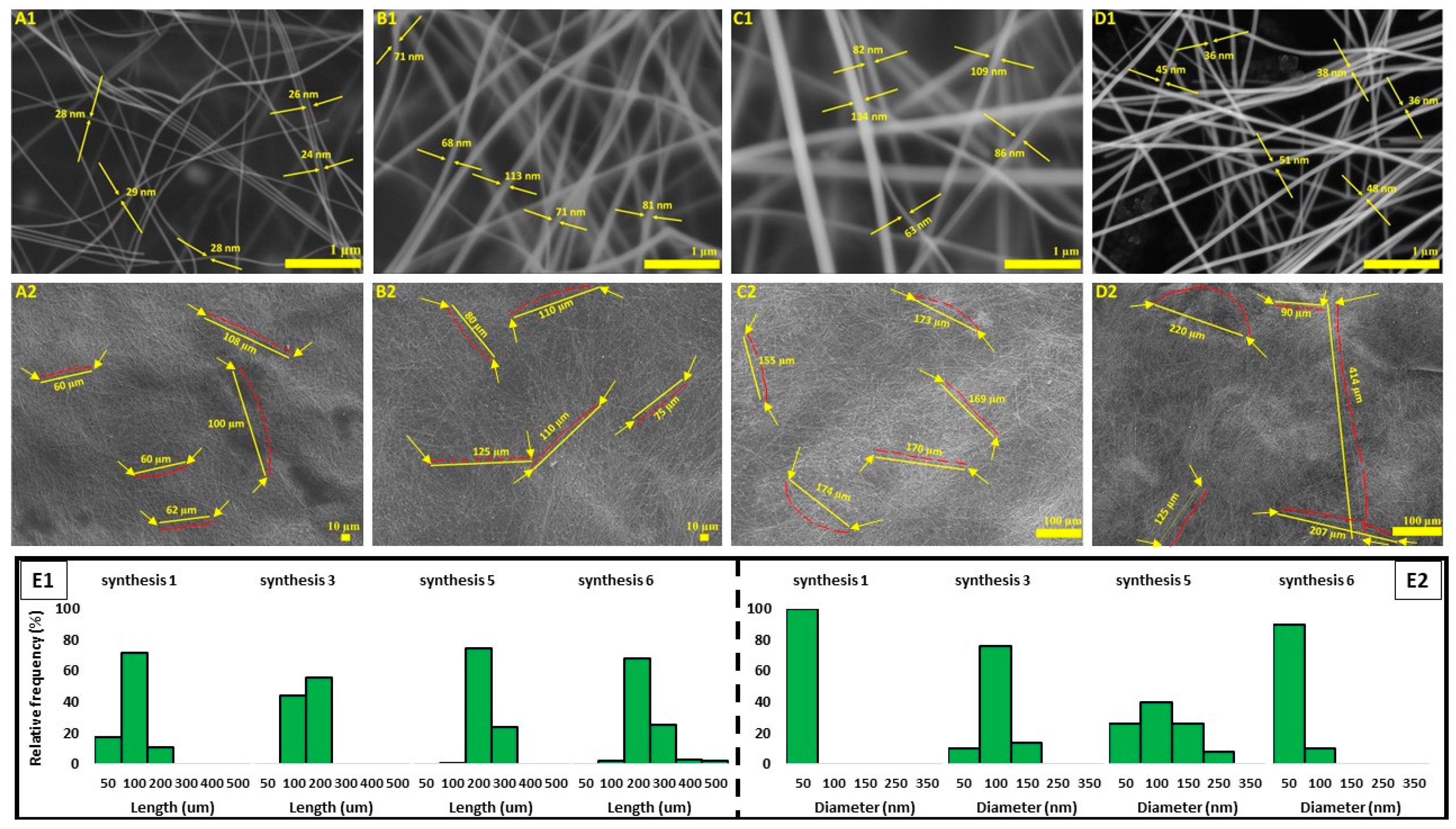
3.2. Entropy, Characterizations, and Analysis
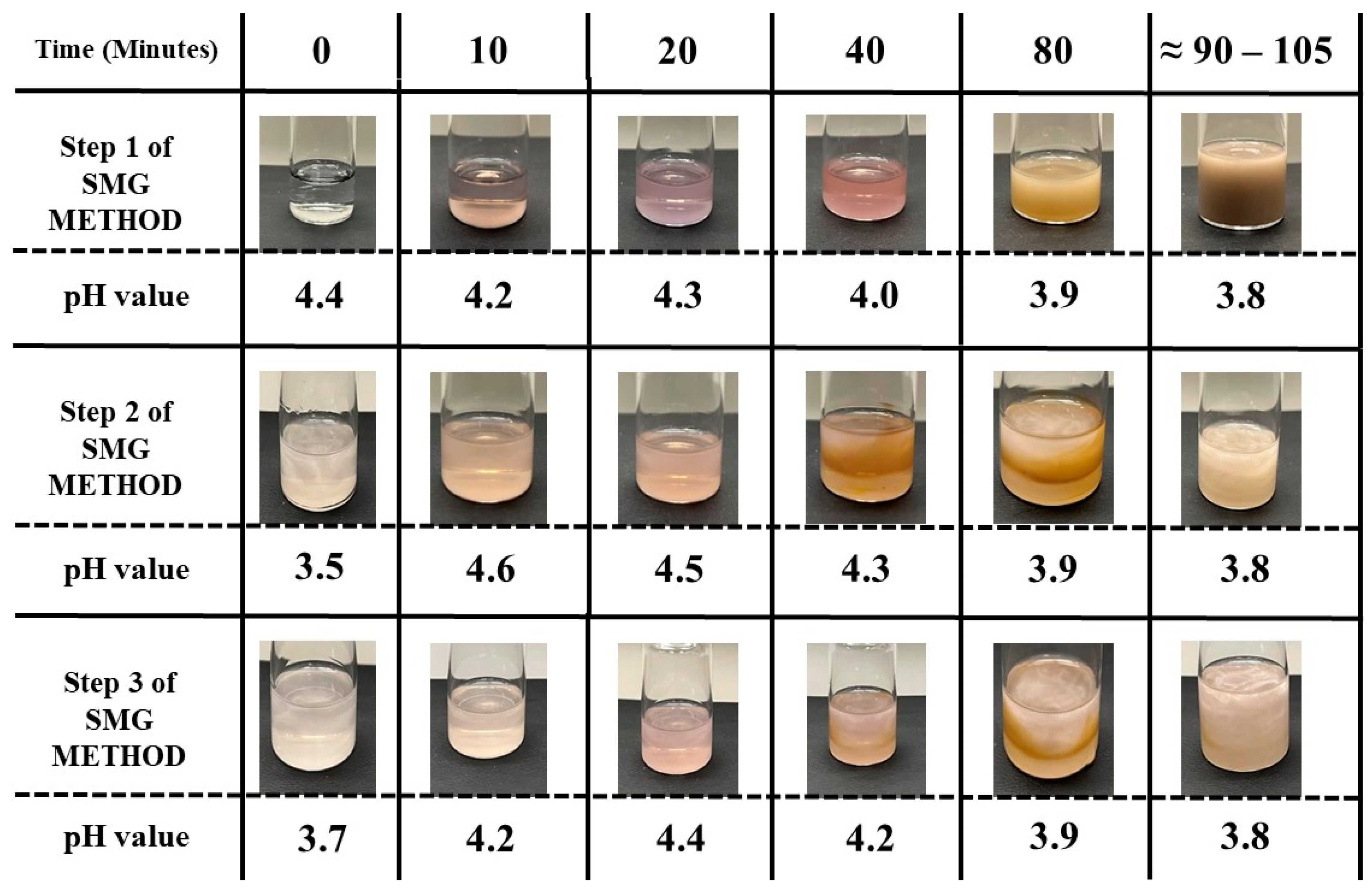
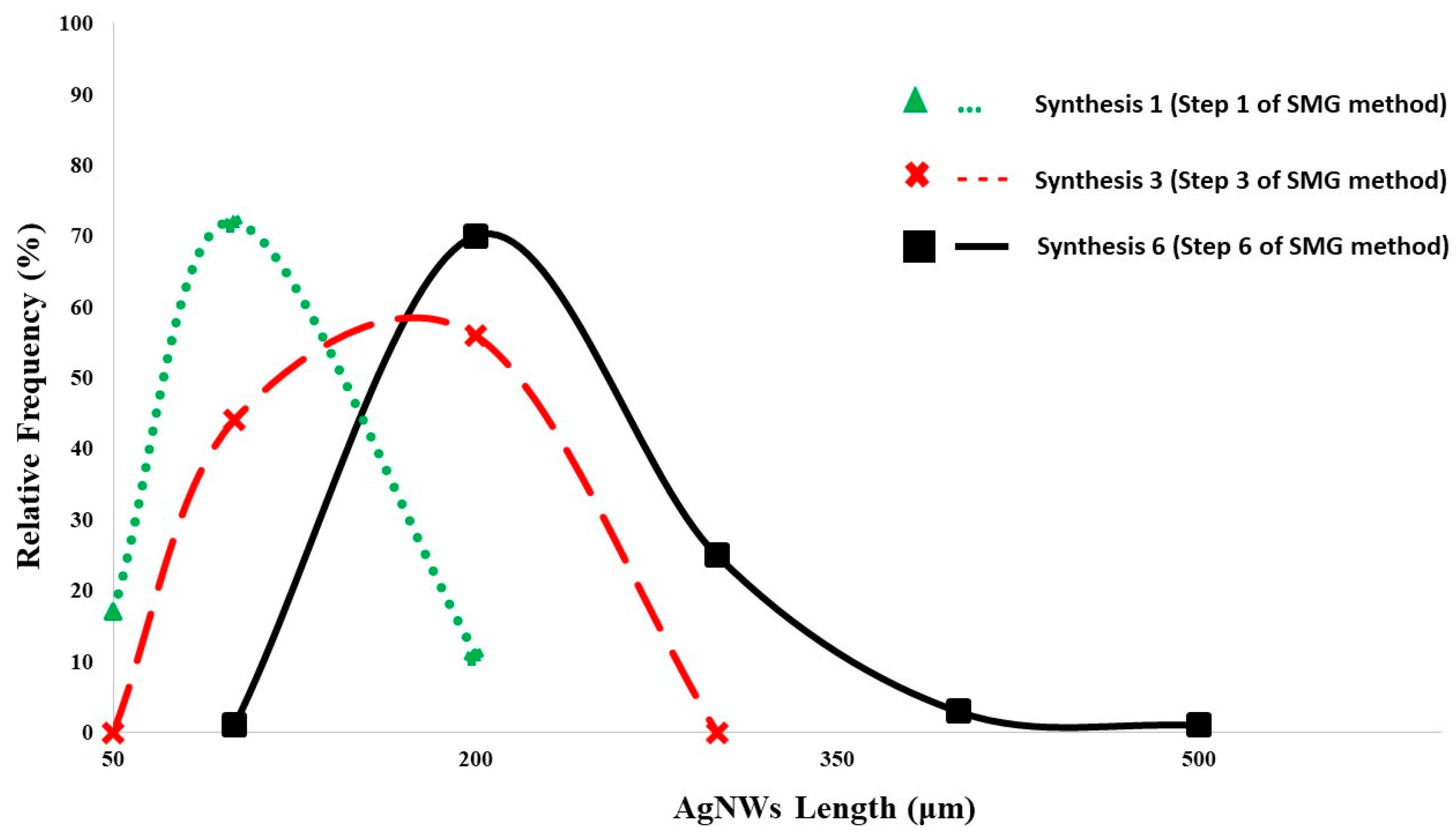
3.3. Successive Multistep Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Li, Y.; Guo, S.; Yang, H.; Chao, Y.; Jiang, S.; Wang, C. One-step synthesis of ultra-long silver nanowires of over 100 μm and their application in flexible transparent conductive films. RSC Adv. 2018, 8, 8057–8063. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Chen, L.; Sowade, E.; Rodriguez, R.D.; Sheremet, E.; Yu, C.M.; Baumann, R.B.; Chen, J.-J. Ultra-uniform and very thin ag nanowires synthesized via the synergy of cl−, br− and Fe3+ for transparent conductive films. Nanomaterials 2020, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, X.; Yang, H.; Chao, Y.; Guo, S.; Wang, C. One-step synthesis of silver nanowires with ultra-long length and thin diameter to make flexible transparent conductive films. Materials 2019, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Chung, C.-H.; Cha, K.C.; Yang, W.; Zheng, Y.B.; Zhou, H.; Song, T.-B.; Chen, C.-C.; Weiss, P.S.; Li, G.; et al. Fused silver nanowires with metal oxide nanoparticles and organic polymers for highly transparent conductors. ACS Nano 2011, 5, 9877–9882. [Google Scholar] [CrossRef]
- Chu, X.; Wang, K.; Tao, J.; Li, S.; Ji, S.; Ye, C. Tackling the stability issues of silver nanowire transparent conductive films through FeCl 3 dilute solution treatment. Nanomaterials 2019, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Su, X.; Luo, H.; Ade, A.; Zhu, H.; Zhang, Y.; Yu, L. Transparent conductive film of silver nanowires employed for SERS detection of mercury ions. Mater. Chem. Phys. 2023, 309, 128335. [Google Scholar] [CrossRef]
- Shi, R.; Lou, Z.; Chen, S.; Shen, G. Flexible and transparent capacitive pressure sensor with patterned microstructured composite rubber dielectric for wearable touch keyboard application. Sci. China Mater. 2018, 61, 1587–1595. [Google Scholar] [CrossRef]
- Sun, S.; Maimaitiyiming, X. Silver nanowire/polyacrylamide/gelatin flexible stress, strain and temperature sensor. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 131919. [Google Scholar] [CrossRef]
- Tu, S.; Ma, Y.; Shi, L.; Li, H.; Chen, M.; Wu, L. Aligned silver Nanowires/Polymer composite films for ultrasensitive and highly stretchable strain sensors. Chem. Eng. J. 2023, 473, 145075. [Google Scholar] [CrossRef]
- Koh, E.H.; Mun, C.; Kim, C.; Park, S.-G.; Choi, E.J.; Kim, S.H.; Dang, J.; Choo, J.; Oh, J.-W.; Kim, D.-H.; et al. M13 Bacteriophage/Silver Nanowire Surface-Enhanced Raman Scattering Sensor for Sensitive and Selective Pesticide Detection. ACS Appl. Mater. Interfaces 2018, 10, 10388–10397. [Google Scholar] [CrossRef]
- Wang, D.; Hua, H.; Liu, Y.; Tang, H.; Li, Y. Single Ag Nanowire Electrodes and Single Pt@Ag Nanowire Electrodes: Fabrication, Electrocatalysis, and Surface-Enhanced Raman Scattering Applications. Anal. Chem. 2019, 91, 4291–4295. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhao, T.; Jiang, W.; Yu, X.; Hu, Y.; Zhu, P.; Zheng, H.; Sun, R.; Wong, C.-P. Highly transparent triboelectric nanogenerator utilizing in-situ chemically welded silver nanowire network as electrode for mechanical energy harvesting and body motion monitoring. Nano Energy 2019, 59, 508–516. [Google Scholar] [CrossRef]
- Kim, S.-W.; Kwon, S.-N.; Na, S.-I. Stretchable and electrically conductive polyurethane- silver/graphene composite fibers prepared by wet-spinning process. Compos. B Eng. 2019, 167, 573–581. [Google Scholar] [CrossRef]
- Dudem, B.; Kim, D.H.; Bharat, L.K.; Yu, J.S. Highly-flexible piezoelectric nanogenerators with silver nanowires and barium titanate embedded composite films for mechanical energy harvesting. Appl. Energy 2018, 230, 865–874. [Google Scholar] [CrossRef]
- Milano, G.; Pedretti, G.; Montano, K.; Ricci, S.; Hashemkhani, S.; Boarino, L.; Ielmini, D.; Ricciardi, C. In materia reservoir computing with a fully memristive architecture based on self-organizing nanowire networks. Nat. Mater. 2022, 21, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Chopin, C.; de Wergifosse, S.; Marchal, N.; Van Velthem, P.; Piraux, L.; Araujo, F.A. Memristive and Tunneling Effects in 3D Interconnected Silver Nanowires. ACS Omega 2023, 8, 6663–6668. [Google Scholar] [CrossRef] [PubMed]
- Crêpellière, J.; Menguelti, K.; Wack, S.; Bouton, O.; Gérard, M.; Popa, P.L.; Pistillo, B.R.; Leturcq, R.; Michel, M. Spray Deposition of Silver Nanowires on Large Area Substrates for Transparent Electrodes. ACS Appl. Nano Mater. 2021, 4, 1126–1135. [Google Scholar] [CrossRef]
- van Berkel, S.; Klitzke, J.S.; Moradi, M.-A.; Hendrix, M.M.; Schmit, P.; van der Schoot, P.; Schrekker, H.S. Spin-coated highly aligned silver nanowire networks in conductive latex-based thin layer films. Thin Solid Films 2021, 724, 138599. [Google Scholar] [CrossRef]
- Sachse, C.; Müller-Meskamp, L.; Bormann, L.; Kim, Y.H.; Lehnert, F.; Philipp, A.; Beyer, B.; Leo, K. Transparent, dip-coated silver nanowire electrodes for small molecule organic solar cells. Org. Electron. 2012, 14, 143–148. [Google Scholar] [CrossRef]
- Karimi-Chaleshtori, R.; Nassajpour-Esfahani, A.; Saeri, M.; Rezai, P.; Doostmohammadi, A. Silver nanowire-embedded PDMS with high electrical conductivity: Nanowires synthesis, composite processing and electrical analysis. Mater. Today Chem. 2021, 21, 100496. [Google Scholar] [CrossRef]
- Giasafaki, D.; Mitzithra, C.; Belessi, V.; Filippakopoulou, T.; Koutsioukis, A.; Georgakilas, V.; Charalambopoulou, G.; Steriotis, T. Graphene-Based Composites with Silver Nanowires for Electronic Applications. Nanomaterials 2022, 12, 3443. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.; Kim, F.; Hess, C.; Goldberger, J.; He, R.; Sun, Y.; Xia, Y.; Yang, P. Langmuir-Blodgett silver nanowire monolayers for molecular sensing using surface-enhanced Raman spectroscopy. Nano Lett. 2003, 3, 1229–1233. [Google Scholar] [CrossRef]
- Lagrange, M.; Langley, D.P.; Giusti, G.; Jiménez, C.; Bréchet, Y.; Bellet, D. Optimization of silver nanowire-based transparent electrodes: Effects of density, size and thermal annealing. Nanoscale 2015, 7, 17410–17423. [Google Scholar] [CrossRef] [PubMed]
- Bergin, S.M.; Chen, Y.-H.; Rathmell, A.R.; Charbonneau, P.; Li, Z.-Y.; Wiley, B.J. The effect of nanowire length and diameter on the properties of transparent, conducting nanowire films. Nanoscale 2012, 4, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Shaikh, M.O.; Chuang, C.-H. Silver nanowire synthesis and strategies for fabricating transparent conducting electrodes. Nanomaterials 2021, 11, 693. [Google Scholar] [CrossRef]
- Ha, H.; Amicucci, C.; Matteini, P.; Hwang, B. Mini review of synthesis strategies of silver nanowires and their applications. Colloid Interface Sci. Commun. 2022, 50, 100663. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, B.; Zhang, H.; Wan, P.; Luo, L.; Kuang, Y.; Sun, X. Probing the seeded protocol for high-concentration preparation of silver nanowires. Nano Res. 2016, 9, 1532–1542. [Google Scholar] [CrossRef]
- Zhang, P.; Wyman, I.; Hu, J.; Lin, S.; Zhong, Z.; Tu, Y.; Huang, Z.; Wei, Y. Silver nanowires: Synthesis technologies, growth mechanism and multifunctional applications. Mater. Sci. Eng. B 2017, 223, 1–23. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, X.; Yang, H.; Chao, Y.; Guo, S.; Wang, C. Solvothermal synthesis of ultra-fine silver nanowires with a diameter about 20 nm and an aspect ratio approximately 2000 for highly conductive flexible transparent film. J. Mater. Sci. Mater. Electron. 2019, 30, 8883–8891. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, X.; Qiu, X.; Chen, J.; Jiang, R. Convenient synthesis of silver nanowires with adjustable diameters via a solvothermal method. J. Colloid Interface Sci. 2010, 344, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, P.; Lee, H.; Lee, D.; Lee, S.S.; Ko, S.H. Very long Ag nanowire synthesis and its application in a highly transparent, conductive and flexible metal electrode touch panel. Nanoscale 2012, 4, 6408–6414. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.J.; Xiong, Y.; Li, Z.-Y.; Yin, Y.; Xia, Y. Right bipyramids of silver: A new shape derived from single twinned seeds. Nano Lett. 2006, 6, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Steeg, E.; Kirmse, H.; Rabe, J.; Kirstein, S. Silver iodide nanowires grown within tubular J-aggregates. J. Colloid Interface Sci. 2018, 530, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Yang, H.J.; Bin Kim, S.; Lee, M.S. Mechanism of growth of colloidal silver nanoparticles stabilized by polyvinyl pyrrolidone in γ-irradiated silver nitrate solution. J. Colloid Interface Sci. 2004, 274, 89–94. [Google Scholar] [CrossRef]
- Whitcomb, D.R.; Clapp, A.R.; Bühlmann, P.; Blinn, J.C.; Zhang, J. New Perspectives on Silver Nanowire Formation from Dynamic Silver Ion Concentration Monitoring and Nitric Oxide Production in the Polyol Process. Cryst. Growth Des. 2016, 16, 1861–1868. [Google Scholar] [CrossRef]
- Coskun, S.; Aksoy, B.; Unalan, H.E. Polyol synthesis of silver nanowires: An extensive parametric study. Cryst. Growth Des. 2011, 11, 4963–4969. [Google Scholar] [CrossRef]
- Ma, J.; Zhan, M. Rapid production of silver nanowires based on high concentration of AgNO3 precursor and use of FeCl3 as reaction promoter. RSC Adv. 2014, 4, 21060–21071. [Google Scholar] [CrossRef]
- Schuette, W.M.; Buhro, W.E. Silver chloride as a heterogeneous nucleant for the growth of silver nanowires. ACS Nano 2013, 7, 3844–3853. [Google Scholar] [CrossRef]
- Wu, K.-J.; Tse, E.C.; Shang, C.; Guo, Z. Nucleation and growth in solution synthesis of nanostructures—From fundamentals to advanced applications. Prog. Mater. Sci. 2022, 123, 100821. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Hsueh, Y.-L.; Huang, J.-J.; Wu, J.-R. Effect of silver nitrate concentration of silver nanowires synthesized using a polyol method and their application as transparent conductive films. Thin Solid Films 2015, 584, 243–247. [Google Scholar] [CrossRef]


| DOE No. | NaCl (µM) | Fe(NO3)3 (µM) | NaBr (µM) | Length (µm) | Diameter (nm) | Aspect Ratio | Entropy | Entropy (STD) |
|---|---|---|---|---|---|---|---|---|
| 1 | 300 | 0.75 | 50 | 40 | 36 | 1100 | 0.0898 | 0.0127 |
| 2 | 300 | 0.75 | 100 | 32 | 39 | 800 | 0.0585 | 0.0104 |
| 3 | 300 | 0.75 | 200 | 19 | 22 | 900 | 0.0843 | 0.0137 |
| 4 | 300 | 1.5 | 50 | 45 | 44 | 1000 | 0.0081 | 0.0060 |
| 5 | 300 | 1.5 | 100 | 40 | 31 | 1300 | 0.0695 | 0.0088 |
| 6 | 300 | 1.5 | 200 | 26 | 26 | 1000 | 0.0968 | 0.0003 |
| 7 | 300 | 2.5 | 50 | 25 | 37 | 700 | 0.0581 | 0.0133 |
| 8 | 300 | 2.5 | 100 | 36 | 30 | 1200 | 0.0551 | 0.0123 |
| 9 | 300 | 2.5 | 200 | 18 | 24 | 750 | 0.0901 | 0.0176 |
| 10 | 600 | 0.75 | 50 | 69 | 49 | 1400 | 0.0119 | 0.0043 |
| 11 | 600 | 0.75 | 100 | 68 | 29 | 2400 | 0.0075 | 0.0062 |
| 12 | 600 | 0.75 | 200 | 44 | 29 | 1500 | 0.0141 | 0.0067 |
| 13 | 600 | 1.5 | 50 | 37 | 44 | 850 | 0.0878 | 0.0243 |
| 14 | 600 | 1.5 | 100 | 58 | 42 | 1400 | 0.0077 | 0.0031 |
| 15 | 600 | 1.5 | 200 | 38 | 36 | 1000 | 0.0619 | 0.0236 |
| 16 | 600 | 2.5 | 50 | 41 | 47 | 900 | 0.0151 | 0.0060 |
| 17 | 600 | 2.5 | 100 | 50 | 46 | 1100 | 0.0581 | 0.0072 |
| 18 | 600 | 2.5 | 200 | 41 | 37 | 1100 | 0.0634 | 0.0178 |
| 19 | 900 | 0.75 | 50 | 27 | 49 | 550 | 0.0729 | 0.0153 |
| 20 | 900 | 0.75 | 100 | 46 | 42 | 1100 | 0.0525 | 0.0083 |
| 21 | 900 | 0.75 | 200 | 46 | 40 | 1150 | 0.1164 | 0.0168 |
| 22 | 900 | 1.5 | 50 | 33 | 43 | 800 | 0.0629 | 0.0122 |
| 23 | 900 | 1.5 | 100 | 39 | 43 | 900 | 0.0781 | 0.0223 |
| 24 | 900 | 1.5 | 200 | 48 | 36 | 1300 | 0.1046 | 0.0088 |
| 25 | 900 | 2.5 | 50 | 32 | 49 | 650 | 0.0798 | 0.0168 |
| 26 | 900 | 2.5 | 100 | 30 | 35 | 850 | 0.0692 | 0.0071 |
| 27 | 900 | 2.5 | 200 | 37 | 38 | 1000 | 0.0837 | 0.0091 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najjari, A.; Namisnak, M.; McCormick, M.; Du, D.; Du, Y. Decoding the Effect of Synthesis Factors on Morphology of Nanomaterials: A Case Study to Identify and Optimize Experimental Conditions for Silver Nanowires. Processes 2024, 12, 1487. https://doi.org/10.3390/pr12071487
Najjari A, Namisnak M, McCormick M, Du D, Du Y. Decoding the Effect of Synthesis Factors on Morphology of Nanomaterials: A Case Study to Identify and Optimize Experimental Conditions for Silver Nanowires. Processes. 2024; 12(7):1487. https://doi.org/10.3390/pr12071487
Chicago/Turabian StyleNajjari, Aryan, Mary Namisnak, Massimo McCormick, Dongping Du, and Yuncheng Du. 2024. "Decoding the Effect of Synthesis Factors on Morphology of Nanomaterials: A Case Study to Identify and Optimize Experimental Conditions for Silver Nanowires" Processes 12, no. 7: 1487. https://doi.org/10.3390/pr12071487






