A Review of the Utilization of CO2 as a Cushion Gas in Underground Natural Gas Storage
Abstract
:1. Introduction
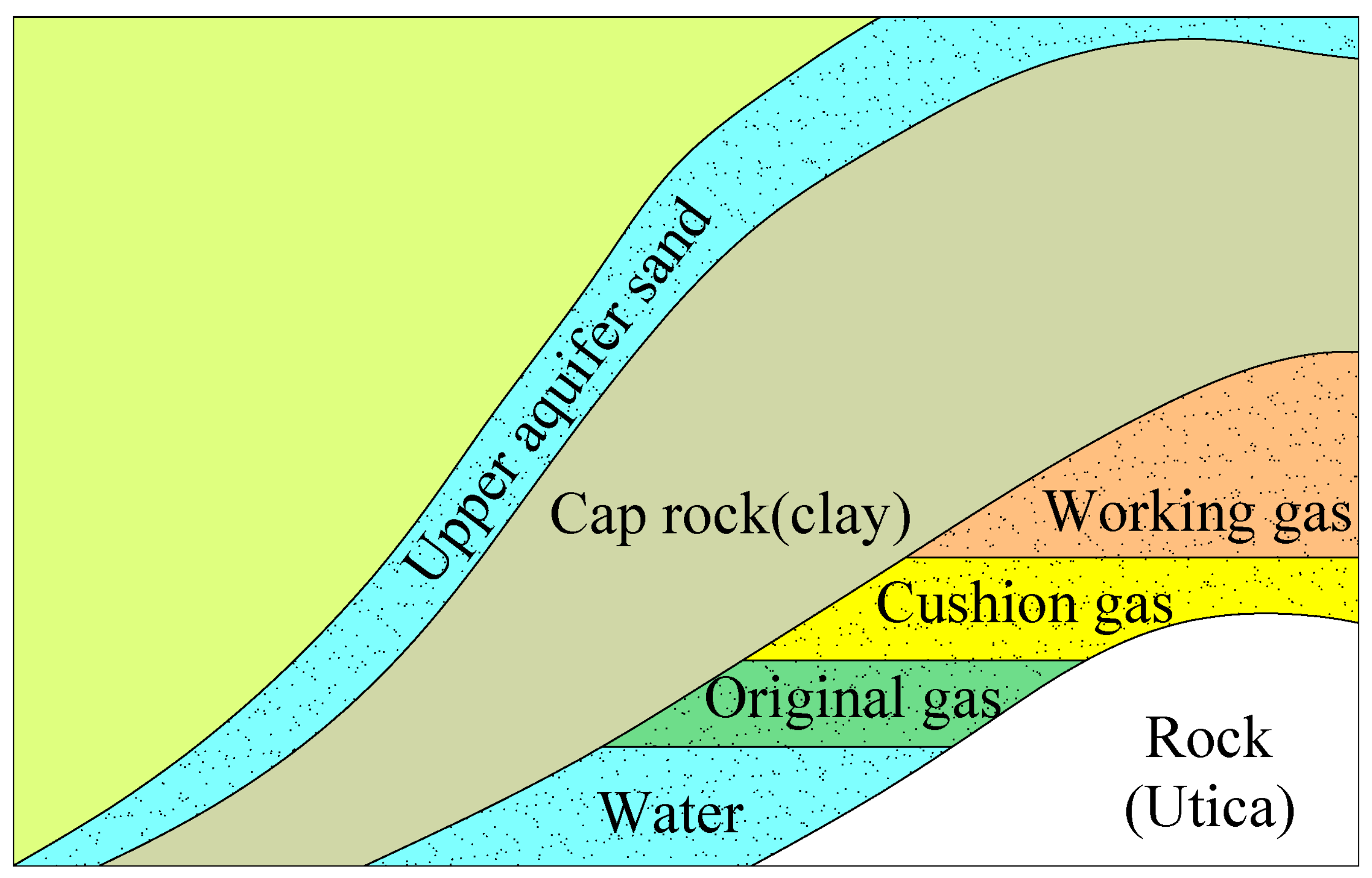
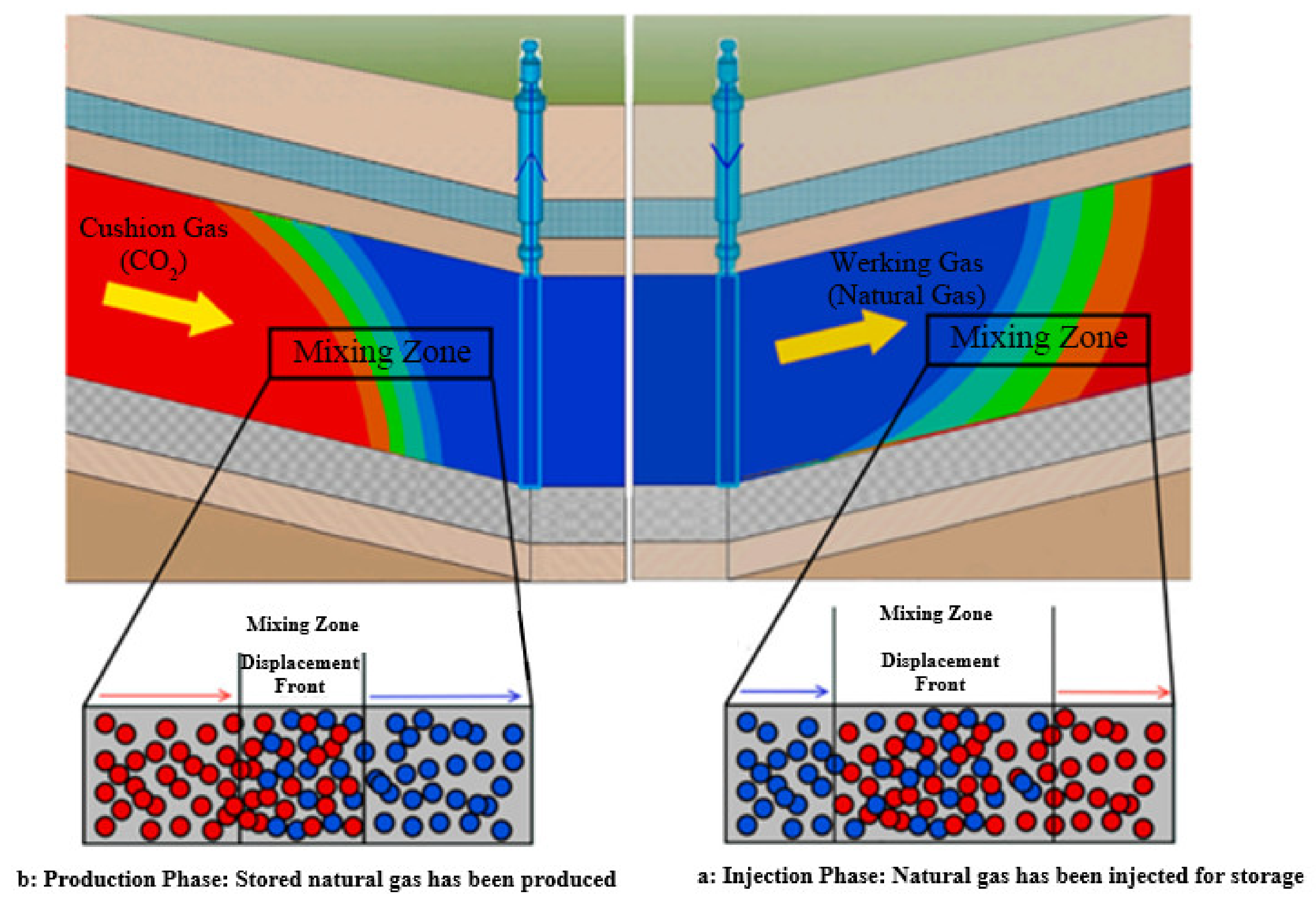
2. The Mechanism of CO2 as a Cushion Gas in Underground Natural Gas Storage
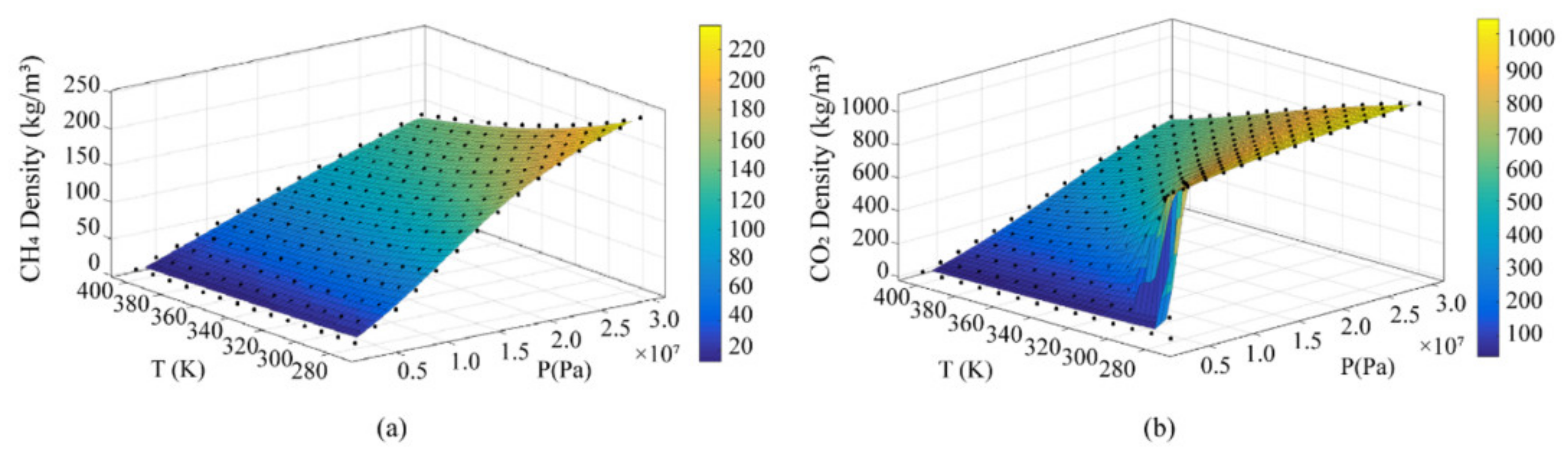
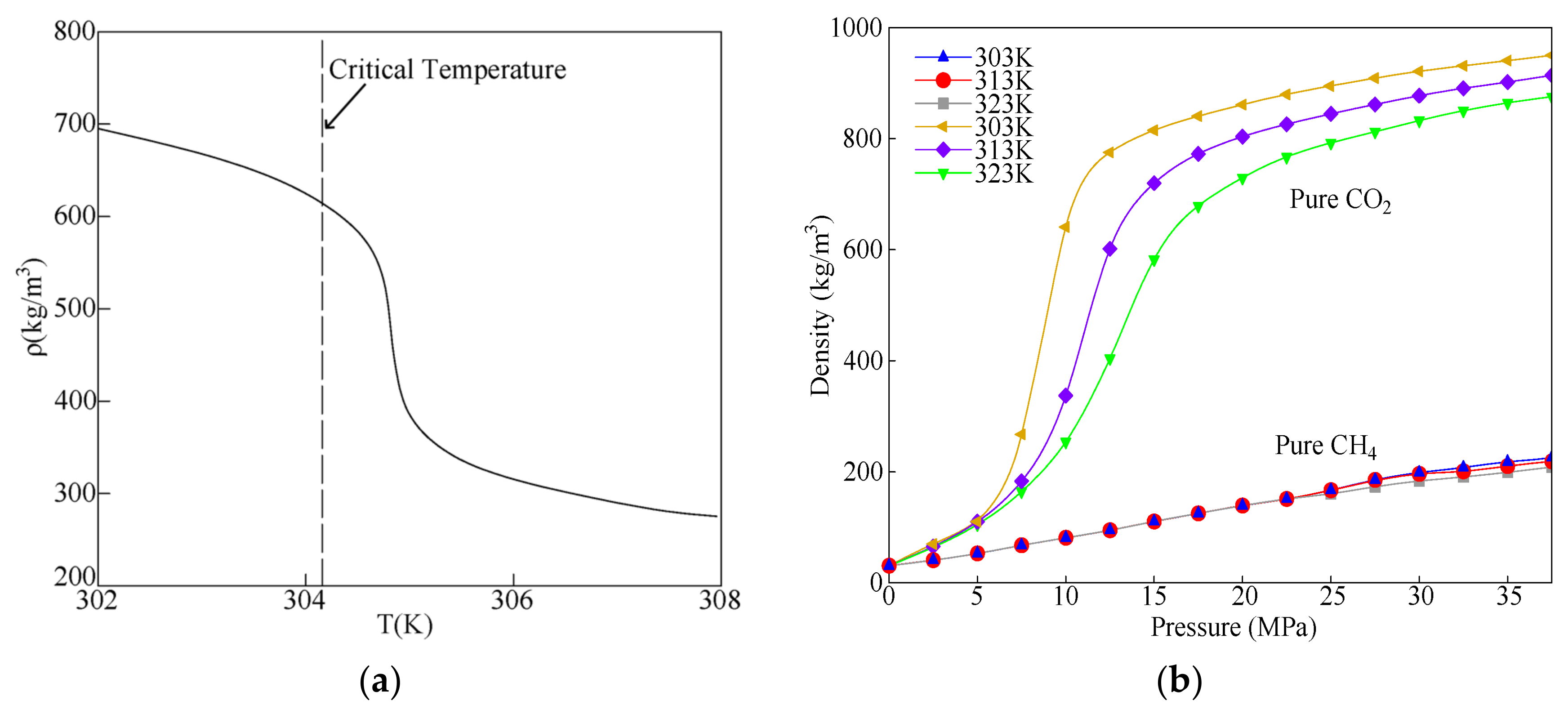
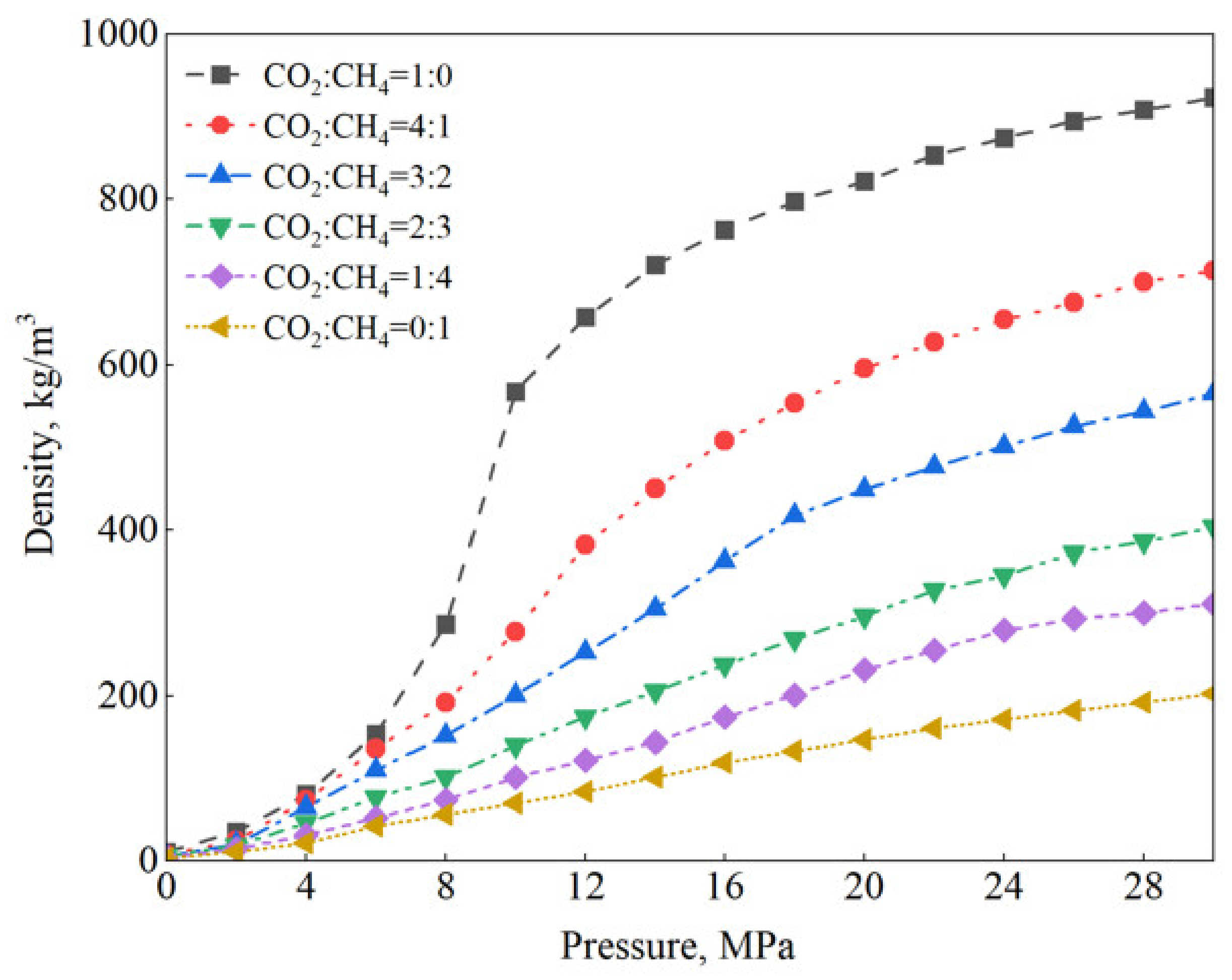
2.1. Density Differences
2.2. Viscosity Differences
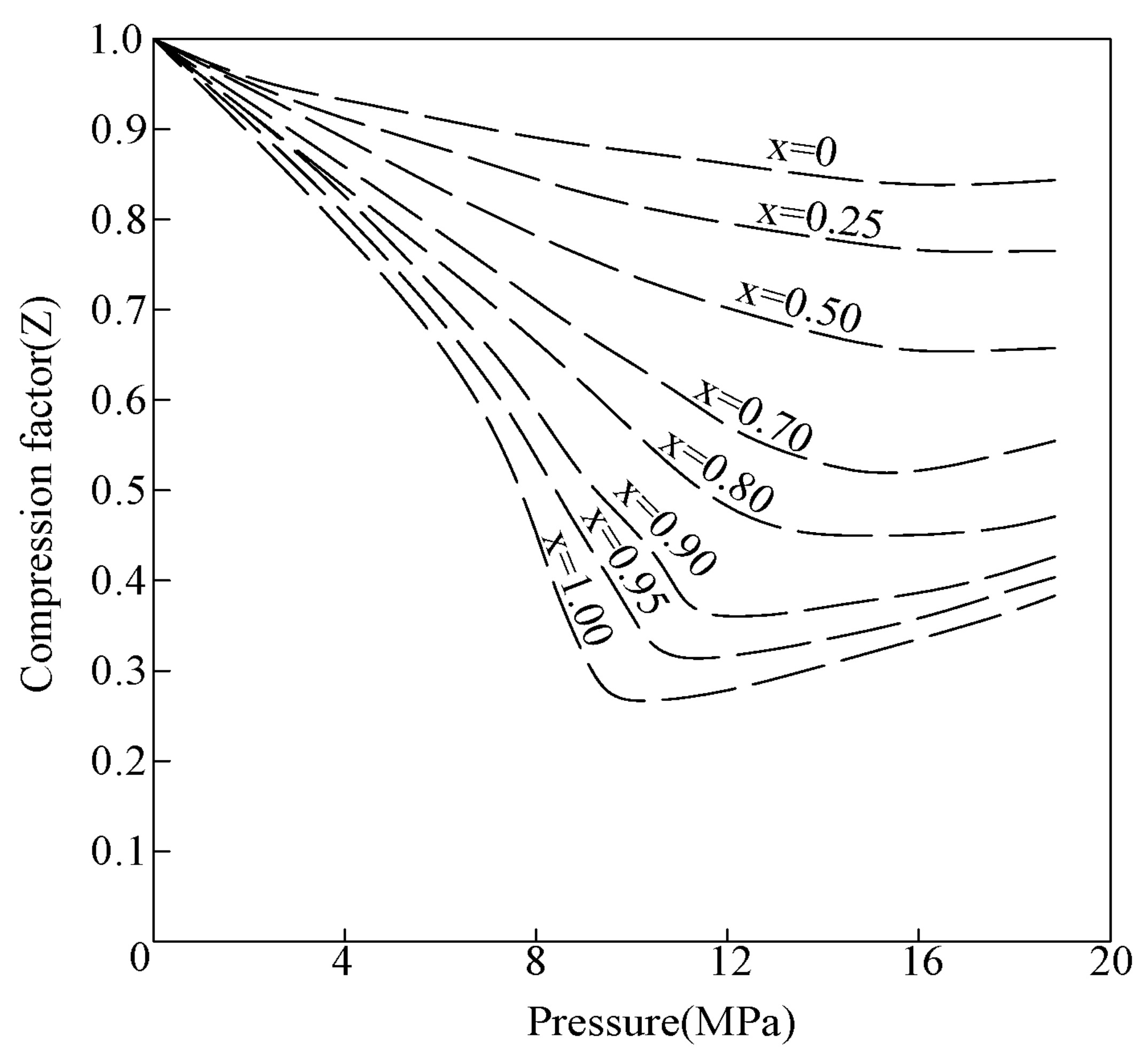
2.3. Compressibility Differences
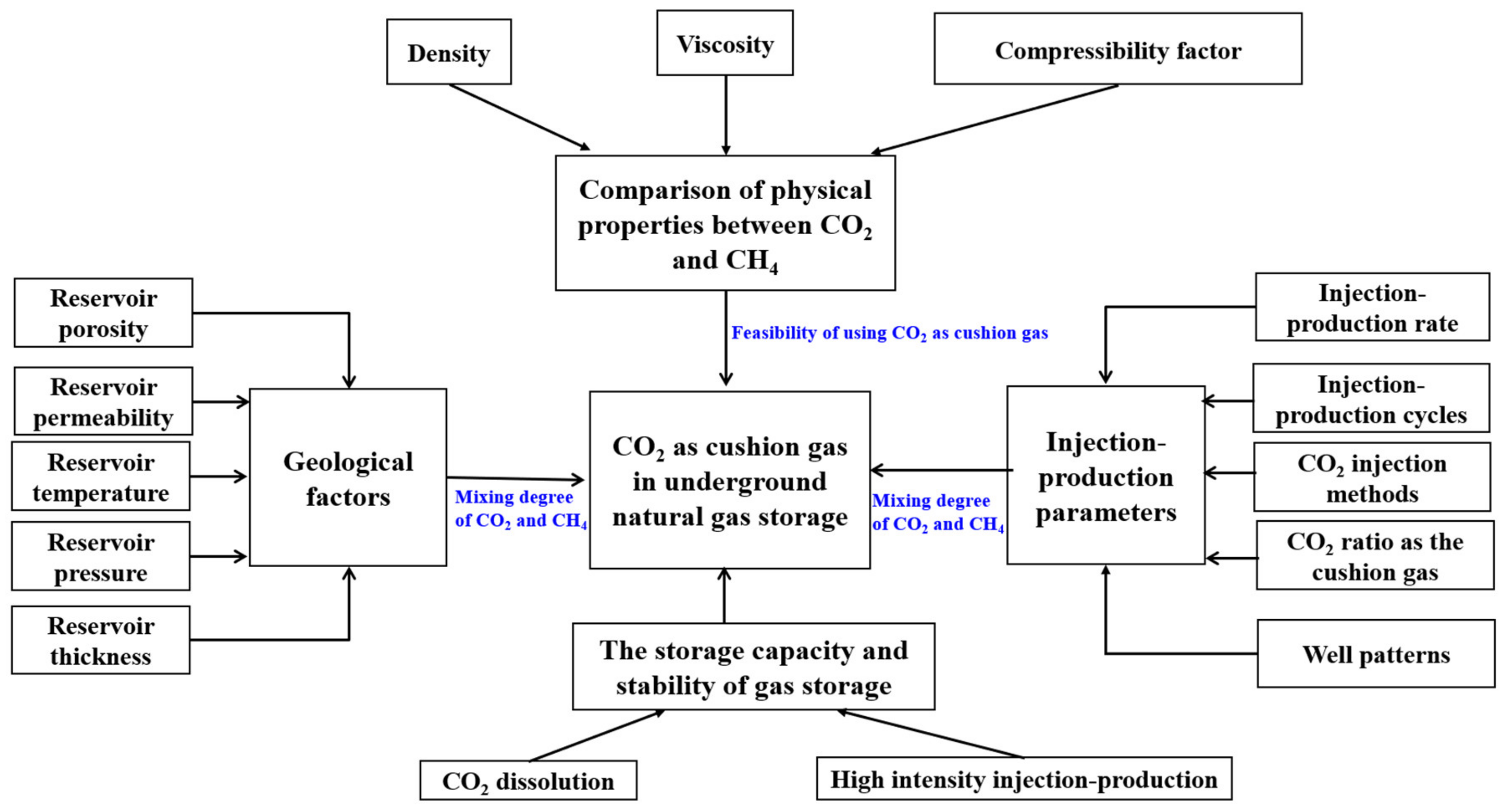
3. Influencing Factors of CO2 as a Cushion Gas to Enhance CH4 Recovery
3.1. Geological Factors
3.1.1. Reservoir Porosity
3.1.2. Reservoir Permeability
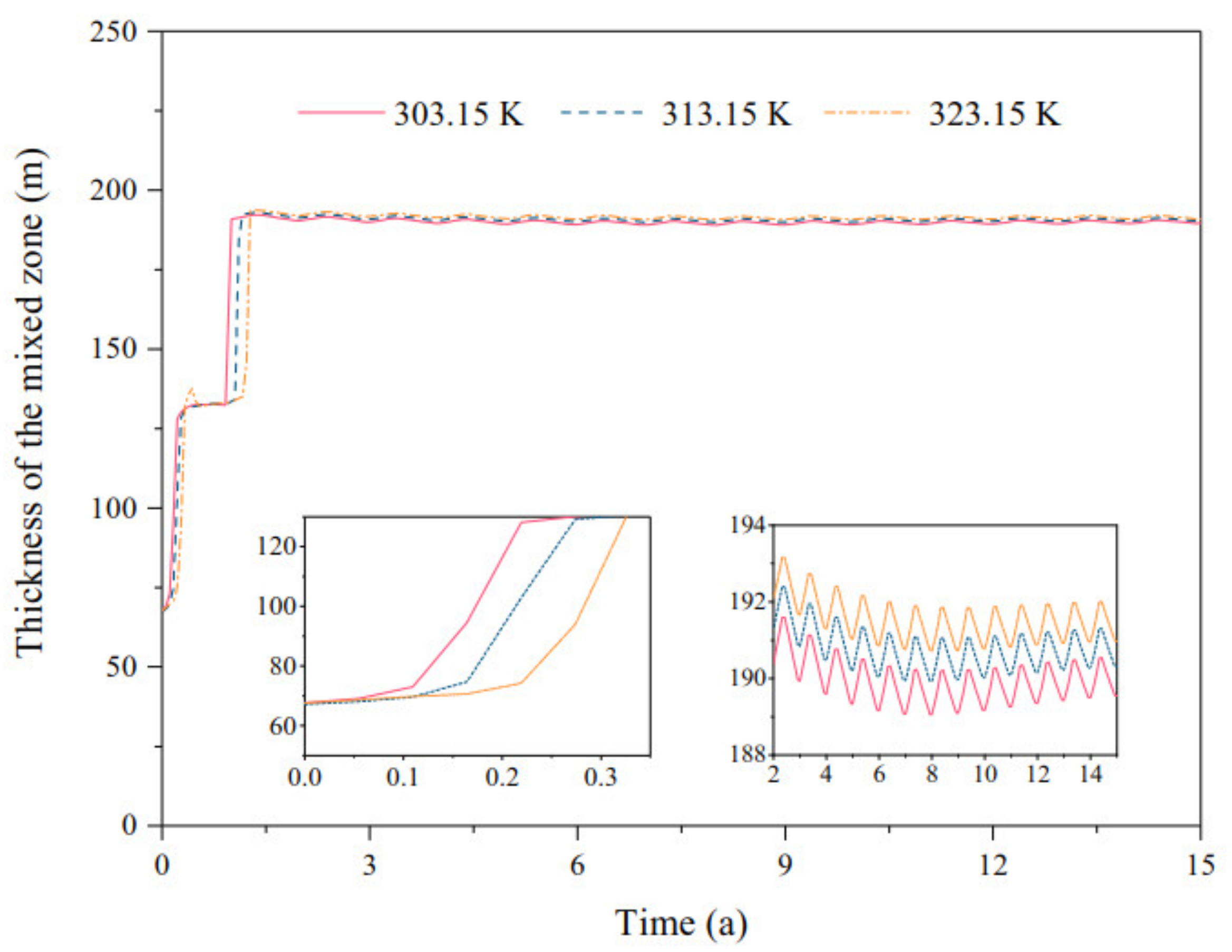
3.1.3. Reservoir Temperature
3.1.4. Reservoir Pressure
3.1.5. Reservoir Thickness
3.2. Injection–Production Parameter Factors
3.2.1. Injection–Production Rate
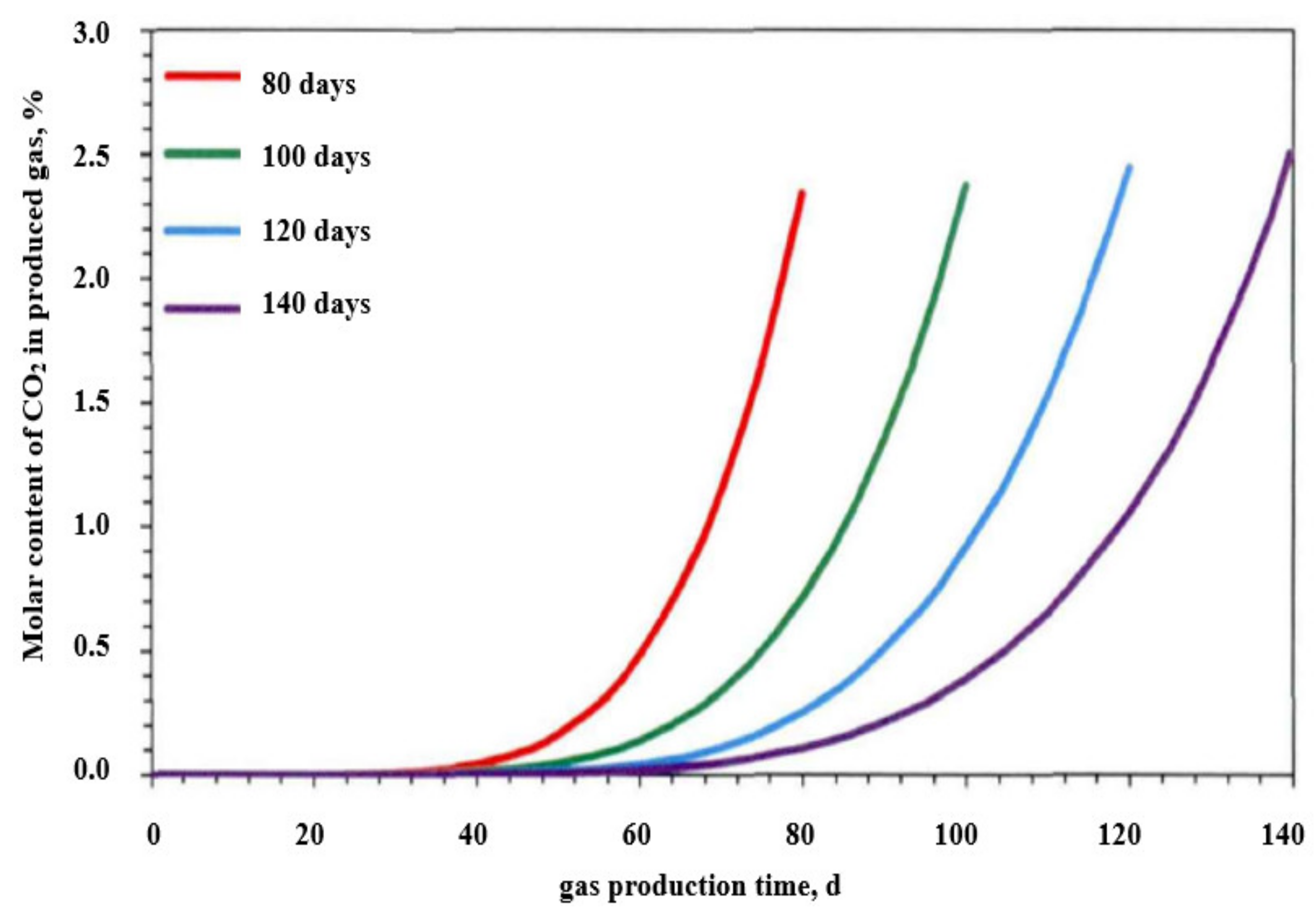
3.2.2. Injection–Production Cycles
3.2.3. CO2 Injection Methods
3.2.4. CO2 Cushion Gas Ratio
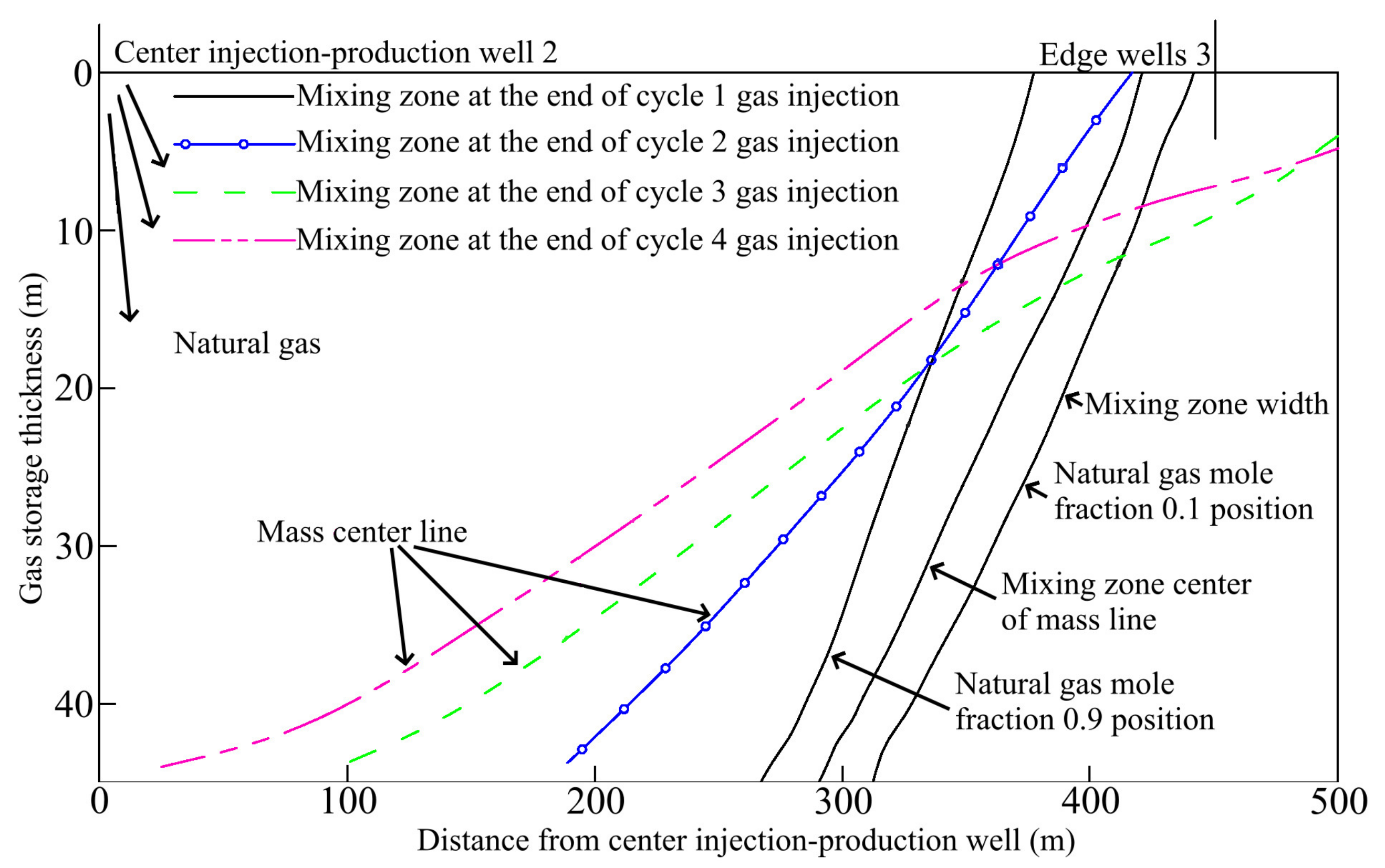
3.2.5. Well Patterns
4. The Storage Capacity and Stability of Gas Storage
5. Conclusions and Challenges
Author Contributions
Funding
Conflicts of Interest
References
- Tian, G. China natural gas development report. Nat. Gas Ind. 2021, 41, 68. [Google Scholar]
- Lei, H. Opportunities and challenges to the construction of underground gas storage in China. Oil Gas Storage Transp. 2018, 37, 728–733. [Google Scholar]
- Wei, G.Q.; Zheng, Y.L.; Qiu, X.S.; Sun, J.C.; Shi, L.; Lai, X. Geological theory and application of underground gas storage in China. Acta Pet. Sin. 2019, 40, 1519–1530. [Google Scholar]
- Bai, M.X.; Sun, J.P.; Song, K.P.; Reinicke, K.M.; Teodoriu, C. Evaluation of mechanical well integrity during CO2 underground storage. Environ. Earth Sci. 2015, 73, 6815–6825. [Google Scholar] [CrossRef]
- Bai, M.X.; Reinicke, K.M.; Song, K.P. An overview of hydrogen underground storage technology and prospects in China. J. Pet. Sci. Eng. 2014, 124, 132–136. [Google Scholar] [CrossRef]
- Zhang, G.X.; Li, B.; Zheng, D.W.; Ding, G.S.; Wei, H.; Qian, P.S.; Li, C. Challenges to and proposals for underground gas storage (UGS) business in China. Nat. Gas Ind. 2017, 37, 153–159. [Google Scholar] [CrossRef]
- Ding, G.S. Developing trend and motives for global underground gas storage. Nat. Gas Ind. 2010, 30, 59–61+117. [Google Scholar]
- Ding, G.S.; Wei, H. Review on 20 years’ UGS construction in China and the prospect. Oil Gas Storage Transp. 2020, 39, 25–31. [Google Scholar]
- Bai, M.X.; Song, K.P.; Li, Y.; Reinicke, K.M. Development of a novel method to evaluate well integrity during CO2 underground storage. SPE J. 2015, 20, 628–641. [Google Scholar] [CrossRef]
- Bai, M.X.; Sun, J.P.; Song, K.P.; Reinicke, K.M.; Li, L.; Qiao, Z. Well Completion and integrity evaluation for CO2 injection wells. Renew. Sustain. Energy Rev. 2015, 45, 556–564. [Google Scholar] [CrossRef]
- Foh, S.E. The Use of Inert Gas as Cushion Gas in Underground Storage: Practical and Economic Issues; Institute of Gas Technology: Chicago, IL, USA, 1991. [Google Scholar]
- Laille, J.P.; Molinard, J.E.; Wents, A. Inert Gas Injection as Part of the Cushion of the Underground Storage of Saint-Clair-Sur-Epte, France; SPE Gas Technology Symposium: Dallas, TX, USA, 1988. [Google Scholar]
- Li, G.T.; Song, G.H.; Zhang, J.; Wen, Q.H.; Zhang, Q. Theoretical discussion of carbon dioxide as cushion gas for underground gas storage tank. Nat. Gas Explor. Dev. 2008, 61–63. [Google Scholar]
- Liu, A. Numerical Simulation of CO2 as Cushion Gas in Aquifer Underground Gas Storage; Southwest Petroleum University: Chengdu, China, 2015. [Google Scholar]
- Wang, Y.J. Feasibility Study on CO2 as Cushion Gas in Depleted Gas Storage; Southwest Petroleum University: Chengdu, China, 2014. [Google Scholar]
- Hu, S.Y.; Li, Y.K.; Wang, Z.W.; Peng, G.Q. Research status and prospect of deplated oil-gas reservoir storage using CO2 as the cushion gas. Oil Gas Storage Transp. 2016, 35, 130–139. [Google Scholar]
- Hitoshi, K.; Yoshiyuki, T.; Yoshikazu, N.; Masaki, I.; Kazuitsu, I.; Yuji, S. Underground storage of carbon dioxide in depleted natural gas reservoirs and in useless aquifers. Eng. Geol. 1993, 34, 175–179. [Google Scholar]
- Jaxek, B.; Czeslaw, R. The gas mixing process in underground gas storages—The “Wierzchowice” UGS case. Arch. Min. Sci. 2008, 53, 53–62. [Google Scholar]
- Tan, Y.F. Numerical solution of gas mixing questions in underground gas storage. Nat. Gas Ind. 2003, 23, 102–105. [Google Scholar]
- Oldenburg, C.M.; Benson, S.M. CO2 Injection for Enhanced Gas Production and Carbon Sequestration; SPE International Petroleum Conference and Exhibition in Mexico: Villahermosa, Mexico, 2002. [Google Scholar]
- Oldenburg, C.M. Carbon Sequestration in Natural Gas Reservoirs: Enhanced Gas Recovery and Natural Gas Storage; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2003; pp. 1–8. [Google Scholar]
- Oldenburg, C.M. Carbon dioxide as cushion gas for natural gas storage. Energy Fuels 2003, 17, 240–246. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.; Park, K. Comparison of nitrogen and carbon dioxide as cushion gas for underground gas storage reservoir. Geosyst. Eng. 2015, 18, 163–167. [Google Scholar] [CrossRef]
- Mu, L.; Liao, X.; Yu, Q.; Hao, L.; Zhang, J.; Zhao, J.; Wu, J. Study on Operation Strategy of Aquifer Underground Gas Storage Using CO2 as Cushion Gas; Carbon Management Technology Conference: Houston, TX, USA, 2019. [Google Scholar]
- Sadeghi, S.; Sedaee, B. Mechanistic simulation of cushion gas and working gas mixing during underground natural gas storage. J. Energy Storage 2022, 46, 103885. [Google Scholar] [CrossRef]
- Helland, J.O.; Friis, H.A.; Assadi, M.; Nagy, S. Machine learning for underground gas storage with cushion CO2 using data from reservoir simulation. IOP Conf. Ser. Mater. Sci. Eng. 2023, 1294, 012058. [Google Scholar] [CrossRef]
- Huang, L. CO2 Geological Storage and Utilization. Atmosphere 2023, 14, 1166. [Google Scholar] [CrossRef]
- Liu, Y.L.; Hu, T.; Rui, Z.H.; Zhang, Z.; Du, K.; Yang, T.; Birol, D.; Erling, H.S.; Farshid, T.; Andrey, A. An integrated framework for geothermal energy storage with CO2 sequestration and utilization. Engineering 2023, 30, 121–130. [Google Scholar] [CrossRef]
- Belonoshko, A.; Saxena, S.K. A molecular dynamics study of the pressure-volume-temperature properties of supercritical fluids: II. CO2, CH4, CO, O2, and H2. Geochim. Cosmochim. Acta 1991, 55, 3191–3208. [Google Scholar] [CrossRef]
- Setzmann, U.; Wagner, W. A new equation of state and tables of thermodynamic properties for methane covering the range from melting line to 625 K at pressures up to 100 MPa. J. Phys. Chem. Ref. Data 1991, 20, 1061–1155. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A New Equation of State for Carbon Dioxide Covering the Fluid Region from the Triple-Point Temperature to 1100 K at Pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Ma, J.L.; Li, Q.; Kempka, T.; Kühn, M. Hydromechanical response and impact of gas mixing behavior in subsurface CH4 storage with CO2-based cushion gas. Energy Fuel 2019, 33, 6527–6541. [Google Scholar] [CrossRef]
- Du, S.Y.; Bai, M.X.; Zhang, Z.C.; Liu, J.Y. Research status of CO2 as cushion gas in underground natural gas storage. Appl. Chem. Ind. 2023, 52, 2387–2391+2396. [Google Scholar]
- Imre, A.; Ramboz, C.; Kraska, T.; Deiters, U.K. Anomalous fluid properties of carbon dioxide in the supercritical region: Application to geological CO2 storage and related hazards. Environ. Earth Sci. 2015, 73, 4373–4384. [Google Scholar] [CrossRef]
- Kunz, O.; Klimeck, R.; Wagner, W.; Jaeschke, M. The GERG-2004 wide-range equation of state for natural gases and other mixtures. In GERG Technical Monograph; Publishing House of the Association of German Engineers: Düsseldorf, Germany, 2007. [Google Scholar]
- Sharif, S.; Hamed, N.; Arezou, J. Utilization of CO2 and N2 as cushion gas in underground gas storage process: A review. J. Energy Storage 2023, 67, 107596. [Google Scholar]
- Nuhu, M.; Abubakar, J.A.; Godpower, C.E.; Salihu, M.S.; Donatus, E.E.; Muhammad, K.A. Alternating N2 gas injection as a potential technique for enhanced gas recovery and CO2 storage in consolidated rocks: An experimental study. J. Pet. Explor. Prod. Technol. 2020, 10, 3883–3903. [Google Scholar]
- Zhang, R.H.; Chen, S.N.; Hu, S.Y.; Zhao, Y.L.; Zhang, B.N.; Wang, R.H. Numerical simulation and laboratory experiments of CO2 sequestration and being as cushion gas in underground natural gas storage reservoirs. J. Nat. Gas Sci. Eng. 2021, 85, 103714. [Google Scholar] [CrossRef]
- Belousov, A.; Lushpeev, V.; Sokolov, A.; Sultanbekov, R.; Tyan, Y.; Ovchinnikov, E.; Shvets, A.; Bushuev, V.; Islamov, S. Hartmann–sprenger energy separation effect for the quasi-isothermal pressure reduction of natural gas: Feasibility analysis and numerical simulation. Energies 2024, 17, 2010. [Google Scholar] [CrossRef]
- Hu, S.Y.; Hu, X.R.; Li, Y.K.; Ma, J.X. Feasibility analysis about taking CO2 as cushion gas for gas storage rebuilt upon depleted gas reservoirs. Reserv. Eval. Dev. 2018, 8, 56–59. [Google Scholar]
- Son, T.N.; Tan, C.N.; Hyunsang, Y.; George, E. Geomechanical study and wellbore stability analysis for potential CO2 storage into devonian and silurian formations of delaware basin. In SPE Oklahoma City Oil and Gas Symposium/Production and Operations Symposium; SPE: Kuala Lumpur, Malaysia, 2023. [Google Scholar]
- Bondarenko, A.V.; Islamov, S.R.; Mardashov, D.V. Features of oil well killing in abnormal carbonate reservoirs operating conditions. In Proceedings of the 15th Conference and Exhibition Engineering and Mining Geophysics. European Association of Geoscientists and Engineers, Gelendzhik, Russia, 22–26 April 2019; pp. 629–633. [Google Scholar]
- Li, Y.K. The Impact of Reservoir Properties and Operating Conditions on Mixing of CO2 Cushion and Natural Gas in Storage Reservoirs; Southwest Petroleum University: Chengdu, China, 2017. [Google Scholar]
- Namdar, H.; Khodapanah, E.; Tabatabaei-Nejad, S.A. Comparison of base gas replacement using nitrogen, flue gas and air during underground natural gas storage in a depleted gas reservoir. Energy Sources Part A 2020, 42, 2778–2793. [Google Scholar] [CrossRef]
- Niu, C.K. Seepage Displacement and Operating Simulation of Fractured Underground Natural Gas Storage Reservoir; Harbin Institute of Technology: Harbin, China, 2016. [Google Scholar]
- Cao, C.; Liao, J.X.; Hou, Z.M.; Xu, H.C.; Faisal, M.; Wu, X.N. Utilization of CO2 as cushion gas for depleted gas reservoir transformed gas storage reservoir. Energies 2020, 13, 576. [Google Scholar] [CrossRef]
- Tan, Y.F.; Zhan, C.H.; Cao, L.; Lin, T. Gas mixing mechanism taking CO2 as cushion gas and feasibility of operation control. Nat. Gas Ind. 2005, 25, 105–107. [Google Scholar]
- Lee, B.; Michael, G.K. A generalized thermodynamic correlation based on three-parameter corresponding states. AIChE J. 1975, 21, 510–527. [Google Scholar] [CrossRef]
- Xiong, Y.; Xu, H.G.; Li, H.; Li, J.D.; Zhu, Y.B. Indoor experimental study on the mechanism of capacity change of gas storage in waste oil reservoir. Sci. Technol. Eng. 2015, 15, 173–176. [Google Scholar]
- Jan, K.; Lukasz, K.; Stanislaw, N. Numerical simulation study on underground gas storage with cushion gas partially replaced with carbon dioxide. Energies 2023, 16, 5248. [Google Scholar] [CrossRef]
- Dumitrache, L.N.; Suditu, S.; Ghețiu, I.; Pană, I.; Brănoiu, G.; Eparu, C. Using numerical reservoir simulation to assess CO2 capture and underground storage, case study on a romanian power plant and its surrounding hydrocarbon reservoirs. Processes 2023, 11, 805. [Google Scholar] [CrossRef]
- Gravanis, E.; Ernestos, S. A working model for estimating CO2-induced uplift of cap rocks under different flow regimes in CO2 sequestration. Geomech. Energy Environ. 2023, 33, 100433. [Google Scholar] [CrossRef]
- Zhao, M.G.; Yang, Y.Z.; Yang, H.Y. Changes of rock properties in CO2 flooding. Sci. Technol. Rev. 2013, 31, 50–52. [Google Scholar]
- Gu, L.B.; Li, Z.P.; Hou, X.L. Experimental research of reservoir physical changes induced by CO2 flooding. J. Oil Gas Technol. 2007, 3, 258–260+513. [Google Scholar]
- Zhao, R.B.; Sun, H.T.; Wu, Y.S.; Zhao, C.F.; Yue, X.A. Laboratory study on the effect of carbon dioxide storage on formation rock. Sci. Sin. 2010, 40, 378–384. [Google Scholar]
- Wang, X.M.; Guo, P.; Jiang, F.G. The physical simulation study on the gas-drive multiphase flow mechanism of aquifer gas storage. Nat. Gas Geosci. 2006, 17, 597–600. [Google Scholar]
- He, X.L.; Huang, S.J.; Sun, C.W.; Xu, J.J.; Zhang, X.J.; Yang, X.P. Gas-water relative permeability variation in multi-cycle injection-production of underground gas storage in flooded depleted gas reservoir. Oil Gas Storage Transp. 2015, 34, 150–153. [Google Scholar]
- Zhang, Q. Experimental study of seepage law of fractured carbonate gas storage injection and production. In Statue of Seepage Fluid Mechanics; Chinese Academy of Science: Beijing, China, 2015. [Google Scholar]
- Ding, Y.H.; Zhang, Q.; Zheng, D.W.; Wang, X.M.; Shi, L.; Li, C.; Xu, H.C. Seepage laws in converting a microfissure-pore carbonate gas reservoir into a UGS. Nat. Gas Ind. 2015, 35, 109–114. [Google Scholar]
- Shi, L.; Liao, G.Z.; Xiong, W.; Gao, S.S.; Geng, T. Gas-water percolation mechanism in an underground storage built on a water-drive sandstone gas reservoir. Nat. Gas Ind. 2012, 32, 85–87. [Google Scholar]
- Xie, J.; Liang, H.Z.; Guo, R.; Wang, J.K.; Wang, M.Q.; Duan, Y.J. Research status of the fluid displacement mechanism during the multi-rounds strong injection-production for the carbonate rock underground gas storage. Period. Ocean. Univ. China 2018, 48, 88–95. [Google Scholar]
- Wang, J.K.; Zhang, J.L.; Ding, F. Initial gas full component simulation experiment of Ban-876 underground gas storage. J. Nat. Gas Sci. Eng. 2014, 18, 131–136. [Google Scholar] [CrossRef]
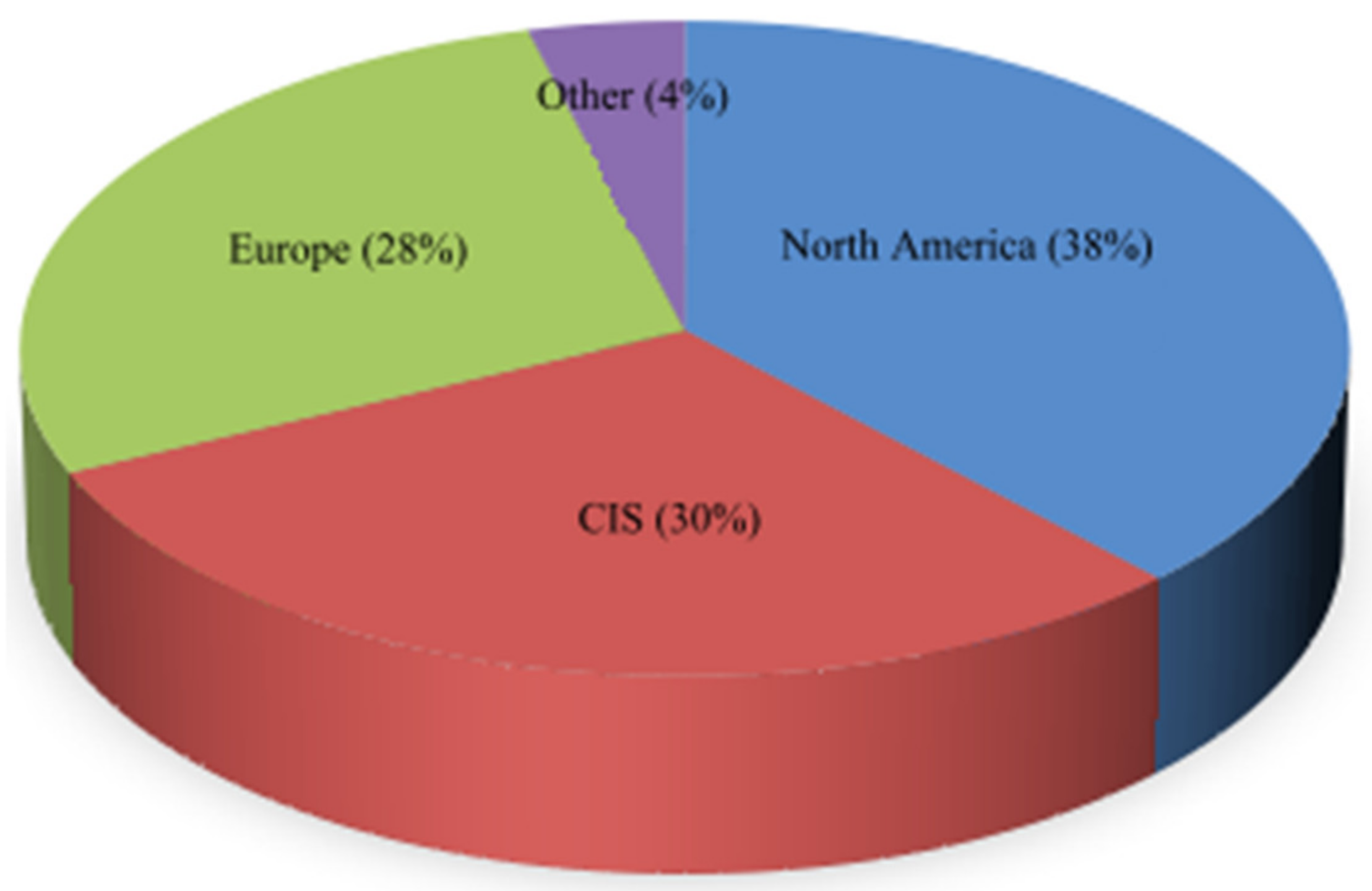




| Region/Year | 2005 | 2010 | 2015 | 2020 | 2025 | 2030 |
|---|---|---|---|---|---|---|
| Commonwealth of Independent States (CIS) Countries | 1360 | 1480 | 1560 | 1650 | 1730 | 1770 |
| North America | 1160 | 1340 | 1530 | 1620 | 1720 | 1870 |
| Europe | 790 | 930 | 1090 | 1210 | 1290 | 1350 |
| Asia–Pacific region | 20 | 20 | 40 | 80 | 100 | 120 |
| Asia | 10 | 20 | 50 | 120 | 200 | 210 |
| Latin America and the Caribbean | 0 | 10 | 10 | 20 | 30 | 40 |
| Middle East | 0 | 0 | 0 | 20 | 30 | 40 |
| Africa | 0 | 0 | 0 | 10 | 20 | 30 |
| Type of Gas Storage | CO2 Cushion Gas Percentage | Reservoir Properties | Storage Conditions | CO2 Content in Recovered Gas /mol% | Reference(s) | |||
|---|---|---|---|---|---|---|---|---|
| Thickness /m | Porosity | Permeability /mD | Temperature /K | Pressure /MPa | ||||
| Aquifer gas storage | 10–30 | 10 | 0.05–0.25 | 100–500 | 313 | 8.4 | 4.1–40.8 | [24] |
| Donghae gas reservoir | 0–20 | 30.48 | 0.2 | Kh: 50–120 Kv: 10 | 303.15–323.15 | 5.17 | 0.2–9.1 | [44] |
| Depleted gas storage | 20 | 30.48 | 0.2 | Kh: 50 Kv: 10 | 366.48 | 5.17 | 6.2 | [23] |
| Depleted gas storage | 10–25 | 7 layers | 0.1–0.4 | 25–500 | 308.15–328.15 | 4 | 0.05–30 | [15] |
| Depleted gas storage | 10–40 | 6 | 0.1–0.25 | 10–50 | 313.15 | 8–12 | 0–24 | [16] |
| Depleted gas storage | 46.6 | 75.6 | 0.21 | 23.7 | 341.48 | 9.29–15.0 | 0.1–1.3 | [36] |
| Aquifer gas storage | Saturation | 10 | 0.2 | 121–1073 | 313.15 | 12 | 4.1–20.4 | [49] |
| Depleted gas storage | Saturation | 22 | 0.3 | 1013 | 313.15 | 0 | Not mentioned specifically | [20,32] |
| Fractured depleted gas storage | 7–91 | 32.4–75.6 | φ: 10.55 φf: 6.07 | 600 | 387.45 | 7.5 | Not mentioned specifically | [45] |
| Aquifer gas storage | 10–40 | 36 grids | 0.17 | 224 | 362.15 | 4 | 1–26 | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Bai, M.; Shi, Y.; Zha, Y.; Yan, D. A Review of the Utilization of CO2 as a Cushion Gas in Underground Natural Gas Storage. Processes 2024, 12, 1489. https://doi.org/10.3390/pr12071489
Du S, Bai M, Shi Y, Zha Y, Yan D. A Review of the Utilization of CO2 as a Cushion Gas in Underground Natural Gas Storage. Processes. 2024; 12(7):1489. https://doi.org/10.3390/pr12071489
Chicago/Turabian StyleDu, Siyu, Mingxing Bai, Yukai Shi, Yuan Zha, and Deng Yan. 2024. "A Review of the Utilization of CO2 as a Cushion Gas in Underground Natural Gas Storage" Processes 12, no. 7: 1489. https://doi.org/10.3390/pr12071489





