Key Takeaways on the Cost-Effective Production of Cellulosic Sugars at Large Scale
Abstract
:1. Introduction
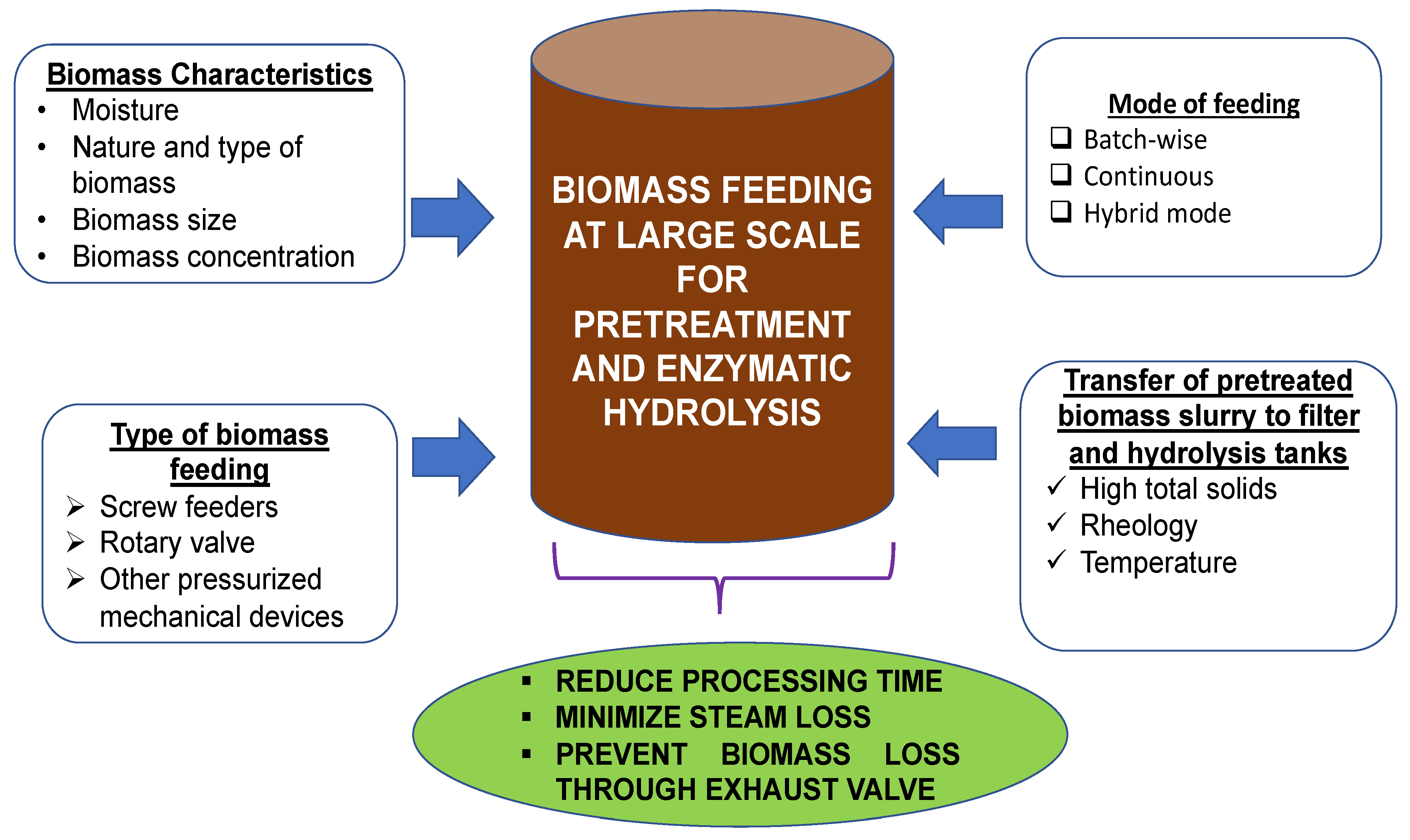
2. Biomass Feeding and Feasible Pretreatment Technology at Commercial Scale
3. Titer, Rate, and Yield (TRY) of Cellulosic Sugar Production
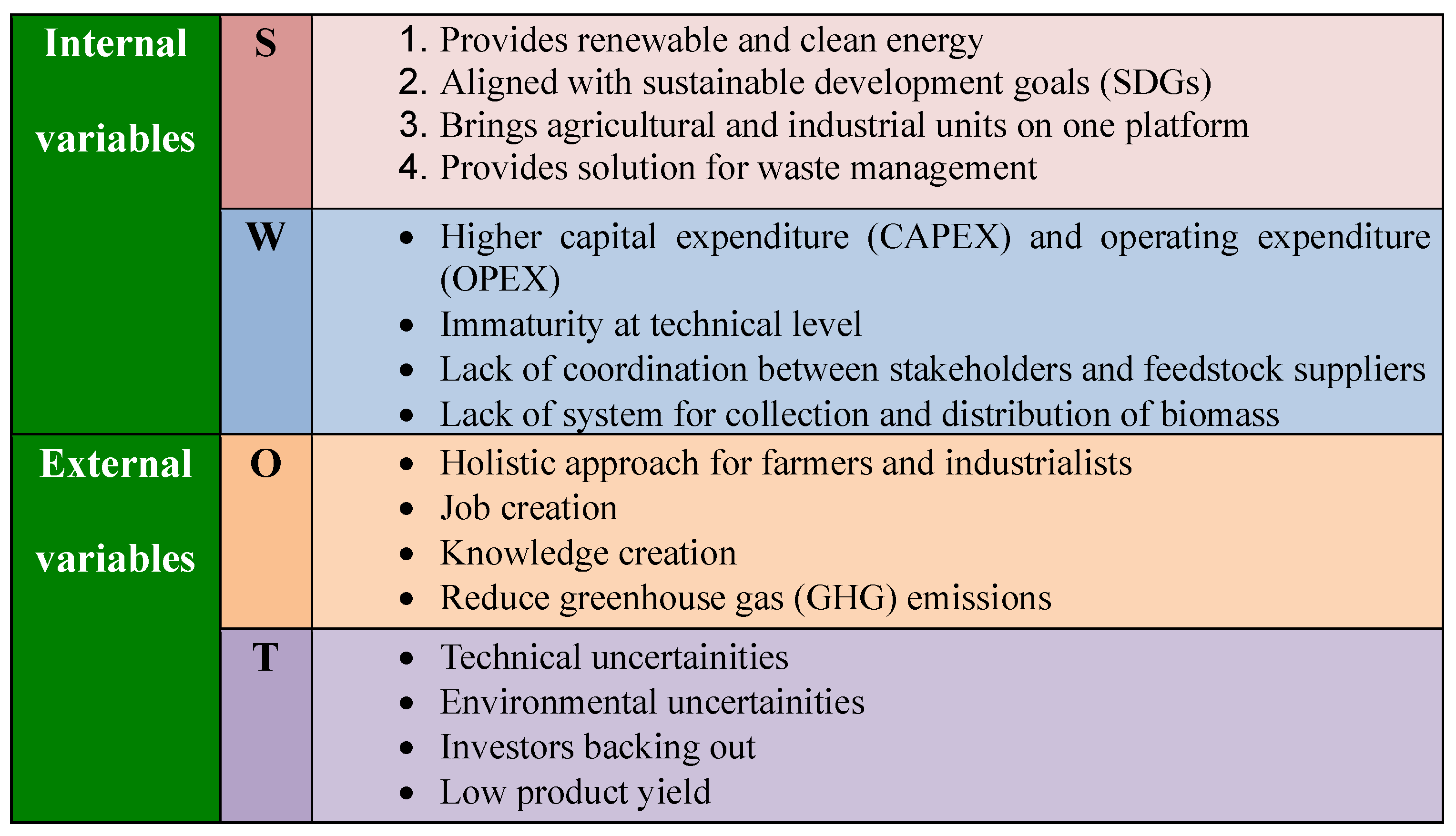
4. SWOT Analysis of 2G Sugar Production at Large Scale
5. Process Viability and Key Areas of Improvement
6. A Brief Case Study of Profitability and the Look Ahead: 1.5G Biorefineries
7. Governing Parameters for Techno-Economic Assessment of Biorefinery
8. Conclusions
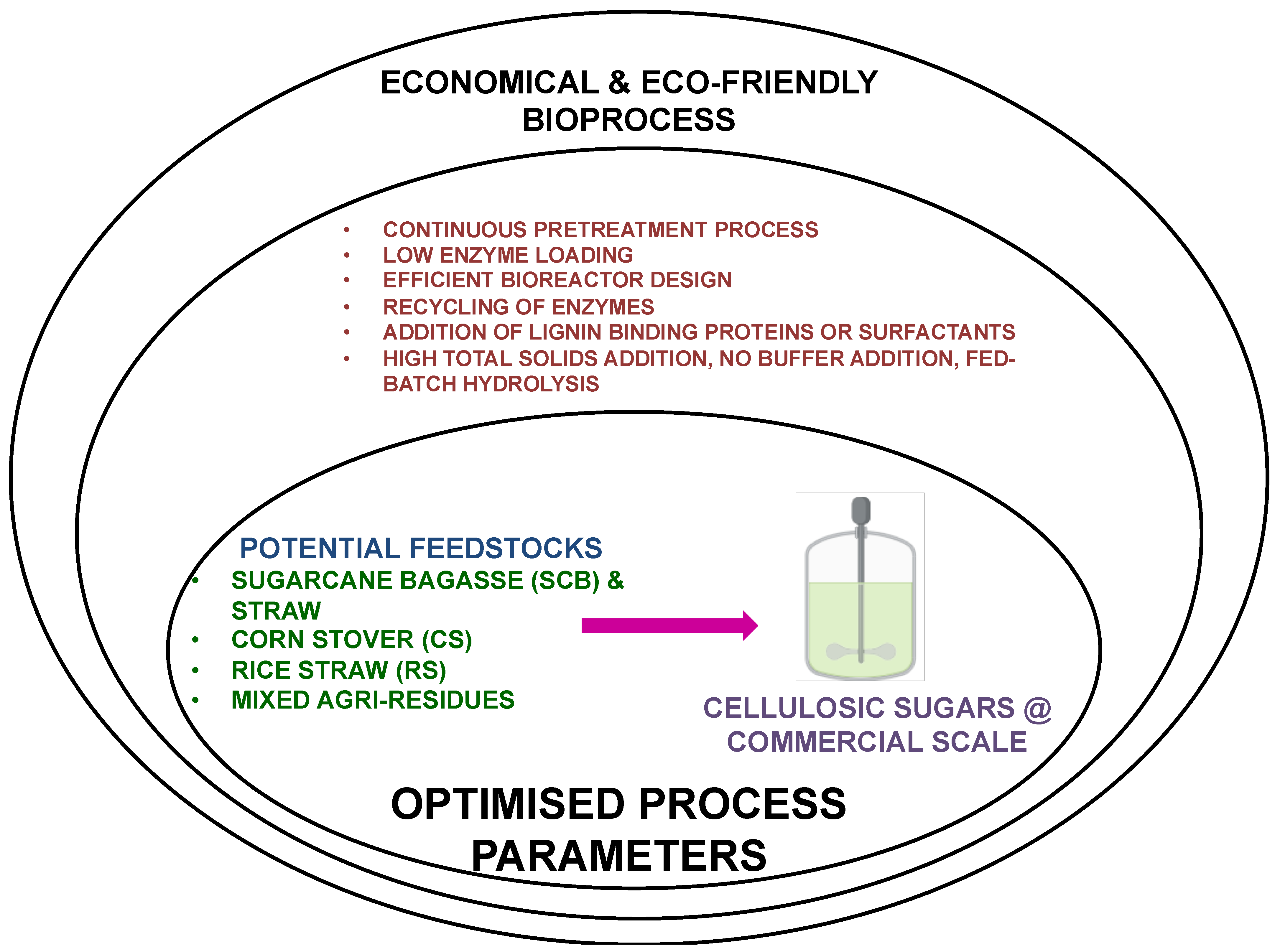
- The feeding of high total solids (more than 50% total solids) in the pretreatment process (steam explosion or ammonia fiber expansion) under continuous mode. Use of biomass received as such from the sugar mills or corn milling processing farms, no further size reduction, screening and washing of biomass before pretreatment
- The robust mechanical feeding of biomass without steam or biomass loss from exhaust valves during pretreatment.
- Fewer chemicals for biomass pretreatment and using less severe process conditions; minimum generation of inhibitors; possible use of mixed feedstock.
- A reduction in the number of filtration and washing steps required for pretreated slurry and biomass.
- The loading of more than 20% total solids during fed-batch enzymatic hydrolysis.
- Avoidance of the use of buffer solutions and use of tap water in pretreatment and hydrolysis.
- Fed-batch enzymatic hydrolysis of pretreated slurry (liquified stream and cellulose solids) without filtration.
- The use of designer and highly potent cellulolytic enzyme cocktails (feedstock-centric) like Cellic Ctec3 or other commercial brands.
- The addition of cheap and easily available proteins or green surfactants into the biomass slurry before enzymatic hydrolysis.
- The use of thermostable cellulolytic enzyme cocktails with LPMOS (lytic polysaccharide monooxygenase), liquefaction enzymes, and ancillary proteins.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reis, C.E.R.; Libardi Junior, N.; Bento, H.B.S.; Carvalho, A.K.F.d.; Vandenberghe, L.P.d.S.; Soccol, C.R.; Aminabhavi, T.M.; Chandel, A.K. Process strategies to reduce cellulase enzyme loading for renewable sugar production in biorefineries. Chem. Eng. J. 2023, 451, 138690. [Google Scholar] [CrossRef]
- Biswas, R.; Persad, A.; Bisaria, V.S. Production of Cellulolytic Enzymes. In Bioprocessing of Renewable Resources to Commodity Bioproducts; Wiley Online Library: Hoboken, NJ, USA, 2014; pp. 105–132. [Google Scholar]
- Arora, R.; Singh, P.; Sarangi, P.K.; Kumar, S.; Chandel, A.K. A critical assessment on scalable technologies using high solids loadings in lignocellulose biorefinery: Challenges and solutions. Crit. Rev. Biotechnol. 2024, 44, 218–235. [Google Scholar] [CrossRef]
- Kaur, J.; Chugh, P.; Soni, R.; Soni, S.K. A low-cost approach for the generation of enhanced sugars and ethanol from rice straw using in-house produced cellulase-hemicellulase consortium from A. niger P-19. Bioresour. Technol. Rep. 2020, 11, 100469. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Shafiei-Alavijeh, R.; Aghbashlo, M.; Tabatabaei, M.; Denayer, J.F.M.; Karimi, K. A critical review of sustainable biorefineries utilizing high-solid processing for industrial crop lignocellulosic wastes valorization. Ind. Crops Prod. 2024, 211, 118236. [Google Scholar] [CrossRef]
- Althuri, A.; Chintagunta, A.D.; Sherpa, K.C.; Banerjee, R. Simultaneous Saccharification and Fermentation of Lignocellulosic Biomass. In Biorefining of Biomass to Biofuels: Opportunities and Perception; Kumar, S., Sani, R.K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 265–285. [Google Scholar]
- Molaverdi, M.; Karimi, K.; Mirmohamadsadeghi, S.; Galbe, M. High titer ethanol production from rice straw via solid-state simultaneous saccharification and fermentation by Mucor indicus at low enzyme loading. Energy Convers. Manag. 2019, 182, 520–529. [Google Scholar] [CrossRef]
- Pratto, B.; dos Santos-Rocha, M.S.R.; Longati, A.A.; de Sousa Júnior, R.; Cruz, A.J.G. Experimental optimization and techno-economic analysis of bioethanol production by simultaneous saccharification and fermentation process using sugarcane straw. Bioresour. Technol. 2020, 297, 122494. [Google Scholar] [CrossRef]
- Wang, Z.; Ning, P.; Hu, L.; Nie, Q.; Liu, Y.; Zhou, Y.; Yang, J. Efficient ethanol production from paper mulberry pretreated at high solid loading in Fed-nonisothermal-simultaneous saccharification and fermentation. Renew. Energy 2020, 160, 211–219. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Li, B.; Feng, Y.; Cui, Q. Consolidated bio-saccharification: Leading lignocellulose bioconversion into the real world. Biotechnol. Adv. 2020, 40, 107535. [Google Scholar] [CrossRef]
- Olguin-Maciel, E.; Singh, A.; Chable-Villacis, R.; Tapia-Tussell, R.; Ruiz, H.A. Consolidated Bioprocessing, an Innovative Strategy towards Sustainability for Biofuels Production from Crop Residues: An Overview. Agronomy 2020, 10, 1834. [Google Scholar] [CrossRef]
- Svoboda, K.; Pohořelý, M.; Hartman, M.; Martinec, J. Pretreatment and feeding of biomass for pressurized entrained flow gasification. Fuel Process. Technol. 2009, 90, 629–635. [Google Scholar] [CrossRef]
- Tyler, L.W.; Damon, S.H. Biomass Handling and Feeding. In Advances in Biofuels and Bioenergy; Madhugiri, N.-R., Jaya, R.S., Eds.; IntechOpen: Rijeka, Croatia, 2018; Chapter 6. [Google Scholar]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; da Silva, S.S. The path forward for lignocellulose biorefineries: Bottlenecks, solutions, and perspective on commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Albarelli, J.Q.; Santos, D.T.; Chundawat, S.P.; Puri, M.; Meireles, M.A.A. Comparative analysis of key technologies for cellulosic ethanol production from Brazilian sugarcane bagasse at a commercial scale. Biofuels Bioprod. Biorefin. 2019, 13, 994–1014. [Google Scholar] [CrossRef]
- Arora, R.; Behera, S.; Kumar, S. Bioprospecting thermophilic/thermotolerant microbes for production of lignocellulosic ethanol: A future perspective. Renew. Sustain. Energy Rev. 2015, 51, 699–717. [Google Scholar] [CrossRef]
- Arora, R.; Behera, S.; Sharma, N.K.; Kumar, S. Bioprospecting thermostable cellulosomes for efficient biofuel production from lignocellulosic biomass. Bioresour. Bioprocess. 2015, 2, 38. [Google Scholar] [CrossRef]
- Arora, R.; Behera, S.; Sharma, N.K.; Kumar, S. A new search for thermotolerant yeasts, its characterization and optimization using response surface methodology for ethanol production. Front. Microbiol. 2015, 6, 889. [Google Scholar] [CrossRef] [PubMed]
- Baral, P.; Munagala, M.; Shastri, Y.; Kumar, V.; Agrawal, D. Cost reduction approaches for fermentable sugar production from sugarcane bagasse and its impact on techno-economics and the environment. Cellulose 2021, 28, 6305–6322. [Google Scholar] [CrossRef]
- Pereira, B.; Arantes, V. Production of cellulose nanocrystals integrated into a biochemical sugar platform process via enzymatic hydrolysis at high solid loading. Ind. Crops Prod. 2020, 152, 112377. [Google Scholar] [CrossRef]
- Nwamba, M.C.; Song, G.; Sun, F.; Mukasekuru, M.R.; Ren, H.; Zhang, Q.; Cao, T.; Wang, H.; Sun, H.; Hong, J. Efficiency enhancement of a new cellulase cocktail at low enzyme loading for high solid digestion of alkali catalyzed atmospheric glycerol organosolvent pre-treated sugarcane bagasse. Bioresour. Technol. 2021, 338, 125505. [Google Scholar] [CrossRef]
- Brondi, M.G.; Elias, A.M.; Furlan, F.F.; Giordano, R.C.; Farinas, C.S. Performance targets defined by retro-techno-economic analysis for the use of soybean protein as saccharification additive in an integrated biorefinery. Sci. Rep. 2020, 10, 7367. [Google Scholar] [CrossRef]
- Lu, M.; Li, J.; Han, L.; Xiao, W. High-solids enzymatic hydrolysis of ball-milled corn stover with reduced slurry viscosity and improved sugar yields. Biotechnol. Biofuels 2020, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhai, R.; Chen, X.; Jiang, X.; Li, C.; Deng, Q.; Xu, Z.; Jin, M. Modeling and design of fed-batch strategies for achieving 255 g/L sugar concentration from high-solid enzymatic hydrolysis of pretreated corn stover. Chem. Eng. J. 2024, 486, 150268. [Google Scholar] [CrossRef]
- Katakojwala, R.; Venkata Mohan, S. Multi-product biorefinery with sugarcane bagasse: Process development for nanocellulose, lignin and biohydrogen production and lifecycle analysis. Chem. Eng. J. 2022, 446, 137233. [Google Scholar] [CrossRef]
- Mukasekuru, M.R.; Kaneza, P.; Sun, H.; Sun, F.F.; He, J.; Zheng, P. Fed-batch high-solids enzymatic saccharification of lignocellulosic substrates with a combination of additives and accessory enzymes. Ind. Crops Prod. 2020, 146, 112156. [Google Scholar] [CrossRef]
- Long, L.; Yang, H.; Ren, H.; Liu, R.; Sun, F.F.; Xiao, Z.; Hu, J.; Xu, Z. Synergism of Recombinant Podospora anserina PaAA9B with Cellulases Containing AA9s Can Boost the Enzymatic Hydrolysis of Cellulosic Substrates. ACS Sustain. Chem. Eng. 2020, 8, 11986–11993. [Google Scholar] [CrossRef]
- Baral, P.; Jain, L.; Kurmi, A.K.; Kumar, V.; Agrawal, D. Augmented hydrolysis of acid pretreated sugarcane bagasse by PEG 6000 addition: A case study of Cellic CTec2 with recycling and reuse. Bioprocess Biosyst. Eng. 2020, 43, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Baral, P.; Kumar, V.; Agrawal, D. Emerging trends in high-solids enzymatic saccharification of lignocellulosic feedstocks for developing an efficient and industrially deployable sugar platform. Crit. Rev. Biotechnol. 2022, 42, 873–891. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Wang, X.; Yuan, W.; Wang, Y.; Zhou, W.; Wang, G.; Liu, Y. Fed-batch enzymatic hydrolysis of alkaline organosolv-pretreated corn stover facilitating high concentrations and yields of fermentable sugars for microbial lipid production. Biotechnol. Biofuels 2020, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.M.; Akter, S.; Dar, M.A.; Tuly, J.A.; Kumar Aswathi, M.; Yun, J.; Li, J.; Qi, X. Enhanced fermentable sugar production in lignocellulosic biorefinery by exploring a novel corn stover and configuring high-solid pretreatment conditions. Bioresour. Technol. 2023, 386, 129498. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Bisen, J.; Bhaduri, D.; Priyadarsini, S.; Munda, S.; Chakraborti, M.; Adak, T.; Panneerselvam, P.; Mukherjee, A.K.; Swain, S.L.; et al. Turn the wheel from waste to wealth: Economic and environmental gain of sustainable rice straw management practices over field burning in reference to India. Sci. Total Environ. 2021, 775, 145896. [Google Scholar] [CrossRef]
- Gabhane, J.; Kumar, S.; Sarma, A.K. Effect of glycerol thermal and hydrothermal pretreatments on lignin degradation and enzymatic hydrolysis in paddy straw. Renew. Energy 2020, 154, 1304–1313. [Google Scholar] [CrossRef]
- Cabrera-Villamizar, L.A.; Ebrahimi, M.; Martínez-Abad, A.; Talens-Perales, D.; López-Rubio, A.; Fabra, M.J. Order matters: Methods for extracting cellulose from rice straw by coupling alkaline, ozone and enzymatic treatments. Carbohydr. Polym. 2024, 328, 121746. [Google Scholar] [CrossRef] [PubMed]
- Maibam, P.D.; Goyal, A. Designing of recombinant hydrolytic enzymes cocktail for effective saccharification of delignified rice straw. Ind. Crops Prod. 2023, 206, 117727. [Google Scholar] [CrossRef]
- Dey, B.; Roy, B.; Datta, S.; Singh, K.G. Comprehensive overview and proposal of strategies for the ethanol sector in India. Biomass Convers. Biorefin. 2023, 13, 4587–4618. [Google Scholar] [CrossRef]
- Dale, B. Time to Rethink Cellulosic Biofuels? Biofuels Bioprod. Biorefin. 2018, 12, 5–7. [Google Scholar] [CrossRef]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin–carbohydrate complexes: Properties, applications, analyses, and methods of extraction: A review. Biotechnol. Biofuels 2018, 11, 269. [Google Scholar] [CrossRef]
- Petridis, L.; Smith, J.C. Molecular-level driving forces in lignocellulosic biomass deconstruction for bioenergy. Nat. Rev. Chem. 2018, 2, 382–389. [Google Scholar] [CrossRef]
- Patiño, M.A.; Ortiz, J.P.; Velásquez, M.; Stambuk, B.U. d-Xylose consumption by nonrecombinant Saccharomyces cerevisiae: A review. Yeast 2019, 36, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.-C.; Hallett, J.P. Recent advances in the pretreatment of lignocellulosic biomass. Curr. Opin. Green Sustain. Chem. 2019, 20, 11–17. [Google Scholar] [CrossRef]
- Valdivia, M.; Galan, J.L.; Laffarga, J.; Ramos, J.-L. Biofuels 2020: Biorefineries based on lignocellulosic materials. Microb. Biotechnol. 2016, 9, 585–594. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J. Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: A review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef]
- Arora, R.; Behera, S.; Sharma, N.K.; Kumar, S. Augmentation of ethanol production through statistically designed growth and fermentation medium using novel thermotolerant yeast isolates. Renew. Energy 2017, 109, 406–421. [Google Scholar] [CrossRef]
- Arora, R.; Bons, H.K.; Kocher, G.S. Fermentative processing of unexploited fruit, karonda (Carissa carandus L.) into alcoholic beverages. S. Afr. J. Bot. 2023, 158, 73–79. [Google Scholar] [CrossRef]
- Pereira, L.M.S.; Milan, T.M.; Tapia-Blácido, D.R. Using Response Surface Methodology (RSM) to optimize 2G bioethanol production: A review. Biomass Bioenergy 2021, 151, 106166. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, N. RSM approach to pre-treatment of lignocellulosic waste and a statistical methodology for optimizing bioethanol production. Waste Manag. Bull. 2024, 2, 49–66. [Google Scholar] [CrossRef]
- Xin, D.; Yang, M.; Chen, X.; Zhang, Y.; Wang, R.; Wen, P.; Zhang, J. Improving cellulase recycling efficiency by decreasing the inhibitory effect of unhydrolyzed solid on recycled corn stover saccharification. Renew. Energy 2020, 145, 215–221. [Google Scholar] [CrossRef]
- Houfani, A.A.; Anders, N.; Spiess, A.C.; Baldrian, P.; Benallaoua, S. Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars—A review. Biomass Bioenergy 2020, 134, 105481. [Google Scholar] [CrossRef]
- Moon, M.; Lee, J.-P.; Park, G.W.; Lee, J.-S.; Park, H.J.; Min, K. Lytic polysaccharide monooxygenase (LPMO)-derived saccharification of lignocellulosic biomass. Bioresour. Technol. 2022, 359, 127501. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Liu, X.; Zhou, S.; Yu, Q.; Xu, Y. Microbial saccharification—Biorefinery platform for lignocellulose. Ind. Crops Prod. 2022, 189, 115761. [Google Scholar] [CrossRef]
- Zhu, J.-Q.; Zong, Q.-J.; Li, W.-C.; Chai, M.-Z.; Xu, T.; Liu, H.; Fan, H.; Li, B.-Z.; Yuan, Y.-J. Temperature profiled simultaneous saccharification and co-fermentation of corn stover increases ethanol production at high solid loading. Energy Convers. Manag. 2020, 205, 112344. [Google Scholar] [CrossRef]
- Akhtar, J.; Hassan, N.; Idris, A.; Ngadiman, N.H.A. Optimization of simultaneous saccharification and fermentation process conditions for the production of succinic acid from oil palm empty fruit bunches. J. Wood Chem. Technol. 2020, 40, 136–145. [Google Scholar] [CrossRef]
- Nalawade, K.; Baral, P.; Patil, S.; Pundir, A.; Kurmi, A.K.; Konde, K.; Patil, S.; Agrawal, D. Evaluation of alternative strategies for generating fermentable sugars from high-solids alkali pretreated sugarcane bagasse and successive valorization to L(+) lactic acid. Renew. Energy 2020, 157, 708–717. [Google Scholar] [CrossRef]
- Shibukawa, V.P.; Reis, C.E.R.; dos Santos, J.C.; Da Rós, P.C.M. Utilization of co-products from corn ethanol industry in a biorefinery context: A review on the biotechnological potential of thin stillage. Braz. J. Chem. Eng. 2023. [Google Scholar] [CrossRef]
- Kordala, N.; Walter, M.; Brzozowski, B.; Lewandowska, M. 2G-biofuel ethanol: An overview of crucial operations, advances and limitations. Biomass Convers. Biorefin. 2024, 14, 2983–3006. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Pavão, L.V.; Ravagnani, M.A.S.S.; Cruz, A.J.G.; Costa, C.B.B. Process integration of a multiperiod sugarcane biorefinery. Appl. Energy 2018, 213, 520–539. [Google Scholar] [CrossRef]
- Rodríguez Carpio, R.; de Carvalho Miyoshi, S.; Elias, A.M.; Furlan, F.F.; de Campos Giordano, R.; Secchi, A.R. Multi-objective optimization of a 1G-2G biorefinery: A tool towards economic and environmental viability. J. Clean. Prod. 2021, 284, 125431. [Google Scholar] [CrossRef]
- Elias, A.M.; Longati, A.A.; de Campos Giordano, R.; Furlan, F.F. Retro-techno-economic-environmental analysis improves the operation efficiency of 1G-2G bioethanol and bioelectricity facilities. Appl. Energy 2021, 282, 116133. [Google Scholar] [CrossRef]
- Brondi, M.G.; Pinto, A.S.S.; Farinas, C.S. Combining additives improves sugars release from hydrothermally pretreated sugarcane bagasse in integrated 1G-2G biorefineries. Bioresour. Technol. Rep. 2021, 15, 100819. [Google Scholar] [CrossRef]
- Marks, C.; König, A.; Mitsos, A.; Viell, J. Minimal viable sugar yield of biomass pretreatment. Biofuels Bioprod. Biorefin. 2020, 14, 301–314. [Google Scholar] [CrossRef]
- Brandt, K.L.; Gao, J.; Wang, J.; Wooley, R.J.; Wolcott, M. Techno-Economic Analysis of Forest Residue Conversion to Sugar Using Three-Stage Milling as Pretreatment. Front. Energy Res. 2018, 6, 77. [Google Scholar] [CrossRef]
- Christensen, A.; Searle, S.; Malins, C.J.I.B. A conversational guide to... renewable identification numbers (RINs) in the US Renewable fuel standard. ICCT Briefing 2014. [Google Scholar]
- Kim, S.; Dale, B.E. A distributed cellulosic biorefinery system in the US Midwest based on corn stover. Biofuels Bioprod. Biorefin. 2016, 10, 819–832. [Google Scholar] [CrossRef]
- Jones, S.; Meyer, P.; Snowden-Swan, L.; Padmaperuma, A.; Tan, E.; Dutta, A.; Jacobson, J.; Cafferty, K. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbon Fuels: Fast Pyrolysis and Hydrotreating Bio-Oil Pathway; NREL: Golden, CO, USA, 2013.
- Dragone, G.; Kerssemakers, A.A.J.; Driessen, J.L.S.P.; Yamakawa, C.K.; Brumano, L.P.; Mussatto, S.I. Innovation and strategic orientations for the development of advanced biorefineries. Bioresour. Technol. 2020, 302, 122847. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.G.W.; Rodrigues, C.; Vandenberghe, L.P.d.S.; Woiciechowski, A.L.; Soccol, C.R. Current advances in on-site cellulase production and application on lignocellulosic biomass conversion to biofuels: A review. Biomass Bioenergy 2020, 132, 105419. [Google Scholar] [CrossRef]
- Slupska, M.; Bushong, D. Lessons from commercialisation of cellulosic ethanol-A Poet perspective. Biofuels Bioprod. Biorefin. 2019, 13, 857–859. [Google Scholar] [CrossRef]
- Atsonios, K.; Kougioumtzis, M.-A.; Panopoulos, K.D.; Kakaras, E. Alternative thermochemical routes for aviation biofuels via alcohols synthesis: Process modeling, techno-economic assessment and comparison. Appl. Energy 2015, 138, 346–366. [Google Scholar] [CrossRef]
- Dutta, A.; Sahir, A.H.; Tan, E.; Humbird, D.; Snowden-Swan, L.J.; Meyer, P.A.; Ross, J.; Sexton, D.; Yap, R.; Lukas, J. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbon Fuels: Thermochemical Research Pathways with In Situ and Ex Situ Upgrading of Fast Pyrolysis Vapors; PNNL: Richland, WA, USA, 2015.
| Feedstock + Composition (% Dry Weight) | Pretreatment Details | Bioprocessing Details | Product Recovery (g/L) | Ref. |
|---|---|---|---|---|
| SCB, cellulose 47.2%, hemicellulose 19.59%, acid-soluble lignin 2.46%, acid-insoluble lignin 25.2% | Alkaline, sodium hydroxide, 2% NaOH, 15% solid loading, 121 °C, 0.5 h | Fed-batch, 20% TS (total solids), Cellic CTec2, 52.5 °C, 48 h, 15 mg protein g−1 glucan | Glucose: 126.80 g/L, Xylose: 51.95 g/L | [20] |
| SCB, cellulose 36.7%, hemicellulose 20.5%, lignin 24.4%, ash 6.4%, extractive 4.8% | Physico-chemical, steam explosion sodium hydroxide and alkaline bleach, steam explosion: Feed rate 10 kg/h saturated steam at a rate of 25−30 kg/h at 15 bar (approx. 190 °C) with a residence time of 15 min; sodium hydroxide: 1% NaOH stirring at 80 rpm 100 °C 1 h; alkaline bleach: 80 °C 2 h 150 rpm 8% H2O2 3% NaOH 5% solid loading | Fed-batch, 20% TS, Cellic CTec2, 50 mM sodium acetate buffer (pH 4.8), 50 °C, 20 rpm, 15.8 mg protein/g glucan | Glucose: 125.00 g/L | [21] |
| SCB, cellulose 41.4%, hemicellulose 23.3%, lignin 22.1% | Physico-chemical, al-AGO, 100 g SCB 1000 g glycerol (99.5% purity) and 2.2 g NaOH [i.e., 0.2% (w/w)] catalyst mixed in a 5-liter round-bottom flask cooked at 240 °C for 30 min | Fed-batch, initial 8% to final 20% TS, LT4, 72 h, 2mg protein g−1 DM | Total sugars: 158 g/L | [22] |
| CS, cellulose 37.5%, hemicellulose 22.7%, lignin 18.2% | Physical, ball milling, 30% solid loading | batch, 30% TS, Cellic CTec2, 48 h, 10 FPU /g DM | Fermentable sugars: 130.5 g/L | [23] |
| CS, 37.5 wt% glucan, 21.6 wt% xylan, 20.4 wt% klason lignin | Physico-chemical, autoclave, 40% solid loading Ca(OH)2 or H2SO4 followed by autoclave | Fed-batch, 20% TS, Cellic CTec3-HS, 72 h, n.d. | Fermentable sugars: 255 g/L | [24] |
| RS, n.d. | Alkaline, sodium hydroxide, 20% solid loading, 42 min, 84 °C | SSF, 10% TS, Cellic CTec2, 50 °C sodium citrate buffer pH 5.5, 24 h, 60 mg enzyme protein/g dry biomass | Reducing sugars: 36.93 g/L | [25] |
| RS, carbohydrates 58% | Thermo-alkaline, Sodium hydroxide and steam, 10% solid loading, 0.25 N NaOH steam, 15 psi, 1 h | batch, 10% TS, enzyme prepared using Aspergillus niger P-19, 50 °C, 200 rpm, 5 days, 5 FPU/g | Free sugars: 70 g/L | [4] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arora, R.; Reis, C.E.R.; Chandel, A.K. Key Takeaways on the Cost-Effective Production of Cellulosic Sugars at Large Scale. Processes 2024, 12, 1496. https://doi.org/10.3390/pr12071496
Arora R, Reis CER, Chandel AK. Key Takeaways on the Cost-Effective Production of Cellulosic Sugars at Large Scale. Processes. 2024; 12(7):1496. https://doi.org/10.3390/pr12071496
Chicago/Turabian StyleArora, Richa, Cristiano E. Rodrigues Reis, and Anuj K. Chandel. 2024. "Key Takeaways on the Cost-Effective Production of Cellulosic Sugars at Large Scale" Processes 12, no. 7: 1496. https://doi.org/10.3390/pr12071496






