Morphological and Structural Characterization of Encapsulated Arginine Systems for Dietary Inclusion in Ruminants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Production of Microencapsulated Systems
2.2. Morphological, Compositional, and Chemical–Structural Characterization
2.3. In Vitro Digestibility
2.4. Statistical Analysis
3. Results and Discussion
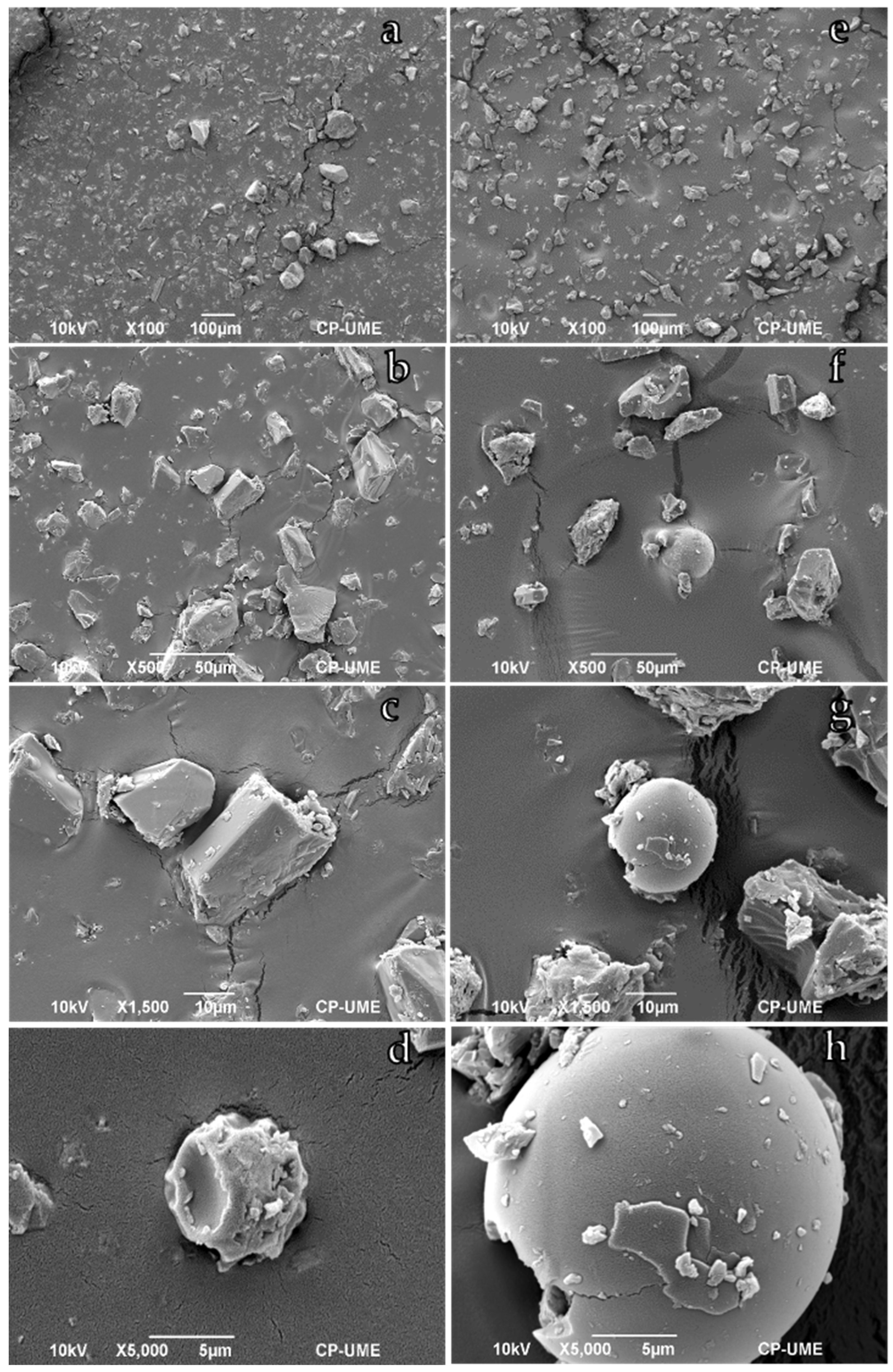
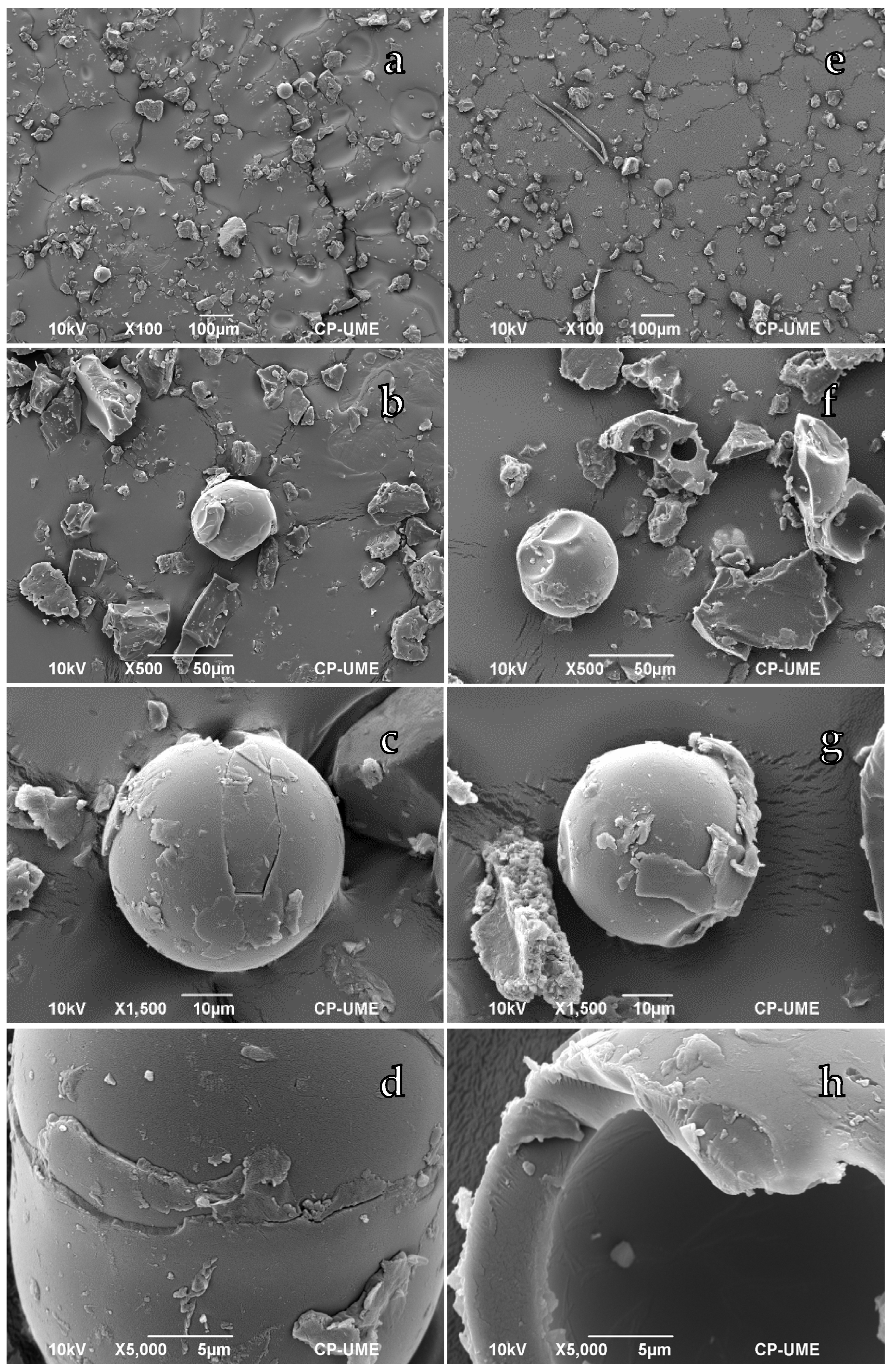
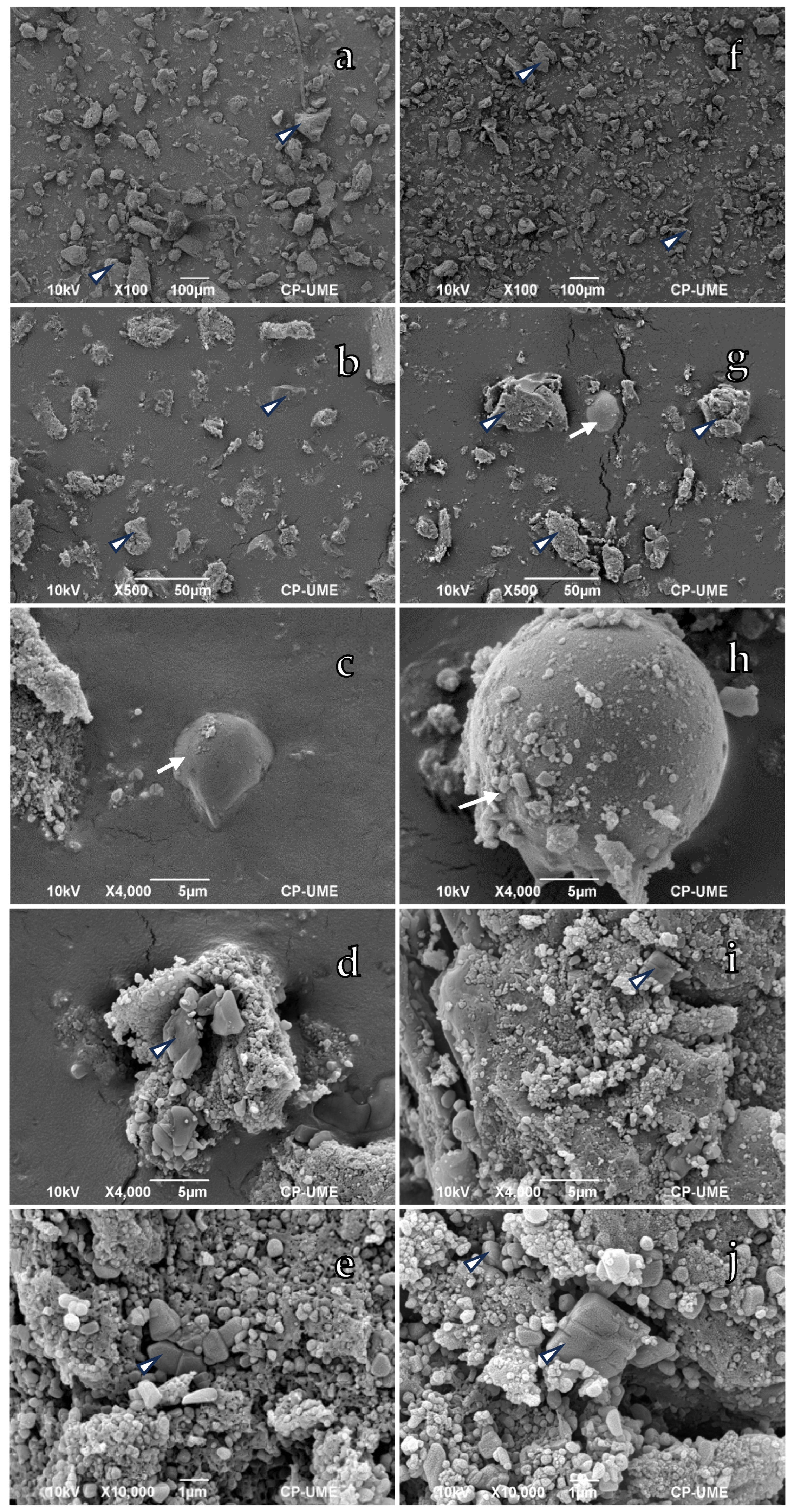
3.1. Morphological Characterization
3.2. Compositional Characterization
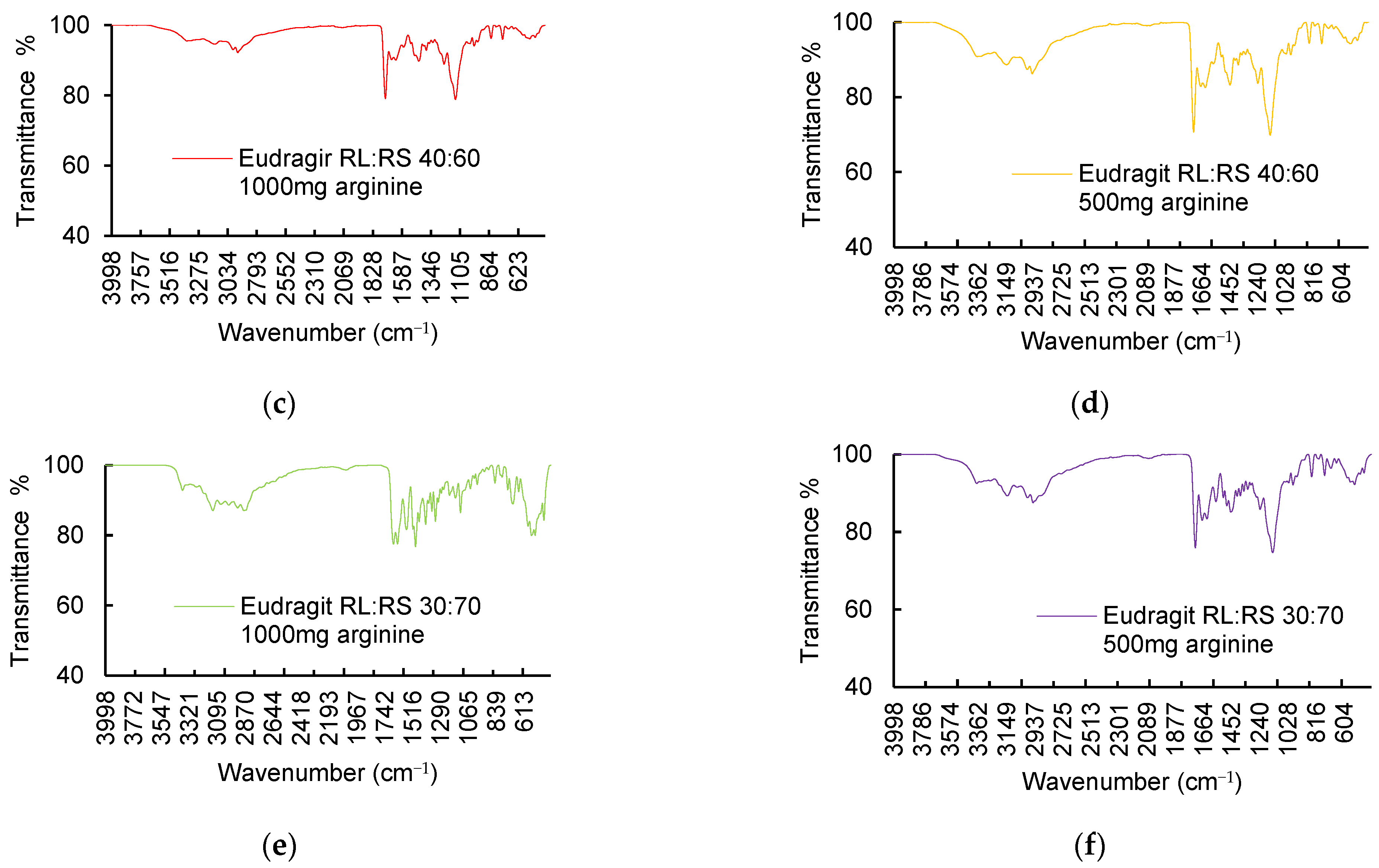
3.3. Chemical–Structural Characterization
3.4. In Vitro Digestibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamalak, A.; Canbolat, Ö.; Gürbüz, Y.; Özay, O. Protected Protein and Amino Acids in Ruminant Nutrition. KMU J. Sci. Eng. 2005, 8, 84–88. [Google Scholar]
- Morris, S.M. Recent advances in arginine metabolism: Roles and regulation of the arginases. Br. J. Pharmacol. 2009, 157, 922–930. [Google Scholar] [CrossRef] [PubMed]
- The National Academies of Sciences, Engineering and Medicine. Nutrient Requirements of Dairy Cattle, 8th ed.; National Academic Press: Washington, DC, USA, 2001. [Google Scholar]
- Wang, W.W.; Qiao, S.Y.; Li, D.F. Amino acids and gut function. Amino Acids 2009, 37, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lyu, Z.; Li, Z.; Liu, L.; Wang, F.; Li, D.; Lai, C. Effects of feeding level and dietary supplementation with crystalline amino acids on digestible, metabolizable and net energy values of corn in growing pigs. Anim. Feed Sci. Technol. 2018, 240, 197–205. [Google Scholar] [CrossRef]
- Pennings, B.; Boirie, Y.; Senden, J.M.G.; Gijsen, A.P.; Kuipers, H.; Van Loon, L.J.C. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gloaguen, M.; Le Floc’h, N.; Corrent, E.; Primot, Y.; van Milgen, J. Providing a diet deficient in valine but with excess leucine results in a rapid decrease in feed intake and modifies the postprandial plasma amino acid and α-keto acid concentrations in pigs. J. Anim. Sci. 2012, 90, 3135–3142. [Google Scholar] [CrossRef] [PubMed]
- Todd, R.D. Microencapsulation and the flavour industry. Flavour Ind. 1970, 1, 768–771. [Google Scholar]
- Piva, A.; Pizzamiglio, V.; Morlacchini, M.; Tedeschi, M.; Piva, G. Lipid microencapsulation allows slow release of organic acids and natural identical flavors along the swine intestine. J. Anim. Sci. 2007, 85, 486–493. [Google Scholar] [CrossRef]
- El-Zaiat, H.M.; Araujo, R.C.; Louvandini, H.; Patiño, H.O.; Abdalla, A.L. Effects of dietary replacement of urea with encapsulated nitrate and cashew nut shell liquid on nutrient digestibility, nitrogen balance, and carcass characteristics in growing lambs. Anim. Feed Sci. Technol. 2020, 266, 114515. [Google Scholar] [CrossRef]
- Alemu, A.W.; Romero-Pérez, A.; Araujo, R.C.; Beauchemin, K.A. Effect of encapsulated nitrate and microencapsulated blend of essential oils on growth performance and methane emissions from beef steers fed backgrounding diets. Animals 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Highstreet, A.; Robinson, P.H.; Robison, J.; Garrett, J.G. Response of Holstein cows to replacing urea with with a slowly rumen released urea in a diet high in soluble crude protein. Livest. Sci. 2010, 129, 179–185. [Google Scholar] [CrossRef]
- Contreras-López, G.; Carrillo-López, L.M.; Vargas-Bello-Pérez, E.; García-Galicia, I.A. Microencapsulation of Feed Additives with Potential in Livestock and Poultry Production: A Systematic Review. Chil. J. Agric. Anim. Sci. 2024, 40, 229–249. [Google Scholar] [CrossRef]
- Meyer, A.M.; Klein, S.I.; Kapphahn, M.; Dhuyvetter, D.V.; Musser, R.E.; Caton, J.S. Effects of rumen-protected arginine supplementation and arginine-HCl injection on site and extent of digestion and small intestinal amino acid disappearance in forage-fed steers. Transí. Anim. Sci. 2018, 2, 205–215. [Google Scholar] [CrossRef]
- Teixeira, P.D.; Tekippe, J.A.; Rodrigues, L.M.; Ladeira, M.M.H.; Pukrop, J.R.; Brad Kim, Y.H.; Schoonmaker, J.P. Effect of ruminally protected arginine and lysine supplementation on serum amino acids, performance, and carcass traits of feedlot steers. J. Anim. Sci. 2019, 97, 3511–3522. [Google Scholar] [CrossRef] [PubMed]
- Baggerman, J.O.; Thompson, A.J.; Jennings, M.A.; Hergenreder, J.E.; Rounds, W.; Smith, Z.K.; Johnson, B.J. Effects of encapsulated methionine on skeletal muscle growth and development and subsequent feedlot performance and carcass characteristics in beef steers. Animals 2021, 11, 1627. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Neto, J.P.; Bezerra, L.R.; da Silva, A.L.; de Moura, J.F.P.; Pereira Filho, J.M.; da Silva Filho, E.C.; Guedes, A.F.; Araújo, M.J.; Edvan, R.L.; Oliveira, R.L. Methionine microencapsulated with a carnauba (Copernicia prunifera) wax matrix for protection from degradation in the rumen. Livest. Sci. 2019, 228, 53–60. [Google Scholar] [CrossRef]
- Romero-Pérez, A.; García-García, E.; Zavaleta-Mancera, A.; Ramírez-Bribiesca, J.E.; Revilla-Vázquez, A.; Hernández-Calva, L.M.; López-Arellano, R.; Cruz-Monterrosa, R.G. Designing and evaluation of sodium selenite nanoparticles in vitro to improve selenium absorption in ruminants. Vet. Res. Commun. 2010, 34, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Valentine, M.E.; Karayilanli, E.; Cherney, J.H.; Cherney, D.J. Comparison of In Vitro Long Digestion Methods and Digestion Rates for Diverse Forages. Crop Sci. 2019, 59, 422–435. [Google Scholar] [CrossRef]
- Carvalho, A.D.; da Silva, A.L.; Silva, A.D.; Netto, A.J.; de Medeiros, T.T.; Araújo Filho, J.M.; Agostini, D.D.; de Oliveira, D.L.; Mazzetto, S.E.; Kotzebue, L.R.; et al. Effect of slow-release urea microencapsulated in beeswax and its inclusion in ruminant diets. Small Rumin. Res. 2019, 179, 56–63. [Google Scholar] [CrossRef]
- Mellema, M.; van Benthum, W.A.J.; Boer, B.; von Harras, J.; Visser, A. Wax encapsulation of water-soluble compounds for application in foods. J. Microencapsul. 2006, 23, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Yuan, Q.; Collins, S. Membrane Emulsification. In Encyclopedia of Surface and Colloid Science, 3rd ed.; Somasundaran, P., Ed.; Taylor & Francis: London, UK, 2010; Volume 1, pp. 1–27. [Google Scholar]
- Collins, S.; York, D.W.; Kazmi, S.; Kaleem Mohammed, A. Formation of wax walled microcapsules via double emulsion using cross membrane emulsification at elevated temperatures. J. Food Eng. 2020, 269, 109739. [Google Scholar] [CrossRef]
- Milanovic, J.; Manojlovic, V.; Levic, S.; Rajic, N.; Nedovic, V.; Bugarski, B. Microencapsulation of flavors in carnauba wax. Sensors 2010, 10, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, T.T.B.; de Azevedo Silva, A.M.; da Silva, A.L.; Bezerra, L.R.; da Silva Agostini, D.L.; de Oliveira, D.L.V.; Mazzetto, S.E.; Kotzebue, L.R.V.; Oliveira, J.R.; Souto, G.S.B.; et al. Carnauba wax as a wall material for urea microencapsulation. J. Sci. Food Agric. 2019, 99, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.X.; Chang, J.; Jia, Y.D. Microencapsulation of crystalline-methionine enclosed with gelatine and sodium alginate by spray-drying. Mater. Rese. Innov. 2015, 19, S5257–S5262. [Google Scholar] [CrossRef]
- Khamanga, S.M.; Parfitt, N.; Nyamuzhiwa, T.; Haidula, H.; Walker, R.B. The Evaluation of Eudragit Microcapsules Manufactured by Solvent Evaporation Using USP Apparatus 1. Dissolut. Technol. 2009, 16, 15–22. [Google Scholar] [CrossRef]
- Quinten, T.; Andrews, G.P.; De Beer, T.; Saerens, L.; Bouquet, W.; Jones, D.S.; Hornsby, P.; Remon, J.P.; Vervaet, C. Preparation and evaluation of sustained-release matrix tablets based on metoprolol and an acrylic carrier using injection moulding. AAPS PharmSciTech 2012, 13, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, S.; Ghanbarzadeh, S.; Mohammadhassani, Z.; Hamishehkar, H. Formulation of gammaoryzanol-loaded nanoparticles for potential application in fortifying food products. Adv. Pharm. Bull. 2014, 4, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Lira-Casas, R.; Ramirez-Bribiesca, J.E.; Zavaleta-Mancera, H.A.; Hidalgo-Moreno, C.; Cruz-Monterrosa, R.G.; Crosby-Galvan, M.M.; Mendez-Rojas, M.A.; Domínguez-Vara, I.A. Designing and evaluation of urea microcapsules in vitro to improve nitrogen slow-release availability in rumen. J. Sci. Food Agric. 2019, 99, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Wolfgong, W.J. Chemical Analysis Techniques for Failure Analysis: Part 1, Common Instrumental Methods; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, N.; Arora, S.; Singla, Y. An overview of multifaceted significance of eudragit polymers in drug delivery systems. Asian J. Pharm. Clin. Res. 2015, 8, 1–6. [Google Scholar]
- Freitas, C.A.S.; de Sousa, P.H.M.; Soares, D.J.; da Silva, J.Y.G.; Benjamin, S.R.; Guedes, M.I.F. Carnauba wax uses in food—A review. Food Chem. 2019, 291, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Guimarães-Inácio, A.; Francisco, C.R.; Rojas, V.M.; de Souza Leone, R.; Valderrama, P.; Bona, E.; Leimann, F.V.; Tanamati, A.A.; Gonçalves, O.H. Evaluation of the oxidative stability of chia oil-loaded microparticles by thermal, spectroscopic and chemometric methods. LWT Food Sci. Technol. 2018, 87, 498–506. [Google Scholar] [CrossRef]
- Huo, J.H.; Peng, Z.G.; Feng, Q. Synthesis and properties of microencapsulated phase change material with a urea–formaldehyde resin shell and paraffin wax core. J. Appl. Polym. Sci. 2020, 137, 48578. [Google Scholar] [CrossRef]
- Kashif, P.M.; Madni, A.; Ashfaq, M.; Rehman, M.; Mahmood, M.A.; Khan, M.I.; Tahir, N. Development of Eudragit RS 100 Microparticles Loaded with Ropinirole: Optimization and In Vitro Evaluation Studies. AAPS PharmSciTech 2017, 18, 1810–1822. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Cremaes, N.; Subra-Paternault, P.; Saurina, J.; Roig, A.; Domingo, C. Compressed antisolvent process for polymer coating of drug-loaded aerogel nanoparticles and study of the release behavior. Colloid. Polym. Sci. 2014, 292, 2475–2484. [Google Scholar] [CrossRef]
- Abdelbagi, M.; Ridwan, R.; Fidriyanto, R.; Rohmatussolihat, R.; Nahrowi, N.; Jayanegara, A. Effects of probiotics and encapsulated probiotics on enteric methane emission and nutrient digestibility in vitro. In Proceedings of the 3rd International Conference of Animal Science and Technology, Makassar, Indonesia, 3–4 November 2020; Volume 788. [Google Scholar] [CrossRef]
- Monteschio, J.O.; Vargas-Junior, F.M.; Almeida, F.L.; Pinto, L.A.; Kaneko, I.N.; Almeida, A.A.; Freitas, L.W.; Alves, S.P.; Bessa, R.J.; Prado, I.N. The effect of encapsulated active principles (eugenol, thymol and vanillin) and clove and rosemary essential oils on the structure, collagen content, chemical composition and fatty acid profile of Nellore heifers muscle. Meat Sci. 2019, 155, 27–35. [Google Scholar] [CrossRef]






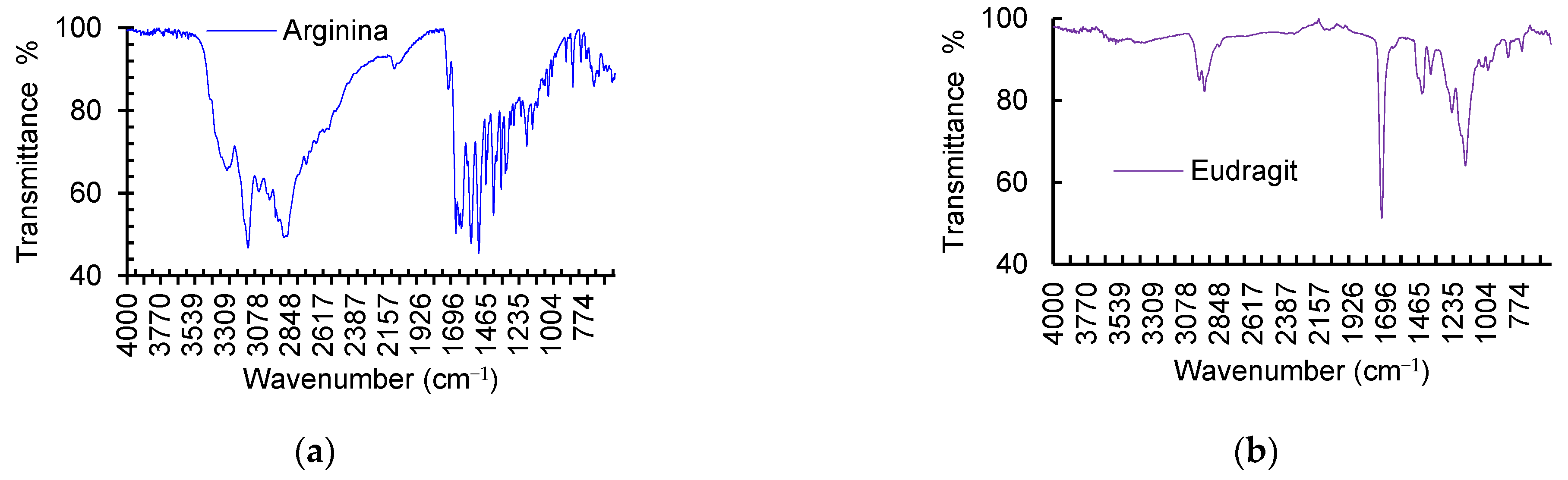
| Sample | % Nitrogen |
|---|---|
| Eudragit RL:RS 30:70 and 500 mg of arginine | 11–16 |
| Eudragit RL: 30:70 and 1000 mg of arginine | 14–18 |
| Eudragit RL:RS 40:60 and 500 mg of arginine | 26–32 |
| Eudragit RL:RS 40:60 and 1000 mg of arginine | 25–35 |
| Wax–arginine 1:1 | 21–24 |
| Wax–arginine 2:1 | 18–23 |
| Wax–arginine 3:1 | 12–16 |
| Wax–arginine 4:1 | 18–21 |
| Treatment | 1 h | 3 h | 5 h |
|---|---|---|---|
| Wax–arginine 1:1 | 42.74 ± 1.42 Ac | 34.5 ± 0.11 Ac | 47.12 ± 0.2 Ab |
| Wax–arginine 2:1 | 22.27 ± 0.05 Bd | 25.86 ± 0.41 Bd | 40.7 ± 0.12 Ac |
| Wax–arginine 3:1 | 15.94 ± 0.79 Be | 11.81 ± 0.07 Be | 21.26 ± 1.75 Ad |
| Wax–arginine 4:1 | 46.14 ± 0.09 Ab | 41.8 ± 12.94 Ab | 41.77 ± 4.25 Ac |
| Arginine | 97.77 ± 0.17 Aa | 97.9 ± 0.52 Aa | 100 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-López, G.; Morales-Rodríguez, S.; Vilchis-Néstor, A.R.; Rentería-Monterrubio, A.L.; Corral-Luna, A.; García-Galicia, I.A.; Carrillo-López, L.M. Morphological and Structural Characterization of Encapsulated Arginine Systems for Dietary Inclusion in Ruminants. Processes 2024, 12, 1498. https://doi.org/10.3390/pr12071498
Contreras-López G, Morales-Rodríguez S, Vilchis-Néstor AR, Rentería-Monterrubio AL, Corral-Luna A, García-Galicia IA, Carrillo-López LM. Morphological and Structural Characterization of Encapsulated Arginine Systems for Dietary Inclusion in Ruminants. Processes. 2024; 12(7):1498. https://doi.org/10.3390/pr12071498
Chicago/Turabian StyleContreras-López, Germán, Simón Morales-Rodríguez, Alfredo R. Vilchis-Néstor, Ana L. Rentería-Monterrubio, Agustín Corral-Luna, Ivan A. García-Galicia, and Luis M. Carrillo-López. 2024. "Morphological and Structural Characterization of Encapsulated Arginine Systems for Dietary Inclusion in Ruminants" Processes 12, no. 7: 1498. https://doi.org/10.3390/pr12071498








