Catalytic Biomass Transformation to Hydrocarbons under Supercritical Conditions over Nickel Supported on Schungite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Schungite-Based Catalyst Preparation
2.3. Catalyst Characterization
2.4. Deoxygenation Procedure
2.5. Liquid Phase Analysis
3. Results and Discussion
3.1. Catalyst Characterization
3.2. Stearic Acid Deoxygenation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asikin-Mijan, N.; Juan, J.C.; Taufiq-Yap, Y.H.; Ong, H.C.; Lin, Y.C.; AbdulKareem-Alsultan, G.; Lee, H.V. Towards sustainable green diesel fuel production: Advancements and opportunities in acid-base catalyzed H2-free deoxygenation process. Catal. Commun. 2023, 182, 106741. [Google Scholar] [CrossRef]
- Belousov, A.S.; Esipovich, A.L.; Kanakov, E.A.; Otopkova, K.V. Recent advances in sustainable production and catalytic transformations of fatty acid methyl esters. Sustain. Energy Fuels 2021, 5, 4512–4545. [Google Scholar] [CrossRef]
- Barbosa, I.V.; Scapim, L.A.; Cavalcante, R.M.; Young, A.F. Industrial production of green diesel in Brazil: Process simulation and economic perspectives. Renew. Energy 2023, 219, 119591. [Google Scholar] [CrossRef]
- Sonthalia, A.; Kumar, N. Hydroprocessed vegetable oil as a fuel for transportation sector: A review. J. Energy Inst. 2019, 92, 1–17. [Google Scholar] [CrossRef]
- Amin, A. Review of diesel production from renewable resources: Catalysis, process kinetics and technologies. Ain Shams Eng. J. 2019, 10, 821–839. [Google Scholar] [CrossRef]
- Cremonez, P.A.; Teleken, J.G.; Weiser Meier, T. Potential of green diesel to complement the Brazilian energy production: A review. Energy Fuels 2021, 35, 176–186. [Google Scholar] [CrossRef]
- Azman, N.S.; Marliza, T.S.; Mijan, N.A.; Yun Hin, T.Y.; Khairuddin, N. Production of Biodiesel from Waste Cooking Oil via Deoxygenation Using Ni-Mo/Ac Catalyst. Processes 2021, 9, 750. [Google Scholar] [CrossRef]
- Alvarez-Galvan, M.C.; Blanko-Brieva, G.; Capel-Sanchez, M.; Morales-delaRosa, S.; Campos-Martin, J.M.; Fierro, J.L.G. Metal phosphide catalysts for the hydrotreatment of non-edible vegetable oils. Catal. Today 2018, 302, 242–249. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Yusup, S.; Ramli, A.; Shahbaz, M.; Aqsha, A. Process optimization of green diesel selectivity and understanding of reaction intermediates. Renew. Energy 2020, 149, 1092–1106. [Google Scholar] [CrossRef]
- Sousa, F.P.; Silva, L.N.; de Rezende, D.B.; de Oliveira, L.C.; Pasa, V.M.D. Simultaneous deoxygenation, cracking and isomerization of palm kernel oil and palm olein over beta zeolite to produce biogasoline, green diesel and biojet-fuel. Fuel 2018, 223, 149–156. [Google Scholar] [CrossRef]
- Oi, L.E.; Choo, M.Y.; Lee, H.V.; Taufiq-Yap, Y.H.; Cheng, C.K.; Juan, J.C. Catalytic deoxygenation of triolein to green fuel over mesoporous TiO2 aided by in situ hydrogen production. Int. J. Hydrog. Energy 2020, 45, 11605–11614. [Google Scholar] [CrossRef]
- Manimaran, R.; Mohanraj, T.; Prabakaran, S. Biodegradable waste-derived biodiesel as a potential green fuel: Optimization of production process and its application in diesel engine. Ind. Crops Prod. 2023, 192, 116078. [Google Scholar] [CrossRef]
- Cavalcanti, C.D.S.; Ravagnani, M.A.S.S.; Stragevitch, L.; Carvalho, F.R.; Pimentel, M.F. Simulation of the soybean oil hydrotreating process for green diesel production. Clean. Chem. Eng. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Ayala-Cortes, A.; Torres, D.; Frecha, E.; Arcelus-Arrillaga, P.; Villafan-Vidales, H.I.; Langoria, A.; Pinilla, J.L.; Suelves, I. Upgrading of biomass-derived solar hydrothermal bio-oils through catalytic hydrodeoxygenation in supercritical ethanol. J. Environ. Chem. Eng. 2023, 11, 111395. [Google Scholar] [CrossRef]
- Zamani, A.S.; Saidi, M.; Najafabadi, A.T. Selective production of diesel-like alkanes via Neem seed oil hydrodeoxygenation over Ni/MgSiO3 catalyst. Renew. Energy 2023, 209, 462–470. [Google Scholar] [CrossRef]
- Jeon, K.W.; Gong, J.H.; Kim, M.J.; Shim, J.O.; Jang, W.J.; Roh, H.S. Review on the production of renewable biofuel: Solvent-free deoxygenation. Renew. Sustain. Energy Rev. 2024, 195, 114325. [Google Scholar] [CrossRef]
- Mahene, W.L.; Kivevele, T.; Machunda, R. The role of textural properties and surface chemistry of activated carbon support in catalytic deoxygenation of triglycerides into renewable diesel. Catal. Commun. 2023, 181, 106737. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Y.; Xu, D.; Guan, Q. A review on recent advances in clean microalgal bio-oil production via catalytic hydrothermal deoxygenation. J. Clean. Prod. 2022, 366, 132978. [Google Scholar] [CrossRef]
- Konwar, L.J.; Mikkola, J.P. Carbon support effects on metal (Pd, Pt and Ru) catalyzed hydrothermal decarboxylation/deoxygenation of triglycerides. Appl. Catal. A Gen. 2022, 638, 118611. [Google Scholar] [CrossRef]
- Zhang, Z.; Okejiri, F.; Li, Y.; Li, J.; Fu, J. Hydrodecarboxylation of Fatty Acids into Liquid Hydrocarbons over Commercial Ru/C Catalyst under Mild Conditions. New J. Chem. 2020, 44, 7642–7646. [Google Scholar] [CrossRef]
- Omer, A.; Kazmi, W.W.; Rahimipetroudi, I.; Syed, M.W.; Rashid, K.; Yang, J.B.; Lee, I.G.; Dong, S.K. Experimental and numerical study on the hexadecanoic acid upgrading kinetics under supercritical ethanol without the use of hydrogen. Renew. Energy 2023, 2019, 119552. [Google Scholar] [CrossRef]
- Lin, M.; Yan, Y.; Li, X.; Li, R.; Wu, Y. Potassium-assisted activation strategy regulating metal-support interaction to promote hydrothermal hydrogenation/deoxygenation of palmitic acid. Fuel Process. Technol. 2023, 250, 107892. [Google Scholar] [CrossRef]
- Baharudin, K.B.; Taufiq-Yap, Y.H.; Hunns, J.; Isaacs, M.; Wilson, K.; Derawi, D. Mesoporous NiO/Al-SBA-15 catalysts for solvent-free deoxygenation of palm fatty acid distillate. Microporous Mesoporous Mater. 2019, 276, 13–22. [Google Scholar] [CrossRef]
- Li, X.; Li, R.; Lin, M.; Yang, M.; Wu, Y. High hydrothermal stability Co@NC catalyst for hydrothermal deoxygenation of algae-based bio-oil model compound. Chem. Eng. Sci. 2024, 284, 119450. [Google Scholar] [CrossRef]
- Gamal, M.S.; Asikin-Mijan, N.; Khalit, W.N.A.W.; Arumugam, M.; Izham, S.M.; Taufiq-Yap, Y.H. Effective catalytic deoxygenation of palm fatty acid distillate for green diesel production under hydrogen-free atmosphere over bimetallic catalyst CoMo supported on activated carbon. Fuel Process. Technol. 2020, 208, 106519. [Google Scholar] [CrossRef]
- Papageridis, K.N.; Charisiou, N.D.; Douvartzides, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKhoori, A.A.; Alkhoori, S.I.; Polychronopoulou, K.; Goula, M.A. Continuous selective deoxygenation of palm oil for renewable diesel production over Ni catalysts supported on Al2O3 and La2O3–Al2O3. RSC Adv. 2021, 11, 8569. [Google Scholar] [CrossRef]
- Mahdi, H.I.; Bazargan, A.; McKay, G.; Azelee, N.I.W.; Meili, L. Catalytic deoxygenation of palm oil and its residue in green diesel production: A current technological review. Chem. Eng. Res. Des. 2021, 174, 158–187. [Google Scholar] [CrossRef]
- Chean, K.W.; Yusup, S.; Loy, A.C.M.; How, B.S.; Skoulou, V.; Taylor, M.J. Recent advances in the catalytic deoxygenation of plant oils and prototypical fatty acid models compounds: Catalysis, process, and kinetics. Mol. Catal. 2022, 523, 111469. [Google Scholar]
- Luo, W.; Cao, W.; Bruijnincx, P.C.A.; Lin, L.; Wang, A.; Zhang, T. Zeolite-supported metal catalysts for selective hydrodeoxygenation of biomass-derived platform molecules. Green Chem. 2019, 21, 3744. [Google Scholar] [CrossRef]
- Obradovic, N.; Gigov, M.; Dordevic, A.; Kern, F.; Dmitrovic, S.; Matovic, B.; Dordevic, A.; Tshantshapanyan, A.; Vlanovic, B.; Petrovic, P.; et al. Shungite—A carbon-mineral rock material: Its sinterability and possible applications. Process. Appl. Ceram. 2019, 13, 89–97. [Google Scholar] [CrossRef]
- Rozhkova, N.N. Schungite Nanocarbons: Structural and Physics Chemical Properties, Activation Mechanisms. Ph.D. Thesis, Institute of Geology, Karelian Science Center, RAS, Saint-Petersburg, Russia, 2013; p. 39. [Google Scholar]
- Frac, M.; Szudek, W.; Szoldra, P.; Pichor, W. The applicability of shungite as an electrically conductive additive in cement composites. J. Build. Eng. 2022, 45, 103469. [Google Scholar] [CrossRef]
- Efremov, S.; Nachiporenko, S.; Tokmurzin, D.; Kaiaidarova, A.; Kalugin, S.; Tassibekov, K. Remediation of soil contaminated by toxic rocket fuel components using modified carbon–mineral adsorbing material produced from shungite rock modified with Mn4+ and Fe3+. Environ. Technol. Innov. 2021, 24, 101962. [Google Scholar] [CrossRef]
- Mooste, M.; Tkesheliadze, T.; Kozlova, J.; Kikas, A.; Kisand, V.; Treshchalov, A.; Tamm, A.; Aruvali, J.; Zagal, J.H.; Kannan, A.M.; et al. Transition metal phthalocyanine-modified shungite-based cathode catalysts for alkaline membrane fuel cell. Int. J. Hydrog. Energy 2021, 46, 4365–4377. [Google Scholar] [CrossRef]
- Balta, Z.; Simsek, E.B. Insights into the photocatalytic behavior of carbon-rich shungite-based WO3/TiO2 catalysts for enhanced dye and pharmaceutical degradation. New Carbon. Mater. 2020, 35, 371–383. [Google Scholar] [CrossRef]
- Stepacheva, A.A.; Markova, M.E.; Schipanskaya, E.O.; Matveeva, V.G.; Sulman, M.G. Highly Effective Schungite-Based Catalyst for Deoxygenation of Biomass Components. Chem. Eng. Trans. 2021, 88, 283–288. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodrigues-Reinoso, F.; Rouquerol, J.; Sing, K.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- He, X.; Xu, Y.; Yao, X.; Zhang, C.; Pu, Y.; Wang, X.; Mao, W.; Du, Y.; Zhong, W. Large exchange bias and enhanced coercivity in strongly-coupled Ni/NiO binary nanoparticles. RSC Adv. 2019, 9, 30195–30206. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Venderbosch, R.H.; He, S.; Bykova, M.V.; Khromova, S.A.; Yakovlev, V.A.; Heeres, H.J. Mono-, bi-, and tri-metallic Ni-based catalysts for the catalytic hydrotreatment of pyrolysis liquids. Biomass-Convers. Biorefinery 2017, 7, 361–376. [Google Scholar] [CrossRef]
- Stepacheva, A.A.; Sidorov, A.I.; Matveeva, V.G.; Sulman, E.M.; Sulman, M.G. Synthesis Fatty acid deoxygenation in supercritical hexane over catalysts synthesized hydrothermally for biodiesel production. Chem. Eng. Technol. 2019, 42, 780–787. [Google Scholar] [CrossRef]
- Stepacheva, A.A.; Bykov, A.V.; Sidorov, A.I.; Sulman, M.G.; Matveeva, V.G.; Sulman, E.M.; Markova, M.E. Ni catalyst synthesized by hydrothermal deposition on the polymer matrix in the supercritical deoxygenation of fatty acids. React. Kinet. Mech. Catal. 2018, 13, 74–81. [Google Scholar]
- Zhang, Z.; Zhao, G.; Sun, W.; Liu, Y.; Lu, Y. Oxidative Dehydrogenation of Ethane: Superior Nb2O5-NiO/Ni-Foam Catalyst Tailored by Tuning Morphology of NiO-Precursors Grown on a Ni-Foam. iScience 2019, 20, 90–99. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Zhang, J.; Luque, R.; Yan, N. Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef]
- L’vov, B.V.; Galwey, A.K. The mechanism and kinetics of NiO reduction by hydrogen. Thermochemical approach. J. Therm. Anal. Calorim. 2012, 110, 601–610. [Google Scholar] [CrossRef]
- Lin, M.; Yan, Y.; Li, X.; Li, R.; Wu, Y. Hydrothermal hydrogenation/deoxygenation of palmitic acid to alkanes over Ni/activated carbon catalyst. Chin. J. Chem. Eng. 2024, 66, 8–18. [Google Scholar] [CrossRef]
- Liu, Y.; Xheng, D.; Yu, H.; Liu, X.; Yu, S.; Wang, X.; Li, L.; Pang, J.; Liu, X.; Yan, Z. Rapid and green synthesis of SAPO-11 for deoxygenation of stearic acid to produce bio-diesel fractions. Microporous Mesoporous Mater. 2020, 303, 110280. [Google Scholar] [CrossRef]
- Jeon, K.W.; Park, H.R.; Lee, Y.L.; Kim, J.E.; Jang, W.J.; Shim, J.O.; Roh, H.S. Deoxygenation of non-edible fatty acid for green diesel production: Effect of metal loading amount over Ni/MgO–Al2O3 on the catalytic performance and reaction pathway. Fuel 2022, 311, 122488. [Google Scholar] [CrossRef]
- Hongloi, N.; Prapainainar, P.; Prapainainar, C. Review of green diesel production from fatty acid deoxygenation over Ni-based catalysts. Mol. Catal. 2022, 523, 111696. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Hafeez, S.; Charisiou, N.D.; Al-Salem, S.M.; Manos, G.; Constantinou, A.; Alkhoori, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; et al. Selective catalytic deoxygenation of palm oil to produce green diesel over Ni catalysts supported on ZrO2 and CeO2–ZrO2: Experimental and process simulation modelling studies. Renew. Energy 2023, 206, 582–596. [Google Scholar] [CrossRef]
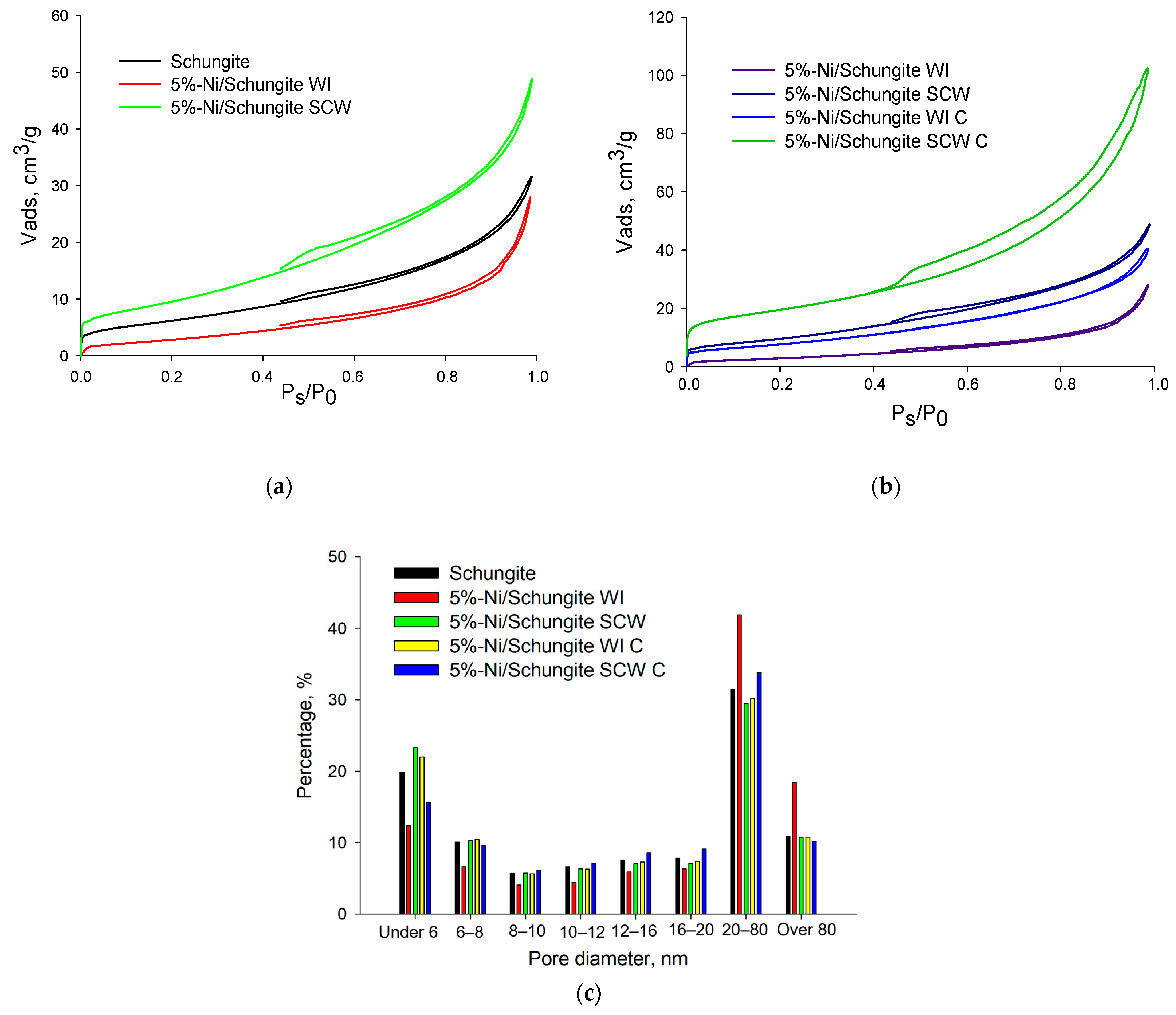
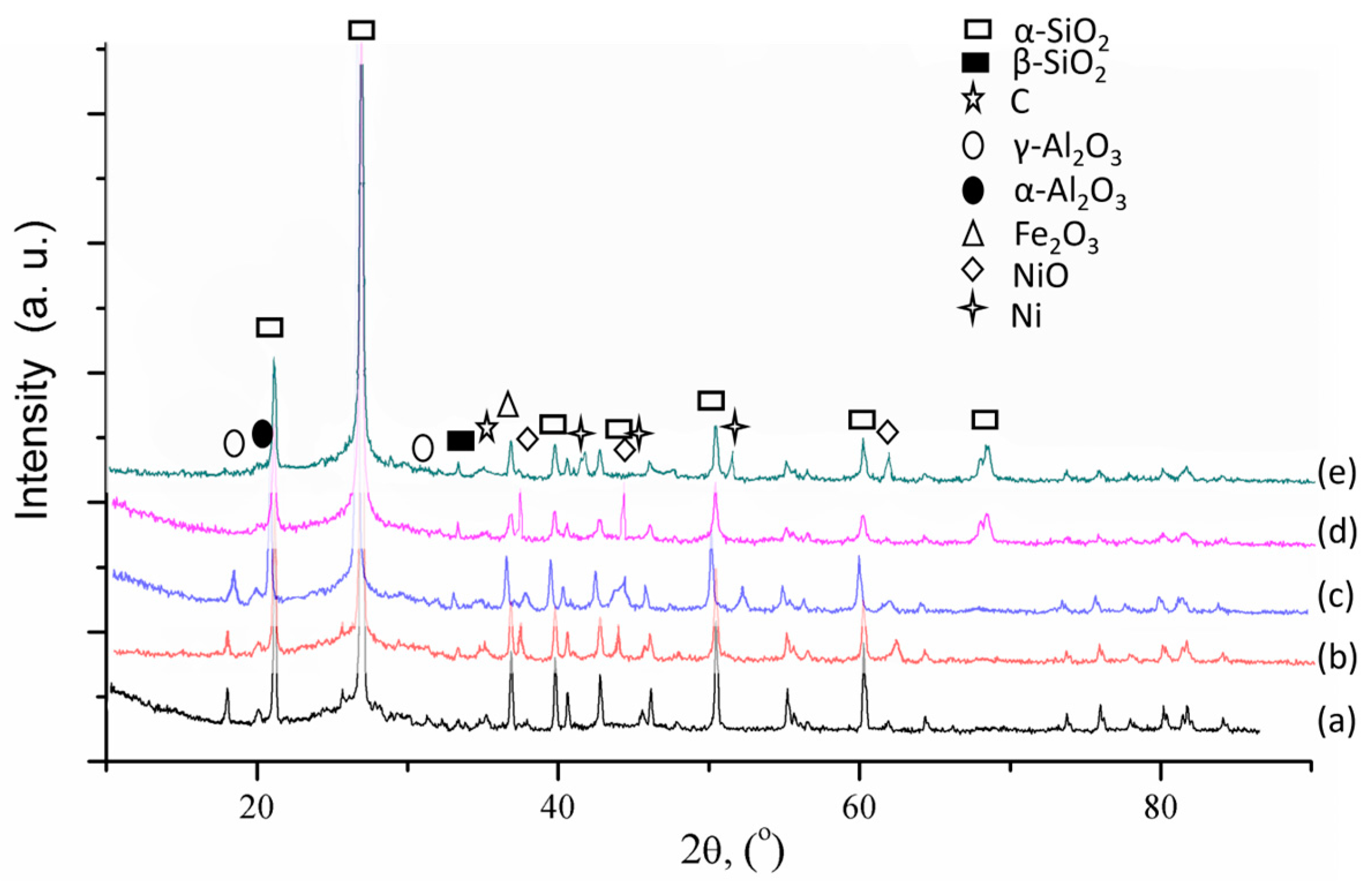
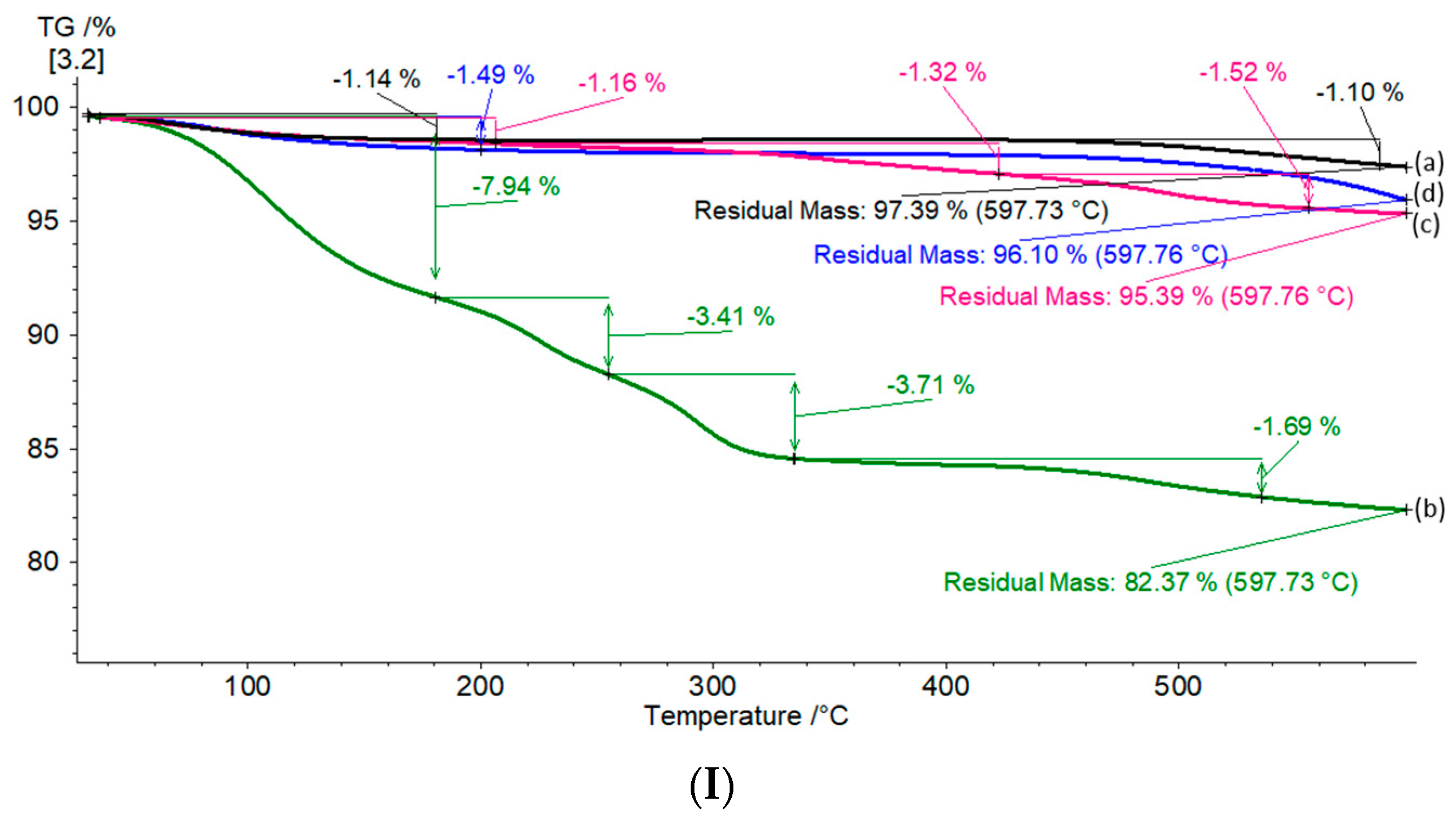

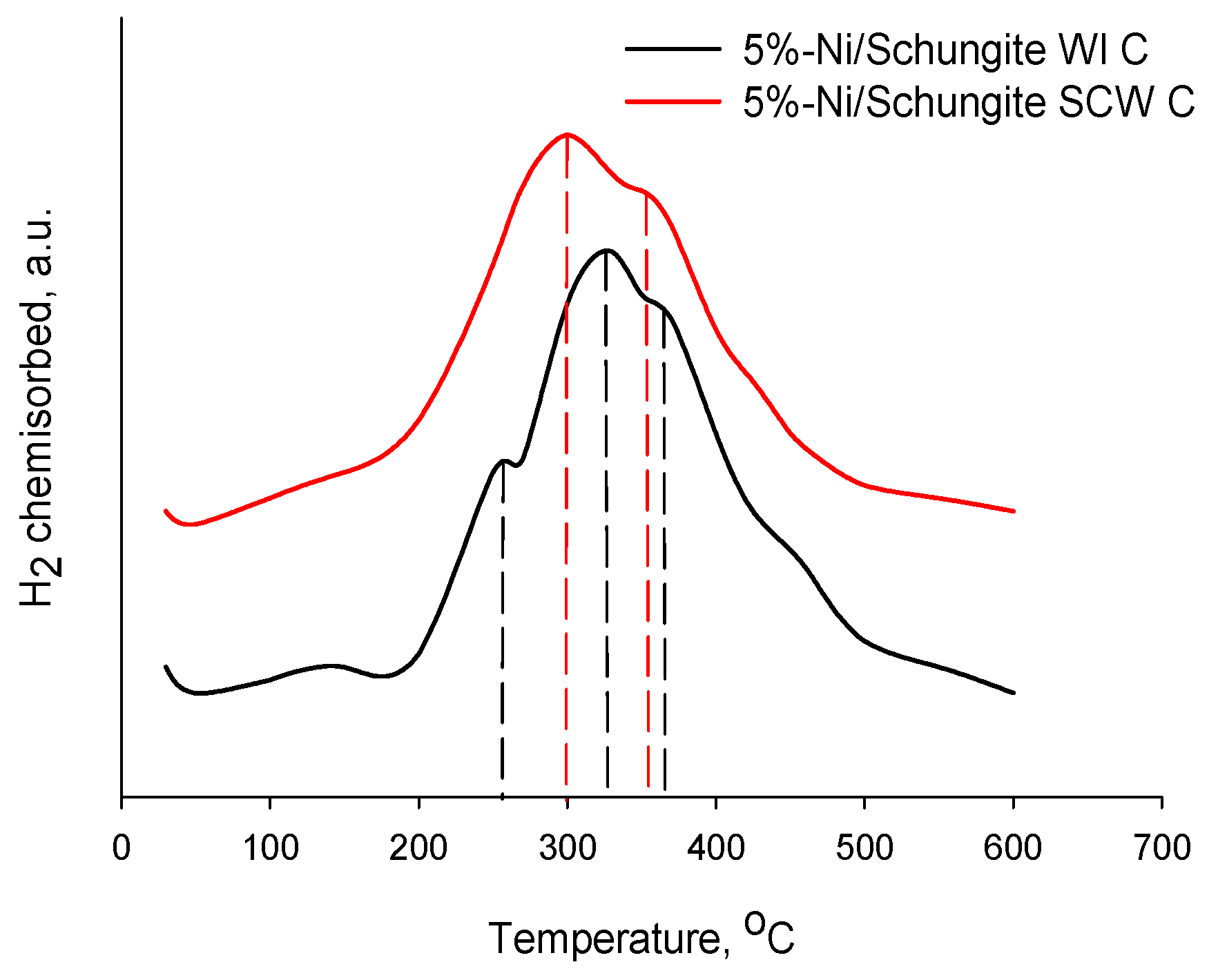
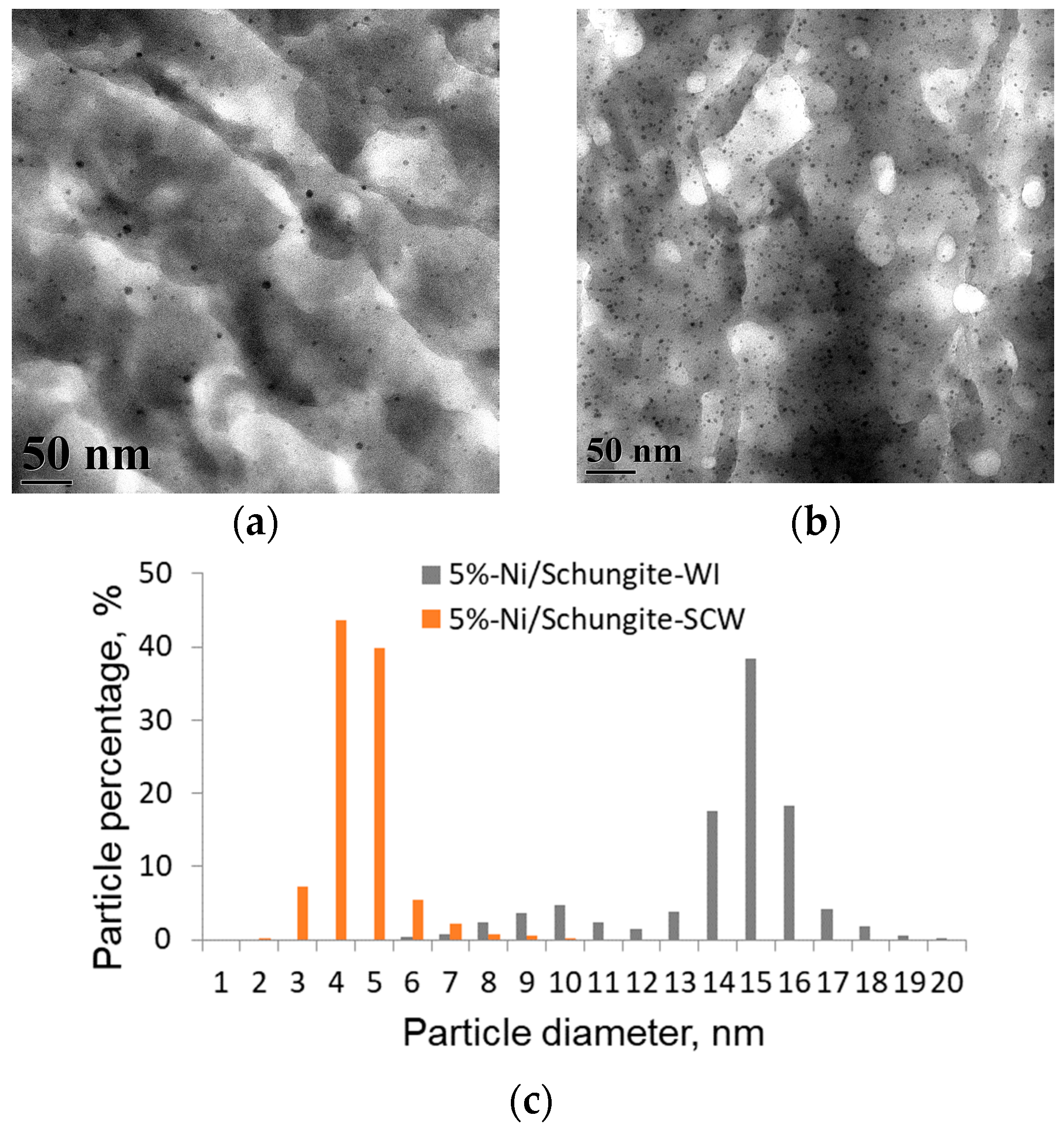
| Sample | Pore Volume, cm3/g | Specific Surface Area, m2/g * | Ni Concentration, wt.% ** | Ni Particle Mean Diameter, nm | Ni Dispersion, % *** |
|---|---|---|---|---|---|
| Schungite | 0.06 ± 0.01 | 22 ± 2 | - | - | - |
| Schungite calcinated | 0.06 ± 0.01 | 29 ± 2 | - | - | - |
| 5%-Ni/schungite-WI | 0.05 ± 0.01 | 10 ± 1 | 3.2 ± 0.1 | 12.2 ± 0.1 | 8.8 ± 0.2 |
| 5%-Ni/schungite-WI calcinated | 0.06 ± 0.01 | 27 ± 2 | 3.2 ± 0.1 | 13.7 ± 0.2 | 7.9 ± 0.1 |
| 5%-Ni/schungite-WI reduced | 0.05 ± 0.01 | 17 ± 1 | 3.2 ± 0.1 | 15.4 ± 0.2 | 6.8 ± 0.1 |
| 5%-Ni/schungite-SCW | 0.08 ± 0.01 | 34 ± 3 | 4.9 ± 0.1 | 4.2 ± 0.1 | 23.4 ± 0.2 |
| 5%-Ni/schungite-SCW calcinated | 0.16 ± 0.02 | 69 ± 3 | 4.9 ± 0.1 | 4.5 ± 0.1 | 22.9 ± 0.2 |
| 5%-Ni/schungite-SCW reduced | 0.15 ± 0.02 | 61 ± 2 | 4.9 ± 0.1 | 4.6 ± 0.1 | 21.3 ± 0.2 |
| Element | Concentration, wt.% * | |
|---|---|---|
| Initial | After Treatment in Subcritical Water | |
| Ba | 0.32 ± 0.01 | 0.15 ± 0.01 |
| Ca | 0.09 ± 0.01 | 0.02 ± 0.01 |
| Mg | 0.34 ± 0.01 | 0.12 ± 0.01 |
| K | 1.02 ± 0.01 | 0.08 ± 0.01 |
| Na | 0.27 ± 0.01 | 0.06 ± 0.01 |
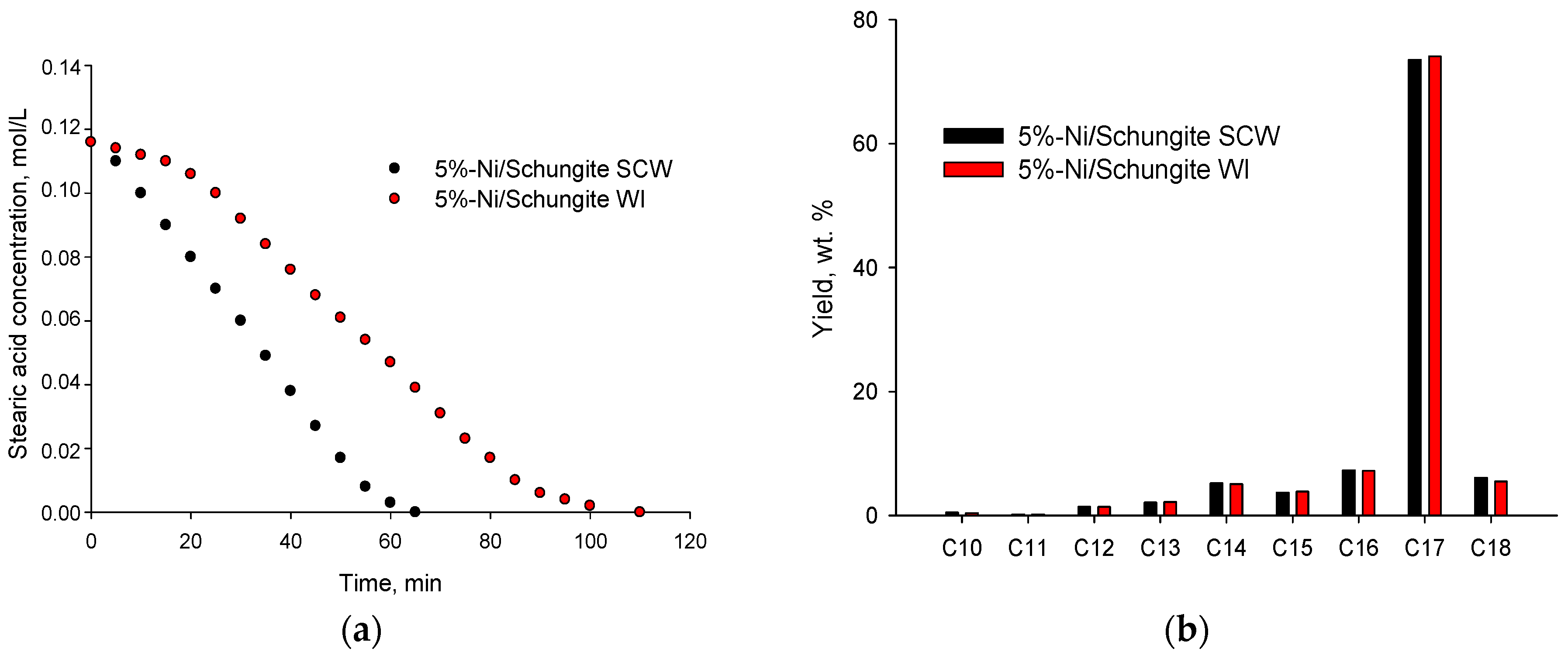
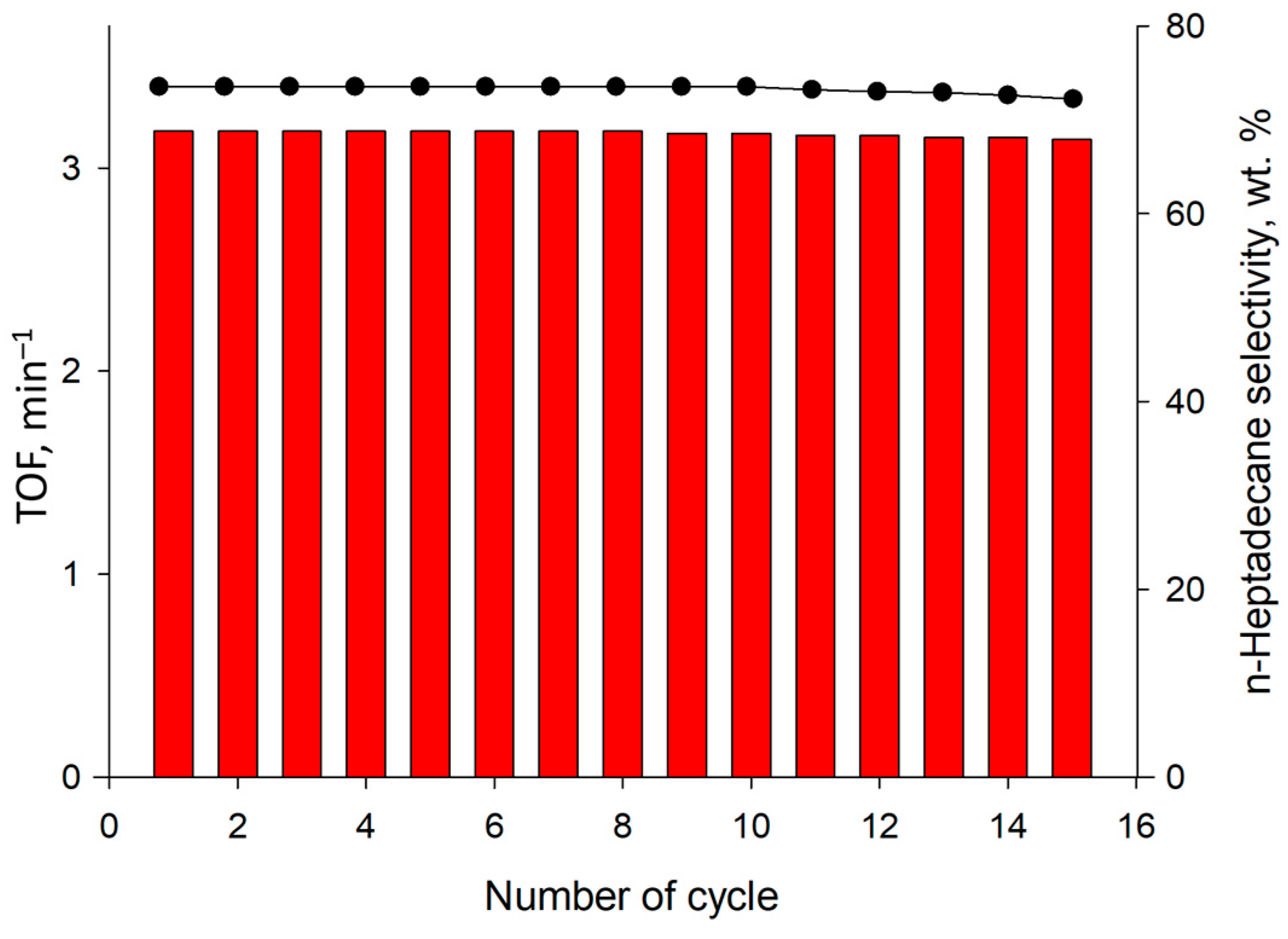
| Ni Precursor | TOF, min−1 | Selectivity to n-Heptadecane, wt.% | Specific Surface Area, m2/g * | Ni Concentration, wt.% ** | Ni Dispersion, % *** |
|---|---|---|---|---|---|
| Ni(NO3)2 | 3.18 ± 0.01 | 73.5 ± 0.1 | 61 ± 2 | 4.9 ± 0.1 | 23.4 ± 0.2 |
| Ni(CH3COO)2 | 3.05 ± 0.01 | 73.0 ± 0.1 | 58 ± 2 | 4.9 ± 0.1 | 20.8 ± 0.2 |
| NiCl2 | 2.48 ± 0.01 | 72.4 ± 0.1 | 46 ± 1 | 4.9 ± 0.1 | 12.5 ± 0.1 |
| Ni Concentration in Catalyst, wt.% * | TOF, min−1 | Selectivity to n-Heptadecane, wt.% | Specific Surface Area, m2/g ** | Ni Dispersion, % *** |
|---|---|---|---|---|
| 2.0 ± 0.1 | 2.55 ± 0.01 | 73.8 ± 0.1 | 65 ± 2 | 27.6 ± 0.2 |
| 4.9 ± 0.1 | 3.18 ± 0.01 | 73.5 ± 0.1 | 61 ± 2 | 23.4 ± 0.2 |
| 9.8 ± 0.1 | 2.48 ± 0.01 | 70.2 ± 0.1 | 39 ± 2 | 11.2 ± 0.1 |
| Parameter | Value | TOF, min−1 | Total Alkane Yield, wt.% | Selectivity to n-Heptadecane, % |
|---|---|---|---|---|
| Temperature, °C (Process conditions: hydrogen partial pressure of 1.5 MPa, catalyst loading 13.2 mol of stearic acid per mol of Ni) | 240 | 1.76 ± 0.01 | 81.1 ± 0.1 | 86.2 ± 0.1 |
| 250 | 2.18 ± 0.01 | 89.4 ± 0.1 | 81.4 ± 0.1 | |
| 260 | 2.52 ± 0.01 | 95.2 ± 0.1 | 76.4 ± 0.1 | |
| 270 | 2.87 ± 0.01 | 98.6 ± 0.1 | 73.8 ± 0.1 | |
| 280 | 3.18 ± 0.01 | 99.9 ± 0.1 | 73.5 ± 0.1 | |
| 290 | 3.75 ± 0.01 | 99.9 ± 0.1 | 67.4 ± 0.1 | |
| 300 | 4.23 ± 0.01 | 99.9 ± 0.1 | 52.6 ± 0.1 | |
| Hydrogen partial pressure, MPa (Process conditions: temperature of 280 °C, catalyst loading 13.2 mol of stearic acid per mol of Ni) | 0.5 | 1.11 ± 0.01 | 62.4 ± 0.1 | 92.2 ± 0.1 |
| 1.0 | 2.38 ± 0.01 | 79.8 ± 0.1 | 81.7 ± 0.1 | |
| 1.5 | 3.18 ± 0.01 | 99.9 ± 0.1 | 73.5 ± 0.1 | |
| 2.0 | 4.56 ± 0.01 | 99.9 ± 0.1 | 72.2 ± 0.1 | |
| 2.5 | 5.48 ± 0.01 | 99.9 ± 0.1 | 70.5 ± 0.1 | |
| 3.0 | 6.92 ± 0.01 | 99.9 ± 0.1 | 68.7 ± 0.1 | |
| Substrate-catalyst ratio, mol stearic acid/mol Ni (Process conditions: temperature of 280 °C, hydrogen partial pressure of 1.5 MPa) | 3.3 | 1.42 ± 0.01 | 99.9 ± 0.1 | 51.3 ± 0.1 |
| 6.6 | 2.23 ± 0.01 | 99.9 ± 0.1 | 68.1 ± 0.1 | |
| 13.2 | 3.18 ± 0.01 | 99.9 ± 0.1 | 73.5 ± 0.1 | |
| 26.4 | 5.06 ± 0.01 | 88.1 ± 0.1 | 86.4 ± 0.1 | |
| 52.8 | 7.25 ± 0.01 | 76.4 ± 0.1 | 98.5 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schipanskaya, E.O.; Stepacheva, A.A.; Markova, M.E.; Bykov, A.V.; Sidorov, A.I.; Matveeva, V.G.; Sulman, M.G.; Kiwi-Minsker, L. Catalytic Biomass Transformation to Hydrocarbons under Supercritical Conditions over Nickel Supported on Schungite. Processes 2024, 12, 1503. https://doi.org/10.3390/pr12071503
Schipanskaya EO, Stepacheva AA, Markova ME, Bykov AV, Sidorov AI, Matveeva VG, Sulman MG, Kiwi-Minsker L. Catalytic Biomass Transformation to Hydrocarbons under Supercritical Conditions over Nickel Supported on Schungite. Processes. 2024; 12(7):1503. https://doi.org/10.3390/pr12071503
Chicago/Turabian StyleSchipanskaya, Elena O., Antonina A. Stepacheva, Mariia E. Markova, Alexey V. Bykov, Alexander I. Sidorov, Valentina G. Matveeva, Mikhail G. Sulman, and Lioubov Kiwi-Minsker. 2024. "Catalytic Biomass Transformation to Hydrocarbons under Supercritical Conditions over Nickel Supported on Schungite" Processes 12, no. 7: 1503. https://doi.org/10.3390/pr12071503





