Competitive Adsorption of Moisture and SO2 for Carbon Dioxide Capture by Zeolites FAU 13X and LTA 5A
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental Procedure
2.2.2. Molecular Sieve Activation and Modification Methods
2.2.3. Distributed Water Distribution Method
2.2.4. Evaluation of CO2 Adsorption Capacity
3. Results and Discussion
3.1. Influencing Parameters on CO2 Adsorption Capacity
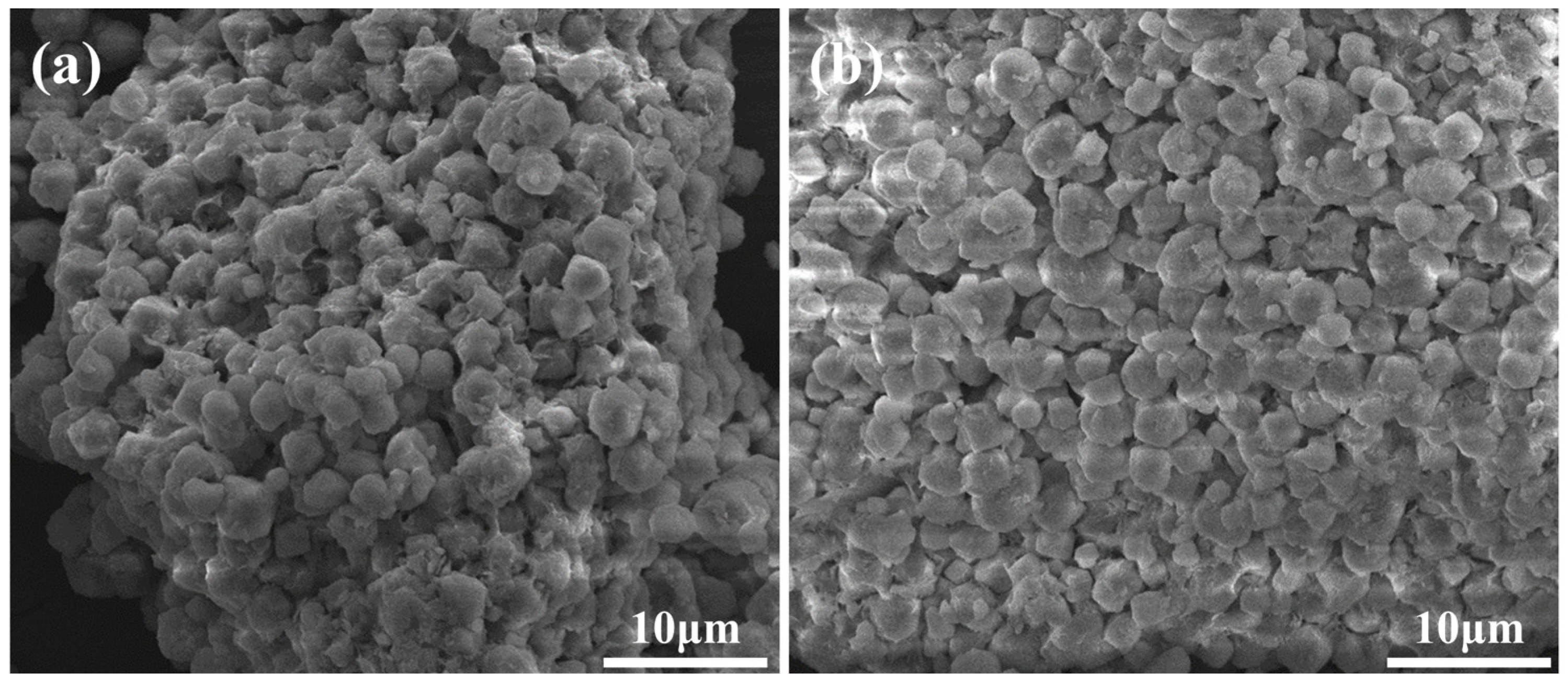
3.1.1. Effect of Molecular Sieve Particle Size
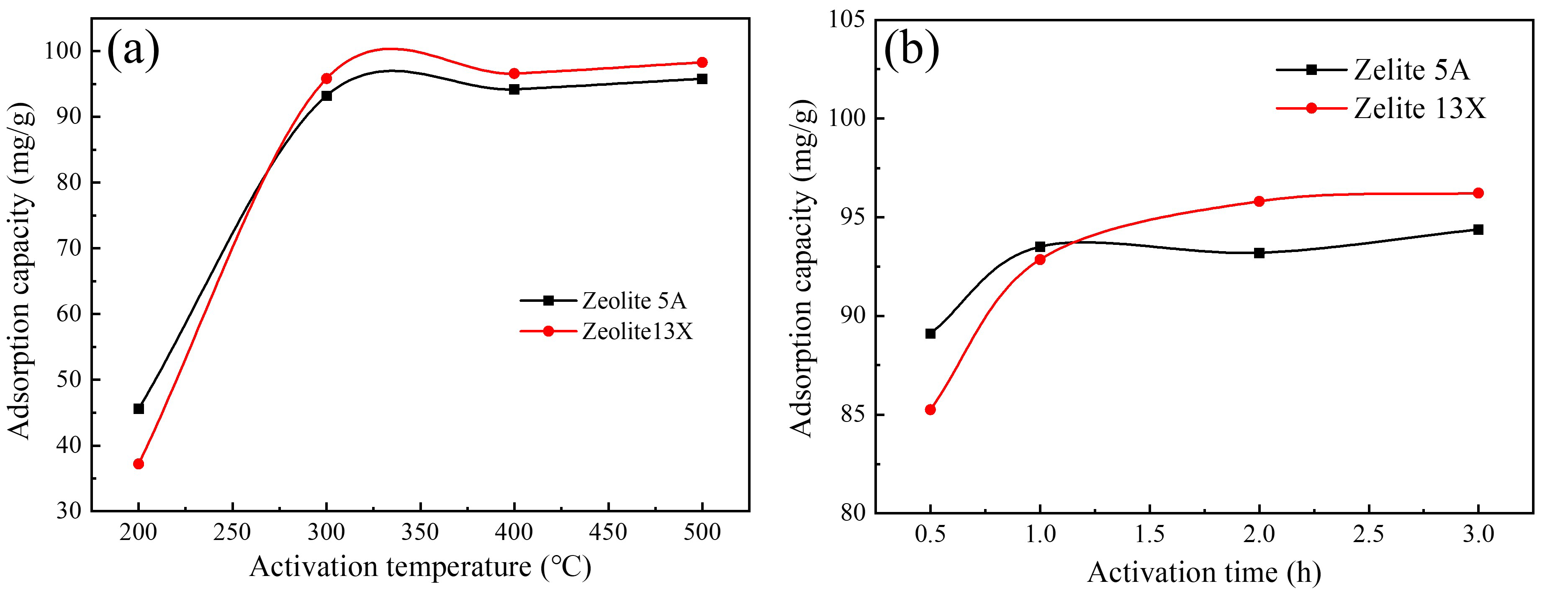
3.1.2. Effect of Activation Temperature and Time
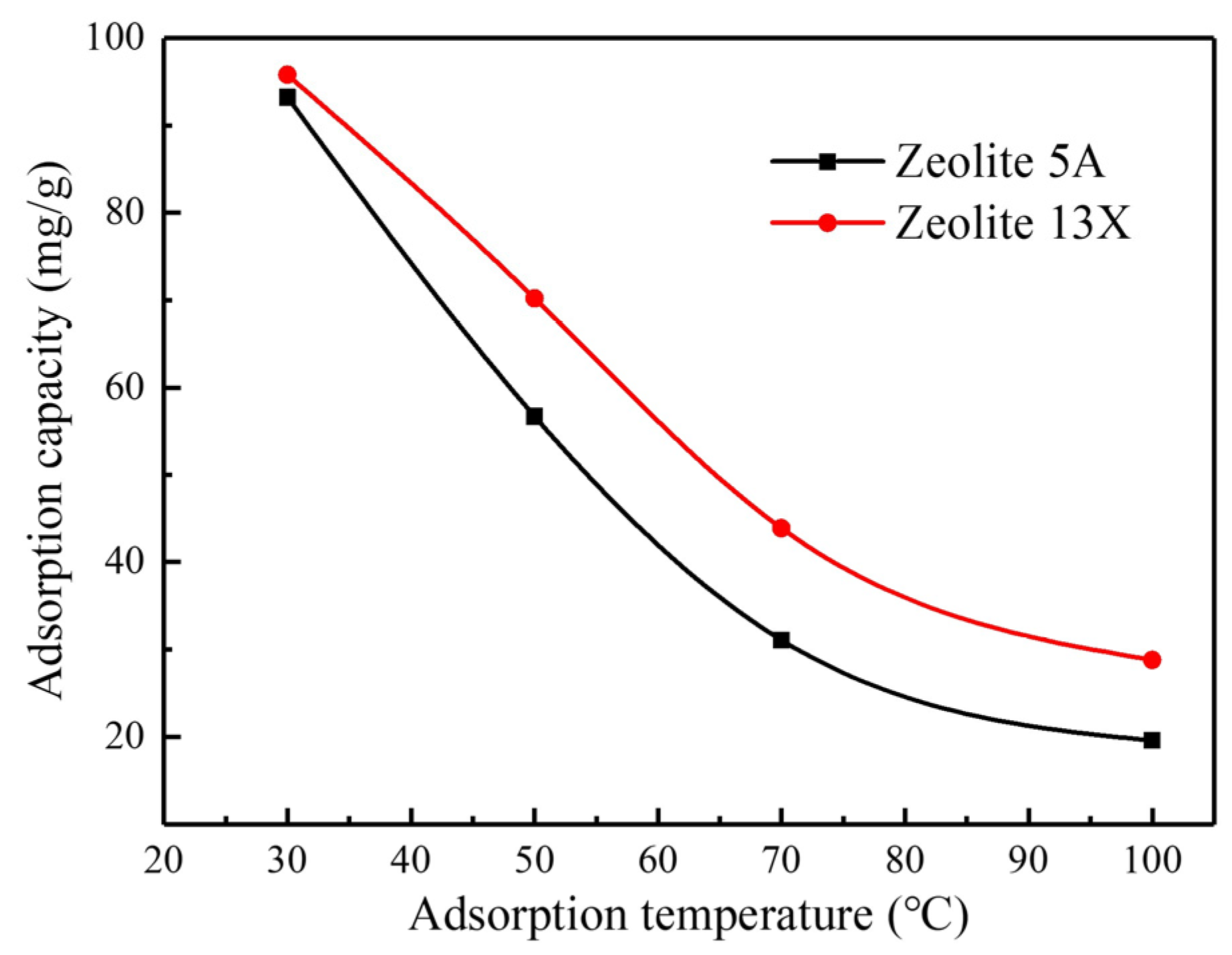
3.1.3. Effect of Adsorption Temperature
3.1.4. Effect of CO2 Concentration
3.1.5. Effect of Acid Cations and SiO2/Al2O3
3.1.6. Regeneration Potential of the Zeolites
3.2. Competitive Adsorption between H2O(g), SO2, and CO2
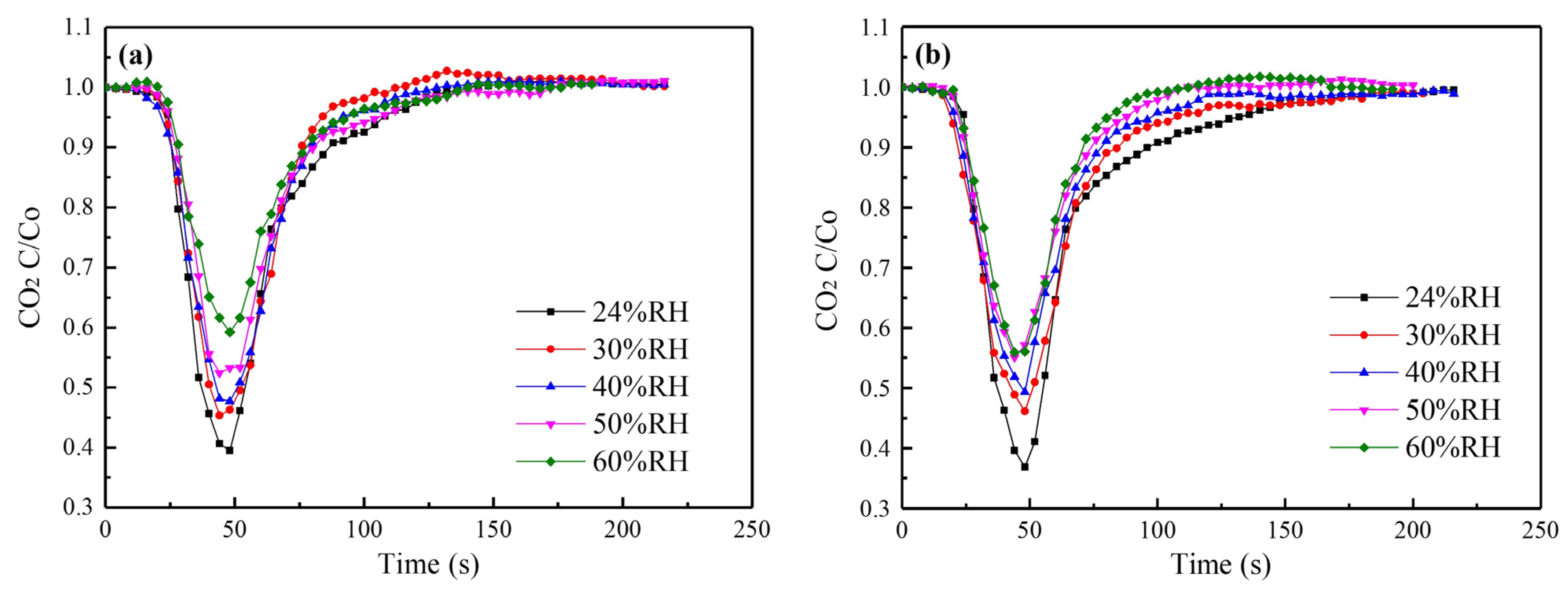
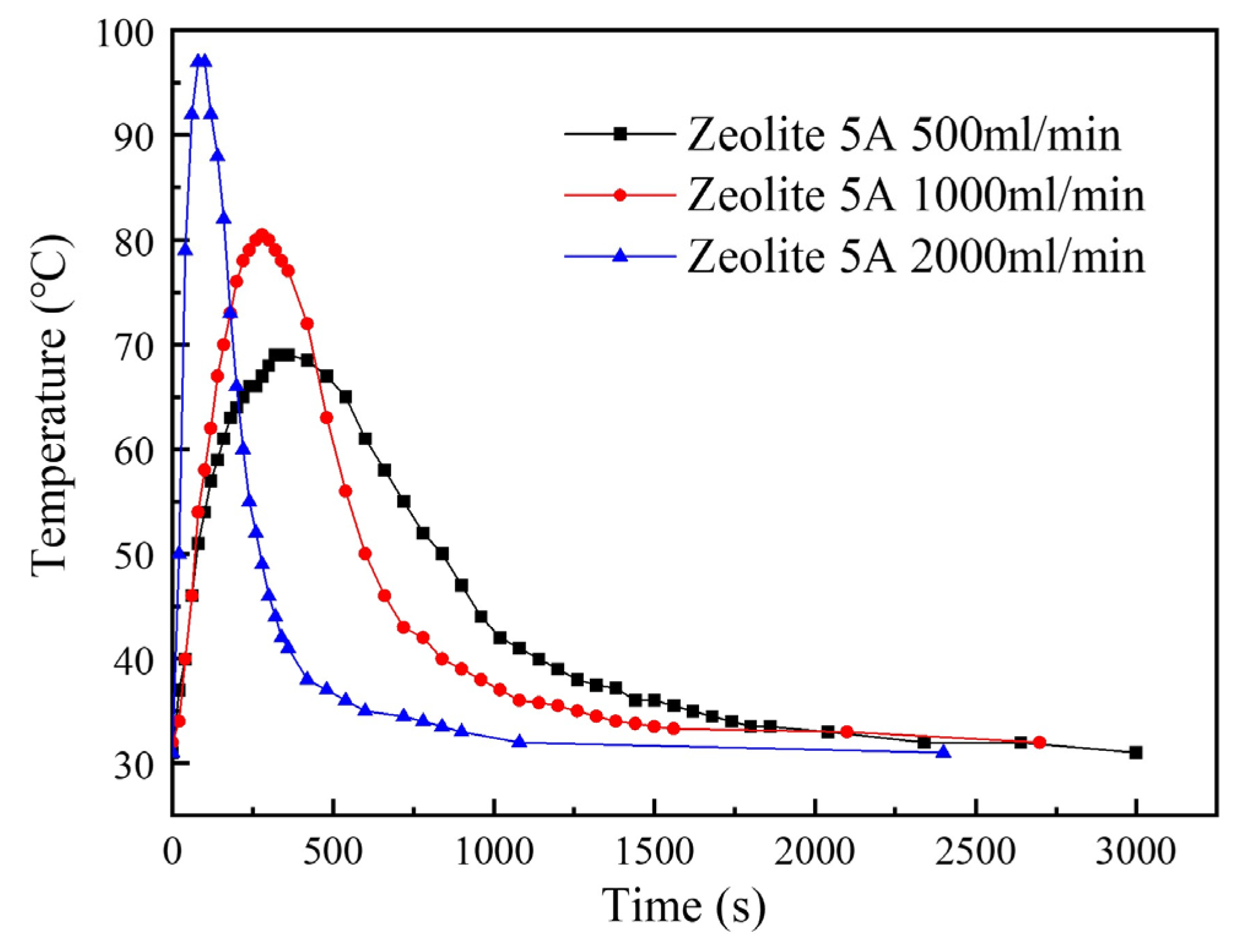
3.2.1. Effects of Moisture Content
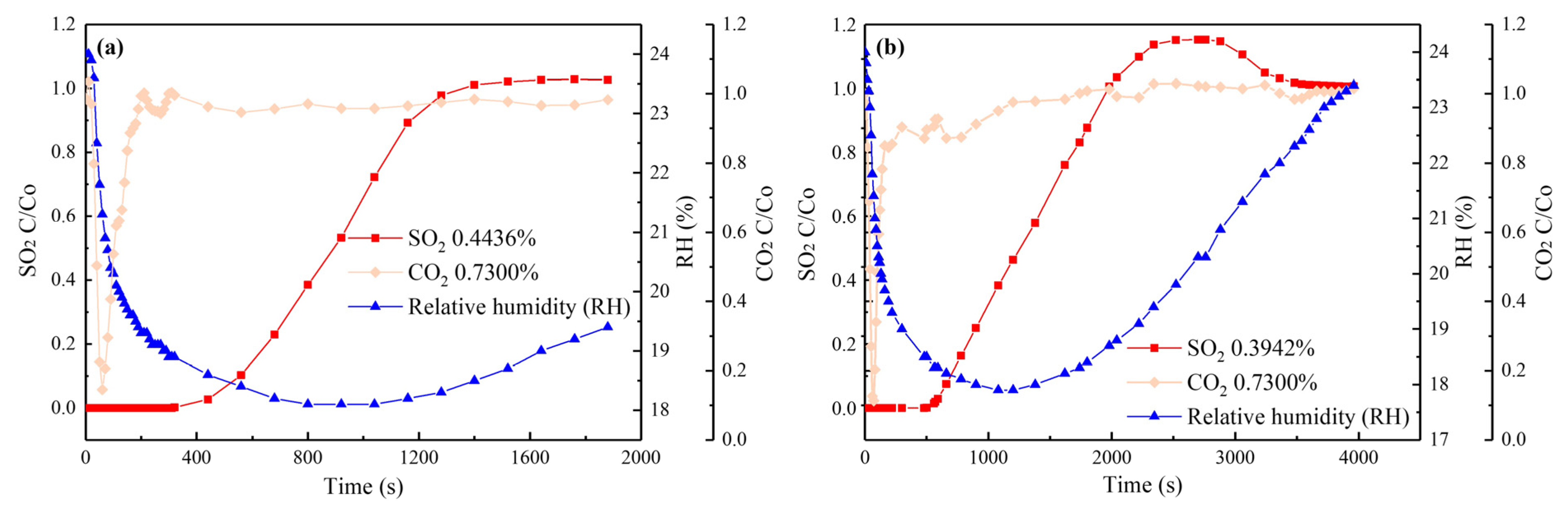
3.2.2. Synergistic Effects of SO2 and H2O(g)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Houghton, J.T.; Ding, Y.; Griggs, D.J.; Noguer, M.; Linden, P.; Dai, X.G.; Maskell, K.; Johnson, C. Climate Change 2001: The Scientific Basis; Cambridge University Press: Cambridge, UK, 2001; Volume 81, p. 208. [Google Scholar]
- Kim, E.J.; Siegelman, R.L.; Jiang, H.Z.H.; Forse, A.C.; Lee, J.H.; Martell, J.D.; Milner, P.J.; Falkowski, J.M.; Neaton, J.B.; Reimer, J.A.; et al. Cooperative carbon capture and steam regeneration with tetraamine-appended metal-organic frameworks. Science 2020, 369, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; AlBahily, K.; Vinu, A. Emerging trends in porous materials for CO2 capture and conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef]
- IEA. Legal and Regulatory Frameworks for CCUS, IEA, Paris. 2022. Available online: https://www.iea.org/reports/legal-and-regulatory-frameworks-for-ccus (accessed on 19 January 2023).
- Mazari, S.A.; Si Ali, B.; Jan, B.M.; Saeed, I.M.; Nizamuddin, S. An overview of solvent management and emissions of amine-based CO2 capture technology. Int. J. Greenh. Gas Control 2015, 34, 129–140. [Google Scholar] [CrossRef]
- Pałka, K.; Pokrowiecki, R. Porous titanium implants: A review. Adv. Eng. Mater. 2018, 20, 15–25. [Google Scholar] [CrossRef]
- Drage, T.C.; Snape, C.E.; Stevens, L.A.; Wood, J.; Wang, J.; Cooper, A.I.; Dawson, R.; Guo, X.; Satterley, C.; Irons, R. Materials challenges for the development of solid sorbents for post-combustion carbon capture. J. Mater. Chem. 2012, 22, 2815–2823. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Giordano, L.; Roizard, D.; Favre, E. Life cycle assessment of post-combustion CO2 capture: A comparison between membrane separation and chemical absorption processes. Int. J. Greenh. Gas Control 2018, 68, 146–163. [Google Scholar] [CrossRef]
- Ho, M.T.; Allinson, G.W.; Wiley, D.E. Reducing the Cost of CO2 Capture from Flue Gases Using Pressure Swing Adsorption. Ind. Eng. Chem. Res. 2008, 47, 4883–4890. [Google Scholar] [CrossRef]
- Harrison, D.P. The role of solids in CO2 capture: A mini review. In Greenhouse Gas Control Technologies 7; Rubin, E.S., Keith, D.W., Gilboy, C.F., Wilson, M., Morris, T., Gale, J., Thambimuthu, K., Eds.; Elsevier Science Ltd.: Oxford, UK, 2005; pp. 1101–1106. [Google Scholar]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. New approach for biogas purification using cryogenic separation and distillation process for CO2 capture. Energy 2018, 156, 328–351. [Google Scholar] [CrossRef]
- Ho, T.M.; Howes, T.; Bhandari, B.R. Encapsulation of gases in powder solid matrices and their applications: A review. Powder Technol. 2014, 259, 87–108. [Google Scholar] [CrossRef]
- Hefti, M.; Marx, D.; Joss, L.; Mazzotti, M. Adsorption equilibrium of binary mixtures of carbon dioxide and nitrogen on zeolites ZSM-5 and 13X. Microporous Mesoporous Mater. 2015, 215, 215–228. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, H.; Geng, X.; Wang, J.; Xiao, J.; Zhu, P. Effect of spray distance on microstructure and tribological performance of suspension plasma-sprayed hydroxyapatite–titania composite coatings. J. Therm. Spray Tech. 2016, 25, 1255–1263. [Google Scholar] [CrossRef]
- Siriwardane, M.S.; Shen, E.P. Fisher Adsorption of CO2 on Zeolites at Moderate Temperatures. Energy Fuels 2005, 19, 1153–1159. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Adsorption Equilibrium of Methane, Carbon Dioxide, and Nitrogen on Zeolite 13X at High Pressures. J. Chem. Eng. Data 2004, 49, 1095–1101. [Google Scholar] [CrossRef]
- Shao, W.; Zhang, L.; Li, L.; Lee, R.L. Adsorption of CO2 and N2 on synthesized NaY zeolite at high temperatures. Adsorption 2009, 15, 497. [Google Scholar] [CrossRef]
- Marcu, I.C.; Sandulescu, I. Study of sulfur dioxide adsorption on Y zeolite. J. Serbian Chem. Soc. 2004, 69, 563–569. [Google Scholar] [CrossRef]
- Boer, D.G.; Langerak, J.; Pescarmona, P.P. Zeolites as Selective Adsorbents for CO2 Separation. ACS Appl. Energy Mater. 2023, 6, 2634–2656. [Google Scholar] [CrossRef]
- Li, G.; Xiao, P.; Webley, P.; Zhang, J.; Singh, R.; Marshall, M. Capture of CO2 from high humidity flue gas by vacuum swing adsorption with zeolite 13X. Adsorption 2008, 14, 415–422. [Google Scholar] [CrossRef]
- Li, G.; Xiao, P.; Webley, P.A.; Zhang, J.; Singh, R. Competition of CO2/H2O in adsorption based CO2 capture. Energy Procedia 2009, 1, 1123–1130. [Google Scholar] [CrossRef]
- Stern, S.A.; DiPaolo, F.S. The Adsorption of Atmospheric Gases on Molecular Sieves at Low Pressures and Temperatures. The Effect of Preadsorbed Water. J. Vac. Sci. Technol. 1967, 4, 347–355. [Google Scholar] [CrossRef]
- Shen, M.; Kong, F.; Guo, W.; Zuo, Z.; Gao, T.; Chen, S.; Tong, L.; Zhang, P.; Wang, L.; Chu, P.K.; et al. Impact of H2O on CO2 adsorption and co-adsorption: Mechanism and high-performance adsorbents for efficient H2O-CO2 capture. Chem. Eng. J. 2024, 479, 147923. [Google Scholar] [CrossRef]
- Walton, K.S.; Abney, M.B.; Douglas LeVan, M. CO2 adsorption in Y and X zeolites modified by alkali metal cation exchange. Microporous Mesoporous Mater. 2006, 91, 78–84. [Google Scholar] [CrossRef]
- Li, G.H.; Wang, Q.S.; Jiang, T.; Luo, J.; Rao, M.J.; Peng, Z.W. Roll-up effect of sulfur dioxide adsorption on zeolites FAU 13X and LTA 5A. Adsorption 2017, 23, 699–710. [Google Scholar] [CrossRef]
- Shah, H.R.; Ahmad, K.; Bashir, M.S.; Shah, S.S.A.; Najam, T.; Ashfaq, M. Metal organic frameworks for efficient catalytic conversion of CO2 and CO into applied products. Mol. Catal. 2022, 517, 112055. [Google Scholar] [CrossRef]
- Ahmad, K.; Nazir, M.A.; Qureshi, A.K.; Hussain, E.; Najam, T.; Javed, M.S.; Shah, S.S.A.; Tufail, M.K.; Hussain, S.; Khan, N.A.; et al. Engineering of Zirconium based metal-organic frameworks (Zr-MOFs) as efficient adsorbents. Mater. Sci. Eng. B 2020, 262, 114766. [Google Scholar] [CrossRef]
- Liu, B.; Lian, Y.; Li, S.; Deng, S.; Zhao, L.; Chen, B.; Wang, D. Experimental investigation on separation and energy-efficiency performance of temperature swing adsorption system for CO2 capture. Sep. Purif. Technol. 2019, 227, 115670. [Google Scholar] [CrossRef]
- Liu, H.; Meng, X.; Dao, T.D.; Zhang, H.; Li, P.; Chang, K.; Wang, T.; Li, M.; Nagao, T.; Ye, J. Conversion of Carbon Dioxide by Methane Reforming under Visible-Light Irradiation: Surface-Plasmon-Mediated Nonpolar Molecule Activation. Angew. Chem. 2015, 54, 11545–11549. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wong, G.; Gan, M.; Chen, X.; Yu, Z.; Ji, Z. Establishment of refined sintering flue gas recirculation patterns for gas pollutant reduction and waste heat recycling. J. Clean. Prod. 2019, 235, 1549–1558. [Google Scholar] [CrossRef]
- Gao, C.; Ge, H.; Lu, Y.; Wang, W.; Zhang, Y. Decoupling of provincial energy-related CO2 emissions from economic growth in China and its convergence from 1995 to 2017. J. Clean. Prod. 2021, 297, 126627. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Q.; Zhang, G.; Wang, Z.; Zhu, P. Effects of sintering flue gas properties on simultaneous removal of SO2 and NO by ammonia-Fe(II)EDTA absorption. J. Energy Inst. 2017, 90, 522–527. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Yi, H.; Tang, X.; Li, Z.; Yu, Q.; Zhao, S.; Gao, F.; Zhou, Y.; Wang, Y. Air pollutant emission and reduction potentials from the sintering process of the iron and steel industry in China in 2017. Environ. Pollut. 2022, 307, 119512. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Q.; Liu, G.R.; Qin, S.; Zhu, T.Y.; Song, J.F.; Xu, W.Q. Emission reduction of PCDD/Fs by flue gas recirculation and activated carbon in the iron ore sintering. Environ. Pollut. 2023, 327, 121520. [Google Scholar] [CrossRef]
- Shen, C.M.; Worek, W.M. Cosorption characteristics of solid adsorbents. Int. J. Heat Mass Transf. 1994, 37, 2123–2129. [Google Scholar] [CrossRef]







| Zeolite (wt. %) | Fe2O3 | Al2O3 | SiO2 | CaO | MgO | Na2O | K2O |
|---|---|---|---|---|---|---|---|
| 5A | 0.97 | 23.75 | 35.75 | 11.84 | 2.08 | 2.95 | 0.30 |
| 13X | 1.22 | 21.33 | 37.79 | 0.94 | 2.05 | 13.39 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wang, Q.; Chen, J.; Liu, H.; Xu, L.; Rao, M. Competitive Adsorption of Moisture and SO2 for Carbon Dioxide Capture by Zeolites FAU 13X and LTA 5A. Processes 2024, 12, 1547. https://doi.org/10.3390/pr12081547
Yang X, Wang Q, Chen J, Liu H, Xu L, Rao M. Competitive Adsorption of Moisture and SO2 for Carbon Dioxide Capture by Zeolites FAU 13X and LTA 5A. Processes. 2024; 12(8):1547. https://doi.org/10.3390/pr12081547
Chicago/Turabian StyleYang, Xiduan, Qishuai Wang, Jing Chen, Huibo Liu, Liangping Xu, and Mingjun Rao. 2024. "Competitive Adsorption of Moisture and SO2 for Carbon Dioxide Capture by Zeolites FAU 13X and LTA 5A" Processes 12, no. 8: 1547. https://doi.org/10.3390/pr12081547
APA StyleYang, X., Wang, Q., Chen, J., Liu, H., Xu, L., & Rao, M. (2024). Competitive Adsorption of Moisture and SO2 for Carbon Dioxide Capture by Zeolites FAU 13X and LTA 5A. Processes, 12(8), 1547. https://doi.org/10.3390/pr12081547






